Processing and Characterization of Spent Nickel–Metal Hydride Type AA Batteries to Recover Valuable Materials (Cobalt, Nickel and Rare Earth Elements)
Abstract
1. Introduction
- Primary or disposable batteries;
- Secondary batteries that can be recharged and reused.
- Anode—nickel/lithium hydride or nickel/lanthanum.
- Cathode—nickel oxide.
2. Materials and Methods
2.1. Processing of Spent Nickel–Metal Hydride Batteries
- Non-ferrous material (metal grid anodes): 4.13 wt.%.
- Ferrous material (metallic casings): 17.95 wt.%.
- Black mass material (anode and cathode powder): 41.91 wt.%.
- Coarse material (metal grid parts with embedded powder content): 26.21 wt.%.
- Non-metallic material (plastic polymers—insulators, separators, paper): 3.66 wt.%.
2.2. Sample Characterization
3. Results and Discussion
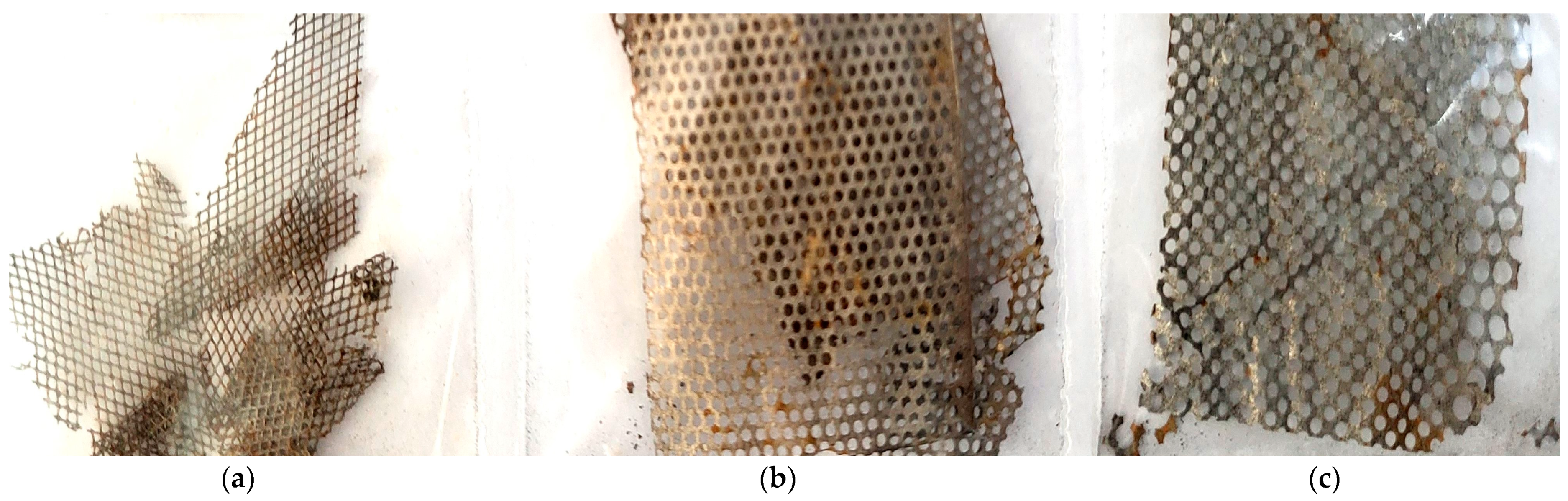
3.1. Characterization of the Metal Grid Anodes
3.1.1. Chemical Composition
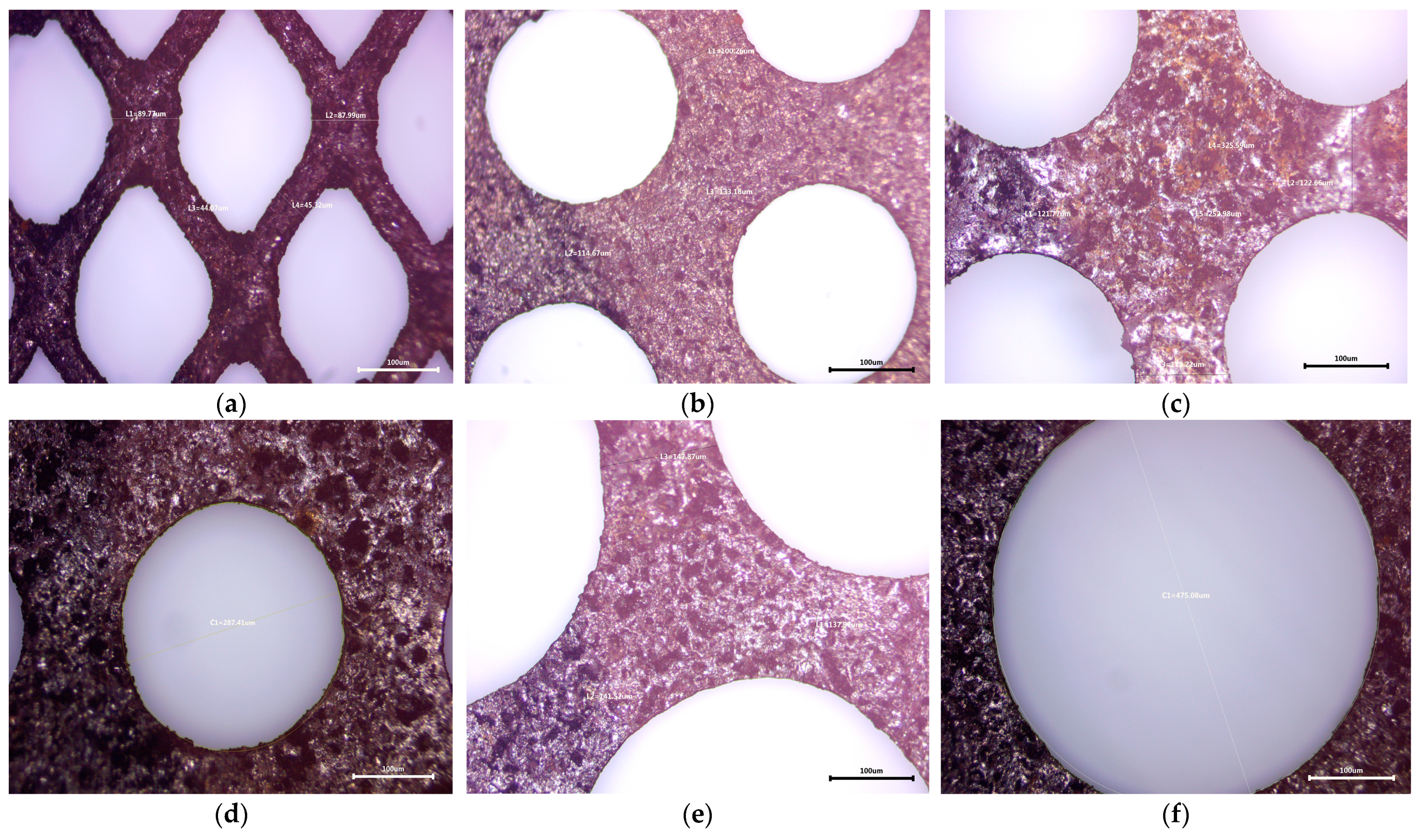
3.1.2. Optical Microscopy
3.2. Characterization of the Black Mass Material (Anode and Cathode Powder)
3.2.1. Chemical Composition
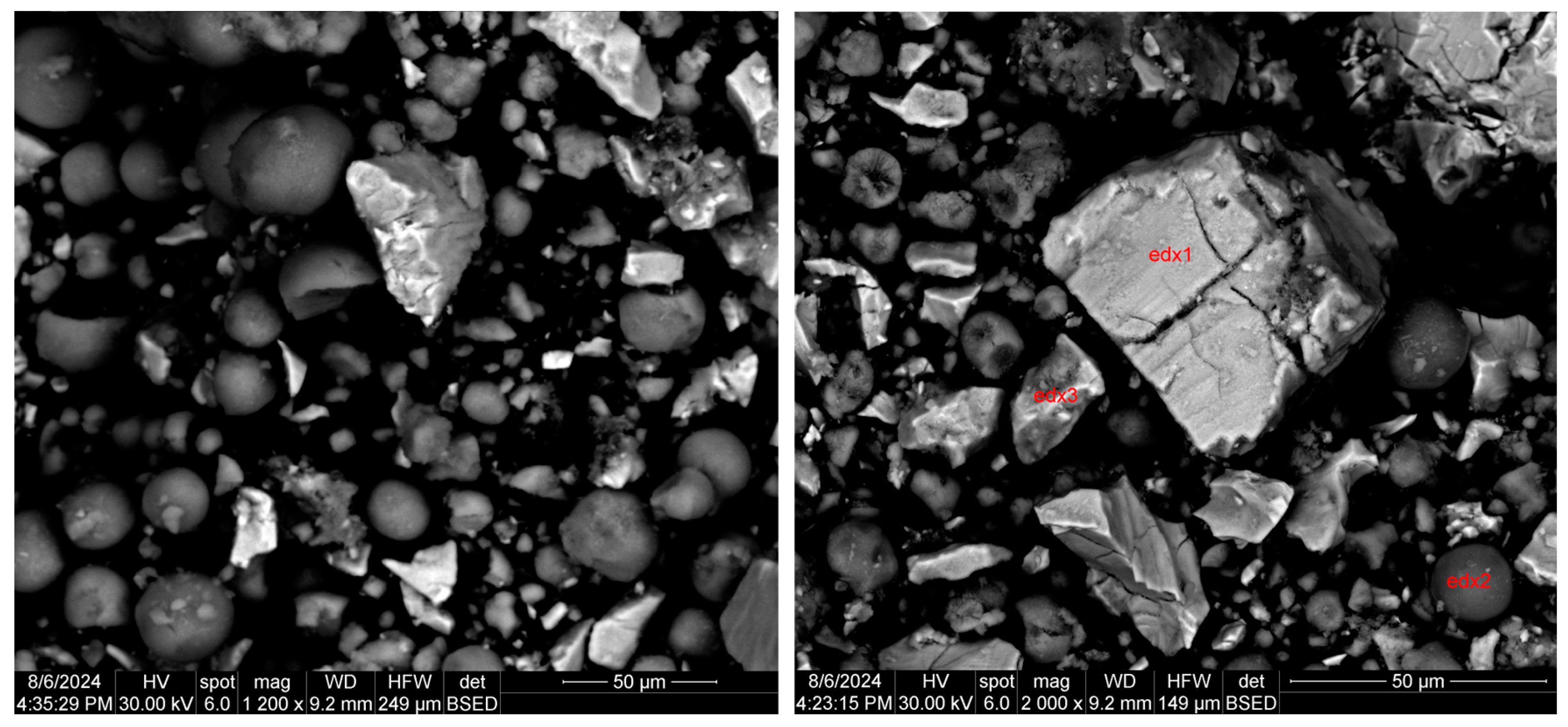
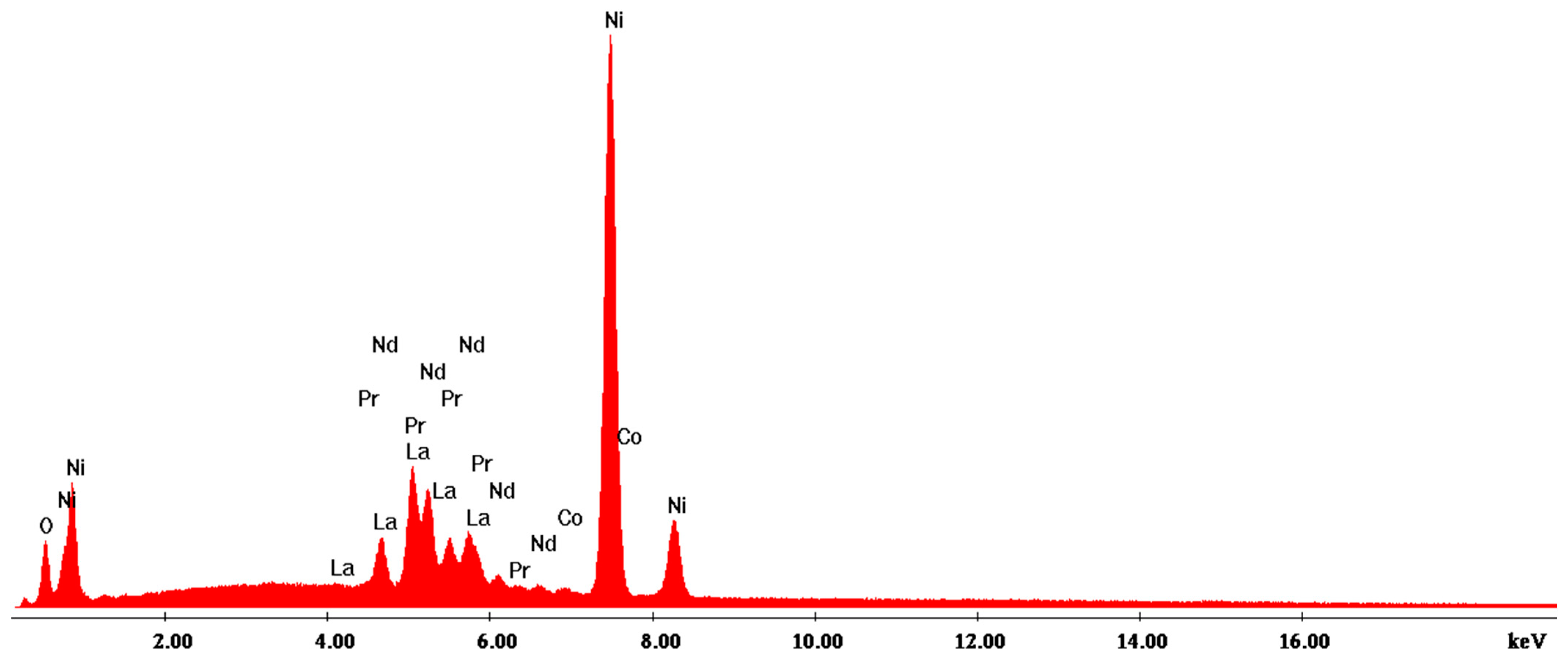
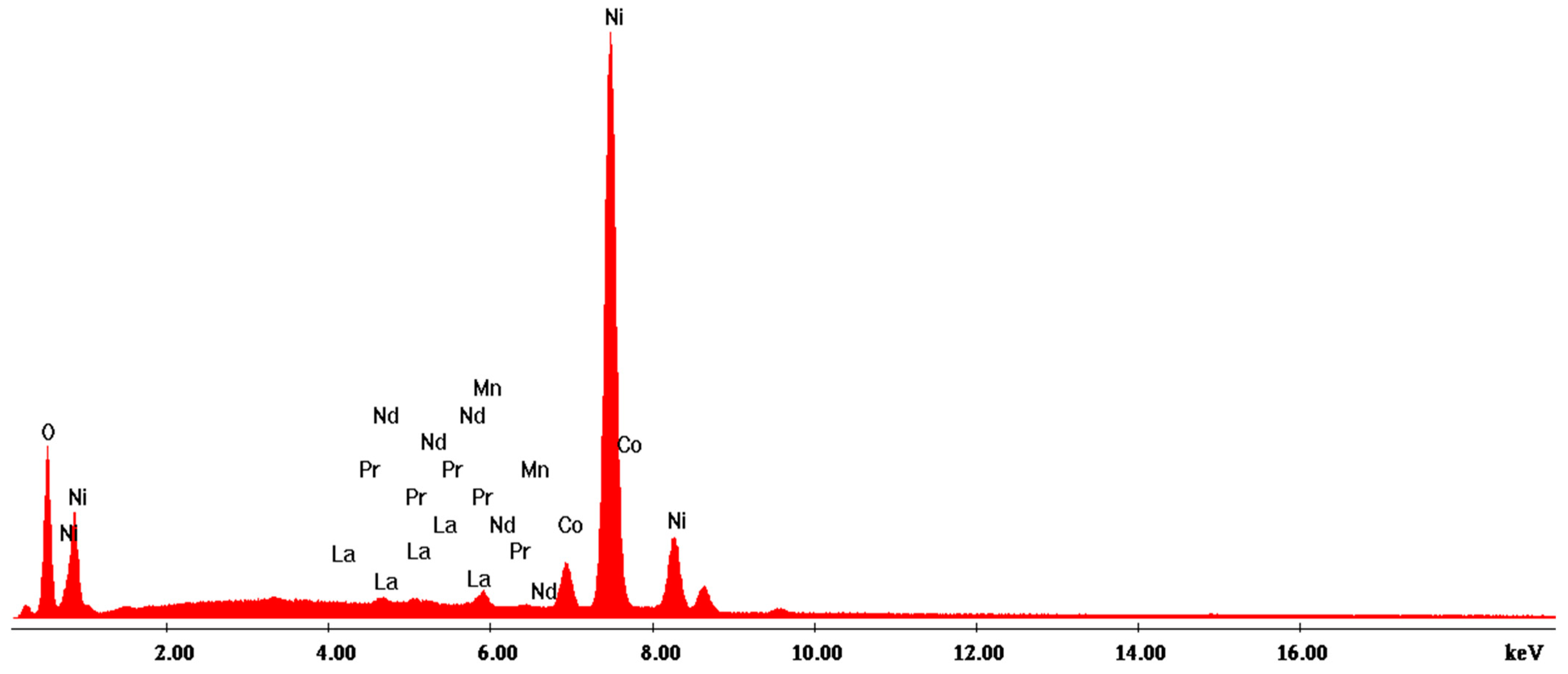
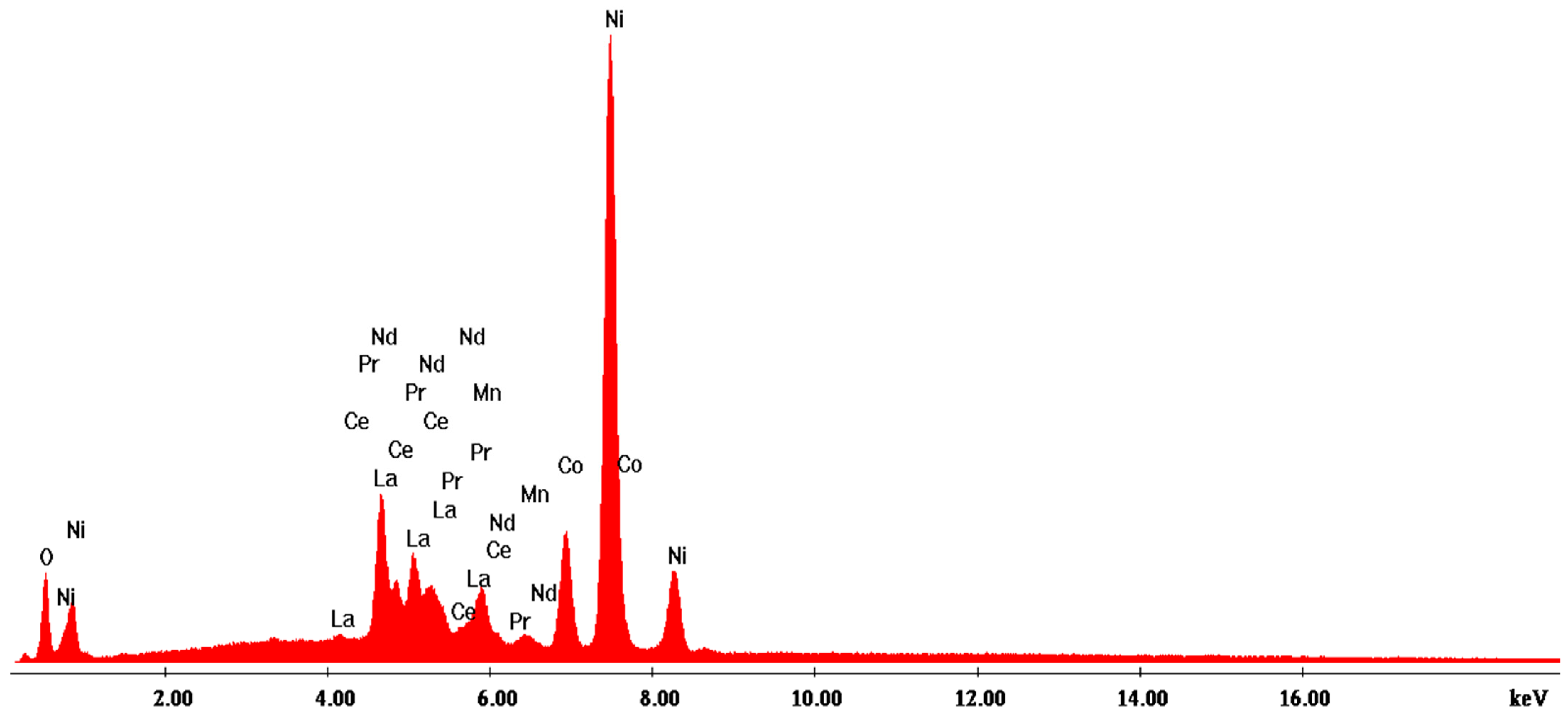
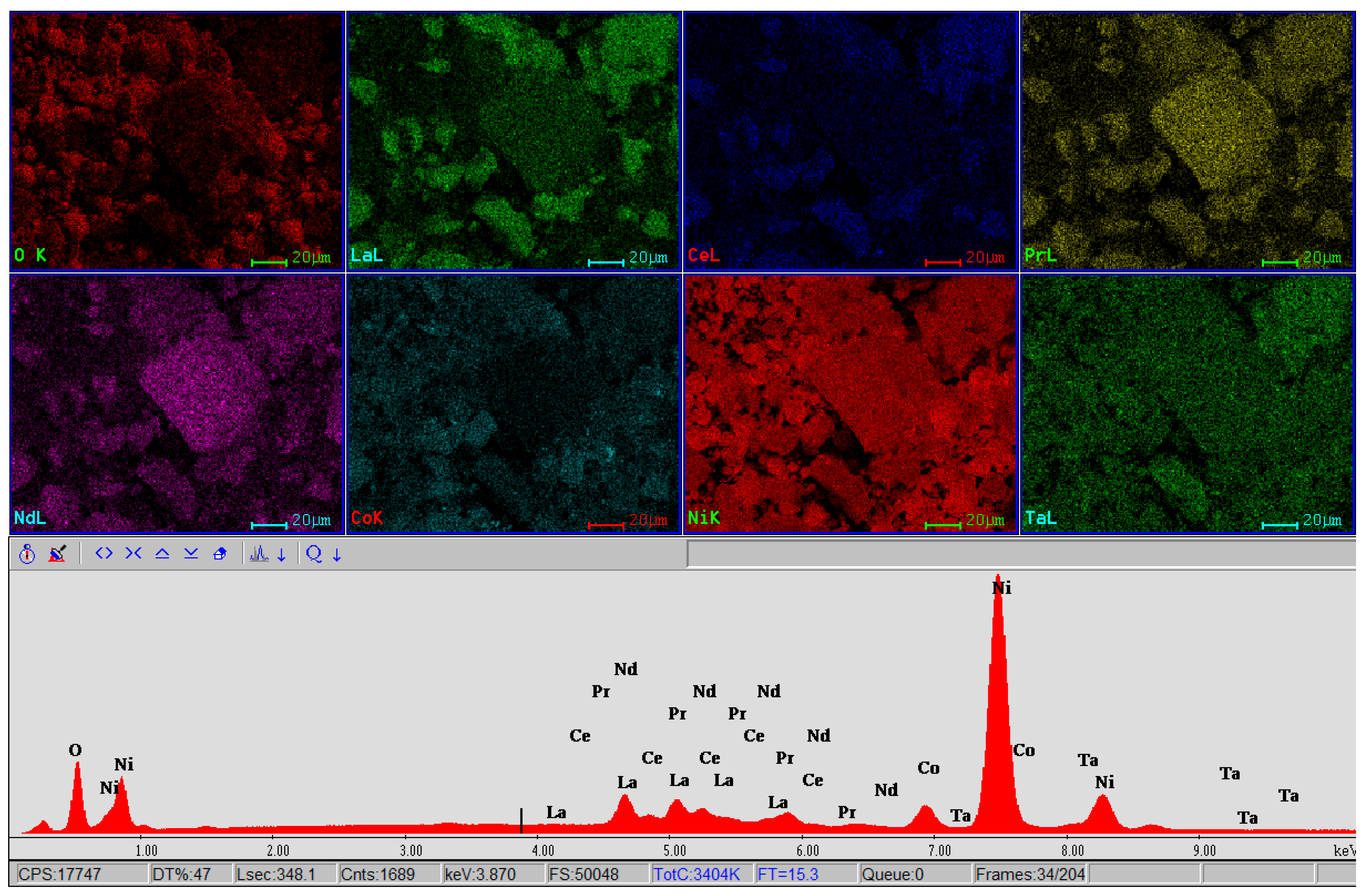
3.2.2. Scanning Electron Microscopy with Energy-Dispersive X-ray Analysis (SEM-EDX)
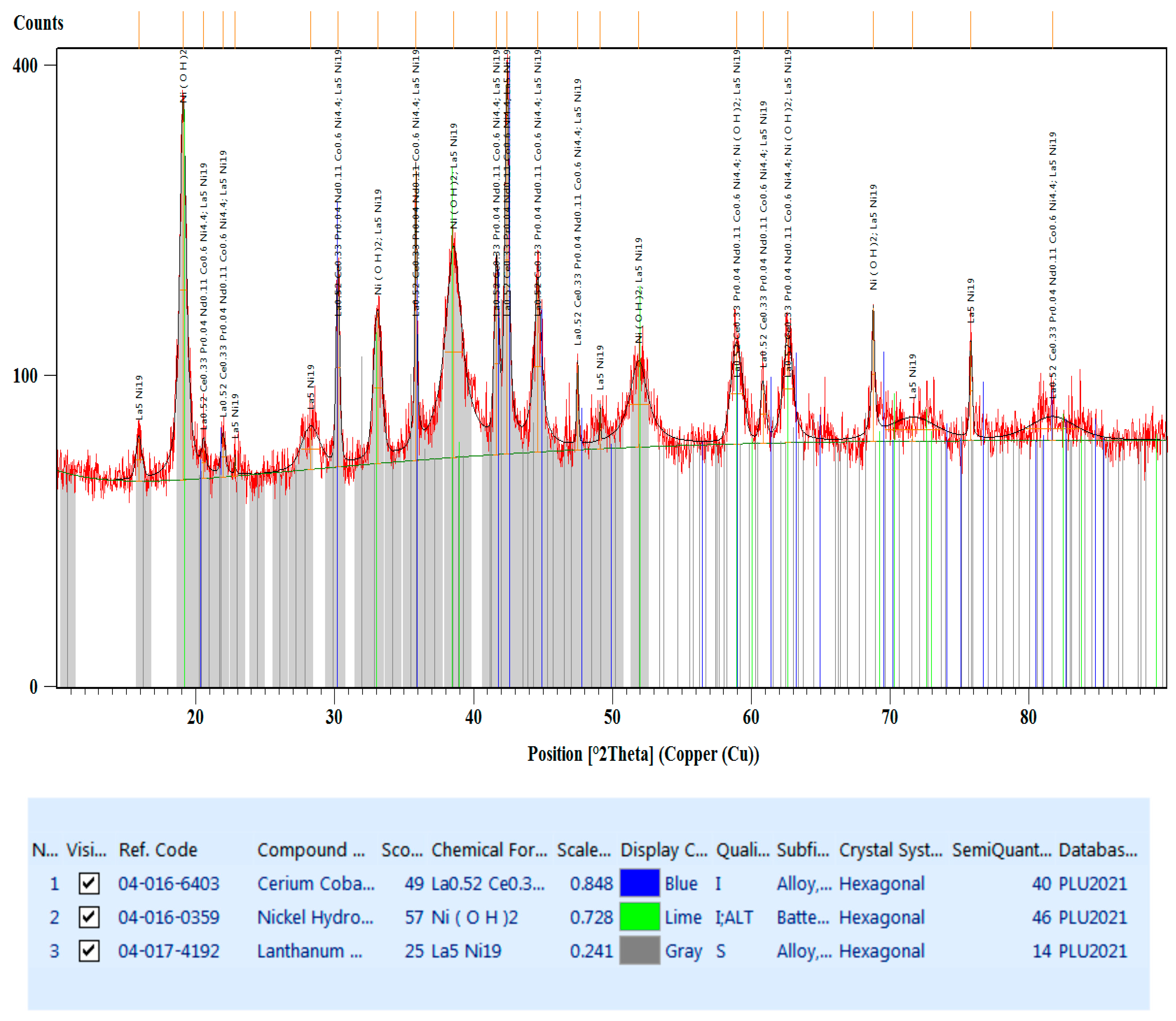
3.2.3. X-ray Diffraction Analysis (XRD)
- La0.52Ce0.33Pr0.04Nd0.11Co0.6Ni4.4, which crystallizes in the hexagonal system, space group P6/mmm (blue lines).
- Ni(OH)2, which crystallizes in the hexagonal system, space group P-3m1 (lime lines).
- La5Ni19, which also crystallizes in the hexagonal system but with a different space group P63/mmc (gray lines).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romanian Association for Recycling—RoRec and Volens Association, No Battery to Waste! Available online: https://www.patruladereciclare.ro/?s=baterii&orderby=post_date&order=desc (accessed on 9 September 2024). (In Romanian).
- Eurostat Statistics Explained, Waste Statistics—Recycling of Batteries and Accumulators. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators (accessed on 10 August 2024).
- Statista, Recycling Collection Rate of Portable Batteries and Accumulators and Volume Put on the Market in the European Union (EU-27) from 2011 to 2021. Available online: https://www.statista.com/statistics/1316387/share-portable-batteries-and-accumulators-collected-for-recycling-eu/ (accessed on 10 August 2024).
- European Portable Battery Association, The Collection of Waste Portable Batteries in Europe in View of the Achievability of the Collection Targets Set by Batteries Directive 2006/66/EC. Available online: https://www.epbaeurope.net/assets/Report-on-the-portable-battery-collection-rates---Short-Update-2022.pdf (accessed on 11 August 2024).
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 2023, 16, 6571. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Townsend, A.; Gouws, R. A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms. Energies 2022, 15, 4930. [Google Scholar] [CrossRef]
- Omar, N.; Firouz, Y.; Monem, M.A.; Samba, A.; Gualous, H.; Coosemans, T.; Van den Bossche, P.; Van Mierlo, J. Analysis of Nickel-Based Battery Technologies for Hybrid and Electric Vehicles. In Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–12. [Google Scholar]
- Mingming, G.; Jianwen, H.; Feng, F.; Derek, O.N. Hydrogen-absorbing alloys for the NICKEL–METAL hydride battery. Int. J. Hydrogen Energy 1998, 23, 1055–1060. [Google Scholar]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, J.; Li, H. Batteries with high theoretical energy densities. Energy Storage Mater. 2020, 26, 46–55. [Google Scholar] [CrossRef]
- Liu, F.; Peng, C.; Porvali, A.; Wang, Z.; Wilson, B.P.; Lundström, M. Synergistic Recovery of Valuable Metals from Spent Nickel−Metal Hydride Batteries and Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16103–16111. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Ruther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Farghal, F.E.; Abdel-Khalek, M.A. Exploitation of spent nickel-metal hydride (Ni-MH) batteries as a source of value-added products. Physicochem. Probl. Miner. Process. 2021, 56, 95–105. [Google Scholar]
- Muhsen, H.; Al-Muhtady, A.; Kadri, A.; Ruziyeh, A. Evaluating and Repurposing of Used Ni-MH Hybrid Batteries. In Proceedings of the International Renewable and Sustainable Energy Conference (IRSEC) 2017, Tangier, Morocco, 4–7 December 2017. [Google Scholar]
- Rodrigues, L.E.O.C.; Mansur, M.B. Hydrometallurgical Separation of Rare Earth Elements, Cobalt and Nickel from Spent Nickel−Metal−Hydride Batteries. J. Power Sources 2010, 195, 3735–3741. [Google Scholar] [CrossRef]
- Müller, T.; Friedrich, B. Development of a Recycling Process for Nickel-Metal Hydride Batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
- Nickel Institute, Nickel in Batteries. Available online: https://nickelinstitute.org/en/nickel-applications/nickel-in-batteries/ (accessed on 14 August 2024).
- Schönfisch, M.; Dasgupta, A.; Wanner, B. Projected Global Demand for Energy Storage. In Emerging Battery Technologies to Boost the Clean Energy Transition; Passerini, S., Barelli, L., Baumann, M., Peters, J., Weil, M., Eds.; The Materials Research Society Series; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Salehi, H.; Maroufi, S.; Mofarah, S.S.; Nekouei, R.K.; Vena, S. Recovery of rare earth metals from Ni-MH batteries: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 178, 1132248. [Google Scholar] [CrossRef]
- Zhi, H.; Ni, S.; Su, X.; Xie, W.; Zhang, H.; Sun, X. Separation and recovery of rare earth from waste nickel-metal hydride batteries by phosphate based extraction-precipitation. J. Rare Earths 2022, 40, 974–980. [Google Scholar] [CrossRef]
- Lin, S.L.; Huang, K.L.; Wang, I.C.; Chou, I.C.; Kuo, Y.M.; Hung, C.H.; Lin, C. Characterization of spent nickel–metal hydride batteries and a preliminary economic evaluation of the recovery processes. J. Air Waste Manag. Assoc. 2016, 66, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Khalid, M.K.; Porvali, A.; Wilson, B.P.; Lundström, M. Recycling of spent NiMH batteries: Integration of battery leach solution into primary Ni production using solvent extraction. Sustain. Mater. Technol. 2019, 22, e00121. [Google Scholar] [CrossRef]
- Rabah, M.A.; Farghaly, F.E.; Abd-El Motaleb, M.A. Recovery of nickel, cobalt and some salts from spent Ni-MH batteries. Waste Manag. 2008, 28, 1159–1167. [Google Scholar] [CrossRef]
- Tang, K.; Ciftja, A.; van der Eijk, C.; Wilson, S.; Tranell, G. Recycling of the rare earth oxides from spent rechargeable batteries using waste metallurgical slags. J. Min. Metall. B Metall. 2013, 49, 233–236. [Google Scholar] [CrossRef]
- Vasile, A.; Ghica, V.G.; Petrescu, M.I.; Niculescu, F.; Turcu, R.N.; Enache, M.E.; Iacob, G. Physical separation and recycling-oriented characterization of metallic material contained in spent NiMH batteries. UPB Sci. Bull. B Chem. Mater. Sci. 2023, 85, 143–154. [Google Scholar]
- Vasile, A.; Ghica, V.G.; Iacob, G.; Petrescu, M.I.; Mihailov, E.; Patroi, D. Recycling Ni-MH batteries used in medical devices. Part I—Investigations on the contents of Ni-MH batteries. UPB Sci. Bull. B Chem. Mater. Sci. 2024, 86, 225–236. [Google Scholar]
- Porvali, A.; Wilson, B.P.; Lundström, M. Lanthanide-Alkali Double Sulfate Precipitation from Strong Sulfuric Acid NiMH Battery Waste Leachate. Waste Manag. 2018, 71, 381–389. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A Review on the Recovery and Separation of Rare Earths and Transition Metals from Secondary Resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Bratosin, I.; Ghica, V.G.; Buzatu, M.; Petrescu, M.I.; Iacob, G.; Kovács, T.A.; Necşulescu, A.D. Separation process optimization of the paste from the aluminum cathode, in the process of recovery of cobalt from used batteries. UPB Sci. Bull. B Chem. Mater. Sci. 2020, 82, 199–210. [Google Scholar]
- Takano, M.; Asano, S.; Goto, M. Recovery of nickel, cobalt and rare-earth elements from spent nickel–metal-hydride battery: Laboratory tests and pilot trials. Hydrometallurgy 2022, 209, 105826. [Google Scholar] [CrossRef]
- Ash, B.; Nalajala, V.S.; Popuri, A.K.; Subbaiah, T.; Minakshi, M. Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes. Nanomaterials 2020, 10, 1878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Miesse, C.M.; Lee, H.B.; Chang, I.W.; Hwang, Y.S.; Jang, J.H.; Cha, S.W. Ultra compact direct hydrogen fuel cell prototype using a metal hydride hydrogen storage tank for a mobile phone. Appl. Energy 2014, 134, 382–391. [Google Scholar] [CrossRef]
- Mansouri, M.; Shtender, V.; Tunsu, C.; Yilmaz, D.; Messaoudi, O.; Ebin, B.; Shalberg, M.; Petranikova, M. Production of AB5 materials from spent Ni-MH batteries with further tests of hydrogen storage suitability. J. Power Sources 2022, 539, 231459. [Google Scholar] [CrossRef]
- Okamoto, H. La-Ni (Lanthanum-Nickel). J. Ph. Equilibr. 2002, 23, 287. [Google Scholar] [CrossRef]
- Yamamoto, T.; Di, Z.; Yamaguchi, M. Microstructures and defect structures in intermetallic compounds in the La–Ni alloy system. J. Alloys Compd. 1999, 293–295, 140–145. [Google Scholar]
- Di, Z.; Yamamoto, T.; Inui, H.; Yamaguchi, M. Characterization of stacking faults on basal planes in intermetallic compounds La5Ni19 and La2Ni7. Intermetallics 2000, 8, 391–397. [Google Scholar] [CrossRef]
- Ferey, A.; Cuevas, F.; Latroche, M.; Knosp, B.; Bernard, P. Elaboration and characterization of magnesium-substituted La5Ni19 hydride forming alloys as active materials for negative electrode in Ni-MH battery. Electrochim. Acta 2009, 54, 1710–1714. [Google Scholar] [CrossRef]
- Glorius, M.; Markovits, M.A.C.; Breitkopf, C. Design of Specific Acid-Base-Properties in CeO2-ZrO2-Mixed Oxides via Templating and Au Modification. Catalysts 2018, 8, 358. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Murphy, E.; Ly, A.; Xu, M.; Matanovic, I.; Pan, X.; Atanassov, P. Robust palladium hydride catalyst for electrocatalytic formate formation with high CO tolerance. Appl. Catal. B Environ. 2022, 316, 121659. [Google Scholar] [CrossRef]
- Neves, F.; Carvalho, M.H.; Trindade, B. Process Development of γ-TiAl Based Alloys and their Consolidation. Mater. Sci. Forum 2004, 455–456, 505–509. [Google Scholar] [CrossRef]
- Shokano, G.; Dehouche, Z.; Galey, B.; Postole, G. Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2. Catalysts 2020, 10, 849. [Google Scholar] [CrossRef]

| Chemical Composition (%) | |||
|---|---|---|---|
| Element | Anode Grid (T1) | Anode Grid (T2) | Anode Grid (T3) |
| Fe | 83.98 | 83.69 | 57.71 |
| Ni | 12.94 | 14.22 | 39.67 |
| Mo | 1.55 | 1.29 | 1.73 |
| Mn | 0.51 | 0.10 | 0.11 |
| Co | 0.47 | 0.37 | 0.33 |
| Ti | 0.23 | 0.12 | 0.12 |
| V | 0.14 | 0.04 | 0.18 |
| Pb | 0.08 | 0.10 | 0.05 |
| Nb | 0.02 | 0.02 | 0.03 |
| Ta | 0.02 | 0.01 | 0.02 |
| Cr | 0.02 | 0.02 | 0.02 |
| W | 0.01 | 0.01 | 0.01 |
| Zr | 0.01 | 0.01 | 0.02 |
| Total | 100 | 100 | 100 |
| Element (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | Fe | Co | Ni | La | Ce | Ta | O.E. * |
| 3.49 | 0.46 | 0.78 | 0.18 | 0.17 | 5.31 | 81.29 | 4.68 | 1.54 | 0.87 | balance |
| Element (% (w/w)) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | Na | Mg | Al | Si | S | K | Mn | Fe | Co | Ni | Cu | Zn | Cd | Ba | La | Ce | Pr | Nd | Ta |
| 0.072 | 1.07 | 0.19 | 0.94 | 0.9 | 0.0214 | 0.36 | 0.89 | 1.14 | 4.78 | 74.17 | 1.52 | 0.27 | 0.0011 | 0.0021 | 6.13 | 4.25 | 0.61 | 1.73 | 0.42 |
| Analysis | Elem | Wt. % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|---|
| EDAX ZAF Quantification (Standardless) Element Normalized SEC Table: Default | OK | 8.21 | 29.39 | 0.0271 | 1.1506 | 0.2861 | 1.0020 |
| LaL | 6.63 | 2.73 | 0.0634 | 0.8709 | 1.0571 | 1.0386 | |
| CeL | 13.07 | 5.31 | 0.1325 | 0.8906 | 1.0792 | 1.0547 | |
| PrL | 13.39 | 5.32 | 0.1373 | 0.8854 | 1.0873 | 1.0647 | |
| CoK | 0.18 | 0.17 | 0.0015 | 0.9912 | 0.8676 | 1.0000 | |
| NiK | 58.52 | 57.08 | 0.5354 | 1.0308 | 0.8875 | 1.0000 | |
| Total | 100.00 | 100.00 |
| Analysis | Elem | Wt. % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|---|
| EDAX ZAF Quantification (Standardless) Element Normalized SEC Table: Default | OK | 23.24 | 52.99 | 0.0844 | 1.0915 | 0.3322 | 1.0023 |
| LaL | 1.05 | 0.27 | 0.0106 | 0.8213 | 1.1204 | 1.0967 | |
| PrL | 0.35 | 0.09 | 0.0038 | 0.8406 | 1.1324 | 1.1384 | |
| NdL | 0.58 | 0.15 | 0.0064 | 0.8357 | 1.1359 | 1.1643 | |
| MnK | 0.85 | 0.57 | 0.0097 | 0.9320 | 0.9809 | 1.2412 | |
| CoK | 4.81 | 2.98 | 0.0447 | 0.9344 | 0.9935 | 1.0000 | |
| NiK | 69.12 | 42.95 | 0.6707 | 0.9705 | 0.9999 | 1.0000 | |
| Total | 100.00 | 100.00 |
| Analysis | Elem | Wt. % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|---|
| EDAX ZAF Quantification (Standardless) Element Normalized SEC Table: Default | OK | 8.46 | 29.13 | 0.0292 | 1.1509 | 0.2994 | 1.0017 |
| LaL | 16.33 | 6.47 | 0.1602 | 0.8703 | 1.0747 | 1.0488 | |
| CeL | 6.45 | 2.54 | 0.0651 | 0.8791 | 1.0854 | 1.0571 | |
| PrL | 1.77 | 0.69 | 0.0183 | 0.8901 | 1.0941 | 1.0642 | |
| NdL | 3.21 | 1.22 | 0.0336 | 0.8849 | 1.1008 | 1.0742 | |
| MnK | 2.73 | 2.74 | 0.0258 | 0.9868 | 0.8745 | 1.0928 | |
| CoK | 9.04 | 8.44 | 0.0789 | 0.9904 | 0.8816 | 1.0000 | |
| NiK | 52.01 | 48.77 | 0.4847 | 1.0298 | 0.9050 | 1.0000 | |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, G.; Ghica, V.-G.; Niculescu, F.; Petrescu, M.-I.; Vasile, A. Processing and Characterization of Spent Nickel–Metal Hydride Type AA Batteries to Recover Valuable Materials (Cobalt, Nickel and Rare Earth Elements). Materials 2024, 17, 4908. https://doi.org/10.3390/ma17194908
Iacob G, Ghica V-G, Niculescu F, Petrescu M-I, Vasile A. Processing and Characterization of Spent Nickel–Metal Hydride Type AA Batteries to Recover Valuable Materials (Cobalt, Nickel and Rare Earth Elements). Materials. 2024; 17(19):4908. https://doi.org/10.3390/ma17194908
Chicago/Turabian StyleIacob, Gheorghe, Valeriu-Gabriel Ghica, Florentina Niculescu, Mircea-Ionuţ Petrescu, and Ana Vasile. 2024. "Processing and Characterization of Spent Nickel–Metal Hydride Type AA Batteries to Recover Valuable Materials (Cobalt, Nickel and Rare Earth Elements)" Materials 17, no. 19: 4908. https://doi.org/10.3390/ma17194908
APA StyleIacob, G., Ghica, V.-G., Niculescu, F., Petrescu, M.-I., & Vasile, A. (2024). Processing and Characterization of Spent Nickel–Metal Hydride Type AA Batteries to Recover Valuable Materials (Cobalt, Nickel and Rare Earth Elements). Materials, 17(19), 4908. https://doi.org/10.3390/ma17194908







