Effects of Thermal Exposure Temperature on Room-Temperature Tensile Properties of Ti65 Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Heat Treatment Process
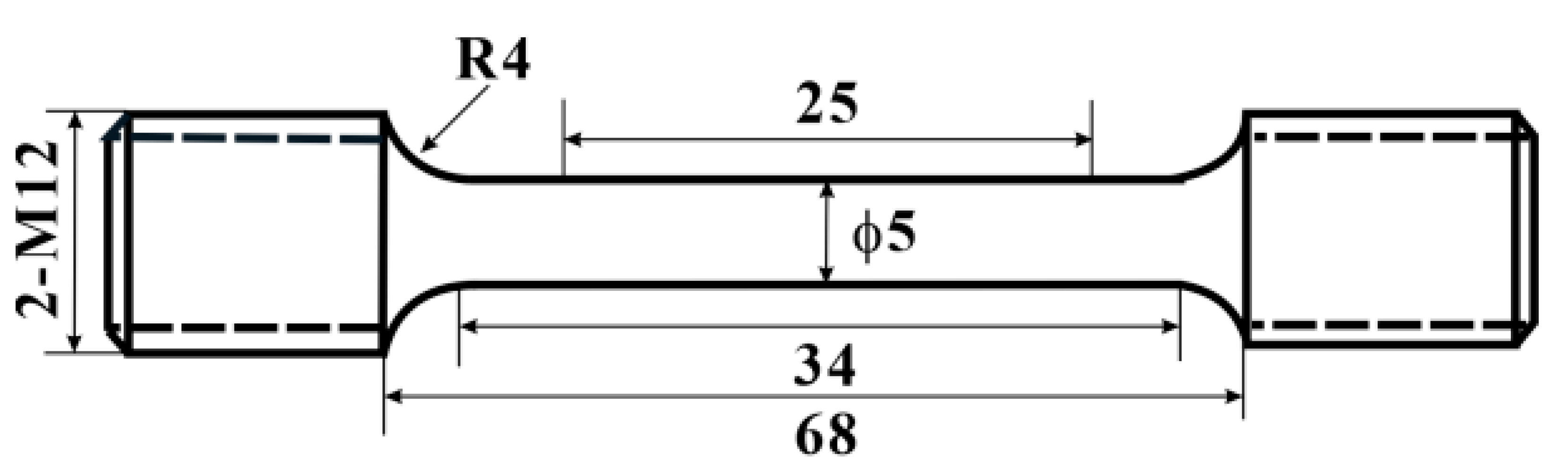
2.2. Thermal Exposure and Tensile Testing
2.3. Microstructural Characterization
3. Results
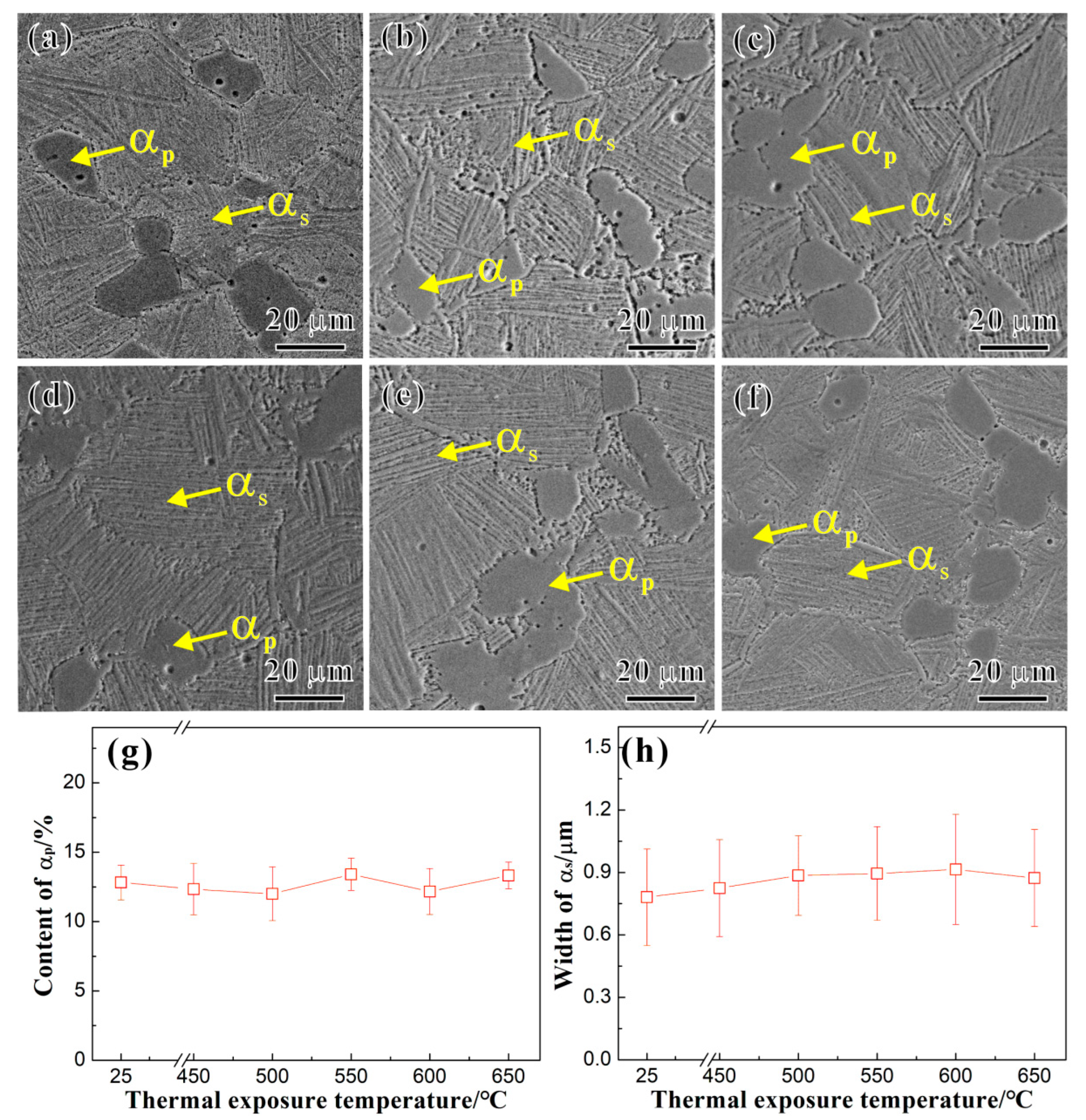
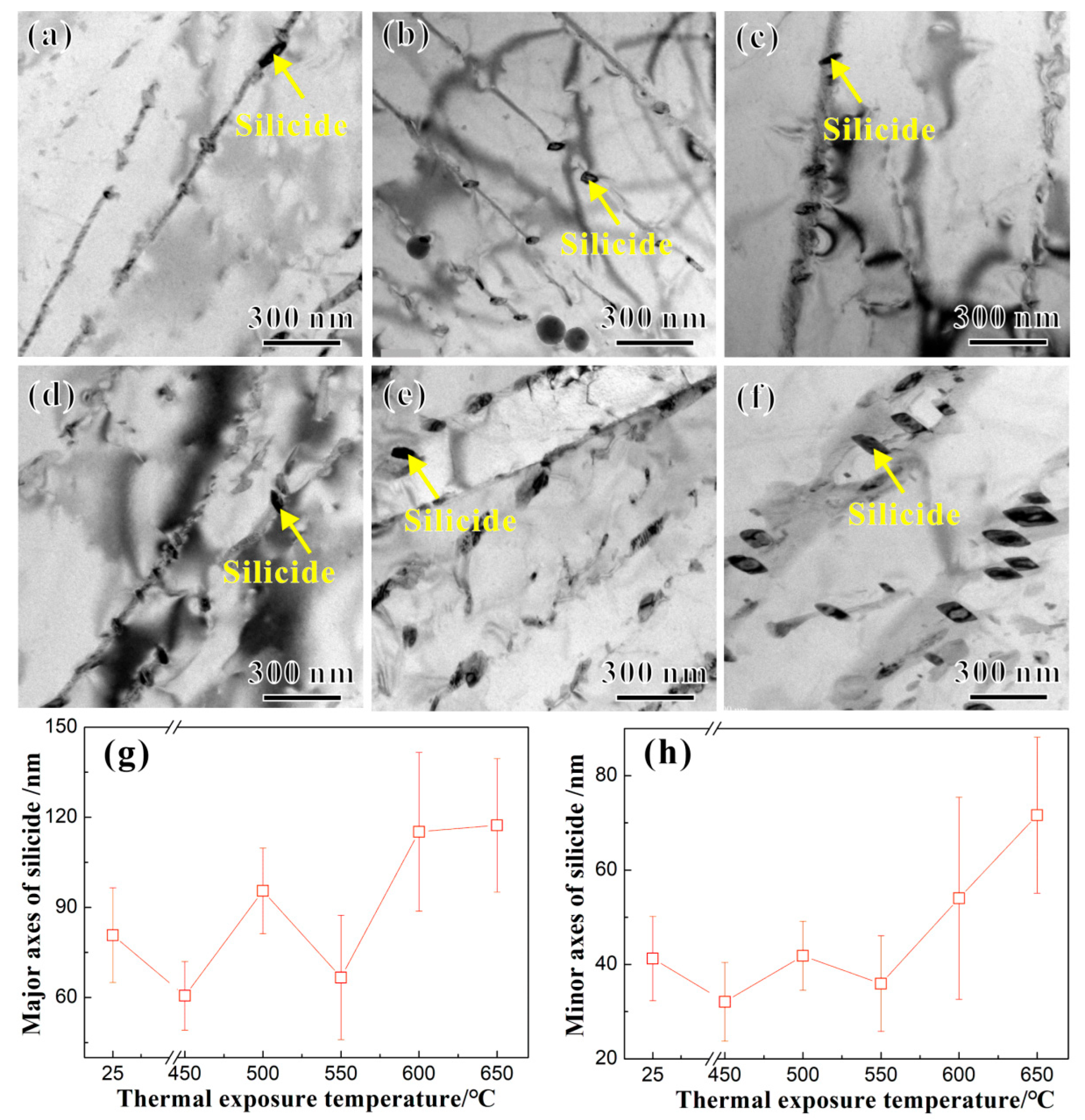
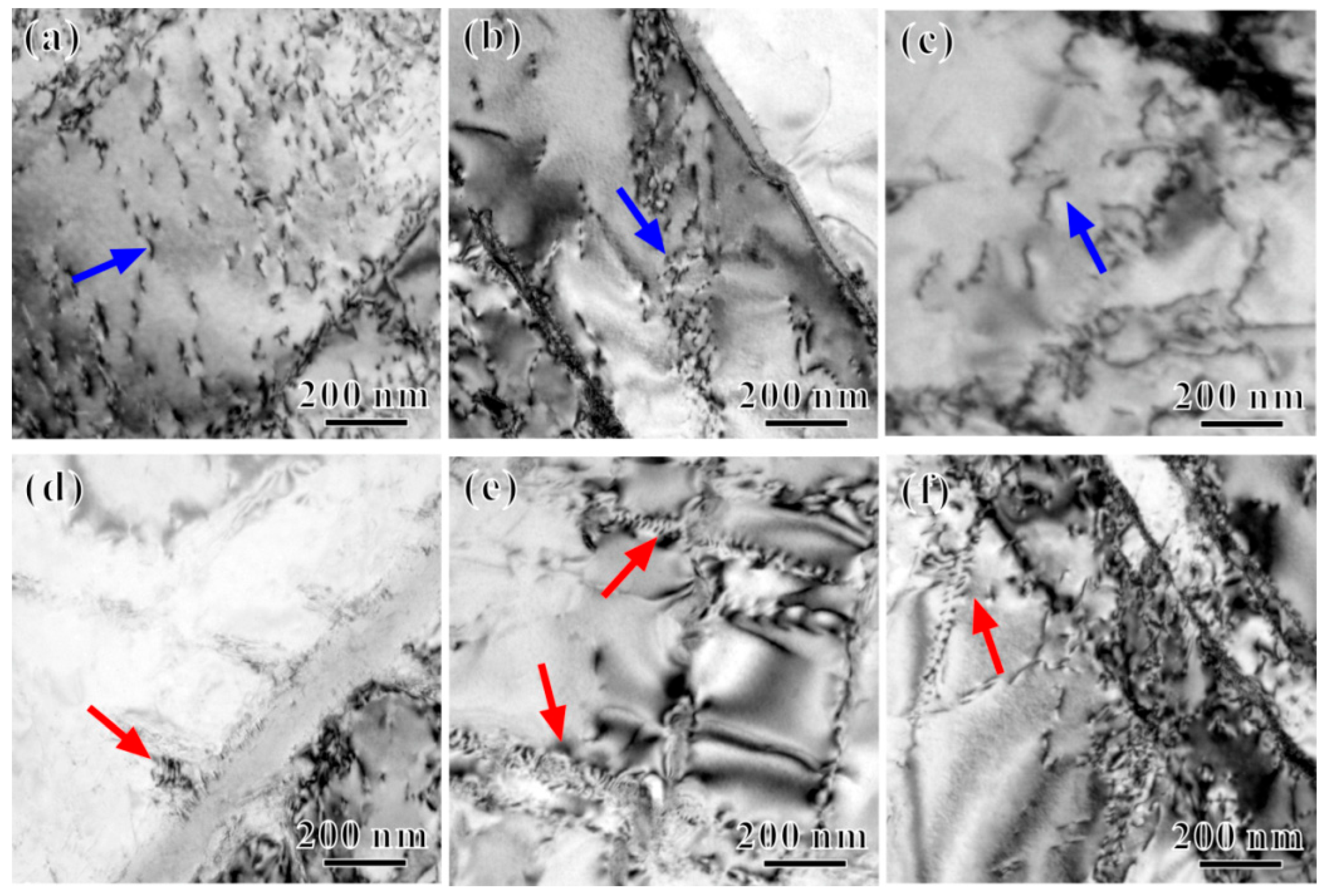
3.1. Microstructure and Precipitated Phases
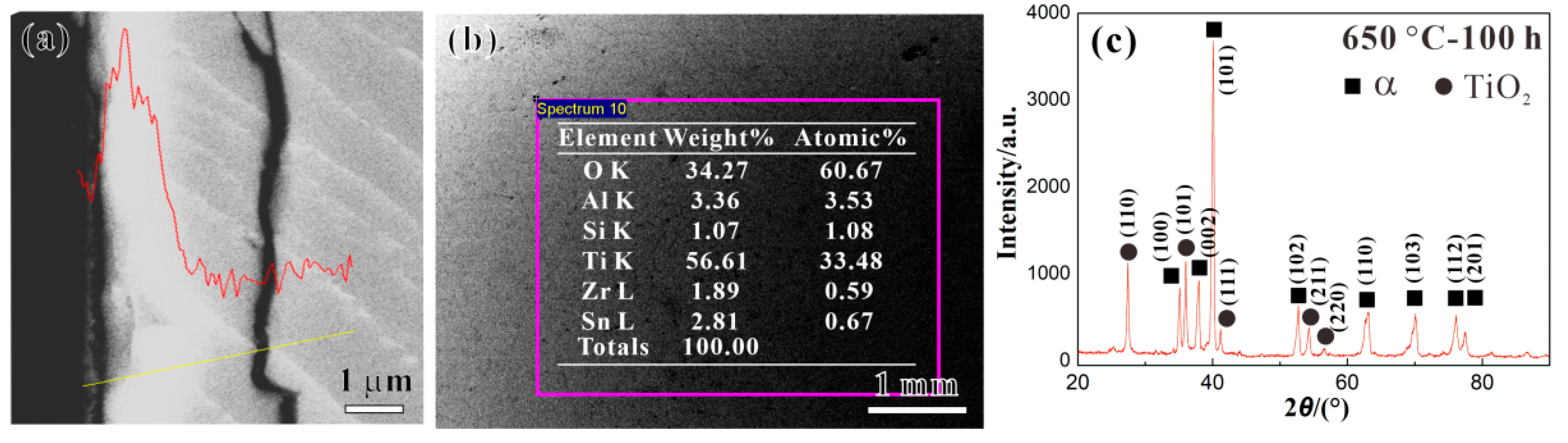
3.2. Characterization of Oxide Layer
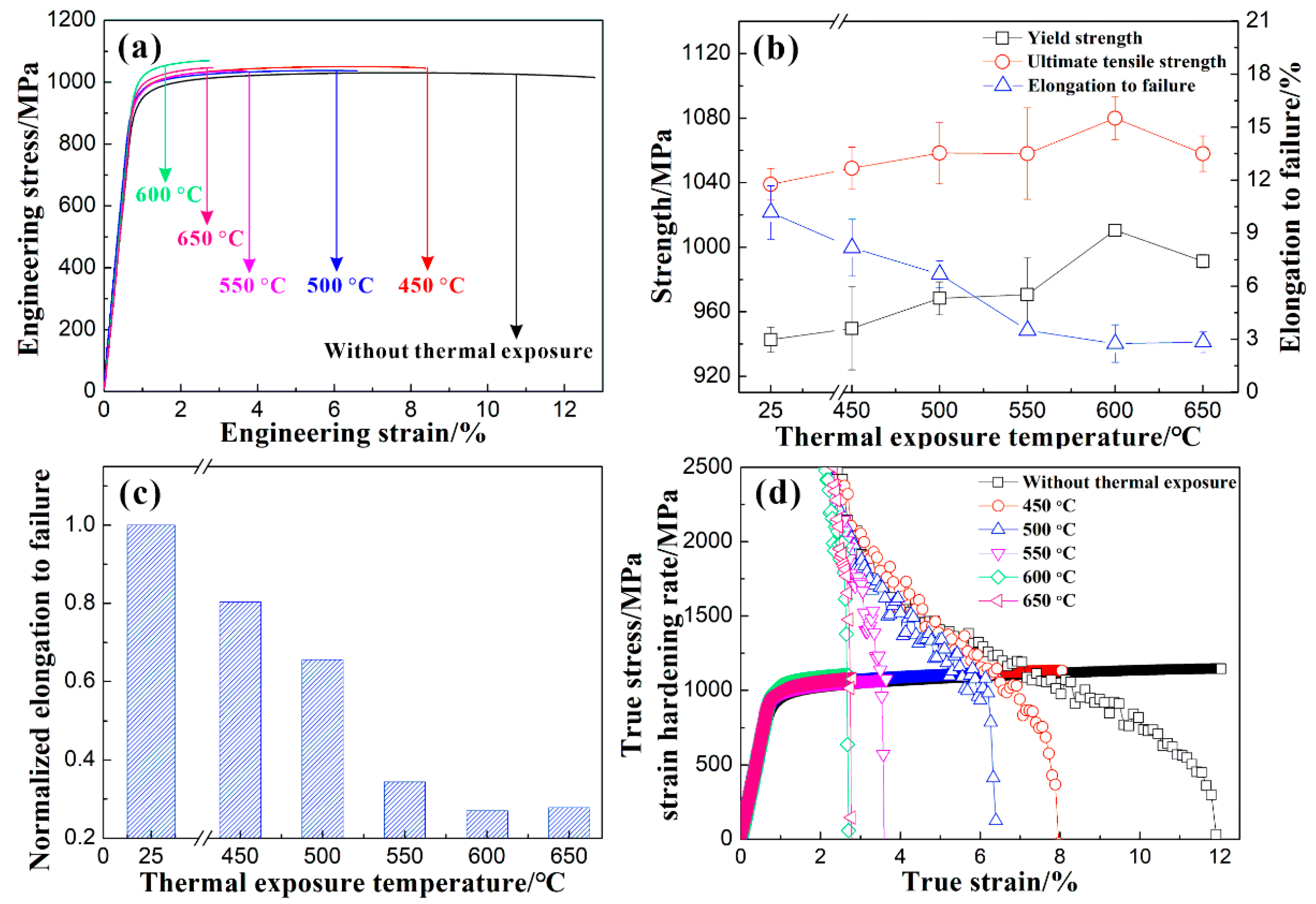
3.3. Tensile Properties
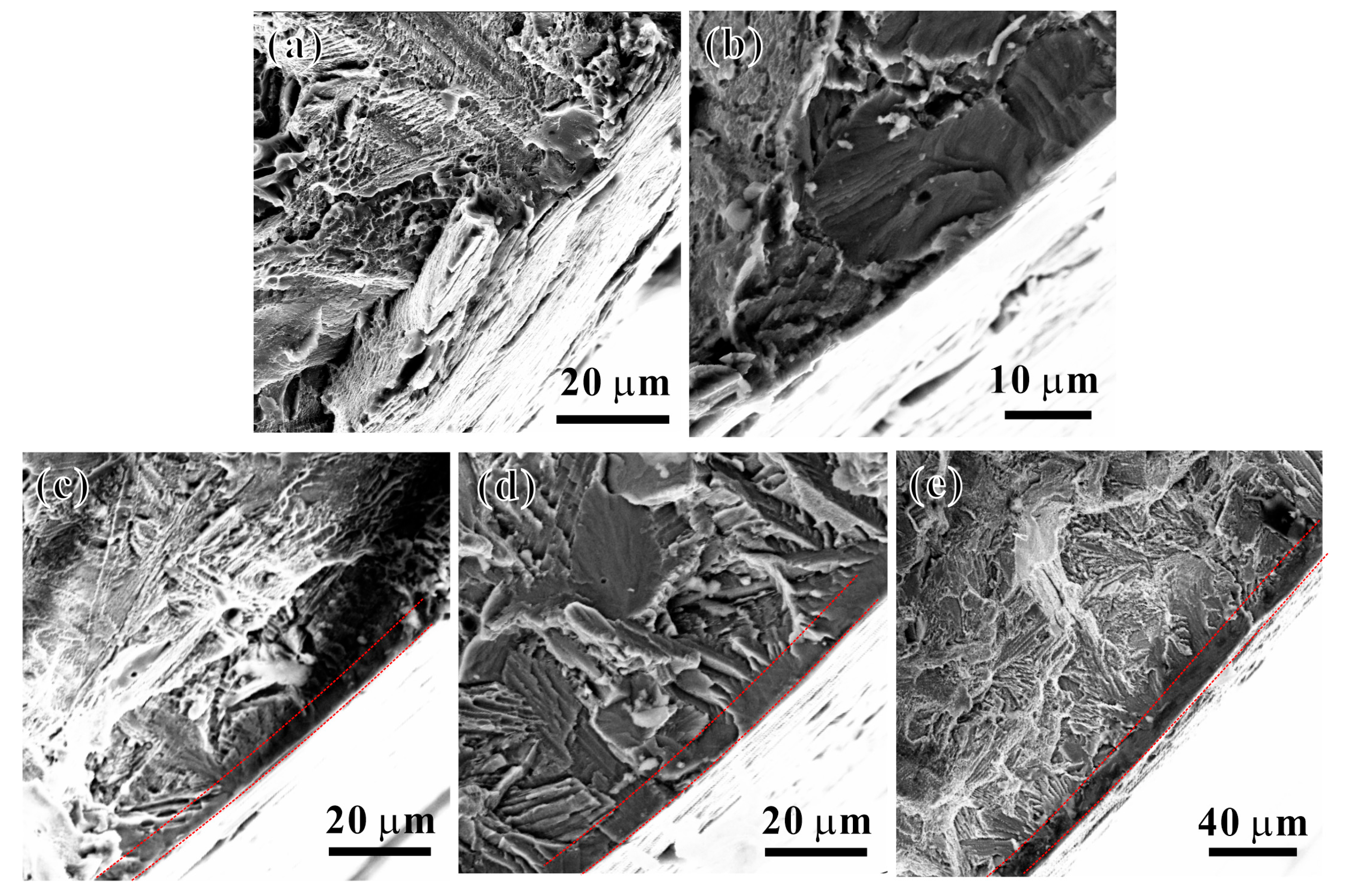
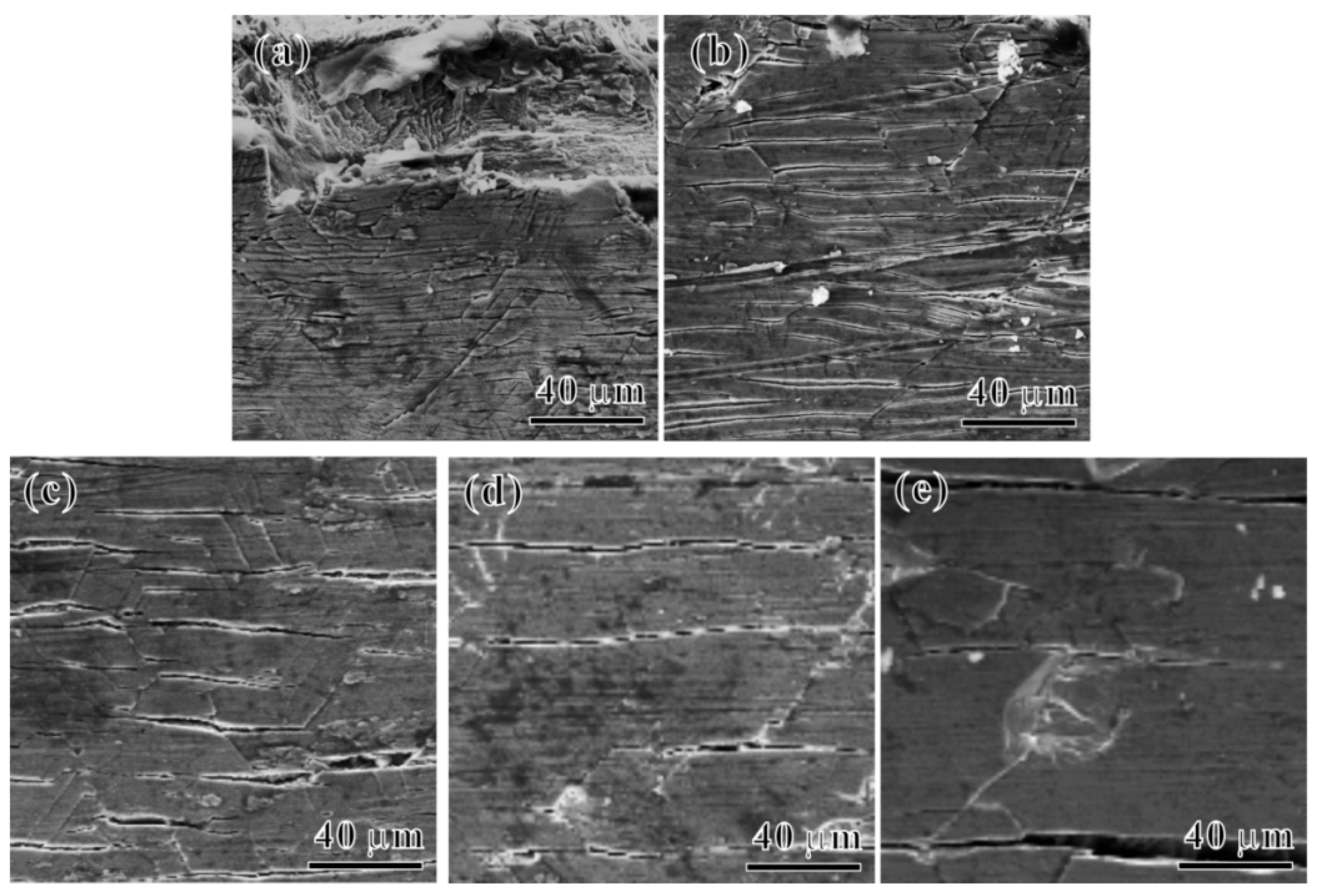
3.4. Tensile Fracture Behavior
4. Discussion
4.1. Influence of Thermal Exposure Temperature on Microstructures
4.2. Effect of Thermal Exposure on the Strength of the Alloy
4.3. Influence of Thermal Exposure on Plasticity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.S. Recent research and development of titanium alloys for aviation application in china. J. Aeronaut. Mater. 2014, 34, 44–50. [Google Scholar]
- Li, M.-Y.; Zhang, B.; Song, Z.-M.; Luo, X.-M.; Zhang, G.-P. Detecting the deformation behavior of bimodal Ti-6Al-4V using a digital image correlation technique. Materials 2022, 15, 7504. [Google Scholar] [CrossRef]
- Hu, B.-L.; Luo, Y.-W.; Zhang, B.; Zhang, G.-P. A comparative investigation of machine learning algorithms for pore-influenced fatigue life prediction of additively manufactured Inconel 718 based on a small dataset. Materials 2023, 16, 6606. [Google Scholar] [CrossRef]
- Paturi, U.M.R.; Palakurthy, S.T.; Cheruku, S.; Darshini, B.V.; Reddy, N.S. Role of machine learning in additive manufacturing of titanium alloys—A review. Arch. Comput. Methods Eng. 2023, 30, 5053–5069. [Google Scholar] [CrossRef]
- Gong, X.Y.; Yabansu, Y.C.; Collins, P.C.; Kalidindi, S.R. Evaluation of Ti-Mn alloys for additive manufacturing using high-throughput experimental assays and gaussian process regression. Materials 2020, 13, 4641. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-M.; Cao, C.-X. Alloy design and application expectation of a new generation 600 °C high temperature titanium alloy. J. Aeronaut. Mater. 2014, 34, 1–26. [Google Scholar]
- Zhao, D.; Fan, J.; Wang, J.; Liang, X.; Wang, Q.; Chen, Z.; Zhang, Z.; Tang, B.; Kou, H.; Li, J. Influence of silicide and texture on the room temperature deformation size effect of Ti65 alloy sheets/foils and its constitutive model modification. Mater. Sci. Eng. A 2022, 856, 143999. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhou, B.; Liu, N.; Chen, L.Q. Effects of microstructure and rare-earth constituent on the oxidation behavior of Ti–5.6Al–4.8Sn–2Zr–1Mo–0.35Si–0.7Nd titanium alloy. Oxid. Met. 2014, 81, 373–382. [Google Scholar] [CrossRef]
- Satko, D.P.; Shaffer, J.B.; Tiley, J.S.; Semiatin, S.L.; Pilchak, A.L.; Kalidindi, S.R.; Kosaka, Y.; Glavicic, M.G.; Salem, A.A. Effect of microstructure on oxygen rich layer evolution and its impact on fatigue life during high-temperature application of α/β titanium. Acta Mater. 2016, 107, 377–389. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, D.; Tang, H.B.; Wang, H.M. Effect of thermal exposure on microstructure and mechanical properties of laser deposited Ti60A high temperature titanium alloy. Rare Met. Mater. Eng. 2014, 43, 1686–1690. [Google Scholar]
- Lunt, D.; Busolo, T.; Xu, X.; da Fonseca, J.Q. Effect of nanoscale α2 precipitation on strain localisation in a two-phase Ti-alloy. Acta Mater. 2017, 129, 72–82. [Google Scholar] [CrossRef]
- Madsen, A.; Ghonem, H. Separating the effects of Ti3Al and silicide precipitates on the tensile and crack growth behavior at room temperature and 593 °C in a near-alpha titanium alloy. J. Mater. Eng. Perform. 1995, 4, 301–307. [Google Scholar] [CrossRef]
- Woodfield, A.P.; Postans, P.J.; Loretto, M.H.; Smallman, R.E. The effect of long-term high temperature exposure on the structure and properties of the titanium alloy Ti 5331S. Acta Mater. 1988, 36, 507–515. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. National Standard of The People’s Republic of China: Beijing, China, 2021.
- Madsen, A.; Andrieu, E.; Ghonem, H. Microstructural changes during aging of a near-α titanium alloy. Mater. Sci. Eng. A 1993, 171, 191–197. [Google Scholar] [CrossRef]
- Ramachandra, C.; Singh, A.K.; Sarma, G.M.K. Microstructural characterisation of near-α titanium alloy Ti-6Al-4Sn-4Zr-0.70Nb-0.50Mo-0.40Si. Metall. Mater. Trans. A 1993, 24, 1273–1280. [Google Scholar] [CrossRef]
- Rajabi, A.; Mashreghi, A.R.; Hasani, S. Non-isothermal kinetic analysis of high temperature oxidation of Ti-6Al-4V alloy. J. Alloys Compd. 2020, 815, 151948. [Google Scholar] [CrossRef]
- Jia, W.J.; Zeng, W.D.; Liu, J.R.; Wang, Q.J.; Zhou, Y.G. Oxidation behavior of Ti60 high temperature titanium alloy. Rare Met. Mater. Eng. 2010, 399, 781–786. [Google Scholar]
- Naydenkin, E.V.; Mishin, I.P.; Ratochka, I.V.; Lykova, O.N. In deformation behavior of near β titanium alloy with oxide layer at room temperature. AIP Conf. Proc. 2020, 2310, 020220. [Google Scholar]
- Zhao, E.; Sun, S.; Zhang, Y. Recent advances in silicon containing high temperature titanium alloys. J. Mater. Res. Technol. 2021, 14, 3029–3042. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Song, X.-Y.; Hui, S.-X.; Ye, W.-J.; Yu, Y.; Li, Y.-F. α2 phase precipitation behavior and tensile properties at room temperature and 650 °C in an (α+β) titanium alloy. Rare Met. 2021, 40, 3261–3268. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Song, X.-Y.; Hui, S.-X.; Ye, W.-J.; Wang, W.-Q. Phase precipitation behavior and tensile property of a Ti–Al–Sn–Zr–Mo–Nb–W–Si titanium alloy. Rare Met. 2015, 37, 1064–1069. [Google Scholar] [CrossRef]
- Zhang, T.J. Electron microscopic study of phase transformation in titanium alloys (VII)—Precipitation of ordered phase Ti3Al in heat-resistant titanium alloys. Rare Met. Mater. Eng. 1990, 2, 74–78. [Google Scholar]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: London, UK, 2008. [Google Scholar]
- Wei, S.-Y.; Shi, W.-M.; Wang, D.-C.; Wang, Q.-J.; Chen, Z.-Y.; Liu, J.-R. Microstructure and mechanical properties of high temperature titanium alloy Ti60 at 600 °C. Chin. J. Nonferrous Met. 2021, 20, 824–829. [Google Scholar]
- Yu, H.-C.; Xie, S.-S.; Zhao, G.-P.; Yang, H.-C. High temperature tensile and creep rupture properties in Ni-based superalloy. J. Mater. Eng. 2003, 9, 3–6. [Google Scholar]
- Krzysztof, A.E.; Adrian, B.; Marian, K. Modelling the structure and mechanical properties of oxide layers obtained on biomedical Ti-6Al-7Nb alloy in the thermal oxidation process. Vacuum 2018, 154, 309–314. [Google Scholar]
- Singh, A.; Balasundar, I.; Gautam, J.P.; Raghu, T. Effect of primary α phase fraction on tensile behavior of IMI 834 alloy. Procedia Struct. Integr. 2019, 14, 78–88. [Google Scholar] [CrossRef]
- Han, D.; Wang, Z.Y.; Yan, Y.; Shi, F.; Li, X.W. A good strength-ductility match in Cu-Mn alloys with high stacking fault energies: Determinant effect of short range ordering. Scr. Mater. 2017, 133, 59–64. [Google Scholar] [CrossRef]




| Element | Al | Sn | Zr | Mo | Si | Nb | Ta | W | C | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 5.75 | 4.00 | 3.50 | 0.50 | 0.40 | 0.30 | 1.00 | 0.80 | 0.05 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Liu, J.-Y.; Liu, J.-R.; Li, W.-Y.; Zhang, B.; Zhang, G.-P. Effects of Thermal Exposure Temperature on Room-Temperature Tensile Properties of Ti65 Alloy. Materials 2024, 17, 4424. https://doi.org/10.3390/ma17174424
Wang Y-C, Liu J-Y, Liu J-R, Li W-Y, Zhang B, Zhang G-P. Effects of Thermal Exposure Temperature on Room-Temperature Tensile Properties of Ti65 Alloy. Materials. 2024; 17(17):4424. https://doi.org/10.3390/ma17174424
Chicago/Turabian StyleWang, Yuan-Chen, Jian-Yang Liu, Jian-Rong Liu, Wen-Yuan Li, Bin Zhang, and Guang-Ping Zhang. 2024. "Effects of Thermal Exposure Temperature on Room-Temperature Tensile Properties of Ti65 Alloy" Materials 17, no. 17: 4424. https://doi.org/10.3390/ma17174424
APA StyleWang, Y.-C., Liu, J.-Y., Liu, J.-R., Li, W.-Y., Zhang, B., & Zhang, G.-P. (2024). Effects of Thermal Exposure Temperature on Room-Temperature Tensile Properties of Ti65 Alloy. Materials, 17(17), 4424. https://doi.org/10.3390/ma17174424







