In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Clamping and Operation Condition
2.2. Catalyst-AST Protocol
2.3. Electrochemical Characterization
2.4. Distribution of Relaxation Times
2.5. SEM–EDS
3. Results and Discussion
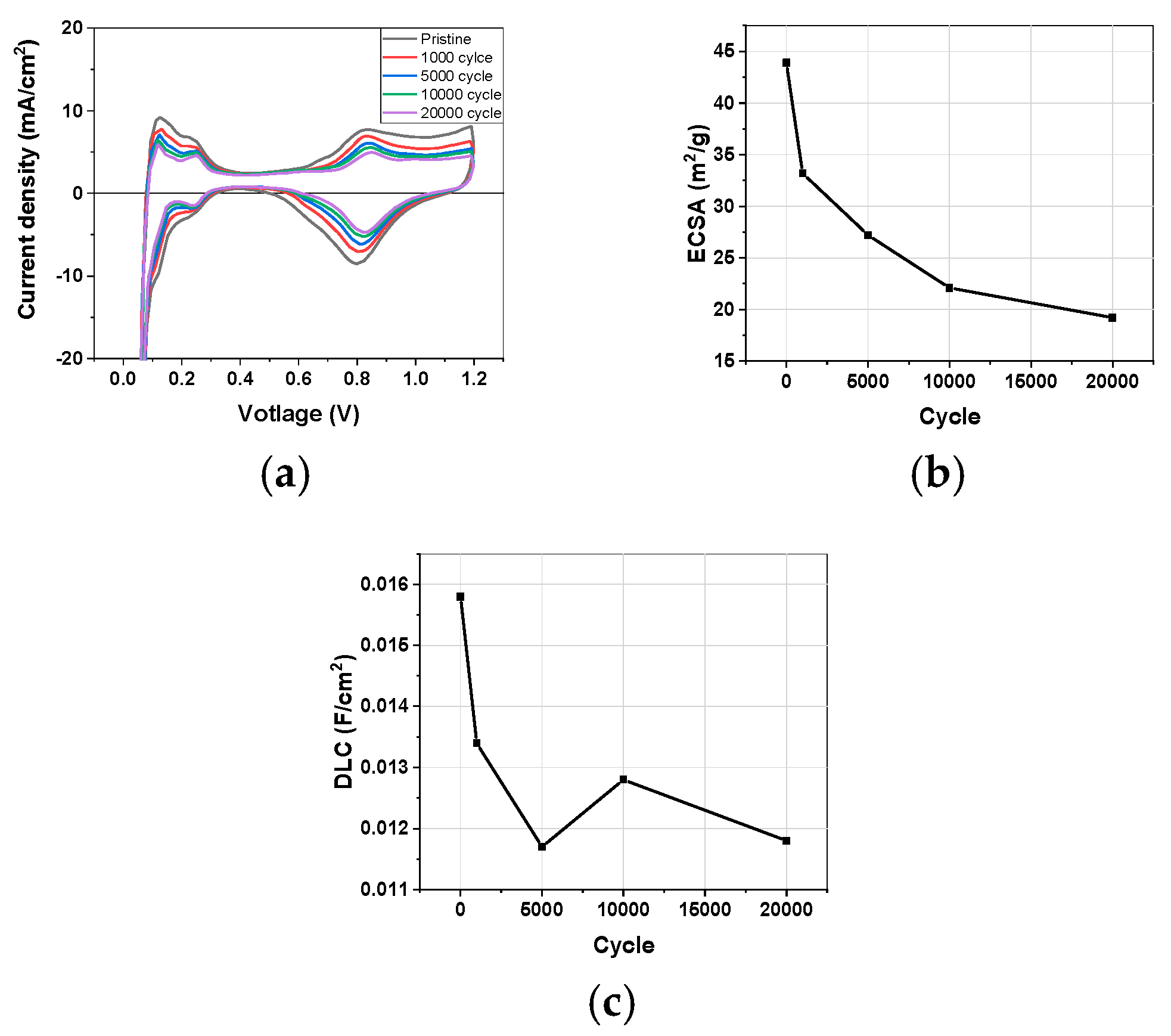
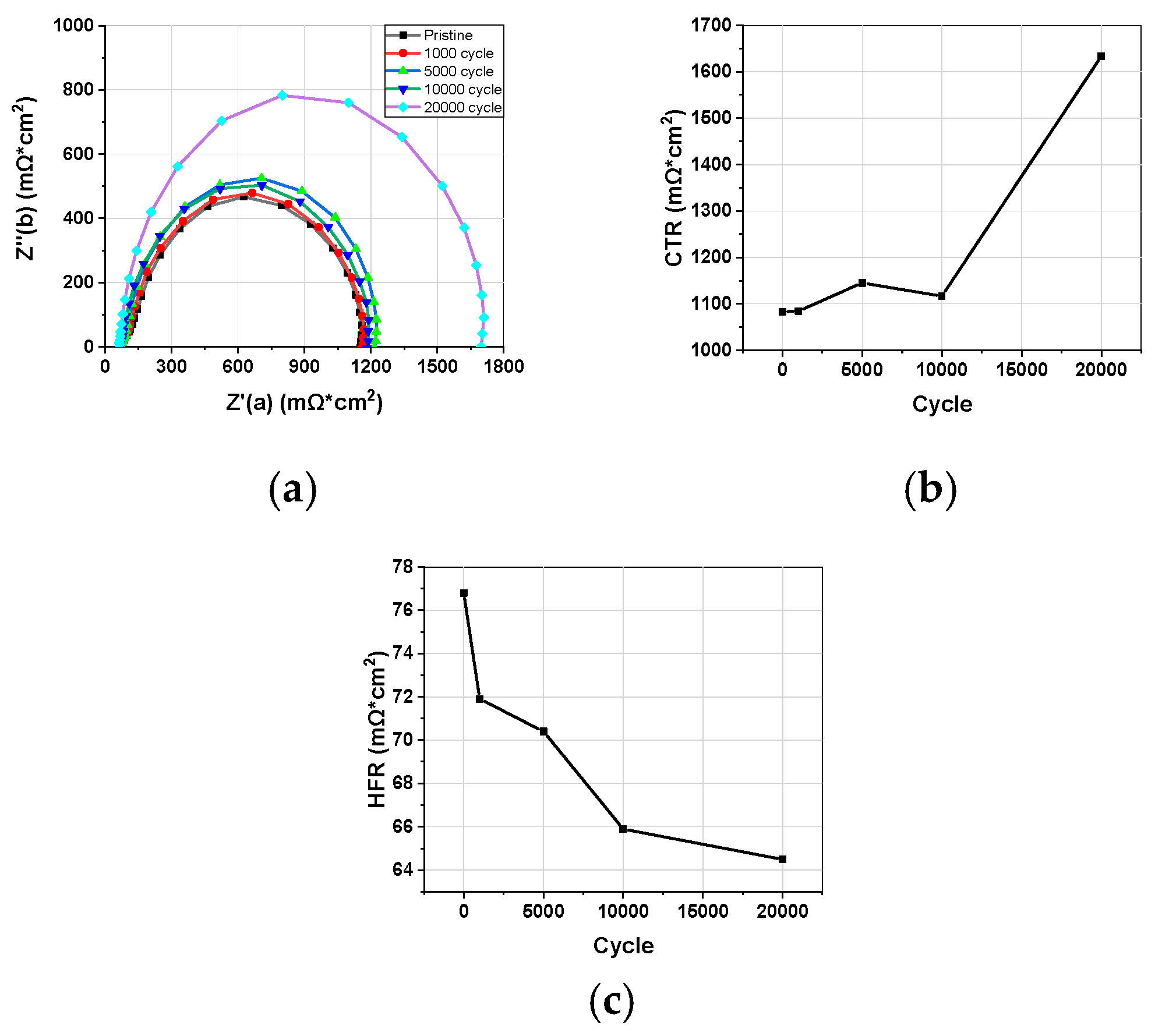
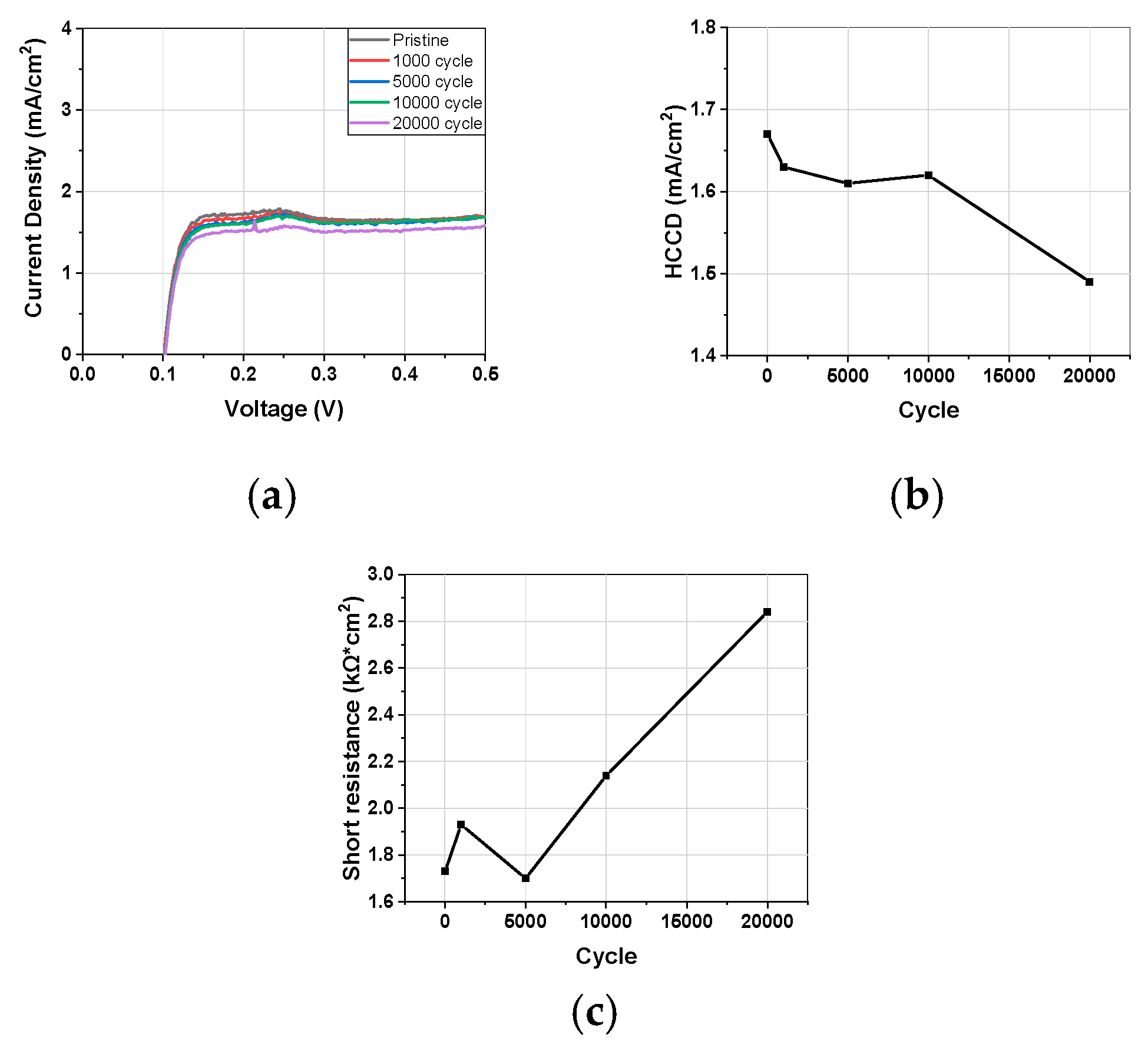
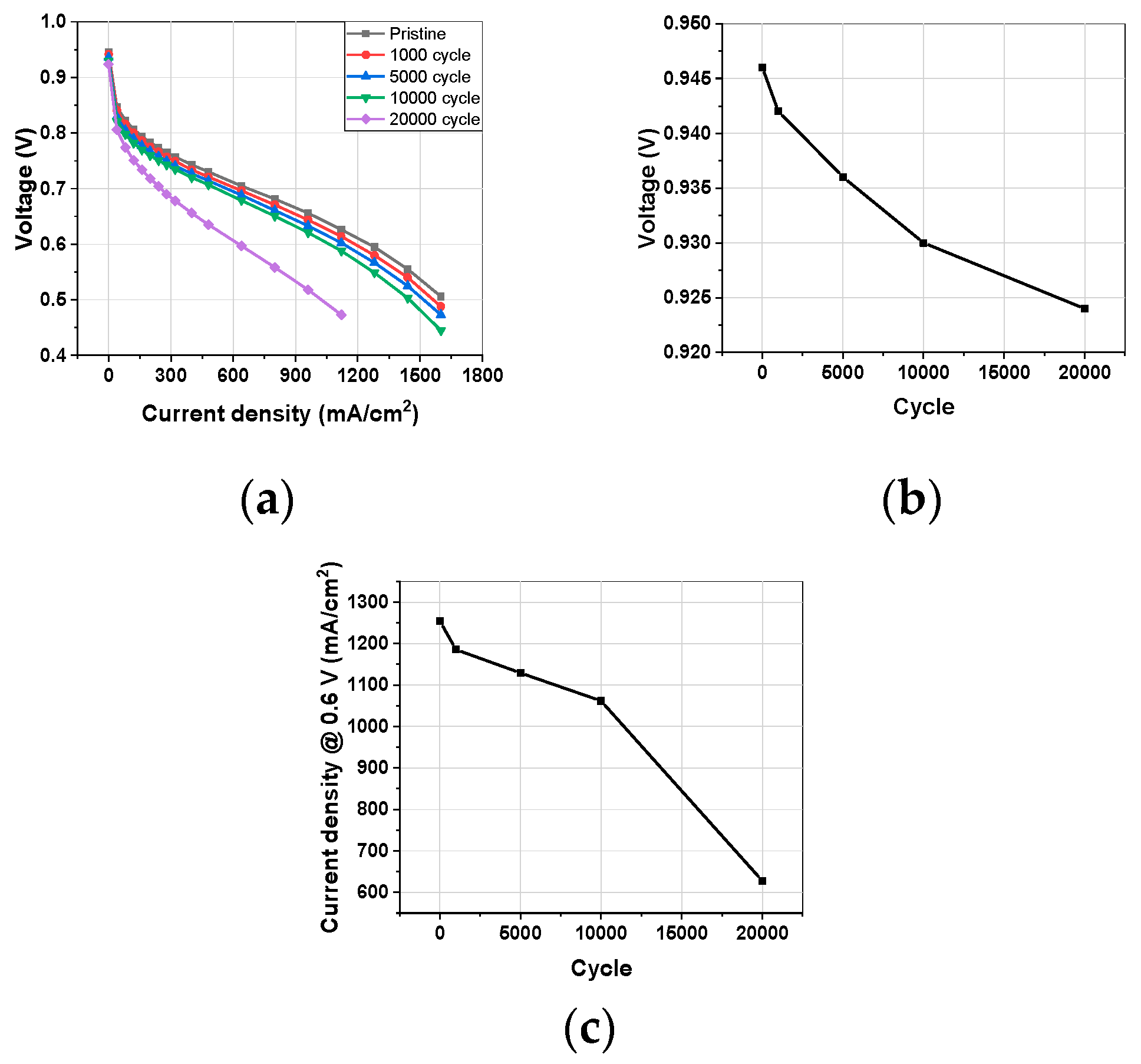
3.1. Electrochemical Characterization during Catalyst-AST
3.1.1. Cyclic Voltammetry
3.1.2. Impedance
3.1.3. Linear Sweep Voltammetry
3.1.4. Polarization Curve
3.2. Change in Binder Resistance
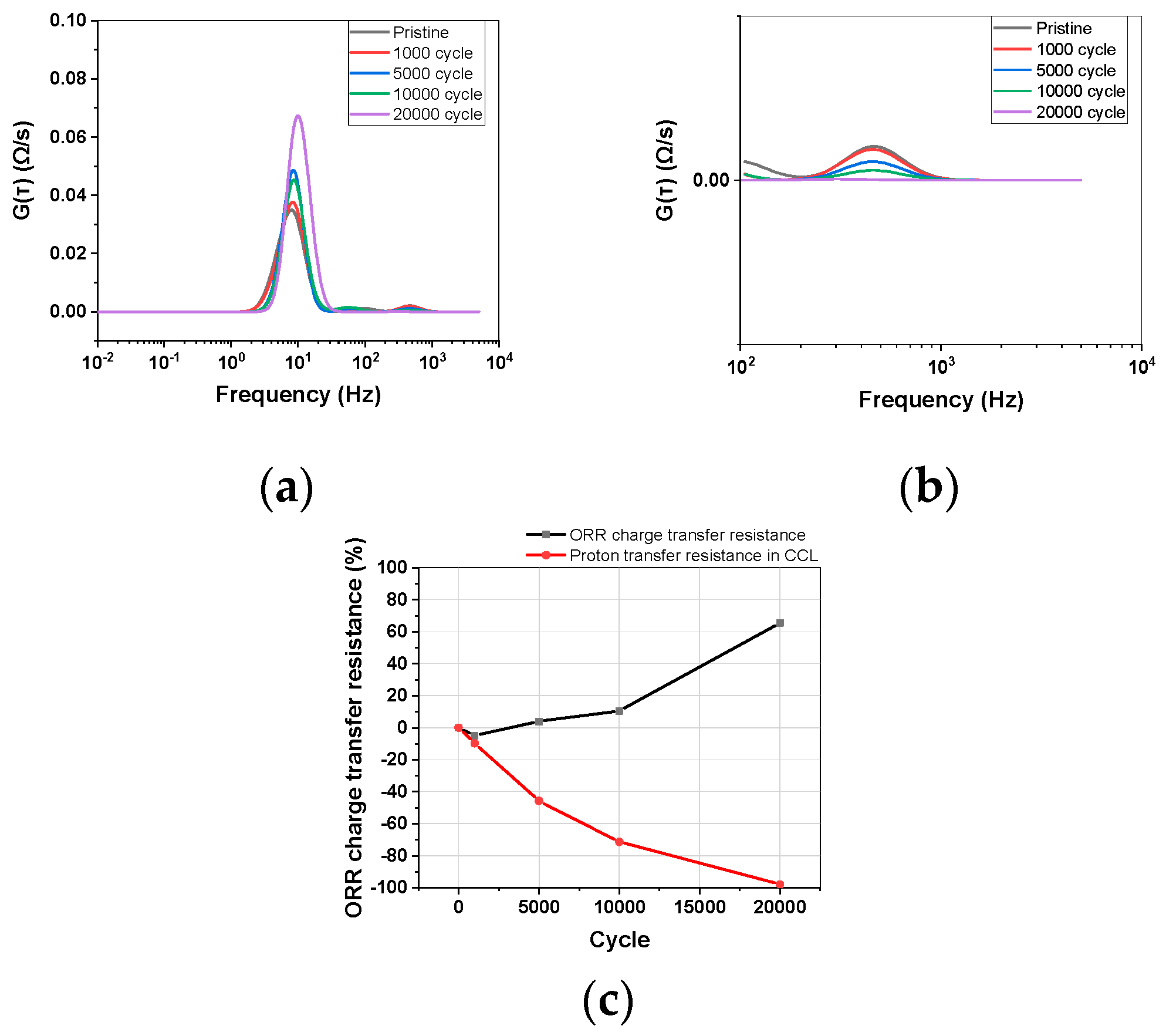
3.2.1. Distribution Relaxation Times
3.2.2. Warburg Like Resistance
3.3. Analysis of Catalyst Layer Degradation
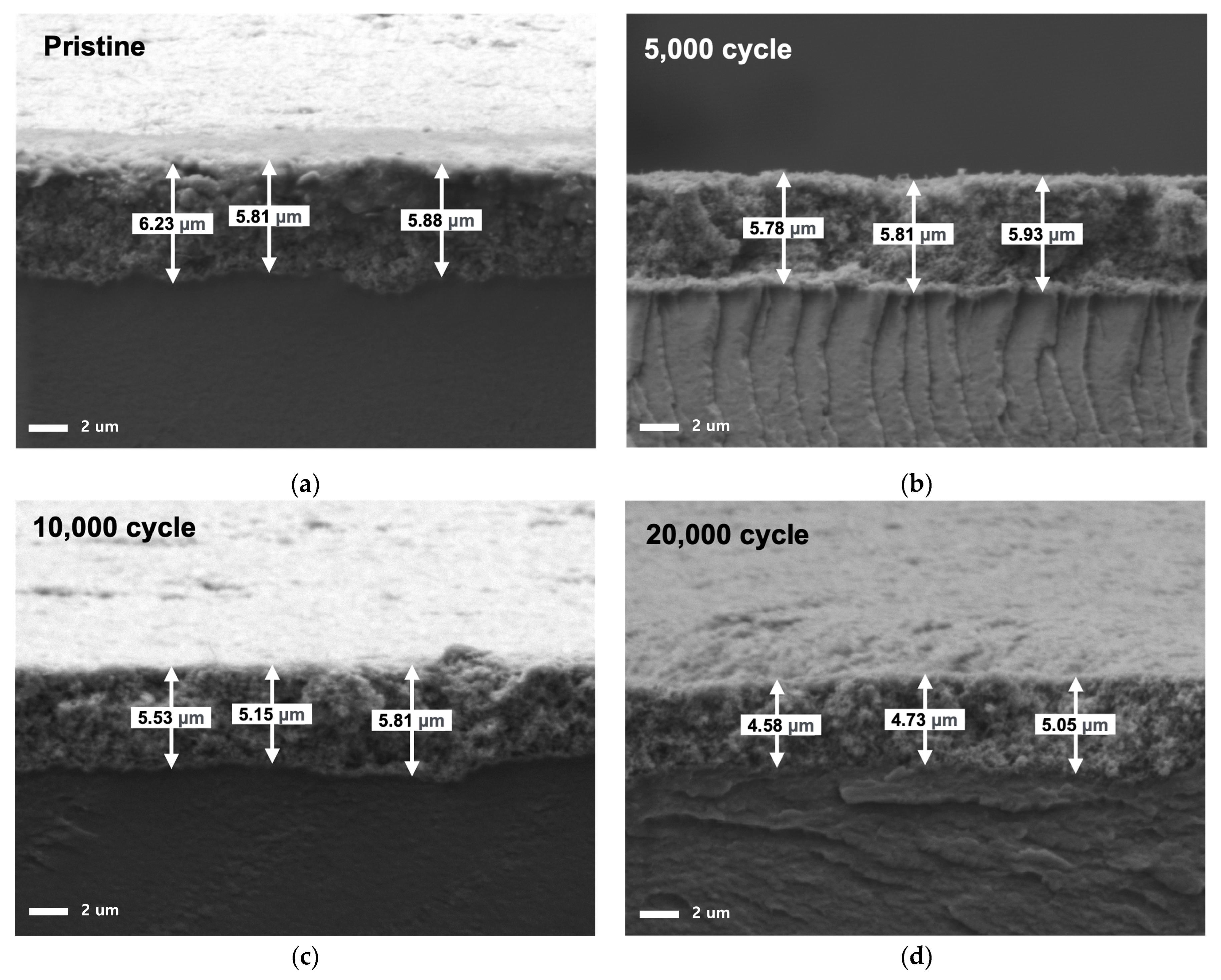
3.3.1. Cross-Sectional SEM of MEA
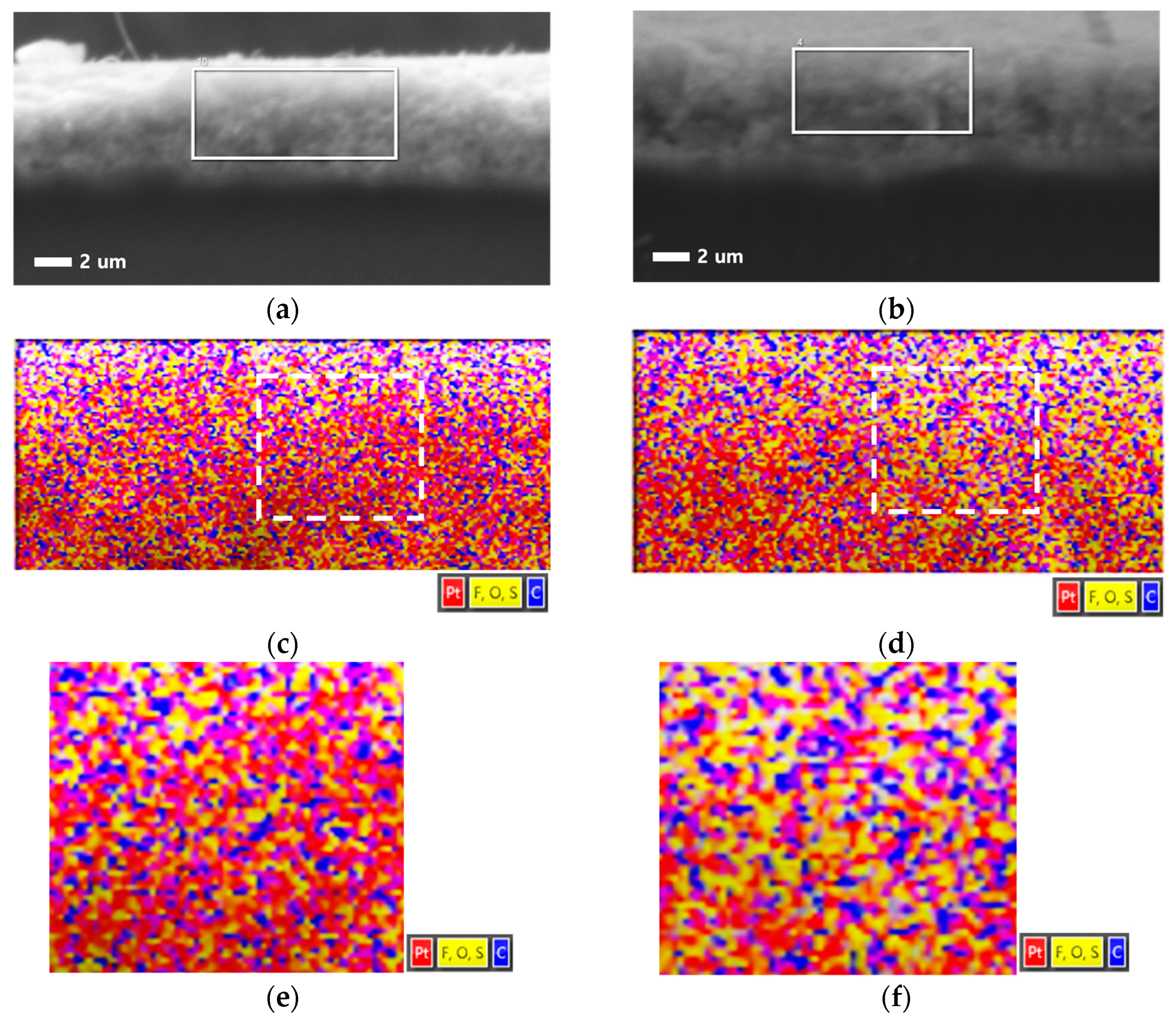
3.3.2. EDS of Catalyst Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst–related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Gittleman, C.S.; Kongkanand, A.; Masten, D.; Gu, W. Materials research and development focus areas for low cost automotive proton–exchange membrane fuel cells. Curr. Opin. Electrochem. 2019, 18, 81–89. [Google Scholar] [CrossRef]
- Kongkanand, A.; Subramanian, N.P.; Yu, Y.; Liu, Z.; Igarashi, H.; Muller, D.A. Achieving high–power PEM fuel cell performance with an ultralow–Pt–content core–shell catalyst. ACS Catal. 2016, 6, 1578–1583. [Google Scholar] [CrossRef]
- Macauley, N.; Papadias, D.D.; Fairweather, J.; Spernjak, D.; Langlois, D.; Ahluwalia, R.; More, K.L.; Mukundan, R.; Borup, R.L. Carbon corrosion in PEM fuel cells and the development of accelerated stress tests. J. Electrochem. Soc. 2018, 165, F3148–F3160. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co intermetallic nanoparticles derived from metal–organic frameworks for oxygen reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Greszler, T.A.; Caulk, D.; Sinha, P. The impact of platinum loading on oxygen transport resistance. J. Electrochem. Soc. 2012, 159, F831. [Google Scholar] [CrossRef]
- Iden, H.; Mashio, T.; Ohma, A. Gas transport inside and outside carbon supports of catalyst layers for PEM fuel cells. J. Electroanal. Chem. 2013, 708, 87–94. [Google Scholar] [CrossRef]
- Jomori, S.; Komatsubara, K.; Nonoyama, N.; Kato, M.; Yoshida, T. An experimental study of the effects of operational history on activity changes in a PEMFC. J. Electrochem. Soc. 2013, 160, F1067. [Google Scholar] [CrossRef]
- Liu, H.; Epting, W.K.; Litster, S. Gas transport resistance in polymer electrolyte thin films on oxygen reduction reaction catalysts. Langmuir 2015, 31, 9853–9858. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El–Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H. The key to high performance low Pt loaded electrodes. J. Electrochem. Soc. 2017, 164, F418. [Google Scholar] [CrossRef]
- Weber, A.Z.; Kusoglu, A. Unexplained transport resistances for low–loaded fuel–cell catalyst layers. J. Mater. Chem. A 2014, 2, 17207–17211. [Google Scholar] [CrossRef]
- Braaten, J.P.; Kariuki, N.N.; Myers, D.J.; Blackburn, S.; Brown, G.; Park, A.; Litster, S. Integration of a high oxygen permeability ionomer into polymer electrolyte membrane fuel cell cathodes for high efficiency and power density. J. Power Sources 2022, 522, 230821. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kodama, K.; Kitano, N.; Suzuki, T.; Minami, S.; Shinozaki, K.; Hasegawa, N.; Shinohara, A. The role of oxygen–permeable ionomer for polymer electrolyte fuel cells. Nat. Commun. 2021, 12, 4956. [Google Scholar] [CrossRef]
- Katzenberg, A.; Chowdhury, A.; Fang, M.; Weber, A.Z.; Okamoto, Y.; Kusoglu, A.; Modestino, M.A. Highly permeable perfluorinated sulfonic acid ionomers for improved electrochemical devices: Insights into structure–property relationships. J. Am. Chem. Soc. 2020, 142, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Macauley, N.; Lousenberg, R.D.; Spinetta, M.; Zhong, S.; Yang, F.; Judge, W.; Nikitin, V.; Perego, A.; Qi, Y.; Pedram, S. Highly durable fluorinated high oxygen permeability ionomers for proton exchange membrane fuel cells. Adv. Energy Mater. 2022, 12, 2201063. [Google Scholar] [CrossRef]
- Rolfi, A.; Oldani, C.; Merlo, L.; Facchi, D.; Ruffo, R. New perfluorinated ionomer with improved oxygen permeability for application in cathode polymeric electrolyte membrane fuel cell. J. Power Sources 2018, 396, 95–101. [Google Scholar] [CrossRef]
- Shimizu, R.; Park, Y.-C.; Kakinuma, K.; Iiyama, A.; Uchida, M. Effects of both oxygen permeability and ion exchange capacity for cathode ionomers on the performance and durability of polymer electrolyte fuel cells. J. Electrochem. Soc. 2018, 165, F3063. [Google Scholar] [CrossRef]
- Yamada, K.; Hommura, S.; Shimohira, T. Effect of high oxygen permeable ionomers on MEA performance for PEFC. ECS Trans. 2013, 50, 1495. [Google Scholar] [CrossRef]
- Marcinkoski, J.; Vijayagopal, R.; Adams, J.; James, B.; Kopasz, J.; Ahluwalia, R. Hydrogen Class 8 Long Haul Truck Targets. Subsection of the Electrified Powertrain Roadmap. Technical Targets for Hydrogen–Fueled Long–Haul Tractor–Trailer Trucks. DOE Adv. Truck Technol 2019, 31. [Google Scholar]
- Balogun, E.O.; Hussain, N.; Chamier, J.; Barendse, P. Performance and durability studies of perfluorosulfonic acid ionomers as binders in PEMFC catalyst layers using Electrochemical Impedance Spectroscopy. Int. J. Hydrog. Energy 2019, 44, 32219–32230. [Google Scholar] [CrossRef]
- El Kaddouri, A.; Flandin, L.; Bas, C. Chemical degradation of PFSA ionomer binder in PEMFC’s catalyst layer. Int. J. Hydrog. Energy 2018, 43, 15386–15397. [Google Scholar] [CrossRef]
- Ren, H.; Teng, Y.; Meng, X.; Fang, D.; Huang, H.; Geng, J.; Shao, Z. Ionomer network of catalyst layers for proton exchange membrane fuel cell. J. Power Sources 2021, 506, 230186. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, M.-S.; Park, J.-S. Effect of dispersing solvents for ionomers on the performance and durability of catalyst layers in proton exchange membrane fuel cells. Electrochim. Acta 2021, 391, 138971. [Google Scholar] [CrossRef]
- Garsany, Y.; Atkinson, R.W.; Sassin, M.B.; Hjelm, R.M.; Gould, B.D.; Swider–Lyons, K.E. Improving PEMFC performance using short–side–chain low–equivalent–weight PFSA ionomer in the cathode catalyst layer. J. Electrochem. Soc. 2018, 165, F381–F391. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, R.; Yang, D.; Li, B.; Ming, P.; Zhang, C. Effect of ionomer content on cathode catalyst layer for PEMFC via molecular dynamics simulations and experiments. Int. J. Hydrog. Energy 2022, 47, 23335–23347. [Google Scholar] [CrossRef]
- DOE Fuel Cell Technologies Office Multi–Year Research, Development, and Demonstration Plan. 3.4 Fuel Cells. 2016. Available online: https://www.energy.gov/sites/prod/files/2014/12/f19/fcto_myrdd_full_document.pdf (accessed on 4 September 2024).
- Litster, S. Durable High-Power-Density Fuel Cell Cathodes for Heavy-Duty Vehicles. In Proceedings of the 2022 Annual Merit Review and Peer Evaluation Report, Virtual, 6–8 June 2022. [Google Scholar]
- 2021 DOE Hydrogen and Fuel Cells Program Review Presentation, Fuel Cell System Analysis; United States Department of Energy: Washington, DC, USA, 2021.
- Takasaki, M.; Nakagawa, Y.; Sakiyama, Y.; Tanabe, K.; Ookubo, K.; Sato, N.; Minamide, T.; Nakayama, H.; Hori, M. Degradation study of perfluorosulfonic acid polymer electrolytes: Approach from decomposition product analysis. J. Electrochem. Soc. 2013, 160, F413. [Google Scholar] [CrossRef]
- Danerol, A.; Bas, C.; Flandin, L.; Claude, E.; Alberola, N. Influence of ageing in fuel cell on membrane/electrodes interfaces. J. Power Sources 2011, 196, 3479–3484. [Google Scholar] [CrossRef]
- Parry, V.; Berthomé, G.; Joud, J.-C.; Lemaire, O.; Franco, A.A. XPS investigations of the proton exchange membrane fuel cell active layers aging: Characterization of the mitigating role of an anodic CO contamination on cathode degradation. J. Power Sources 2011, 196, 2530–2538. [Google Scholar] [CrossRef]
- Zhang, F.-Y.; Advani, S.G.; Prasad, A.K.; Boggs, M.E.; Sullivan, S.P.; Beebe, T.P., Jr. Quantitative characterization of catalyst layer degradation in PEM fuel cells by X–ray photoelectron spectroscopy. Electrochim. Acta 2009, 54, 4025–4030. [Google Scholar] [CrossRef]
- Morawietz, T.; Handl, M.; Oldani, C.; Gazdzicki, P.; Hunger, J.; Wilhelm, F.; Blake, J.; Friedrich, K.A.; Hiesgen, R. High–resolution analysis of ionomer loss in catalytic layers after operation. J. Electrochem. Soc. 2018, 165, F3139. [Google Scholar] [CrossRef]
- Morawietz, T.; Handl, M.; Oldani, C.; Friedrich, K.A.; Hiesgen, R. Quantitative in situ analysis of ionomer structure in fuel cell catalytic layers. ACS Appl. Mater. Interfaces 2016, 8, 27044–27054. [Google Scholar] [CrossRef]
- Lefebvre, M.C.; Martin, R.B.; Pickup, P.G. Characterization of ionic conductivity profiles within proton exchange membrane fuel cell gas diffusion electrodes by impedance spectroscopy. Electrochem. Solid–State Lett. 1999, 2, 259. [Google Scholar] [CrossRef]
- Reshetenko, T.; Kulikovsky, A. Understanding the distribution of relaxation times of a low–Pt PEM fuel cell. Electrochim. Acta 2021, 391, 138954. [Google Scholar] [CrossRef]
- Schichlein, H.; Müller, A.C.; Voigts, M.; Krügel, A.; Ivers–Tiffée, E. Deconvolution of electrochemical impedance spectra for the identification of electrode reaction mechanisms in solid oxide fuel cells. J. Appl. Electrochem. 2002, 32, 875–882. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.G.; Jung, H.-S.; Pak, C. Unveiling the Resistance Component on Fuel Cell Electrodes by Ionic Liquid Adsorbed PtCo/C Catalyst through Distribution of Relaxation Time. Appl. Surf. Sci. 2024, 657, 159797. [Google Scholar] [CrossRef]
- Heinzmann, M.; Weber, A.; Ivers–Tiffée, E. Advanced impedance study of polymer electrolyte membrane single cells by means of distribution of relaxation times. J. Power Sources 2018, 402, 24–33. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Yoo, D.; Hwang, B.; Oh, S.; Park, K. Acceleration of electrolyte membrane degradation by frequent activation in PEMFC electrochemical durability evaluation. Korean J. Chem. Eng. 2023, 40, 2004–2009. [Google Scholar] [CrossRef]
- Danilczuk, M.; Lancucki, L.; Schlick, S.; Hamrock, S.J.; Haugen, G.M. In–depth profiling of degradation processes in a fuel cell: 2D spectral–spatial FTIR spectra of Nafion membranes. ACS Macro Lett. 2012, 1, 280–285. [Google Scholar] [CrossRef]
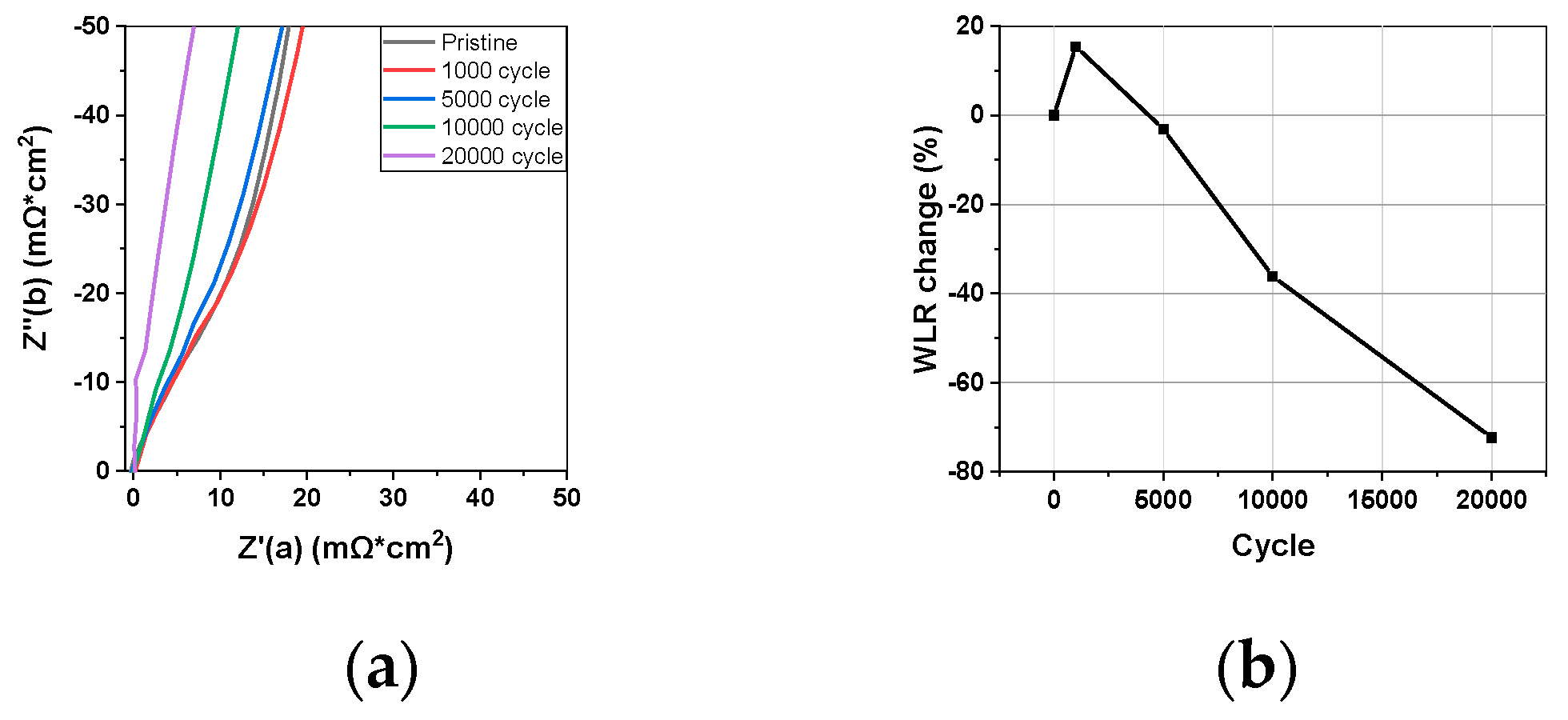
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Park, S.; Oh, S.; Kim, M.P.; Park, K. In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells. Materials 2024, 17, 4425. https://doi.org/10.3390/ma17174425
Yoo D, Park S, Oh S, Kim MP, Park K. In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells. Materials. 2024; 17(17):4425. https://doi.org/10.3390/ma17174425
Chicago/Turabian StyleYoo, Donggeun, Sujung Park, Sohyeong Oh, Minsoo P. Kim, and Kwonpil Park. 2024. "In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells" Materials 17, no. 17: 4425. https://doi.org/10.3390/ma17174425
APA StyleYoo, D., Park, S., Oh, S., Kim, M. P., & Park, K. (2024). In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells. Materials, 17(17), 4425. https://doi.org/10.3390/ma17174425








