Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
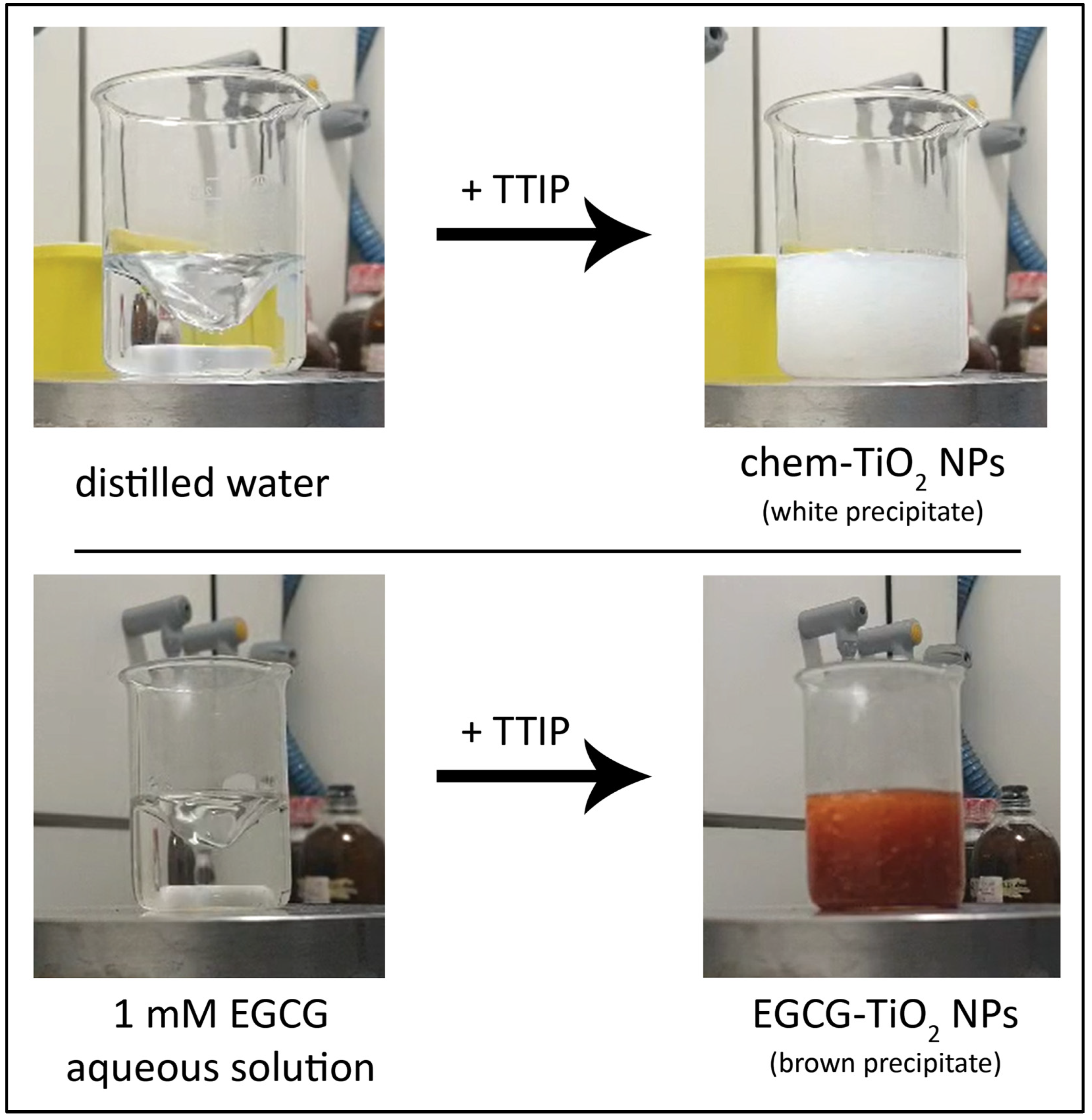
2.1. Chemical Synthesis of TiO2 NPs
2.2. Green Synthesis of TiO2 NPs
2.3. Physicochemical Characterization of TiO2 NPs
3. Results
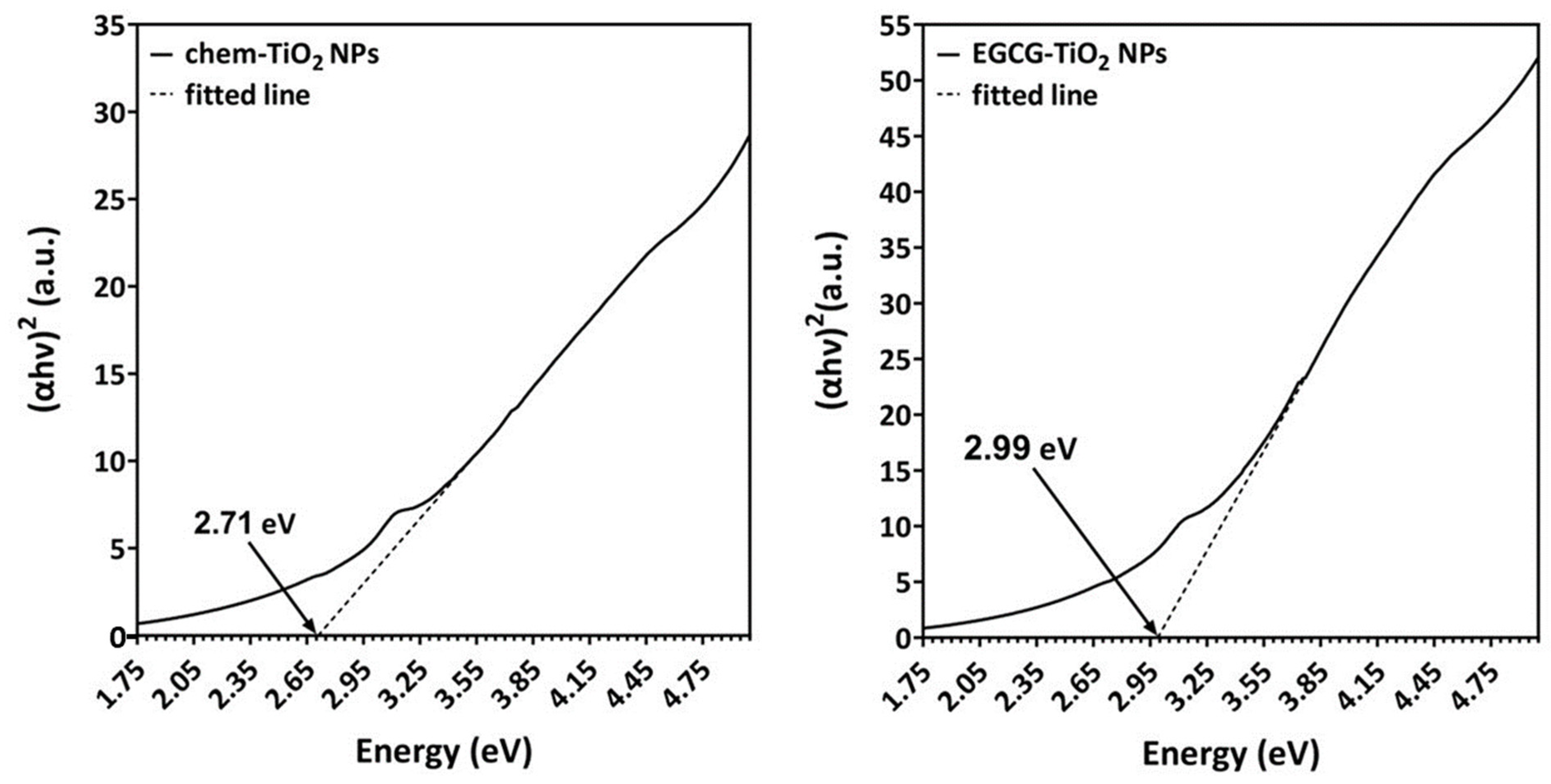
3.1. Optical Properties of Green Synthesised TiO2 NPs
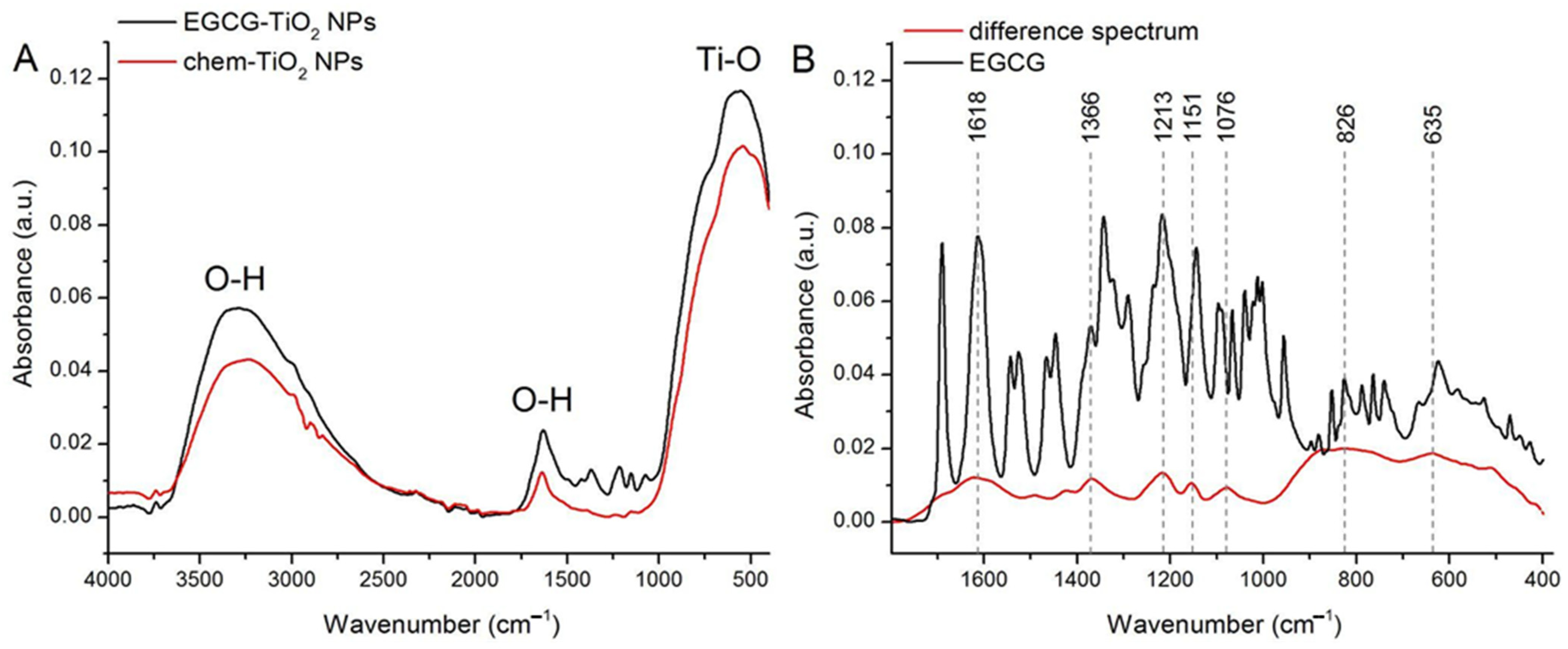
3.2. Determination of the Functional Groups on the Surface of Green Synthesised TiO2 NPs
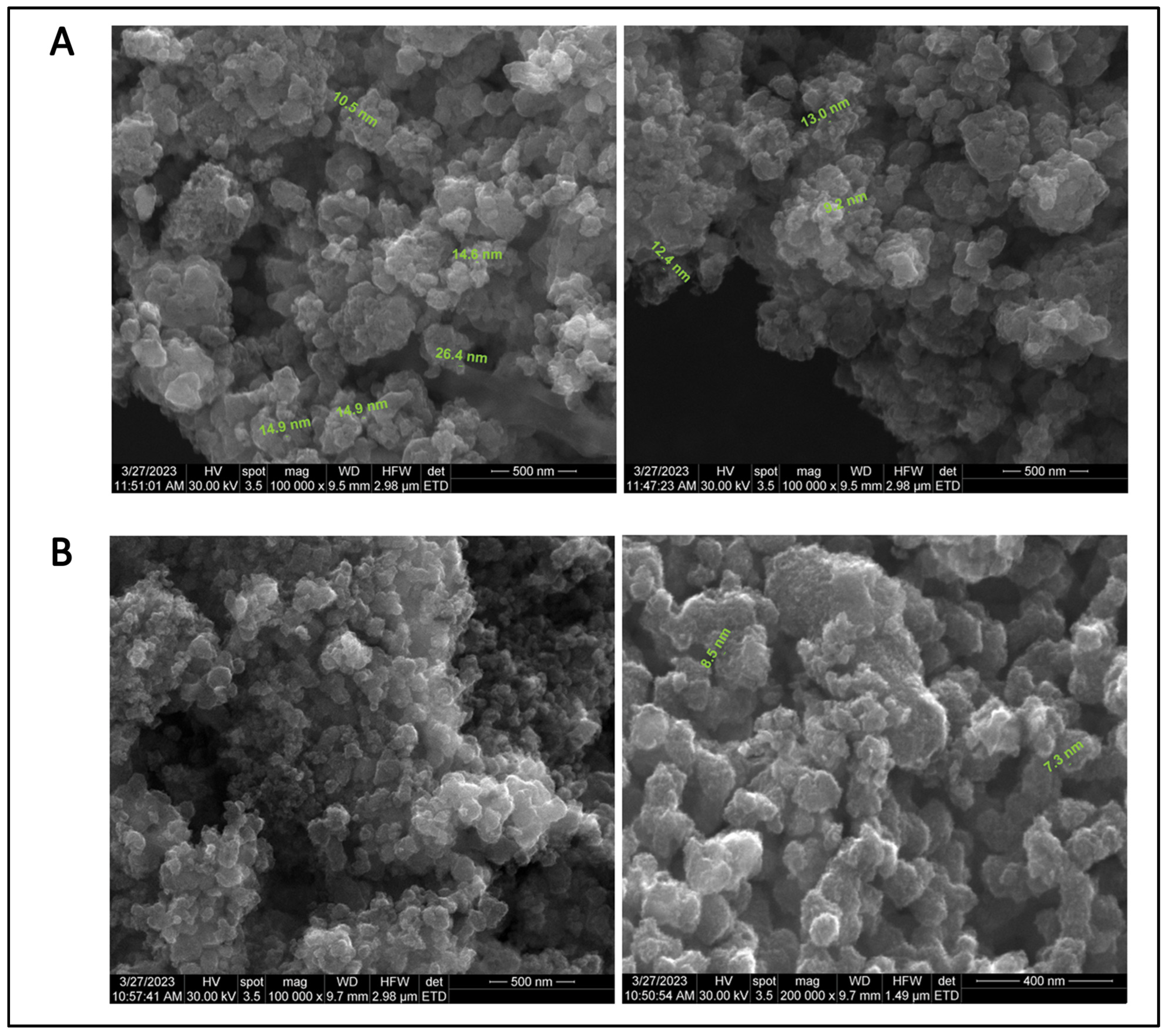
3.3. SEM Analysis of Green Synthesised TiO2 NPs
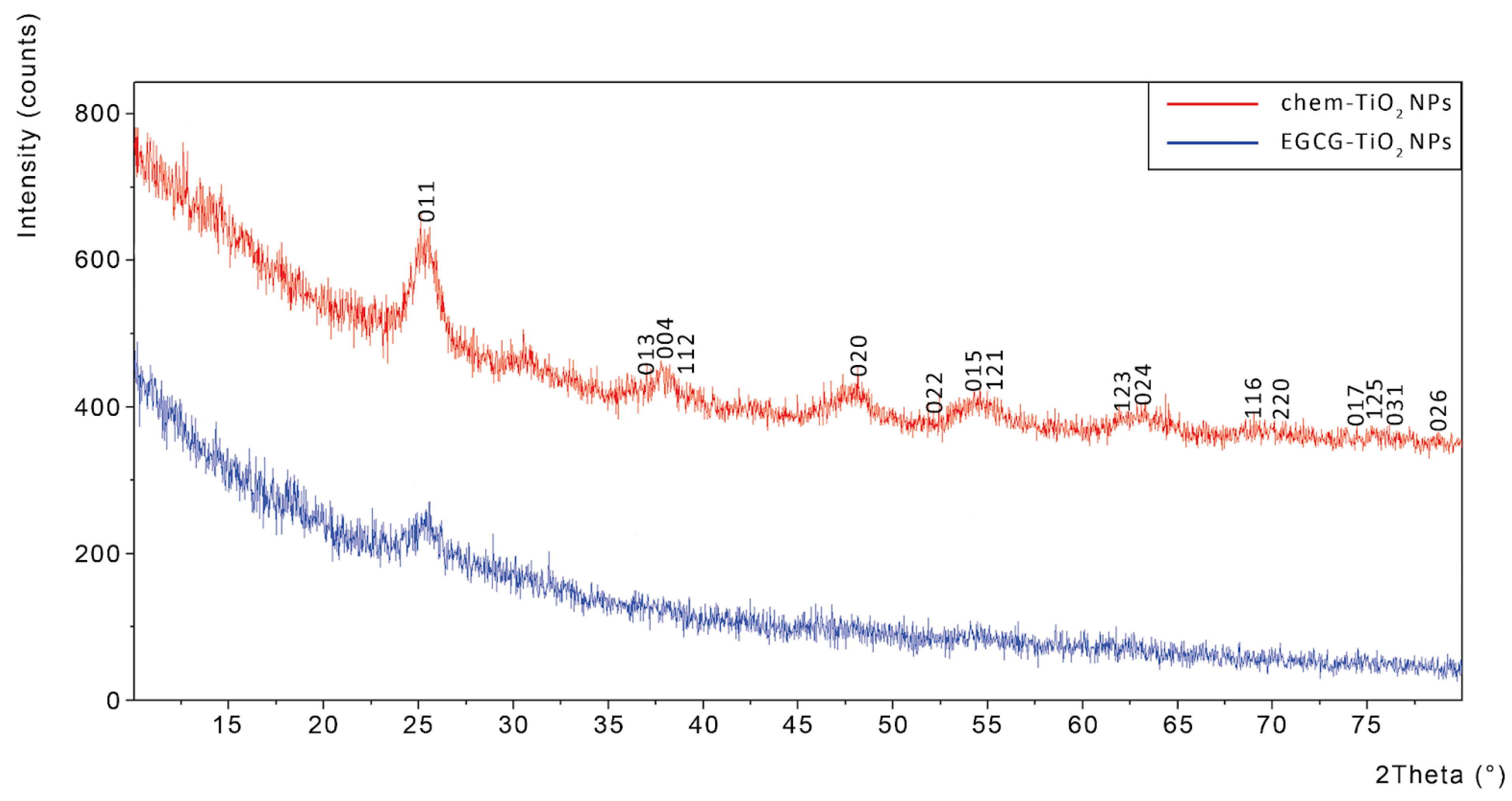
3.4. Crystallographic Structure of Green Synthesised TiO2 NPs
3.5. Hydrodynamic Diameter and Zeta-Potential of Green Synthesised TiO2 NPs
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.T.; John, H. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Ganguli, P.; Chaudhuri, S. Nanomaterials in antimicrobial paints and coatings to prevent biodegradation of man-made surfaces: A review. Mater. Today Proc. 2021, 45, 3769–3777. [Google Scholar] [CrossRef]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of inorganic nanoparticles in food packaging: A comprehensive review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr. Environ. Assess. Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef]
- Abdallah, B.B.; Zhang, X.; Andreu, I.; Gates, B.D.; El Mokni, R.; Rubino, S.; Landoulsi, A.; Chatti, A. Differentiation of nanoparticles isolated from distinct plant species naturally growing in a heavy metal polluted site. J. Hazard. Mater. 2020, 386, 121644. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miu, B.A.; Dinischiotu, A. New green approaches in nanoparticles synthesis: An overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green tea catechins: Their use in treating and preventing infectious diseases. Biomed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Gavlighi, H.A. Antioxidant and antimicrobial activities of (-)-epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Zhang, H.; Li, L.; Liu, Z. Effects of green tea extract epigallocatechin-3-gallate (EGCG) on oral disease-associated microbes: A review. J. Oral Microbiol. 2022, 14, 2131117. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int. J. Nanomed. 2012, 7, 4263–4267. [Google Scholar] [CrossRef]
- Widatalla, H.A.; Yassin, L.F.; Alrasheid, A.A.; Ahmed, S.A.R.; Widdatallah, M.O.; Eltilib, S.H.; Mohamed, A.A. Green synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity. Nanoscale Adv. 2022, 4, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: Catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Tharani, M.; Sivaperumal, P.; Lakshmi, T. Green synthesis of selenium nanoparticles using black tea (Camellia sinensis) and its antioxidant and antimicrobial activity. J. Complement. Med. Res. 2020, 11, 75–82. [Google Scholar]
- Khan, A.; Shabir, D.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia sinensis leaves extract. Mater. Res. Express 2021, 8, 015402. [Google Scholar] [CrossRef]
- Jeronsia, J.E.; Joseph, L.A.; Vinosha, P.A.; Mary, A.J.; Das, S.J. Camellia sinensis leaf extract mediated synthesis of copper oxide nanostructures for potential biomedical applications. Mater. Today Proc. 2019, 8, 214–222. [Google Scholar] [CrossRef]
- Franco, R.T.; Silva, A.L.; Licea, Y.E.; Serna, J.D.P.; Alzamora, M.; Sánchez, D.R.; Carvalho, N.M.F. Green synthesis of iron oxides and phosphates via thermal treatment of iron polyphenols synthesized by a Camellia sinensis extract. Inorg. Chem. 2021, 60, 5734–5746. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; Alkathiri, T.A.; Alfarraj, A.E.; Alatawi, O.M.; Alkathiri, A.S.; Panneerselvam, C.; Vanaraj, S.; Darwish, A.A.A.; Hamdalla, T.A.; Pasha, A.; et al. Green synthesis and characterization of zinc oxide nanoparticles using Camellia sinensis tea leaf extract and their antioxidant, antibacterial and anticancer efficacy. Res. Chem. Intermed. 2022, 48, 4769–4783. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Dessi, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.-L.; Tang, J.; Chu, C.-H. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Suresh, D.; Udayabhanu; Nethravathi, P.C.; Lingaraju, K.; Rajanaika, H.; Sharma, S.C.; Nagabhushana, H. EGCG assisted green synthesis of ZnO nanopowders: Photodegradative, antimicrobial and antioxidant activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A.A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of nanosuspensions in bottom-up approach: Theories and optimization. DARU J. Pharm. Sci. 2019, 27, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.E.; Søgaard, E.G. Sol–gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef]
- Mahshid, S.; Ghamsari, M.S.; Askari, M.; Afshar, N.; Lahuti, S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. Semicond. Phys. Quantum Electron. Optoelectron. 2006, 9, 65–68. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Pereira, J.C.; Matos, J.C.; Vasconcelos, H.C. Photonic band gap and bactericide performance of amorphous sol-gel titania: An alternative to crystalline TiO2. Molecules 2018, 23, 1677. [Google Scholar] [CrossRef]
- Ngo, H.M.; Pawar, A.U.; Tang, J.; Zhuo, Z.; Lee, D.K.; Ok, K.M.; Kang, Y.S. Synthesis of uniform size rutile TiO2 microrods by simple molten-salt method and its photoluminescence activity. Nanomaterials 2022, 12, 2626. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, C.C.; Lin, H.P.; Shih, W.A.; Hsieh, W.L.; Lai, C.H.; Takeuchi, Y.; Chen, Y.W. A functional chitosan membrane with grafted epigallocatechin-3-gallate and lovastatin enhances periodontal tissue regeneration in dogs. Carbohydr. Polym. 2016, 151, 790–802. [Google Scholar] [CrossRef]
- Jawed, A.; Golder, A.K.; Pandey, L.M. Synthesis of iron oxide nanoparticles mediated by Camellia sinensis var. Assamica for Cr(VI) adsorption and detoxification. Bioresour. Technol. 2023, 376, 128816. [Google Scholar] [CrossRef]
- Li, Q.; Duan, M.; Liu, L.; Chen, X.; Fu, Y.; Li, J.; Zhao, T.; McClements, D.J. Impact of polyphenol interactions with titanium dioxide nanoparticles on their bioavailability and antioxidant activity. J. Agric. Food Chem. 2021, 69, 9661–9670. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, C.; Gao, Z.; Zheng, J.; He, L.; McClements, D.J.; Xiao, H. Characterization of the interactions between titanium dioxide nanoparticles and polymethoxyflavones using surface-enhanced Raman spectroscopy. J. Agric. Food Chem. 2016, 64, 9436–9441. [Google Scholar] [CrossRef] [PubMed]
- Hudlikar, M.; Joglekar, S.; Dhaygude, M.; Kodam, K. Green synthesis of TiO2 nanoparticles by using aqueous extract of Jatropha curcas L. latex. Mater. Lett. 2012, 75, 196–199. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; El-Azim, M.H.M.A. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Saranya, K.S.; Padil, V.; Senan, C.; Pilankatta, R.; Saranya, K.; George, B.; Wacławek, S.; Černík, M. Green synthesis of high temperature stable anatase titanium dioxide nanoparticles using gum kondagogu: Characterization and solar driven photocatalytic degradation of organic dye. Nanomaterials 2018, 8, 1002. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, R.; Das, A.; Sarma, H.; et al. Green synthesis of titanium dioxide nanoparticles using Ocimum sanctum leaf extract: In vitro characterization and its healing efficacy in diabetic wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef]
- Lu, L.Y.; Ou, N.; Lu, Q.-B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Tahir, M.B. Green synthesis of TiO2 nanoparticle using cinnamon powder extract and the study of optical properties. J. Inorg. Organomet. Polym. 2020, 30, 1425–1429. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.I.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green synthesis of TiO2 nanoparticles using Acorus calamus leaf extract and evaluating its photocatalytic and in vitro antimicrobial activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.Y.; Kominami, H.; Kera, Y.; Ikeda, S.; Noguchi, H.; Uosaki, K.; Ohtani, B. Evaluation of electron–hole recombination properties of titanium(IV) oxide particles with high photocatalytic activity. Res. Chem. Intermed. 2007, 33, 285–296. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP) NTP Technical Report on the Toxicology Studies of Green Tea Extract in F344/NTac Rats and B6C3F1/n Mice and Toxicology and Carcinogenesis Studies of Green Tea Extract in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/n Mice (Gavage Studies). Available online: https://www.ncbi.nlm.nih.gov/books/NBK561012/figure/fig-2-I.1/ (accessed on 10 August 2023).
- Wang, D.; Kim, D.; Shin, C.-H.; Zhao, Y.; Park, J.-S.; Ryu, M. Evaluation of epigallocatechin gallate (EGCG) to remove Pb(II) using spectroscopic and quantum chemical calculation method. Environ. Earth Sci. 2019, 78, 138. [Google Scholar] [CrossRef]
- Göl, F.; Aygün, A.; Seyrankaya, A.; Gür, T.; Yenikaya, C.; Şen, F. Green synthesis and characterization of Camellia sinensis mediated silver nanoparticles for antibacterial ceramic applications. Mater. Chem. Phys. 2020, 250, 123037. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-containing nanoparticles: Synthesis, properties, and therapeutic delivery. Adv. Mater. 2021, 33, e2007356. [Google Scholar] [CrossRef]
- Sunny, N.E.; Mathew, S.S.; Chandel, N.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Vasseghian, Y.; Rajamohan, N.; Kumar, S.V. Green synthesis of titanium dioxide nanoparticles using plant biomass and their applications—A review. Chemosphere 2022, 300, 134612. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Chironi, I.; Karatchevtseva, I.; Triani, G.; Sorrell, C.C. Single and mixed phase TiO2 powders prepared by excess hydrolysis of titanium alkoxide. Adv. Appl. Ceram. 2012, 111, 149–158. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Shin, S.-W.; Kim, K.; Park, Y. Facile green synthesis of titanium dioxide nanoparticles by upcycling mangosteen (Garcinia mangostana) pericarp extract. Nanoscale Res. Lett. 2022, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Rizwana, K.; Shivashangari, K.S.; Ravikumar, V. Ultra-rapid photocatalytic activity of Azadirachta indica engineered colloidal titanium dioxide nanoparticles. Appl. Nanosci. 2015, 5, 731–736. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Synthesis and characterization of titanium dioxide nanoparticles using Euphorbia heteradena Jaub root extract and evaluation of their stability. Ceram. Int. 2015, 41, 14435–14439. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin gallate-gold nanoparticles exhibit superior antitumor activity compared to conventional gold nanoparticles: Potential synergistic interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]
- Prema, P.; Veeramanikandan, V.; Rameshkumar, K.; Gatasheh, M.K.; Hatamleh, A.A.; Balasubramani, R.; Balaji, P. Statistical optimization of silver nanoparticle synthesis by green tea extract and its efficacy on colorimetric detection of mercury from industrial waste water. Environ. Res. 2022, 204, 111915. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Singh, J.; Rawat, M.; Kumar, S. Expanding horizon: Green synthesis of TiO2 nanoparticles using Carica papaya leaves for photocatalysis application. Mater. Res. Express 2019, 6, 095034. [Google Scholar] [CrossRef]
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green synthesis of anatase titanium dioxide nanoparticles using Cuminum cyminum seed extract; effect on mung bean (Vigna radiata) seed germination. Inorg. Chem. Commun. 2021, 126, 108485. [Google Scholar] [CrossRef]

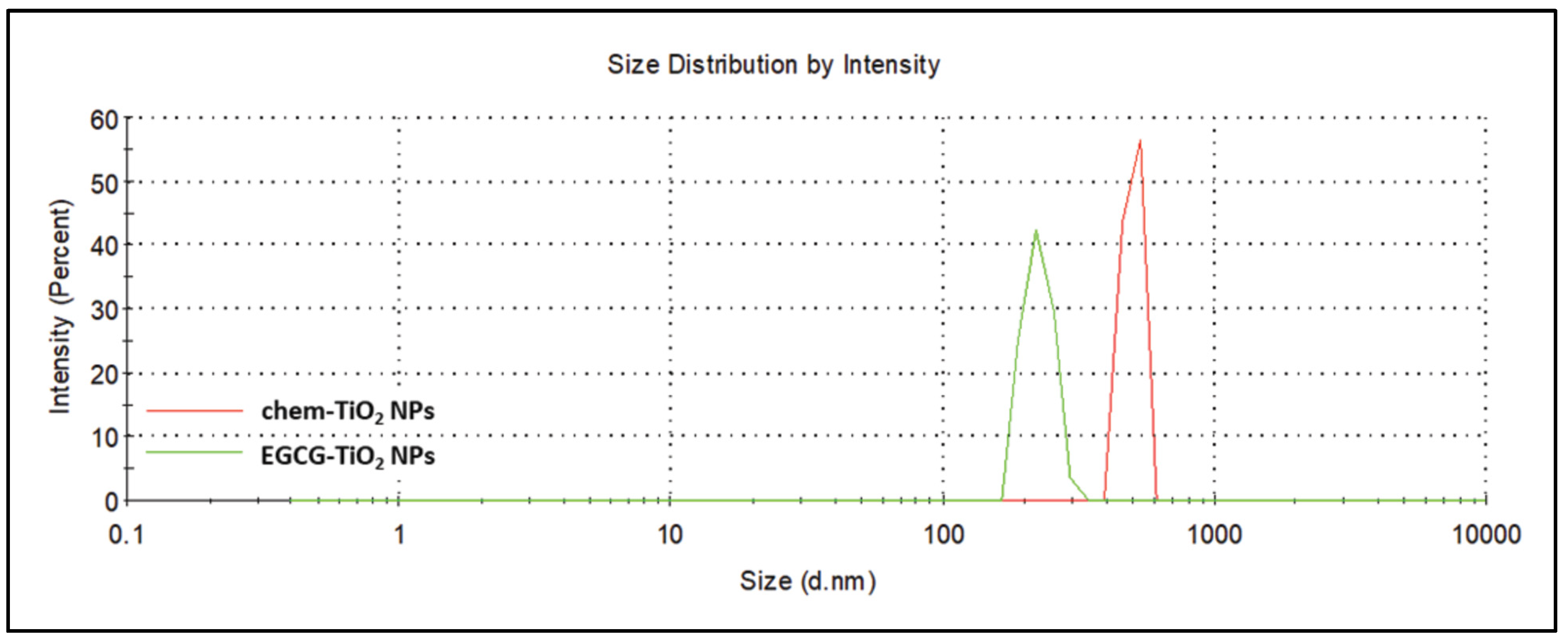
| Sample | Z-Average (d. nm) | Size Peak (d. nm) | Polydispersity Index (PdI) | Zeta Potential (mV) |
|---|---|---|---|---|
| chem-TiO2 NPs | 1469 ± 101.8 | 589.9 ± 211.6 | 0.747 ± 0.271 | −20.3 ± 1.27 |
| EGCG–TiO2 NPs | 438.5 ± 22.56 | 245.5 ± 18.28 | 0.469 ± 0.027 | −56.5 ± 6.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miu, B.A.; Stan, M.S.; Mernea, M.; Dinischiotu, A.; Voinea, I.C. Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles. Materials 2024, 17, 275. https://doi.org/10.3390/ma17020275
Miu BA, Stan MS, Mernea M, Dinischiotu A, Voinea IC. Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles. Materials. 2024; 17(2):275. https://doi.org/10.3390/ma17020275
Chicago/Turabian StyleMiu, Bogdan Andrei, Miruna Silvia Stan, Maria Mernea, Anca Dinischiotu, and Ionela Cristina Voinea. 2024. "Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles" Materials 17, no. 2: 275. https://doi.org/10.3390/ma17020275
APA StyleMiu, B. A., Stan, M. S., Mernea, M., Dinischiotu, A., & Voinea, I. C. (2024). Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles. Materials, 17(2), 275. https://doi.org/10.3390/ma17020275









