Ion Implantation Combined with Heat Treatment Enables Excellent Conductivity and Corrosion Resistance of Stainless Steel Bipolar Plates for Hydrogen Fuel Cells
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Preparation
2.2. Surface Characterization
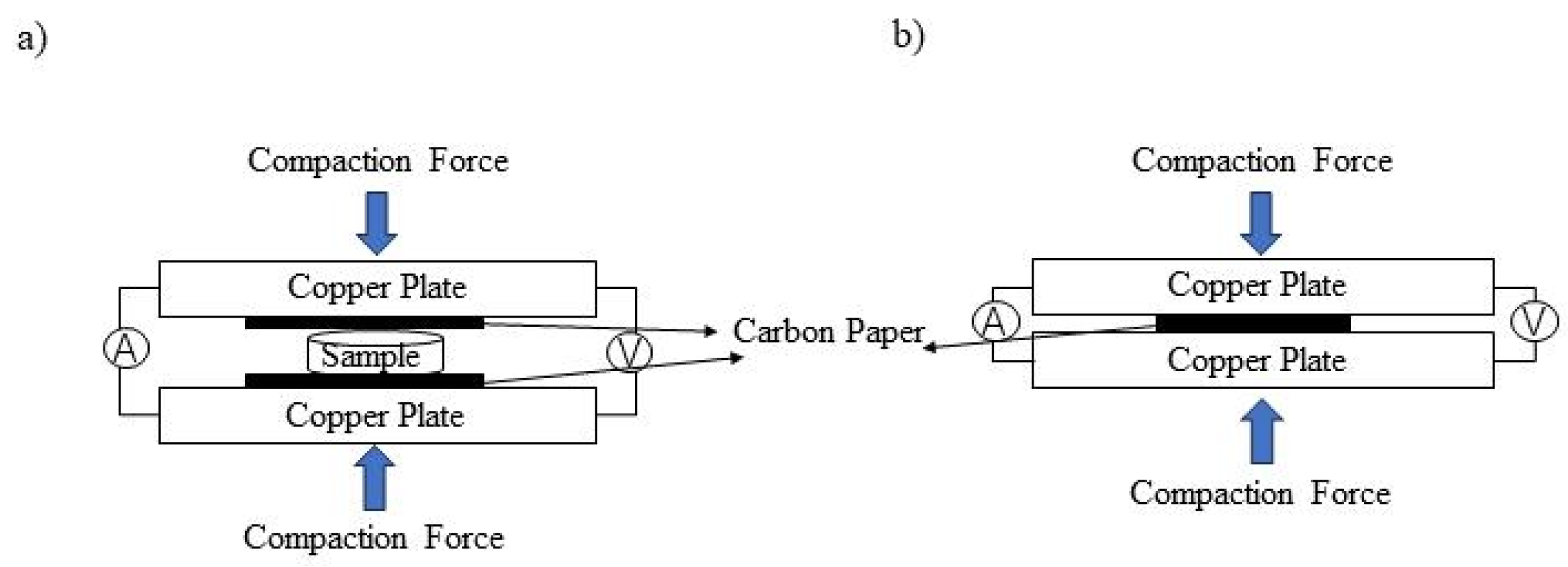
2.3. ICR Measurements
2.4. Electrochemical Measurements
3. Results and Discussion
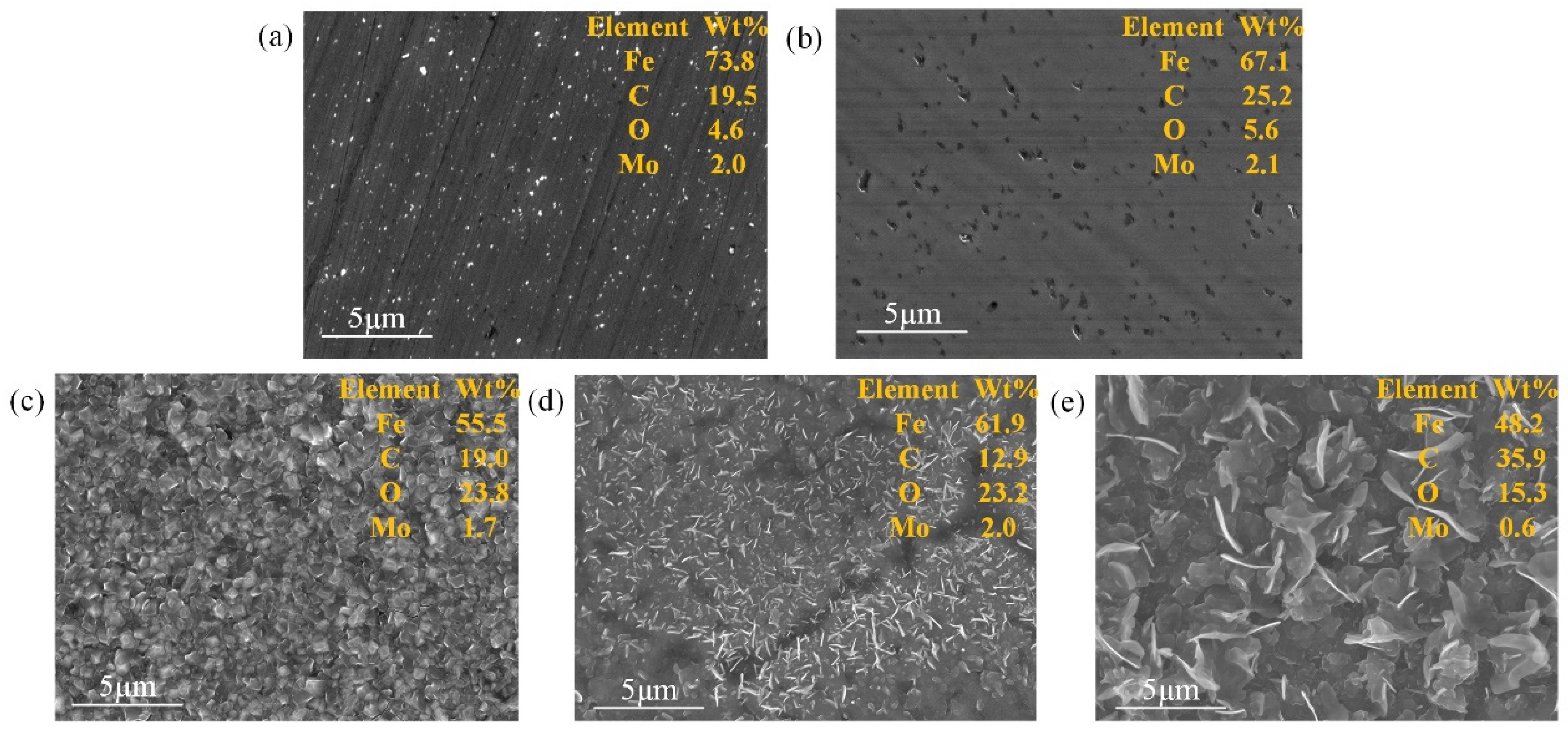
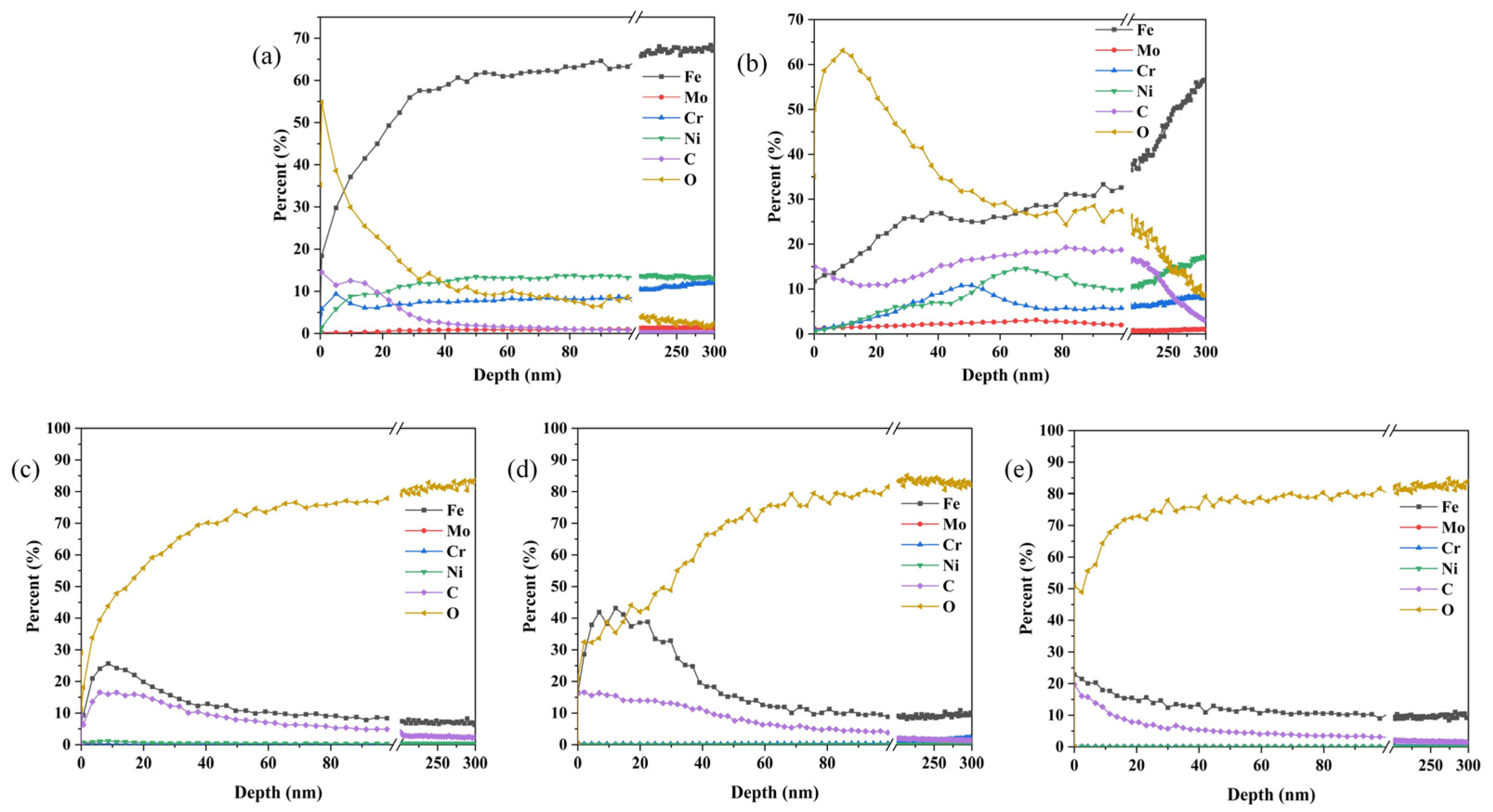
3.1. Characterization of the Modified Layer
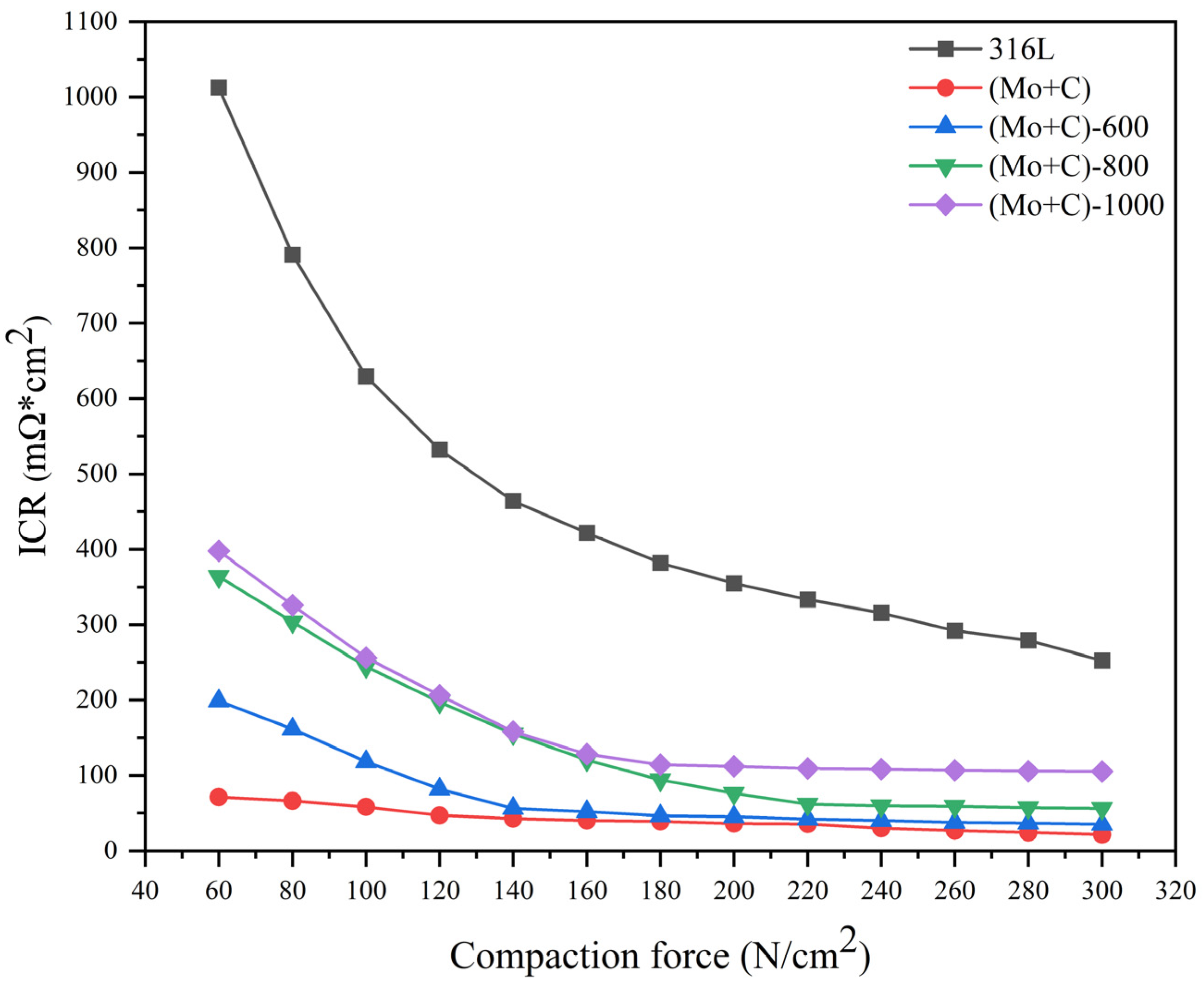
3.2. Interfacial Contact Resistance
3.3. Potentiodynamic Polarization Test
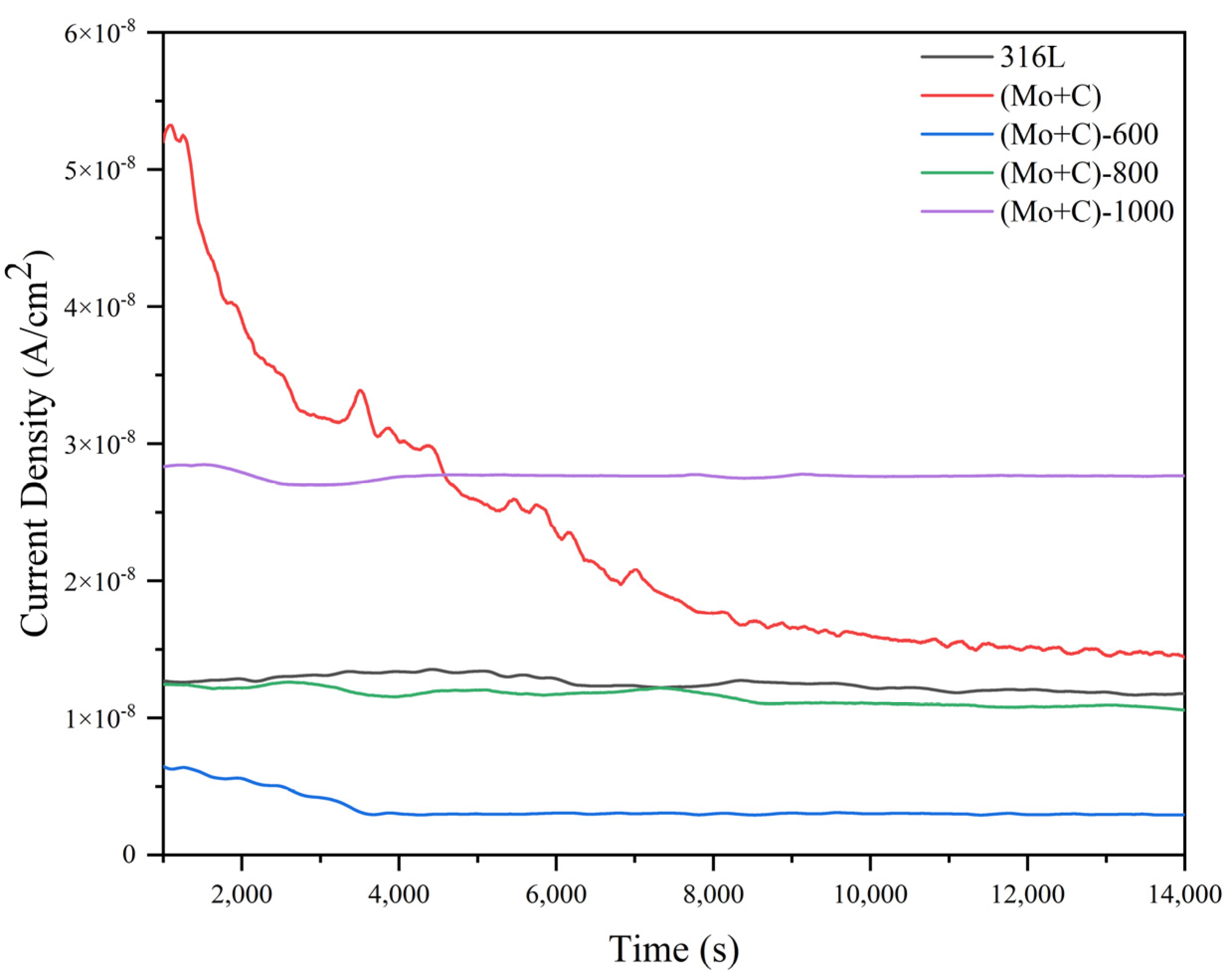
3.4. Potentiostatic Curves of SS 316 L Substrate and Modified Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cropper, M.A.J.; Geiger, S.; Jollie, D.M. Fuel cells: A survey of current developments. J. Power Sources 2004, 131, 57–61. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Lee, S.H.; Woo, S.P.; Kakati, N.; Lee, Y.N.; Yoon, Y.S. Corrosion and electrical properties of carbon/ceramic multilayer coated on stainless steel bipolar plates. Surf. Coat. Technol. 2016, 303, 162–169. [Google Scholar] [CrossRef]
- Bohackova, T.; Ludvik, J.; Kouril, M. Metallic Material Selection and Prospective Surface Treatments for Proton Exchange Membrane Fuel Cell Bipolar Plates—A Review. Materials 2021, 14, 2682. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Kobayashi, O. Mass production cost of PEM fuel cell by learning curve. Int. J. Hydrogen Energy 2004, 29, 985–990. [Google Scholar] [CrossRef]
- Avram, D.N.; Davidescu, C.M.; Hulka, I.; Dan, M.L.; Stanciu, E.M.; Pascu, A.; Mirza-Rosca, J.C. Corrosion Behavior of Coated Low Carbon Steel in a Simulated PEMFC Environment. Materials 2023, 16, 3056. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.L.; Kellenberger, A.; Duca, D.; Vaszilcsin, N.; Craciunescu, C.M.; Mitelea, I.; Ercuta, A.; Lædre, S.; Khoza, T. Corrosion Resistance of AISI 442 and AISI 446 Ferritic Stainless Steels as a Support for PEMWE Bipolar Plates. Materials 2023, 16, 1501. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A review of metallic bipolar plates for proton exchange membrane fuel cells: Materials and fabrication methods. Adv. Mater. Sci. Eng. 2012, 2012, 828070. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.D.; Ett, G.; Ett, V. Carbon materials in composite bipolar plates for polymer electrolyte membrane fuel cells: A review of the main challenges to improve electrical performance. J. Power Sources 2011, 196, 2945–2961. [Google Scholar] [CrossRef]
- Hodgson, D.R.; May, B.; Adcock, P.I. New lightweight bipolar plate system for polymer electrolyte membrane fuel cells. J. Power Sources 2001, 96, 233–235. [Google Scholar] [CrossRef]
- Hentall, P.L.; Lakeman, J.B.; Mepsted, G.O.; Adcok, P.L.; Moore, J.M. New materials for polymer electrolyte membrane fuel cell current collectors. J. Power Sources 1999, 80, 235–241. [Google Scholar] [CrossRef]
- Chu, D.; Jiang, R. Performance of polymer electrolyte membrane fuel cell (PEMFC) stacks: Part I. Evaluation and simulation of an air-breathing PEMFC stack. J. Power Sources 1999, 83, 128–133. [Google Scholar] [CrossRef]
- Makkus, R.C.; Janssen, A.H.H.; Bruijn, F.A.D.; Mallant, R.K.A.M. Use of stainless steel for cost competitive bipolar plates in the SPFC. J. Power Sources 2000, 86, 274–282. [Google Scholar] [CrossRef]
- Wang, L.X.; Sun, J.C. Molybdenum modified AISI 304 stainless steel bipolar plate for proton exchange membrane fuel cell. J. Renew. Sustain. Energy 2013, 5, 021407. [Google Scholar] [CrossRef]
- Lee, S.B.; Cho, K.H.; Lee, W.G.; Jiang, H. Improved corrosion resistance and interfacial contact resistance of 316L stainless steel for proton exchange membrane fuel cell bipolar plates by chromizing surface treatment. J. Power Sources 2009, 187, 318–323. [Google Scholar] [CrossRef]
- Viviente, J.L.; Garcia, A.; Loinaz, A.; Alonso, F.; Oñate, J.I. Carbon layers formed on steel and Ti alloys after ion implantation of C+ at very high doses. Vacuum 1999, 52, 141–146. [Google Scholar] [CrossRef]
- Liang, H.; Ma, F.R.; Zhang, T.H.; Zhu, H.; Wu, X.Y.; Zhang, H.X. Surface structure and corrosion properties of binary Ti-C and Mo-C coatings co-deposited by filtered vacuum arc plasma deposition system. Surf. Coat. Technol. 2000, 131, 58–61. [Google Scholar] [CrossRef]
- Kiniger, M.; Isenmenger-Sittner, C.; Hell, J.; Schwarz, B.; Hutter, H.; Puchner, S. Carbide formation upon heat treatment of molybdenum layers deposited on carbon substrates: Comparison of experimental data with a cellular automaton model. Surf. Interface Anal. 2008, 40, 786–789. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, J.H.; Kim, J.H. Effect of chemical and heat treatment on the interfacial contact resistance and corrosion resistance of 446M ferritic stainless steel as a bipolar plate for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 9926–9935. [Google Scholar] [CrossRef]
- Li, D.G.; Chen, D.R.; Liang, P. Influence of heat treatment on corrosion behaviors of 316L stainless steel in simulated cathodic environment of proton exchange membrane fuel cell (PEMFC). Int. J. Hydrogen Energy 2020, 45, 30101–30112. [Google Scholar] [CrossRef]
- Salman, O.O.; Gammer, C.; Chaubey, A.K.; Eckert, J.; Scudino, S. Effect of heat treatment on microstructure and mechanical properties of 316L steel synthesized by selective laser melting. Mater. Sci. Eng. 2019, 748, 205–212. [Google Scholar] [CrossRef]
- Chen, X.H.; Jia, L.; Cheng, X.; Wang, H.; Huang, Z. Effect of heat treatment on microstructure, mechanical and corrosion properties of austenitic stainless steel 316L using arc additive manufacturing. Mater. Sci. Eng. 2018, 715, 307–314. [Google Scholar] [CrossRef]
- Yari, M.; Larijani, M.; Afshar, A.; Eshghabadi, M.; Shokouhy, A. Physical properties of sputtered amorphous carbon coating. J. Alloys Compd. 2012, 513, 135–138. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Singh, R.S.; Gautam, A.; Rai, V. Graphene-based bipolar plates for polymer electrolyte membrane fuel cells. Front. Mater. Sci. 2019, 13, 217–241. [Google Scholar] [CrossRef]
- Xiang, M.L.; Li, D.B.; Li, W.H.; Zhong, B.; Sun, Y.H. Potassium and nickel doped β-Mo2C catalysts for mixed alcohols synthesis via syngas. Catal. Commun. 2007, 8, 513–518. [Google Scholar] [CrossRef]
- Wang, S.N.; Liao, L.; Shi, Z.P.; Xiao, J.J.; Gao, Q.S.; Zhang, Y.H.; Liu, B.H.; Tang, Y. Mo2C/Reduced-Graphene-Oxide nanocomposite: An efficient electrocatalyst for the hydrogen evolution reaction. Chemelectrochem 2016, 3, 2110–2115. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X.L. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.B.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.K.; Zhang, Z.; Wang, Y.J.; Feng, Q.; Wang, H.J.; Li, H. Molybdenum carbide coated 316L stainless steel for bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 4940–4950. [Google Scholar] [CrossRef]
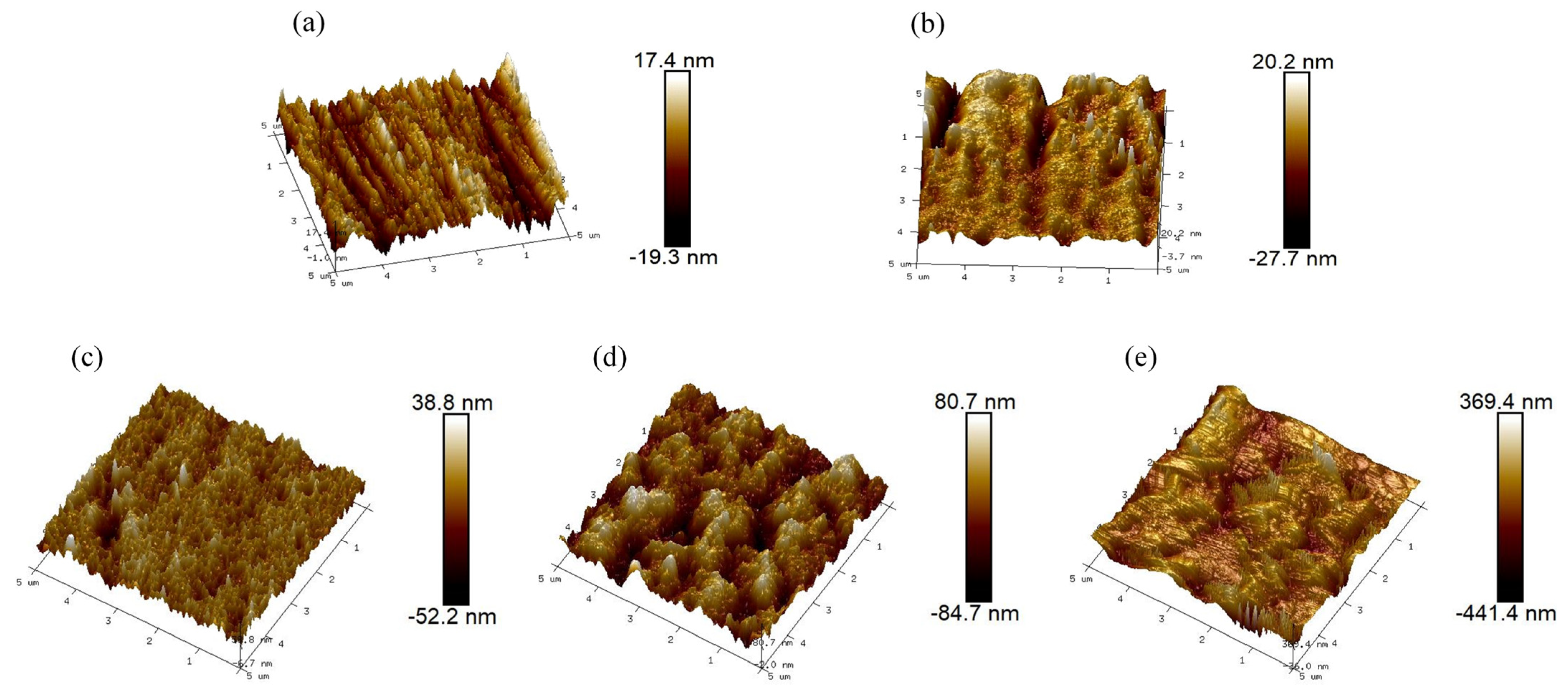


| Sample Code | Surface Roughness (Image Ra, nm) | Surface Roughness (Image Rq, nm) |
|---|---|---|
| 316 L | 4.68 | 5.87 |
| (Mo+C) | 5.38 | 7.70 |
| (Mo+C)-600 | 6.87 | 8.96 |
| (Mo+C)-800 | 23.20 | 28.80 |
| (Mo+C)-1000 | 100.00 | 127.00 |
| Samples | Ecorr (V) | Icorr (A/cm2) | Etran (V) |
|---|---|---|---|
| 316 L | −0.743 | 7.01 × 10−3 | 0.484 |
| (Mo+C) | −0.731 | 5.33 × 10−3 | 0.494 |
| (Mo+C)-600 | −0.369 | 5.09 × 10−5 | 0.462 |
| (Mo+C)-800 | −0.747 | 13.84 × 10−3 | 0.477 |
| (Mo+C)-1000 | −0.766 | 4.08 × 10−4 | 0.497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ding, L.; Pan, Y.; Zhang, X.; Yang, M.; Zhu, C. Ion Implantation Combined with Heat Treatment Enables Excellent Conductivity and Corrosion Resistance of Stainless Steel Bipolar Plates for Hydrogen Fuel Cells. Materials 2024, 17, 779. https://doi.org/10.3390/ma17040779
Wang R, Ding L, Pan Y, Zhang X, Yang M, Zhu C. Ion Implantation Combined with Heat Treatment Enables Excellent Conductivity and Corrosion Resistance of Stainless Steel Bipolar Plates for Hydrogen Fuel Cells. Materials. 2024; 17(4):779. https://doi.org/10.3390/ma17040779
Chicago/Turabian StyleWang, Ruijuan, Li Ding, Yong Pan, Xin Zhang, Meng Yang, and Chengfei Zhu. 2024. "Ion Implantation Combined with Heat Treatment Enables Excellent Conductivity and Corrosion Resistance of Stainless Steel Bipolar Plates for Hydrogen Fuel Cells" Materials 17, no. 4: 779. https://doi.org/10.3390/ma17040779
APA StyleWang, R., Ding, L., Pan, Y., Zhang, X., Yang, M., & Zhu, C. (2024). Ion Implantation Combined with Heat Treatment Enables Excellent Conductivity and Corrosion Resistance of Stainless Steel Bipolar Plates for Hydrogen Fuel Cells. Materials, 17(4), 779. https://doi.org/10.3390/ma17040779







