Geothermal Nano-SiO2 Waste as a Supplementary Cementitious Material for Concrete Exposed at High Critical Temperatures
Abstract
:1. Introduction
2. Materials and Methods
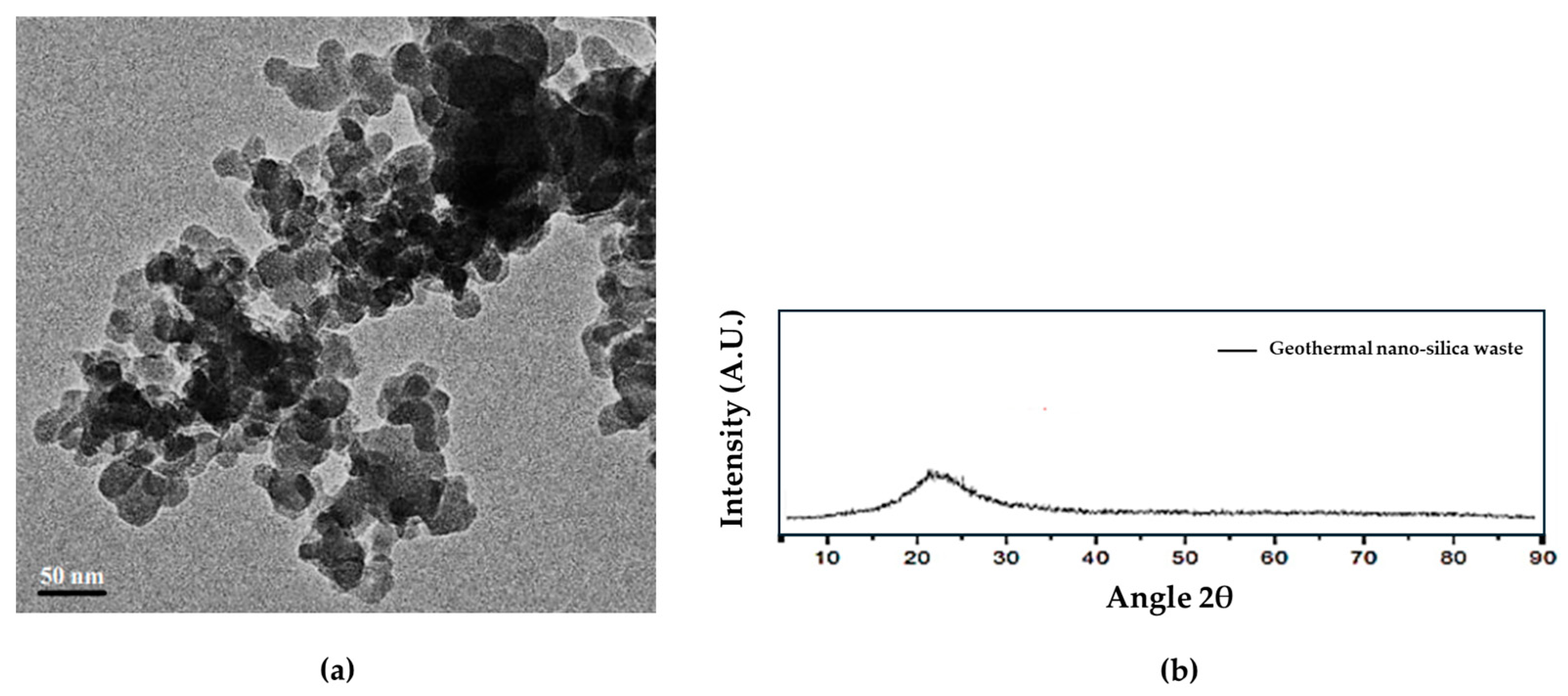
2.1. Material Characterization
2.2. Mix Design
2.3. Heat Treatment of Concrete Samples
2.4. Instrumental Methods
3. Results and Discussion
3.1. Physical Properties
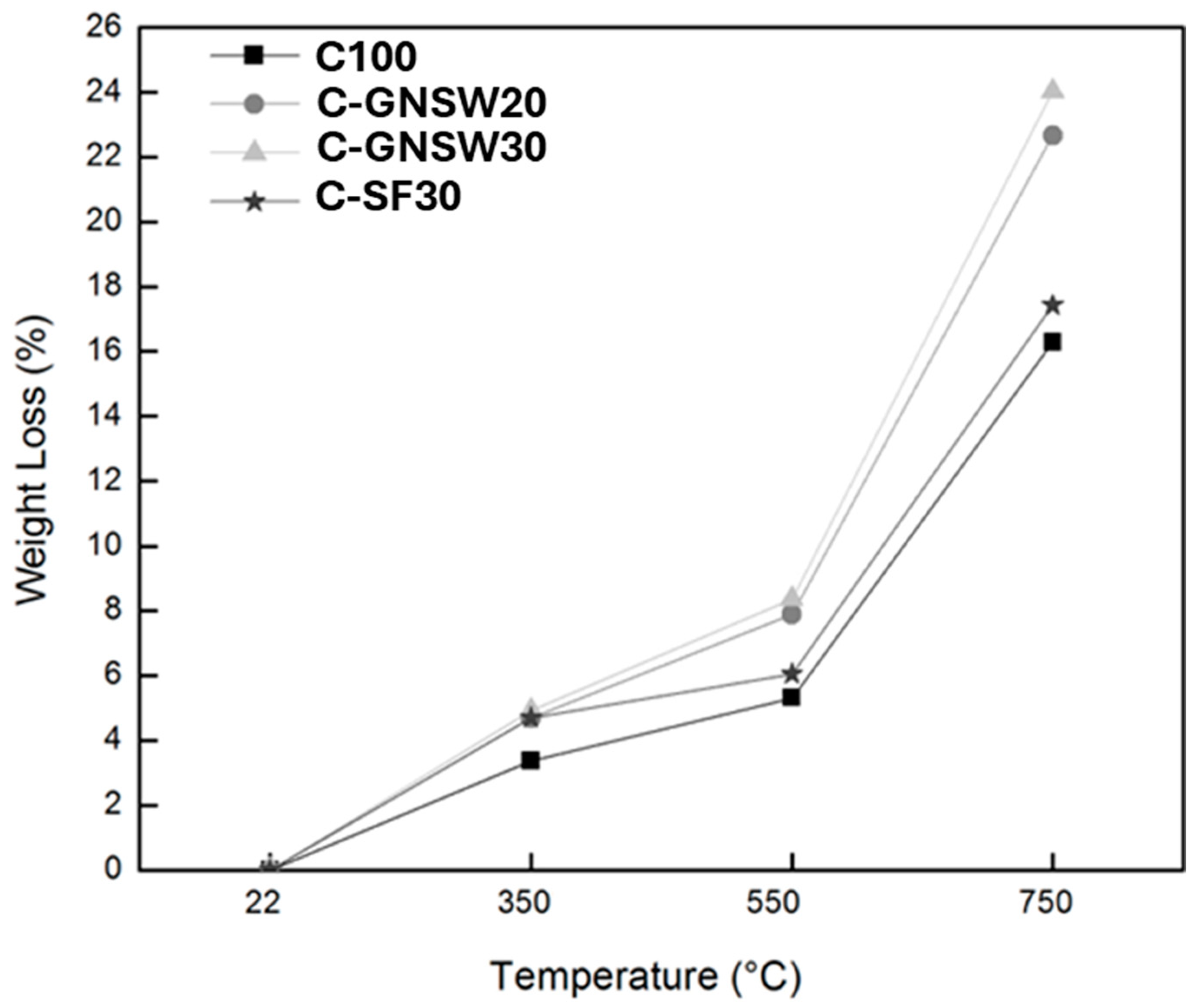
3.2. Weight Loss
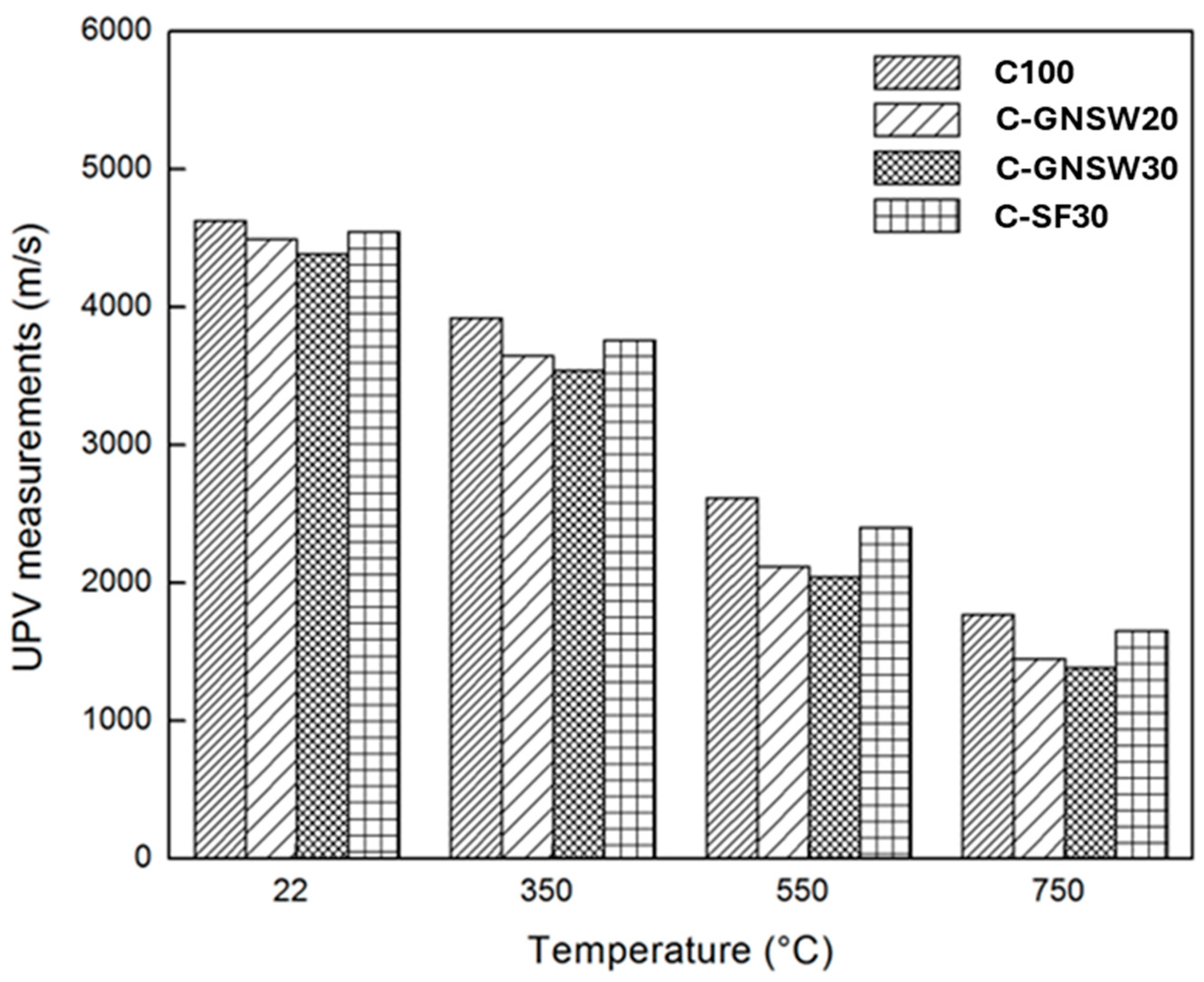
3.3. Ultrasonic Pulse Velocity (UPV)
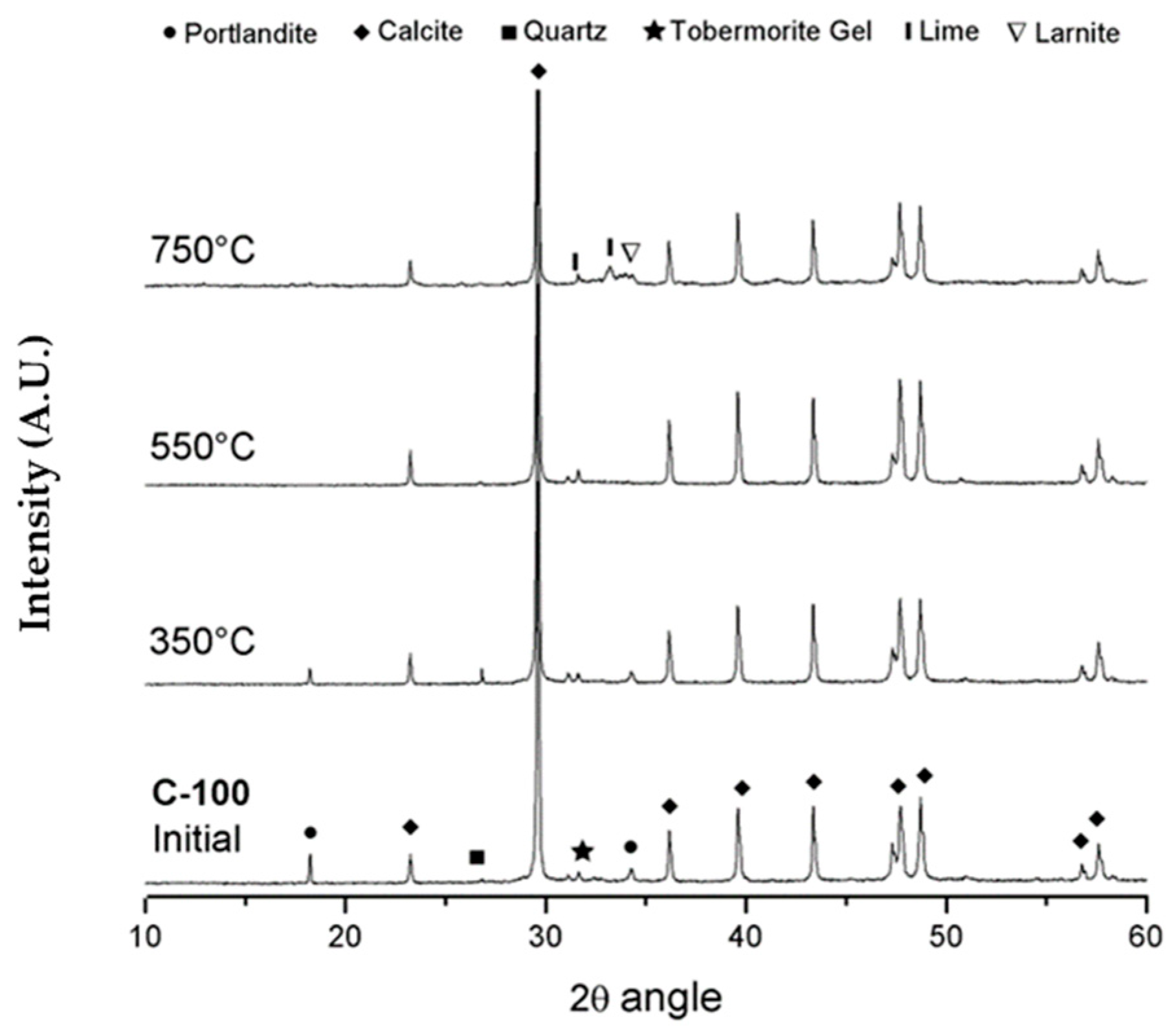
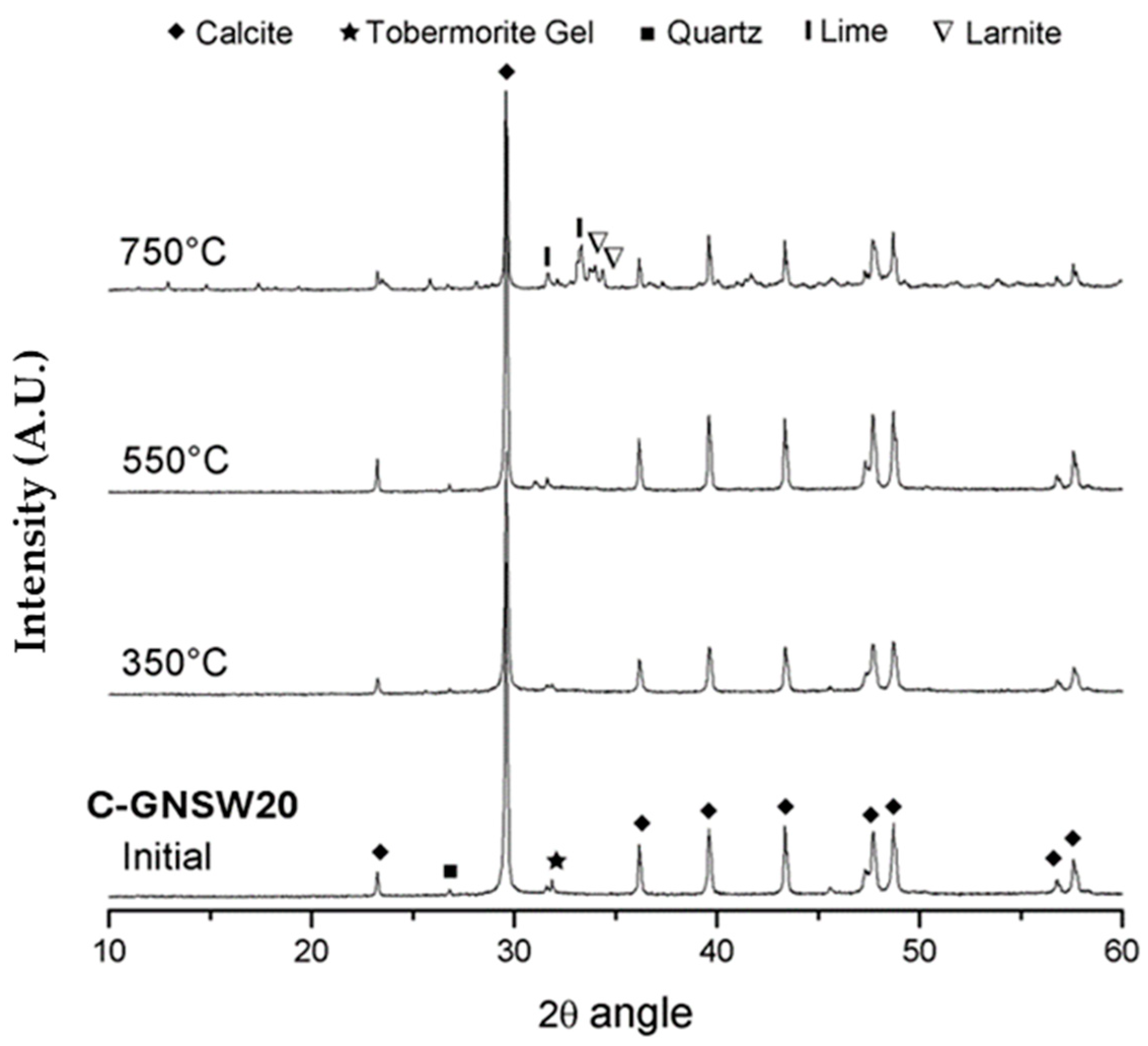
3.4. X-ray Diffraction (XRD)
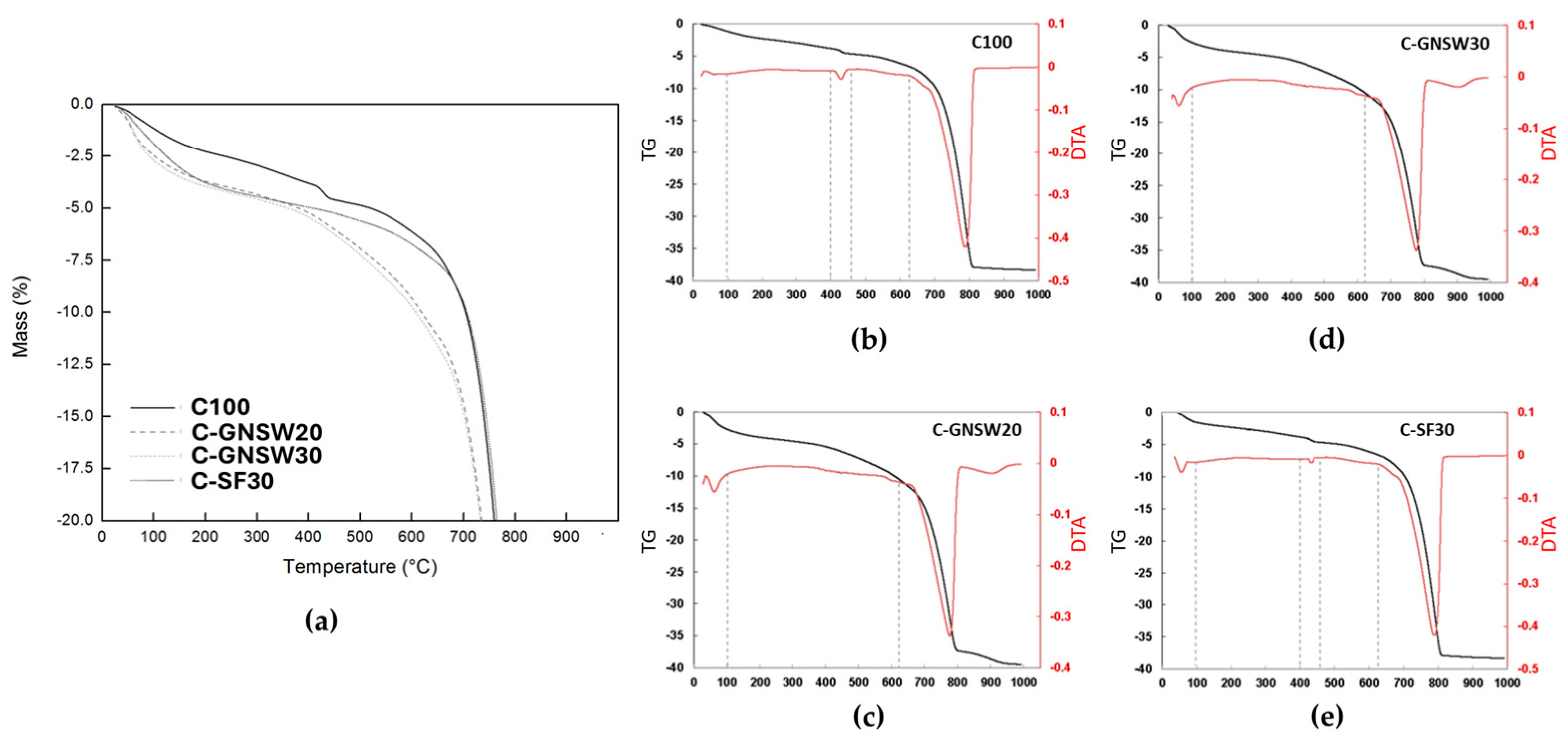
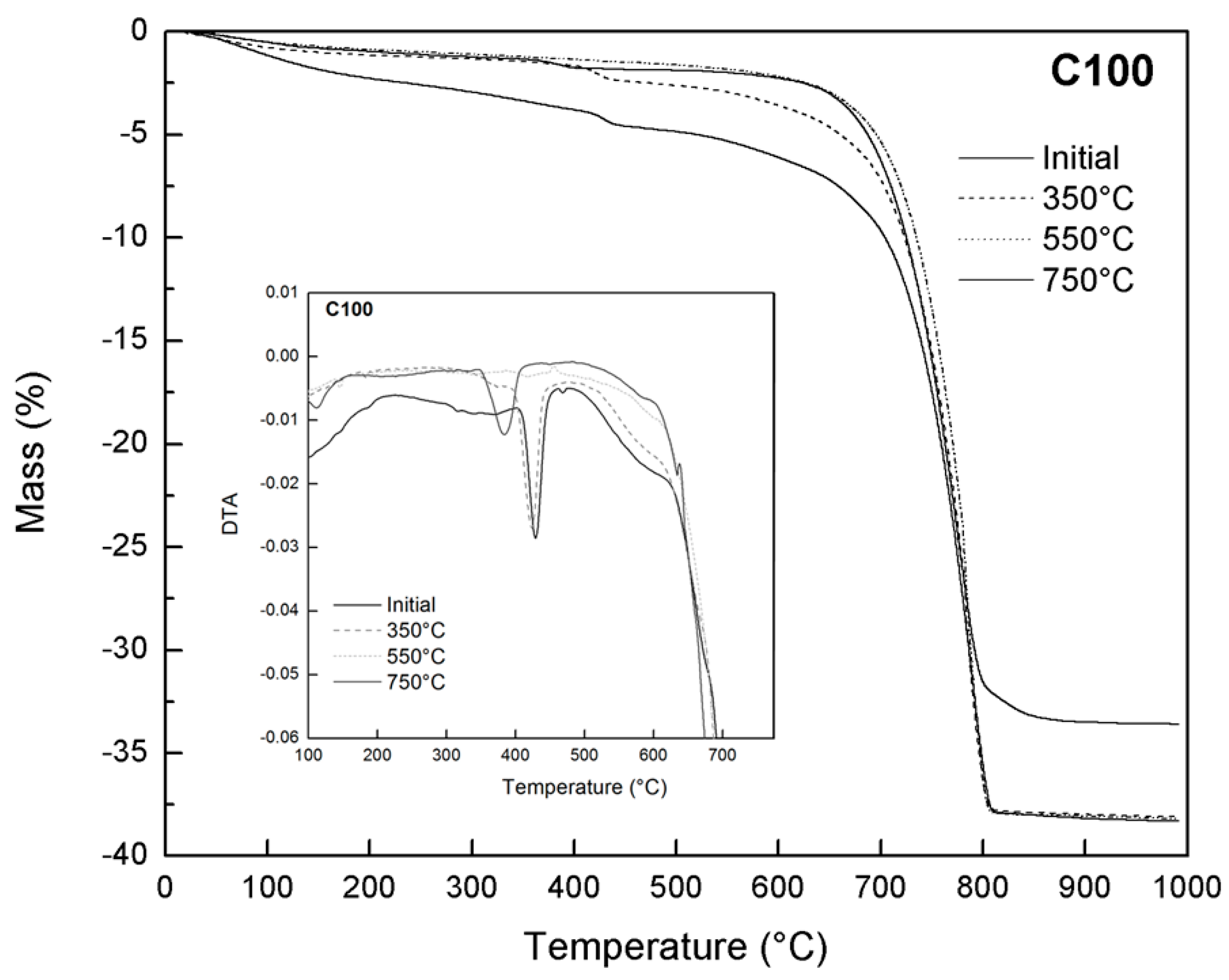
3.5. Thermal Analysis (TG-DTA)
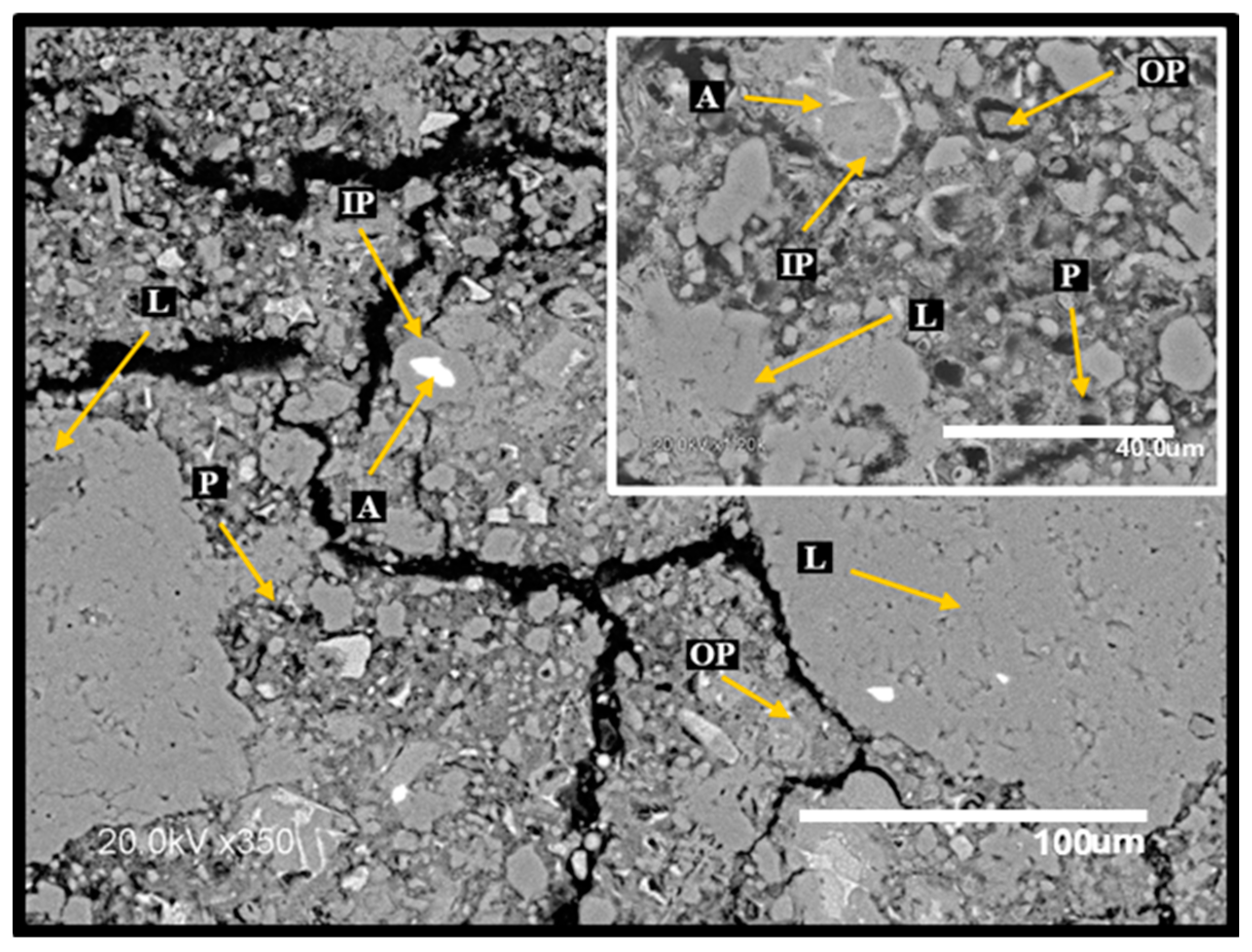
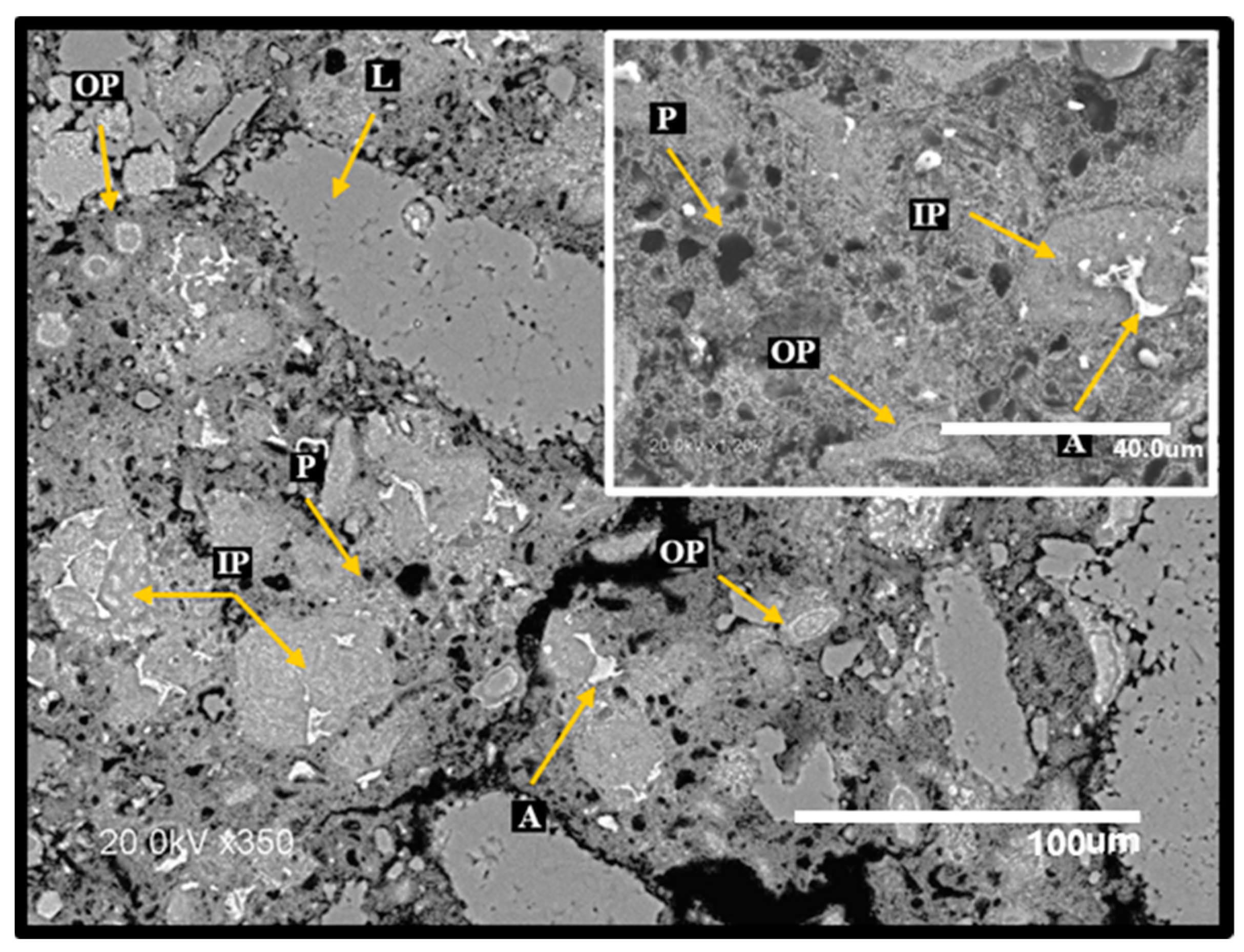
3.6. Surface Inspection and Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Environment; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Neville, A.M.; Brooks, J.J. Concrete Technology, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Montoya, A.S.; Chung, C.-W.; Kim, J.-H. High Performance Concretes with Highly Reactive Rice Husk Ash and Silica Fume. Materials 2023, 16, 3903. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.-M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emission from global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; de Gutiérrez, R.M.; Martínez, E. Life cycle assessment (LCA) of an alkali-activated binary concrete based on natural volcanic pozzolan: A comparative analysis to OPC concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, Q. Upcycling carbon dioxide to improve mechanical strength of Portland cement. J. Clean. Prod. 2018, 196, 726–738. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Magallanes-Rivera, R.X.; Gorokhovsky, A. Waste gypsum–blast furnace slag cement in mortars with granulated slag and silica sand as aggregates. Constr. Build. Mater. 2009, 23, 2851–2855. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.; Sarker, P.K. Value added utilization of by-product electric furnace ferronickel slag as construction materials: A review. Resour. Conserv. Recycl. 2018, 134, 10–24. [Google Scholar] [CrossRef]
- Gartner, E.M.; Macphee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Miner. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Supplementary cementitious materials and blended cements. In Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 53–73. [Google Scholar]
- Papadakis, V.G.; Tsimas, S. Supplementary cementig materials in concrete: Part I: Efficiency and design. Cem. Concr. Res. 2002, 32, 1525–1532. [Google Scholar] [CrossRef]
- Megat Johari, M.A.; Brooks, J.J.; Kabir, S.; Rivard, P. Influence of supplementary cementitious materials on engineering properties of high strength concrete. Constr. Build. Mater. 2011, 25, 2639–2648. [Google Scholar] [CrossRef]
- Juenger, M.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Ali, M.; Saidur, R.; Hossain, M. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Higuchi, T.; Morioka, M.; Yoshioka, I.; Yokozeki, K. Development of a new ecological concrete with CO2 emission below zero. Constr. Build. Mater. 2014, 67, 338–343. [Google Scholar] [CrossRef]
- Heikal, M.; Al-Duaij, O.; Ibrahim, N. Microstructure of composite cements containing blast-furnace slag and silica nanoparticles subjected to elevated thermally treatment temperature. Constr. Build. Mater. 2015, 93, 1067–1077. [Google Scholar] [CrossRef]
- Morsy, M.S.; Al-Salloum, Y.A.; Abbas, H.; Alsayed, S.H. Behavior of blended cement mortars containing nano-metakaolin at elevated temperatures. Constr. Build. Mater. 2012, 35, 900–905. [Google Scholar] [CrossRef]
- Kong, D.; Du, X.; Wei, S.; Zhang, H.; Yang, Y.; Shah, S. Influence of nano-silica agglomeration on microstructure and properties of the hardened cement-based materials. Constr. Build. Mater. 2012, 37, 707–715. [Google Scholar] [CrossRef]
- Heikal, M.; Ali, A.I.; Ismail, M.N.; Awad, S.; Ibrahim, N.S. Behavior of composite cement pastes containing silica nano-particles at elevated temperatures. Constr. Build. Mater. 2014, 70, 339–350. [Google Scholar] [CrossRef]
- Al Tawaiha, H.; Alhomaidat, F.; Eljufout, T. A Review of the Effect of Nano-Silica on the Mechanical and Durability Properties of Cementitious Composites. Infrastructures 2023, 8, 132. [Google Scholar] [CrossRef]
- Barbhuiya, G.H.; Moiz, M.A.; Hasan, S.D.; Zaheer, M.M. Effects of the nanosilica addition on cement concrete: A review. Mater. Today Proc. 2020, 32, 560–566. [Google Scholar] [CrossRef]
- Karlina, A.I.; Karlina, Y.I.; Gladkikh, V.A. Analysis of Experience in the Use of Micro- and Nanoadditives from Silicon Production Waste in Concrete Technologies. Minerals 2023, 13, 1525. [Google Scholar] [CrossRef]
- Abhilash, P.P.; Nayak, D.K.; Sangoju, B.; Kumar, R.; Kumar, V. Effect of nano silica in concrete; a review. Constr. Build. Mater. 2021, 278, 122347. [Google Scholar]
- Morteza, B.; Mazyar, B.; Farhad, A. Performance of nano silica modified high strength concrete at elevated temperatures. Constr. Build. Mater. 2014, 68, 402–408. [Google Scholar]
- Singh, L.; Karade, S.; Bhattacharyya, S.; Yousuf, M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials. A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Effect of pumice and fly ash incorporation on high temperature resistance of cement based mortars. Cem. Concr. Res. 2007, 37, 988–995. [Google Scholar] [CrossRef]
- Ibrahim, R.; Hamid, R.; Taha, M. Fire resistance of high volume fly ash mortars with nanosilica addition. Constr. Build. Mater. 2012, 36, 779–786. [Google Scholar] [CrossRef]
- Iñiguez-Sánchez, C.; Gómez-Zamorano, L.; Alonso, M. Impact of nano-geothermal silica waste and chloride content on pore solution, microstructure, and hydration products in Portland cement pastes. J. Mater. Sci. 2012, 47, 3639–3647. [Google Scholar] [CrossRef]
- Gómez-Zamorano, L.Y.; Escalante-García, J.I.; Mendoza-Suárez, G. Geothermal waste: An alternative replacement material of portland cement. J. Mater. Sci. 2004, 39, 4021–4025. [Google Scholar] [CrossRef]
- Escalante, J.; Mendoza, G.; Mancha, H.; López, J.; Vargas, G. Pozzolanic properties of a geothermal silica waste material. Cem. Concr. Res. 1999, 29, 623–625. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Li, X.; Bai, C.; Qiao, Y.; Wang, X.; Yang, K.; Colombo, P. Preparation, properties and applications of fly ash-based porous geopolymers: A review. J. Clean. Prod. 2022, 359, 132043. [Google Scholar] [CrossRef]
- Barboza-Chavez, A.C.; Gómez-Zamorano, L.Y.; Acevedo-Dávila, J.L. Synthesis and Characterization of a Hybrid Cement Based on Fly Ash, Metakaolin and Portland Cement Clinker. Materials 2020, 13, 1084. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Ni, W.; Wang, H.-J.; Liu, H.-J. Activated fly ash/slag blended cement. Resour. Conserv. Recycl. 2007, 52, 303–313. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Khalid, N.H.A.; Fediuk, R.; Ozbakkaloglu, T.; Lee, Y.H.; Haruna, S.; Lee, Y.Y. Slag uses in making an ecofriendly and sustainable concrete: A review. Constr. Build. Mater. 2021, 272, 121942. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Cementos híbridos de bajo impacto ambiental: Reducción del factor Clinker. Rev. ALCONPAT 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Guerra-Cossío, M.A.; González-López, J.R.; Magallanes-Rivera, R.X.; Zaldívar-Cadena, A.A.; Figueroa Torres, M.Z. Anhydrite, blast-furnace slag and silica fume composites: Properties and reaction products. Adv. Cem. Res. 2019, 31, 362–369. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Batuecas, E.; Ramón-Álvarez, I.; Sánchez-Delgado, S.; Torres-Carrasco, M. Carbon footprint and water use of alkali-activated and hybrid cement mortars. J. Clean. Prod. 2021, 319, 128653. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.V.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jimenez, A.M. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Qiu, J.; Xing, J. Review: Alkali-activated blast furnace slag for eco-friendly binders. J. Mater. Sci. 2022, 57, 1599–1622. [Google Scholar] [CrossRef]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Daci’c, A.; Kopecskó, K.; Fenyvesi, O.; Merta, I. The Obstacles to a Broader Application of Alkali-Activated Binders as a Sustainable Alternative—A Review. Materials 2023, 16, 3121. [Google Scholar] [CrossRef]
- John, V.M.; Damineli, B.L.; Quattrone, M.; Pileggi, R.G. Fillers in cementitious materials—Experience, recent advances and future potential. Cem. Concr. Res. 2018, 114, 65–78. [Google Scholar] [CrossRef]
- Bijen, J. Benefits of slag and fly ash. Constr. Build. Mater. 1996, 10, 309–314. [Google Scholar] [CrossRef]
- Jamali, M.; Gupta, S. Utilization of silica fume with fly ash and properties of Portland cement-silica fume-fly ash-concrete. AIP Conf. Proc. 2023, 2558, 020025. [Google Scholar]
- Gomez-Zamorano, L.Y.; Escalante-Garcia, J.I. Hydration and microstructure of Portland cement partially substituted with ultrafine silica. Mater. Constr. 2009, 59, 5–16. [Google Scholar] [CrossRef]
- León-Malacara, B. Effect of Adittion of Geothermal Silica Waste with Different Concentrations of Chlorides in Portland Cement Mortars (Efecto de la Adición de Desecho Geotérmico con Diferentes Concentraciones de Cloruros en Morteros de Cemento Portland). Master’s Thesis, CINVESTAV, Mexico City, Mexico, 2007. (In Spanish). [Google Scholar]
- Lozano-Vargas, J.; Gomez-Zamorano, L.Y. Investigation of the behavior of composite cements with ground granulated blast furnace slag. In Proceedings of the XIII International Congress on the Cement Chemistry, Madrid, Spain, 3–8 July 2011; ISBN 978-84-7292399-7. [Google Scholar]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. One-part geopolymer mixes from geothermal silica and sodium aluminate. Ind. Eng. Chem. Res. 2008, 47, 9396–9405. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Gorokhovsky, A.V.; Mendoza, G.; Fuentes, A.F. Effect of geothermal waste on strength and microstructure of alkali-activated slag cement mortars. Cem. Concr. Res. 2003, 33, 1567–1574. [Google Scholar] [CrossRef]
- Gomez-Zamorano, L.Y.; Vega-Cordero, E.; Struble, L. Composite geopolymers of metakaolin and geothermal nanosilica waste. Constr. Build. Mater. 2016, 115, 269–276. [Google Scholar] [CrossRef]
- Puente, R.; Gómez-Zamorano, L.; Alonso, M.C. Mortars modified with geothermal nanosilica waste: Effect on the electrochemical properties of embedded steel rods. Int. J. Electrochem. Sci. 2012, 7, 136–149. [Google Scholar] [CrossRef]
- Puente, R. Efecto de la Adición del Residuo Nanosílice Geotérmica sobre la Durabilidad de Morteros y Concretos base Cemento Portland. Doctoral’s Thesis, Programa Doctoral en Ingeniería de Materiales, Universidad Autónoma de Nuevo Leon, San Nicolás de los Garza, Nuevo León, Mexico, 2012. [Google Scholar]
- Yang, H.; Lin, Y.; Hsiao, C.; Liu, J. Evaluating residual compressive strength of concrete at elevated temperatures using ultrasonic pulse velocity. Fire Saf. J. 2009, 44, 121–130. [Google Scholar] [CrossRef]
- Arioz, O. Effects of elevated temperatures on properties of concrete. Fire Saf. J. 2007, 42, 516–522. [Google Scholar] [CrossRef]
- Handoo, S.K.; Agarwal, S.; Agarwal, S.K. Physicochemical, mineralogical, and morphological characteristics of concrete exposed to elevated temperatures. Cem. Concr. Res. 2012, 32, 1009–1018. [Google Scholar] [CrossRef]
- Demirel, B.; Kelestemur, O. Effect of elevated temperature on the mechanical properties of concrete produced with finely ground pumice and silica fume. Fire Saf. J. 2010, 45, 385–391. [Google Scholar] [CrossRef]
- Ali, B.; Hasan, Z. Effect of silica fume addition and water to cement ratio on the properties of high strength concrete after exposure to high temperatures. Cem. Concr. Compos. 2008, 30, 106–122. [Google Scholar]
- Abdul, A.; Shehu, I.; Ismail, M. Effect of cooling regime on the residual performance of high-volume palm oil fuel ash concrete exposed to high temperatures. Constr. Build. Mater. 2015, 98, 875–883. [Google Scholar]
- Ma, Q.; Guo, R.; Zhao, Z.; Lin, Z.; He, K. Mechanical properties of concrete at high temperature—A review. Constr. Build. Mater. 2015, 93, 371–383. [Google Scholar] [CrossRef]
- Cree, D.; Green, M.; Noumowe, A. Residual strength of concrete containing recycled materials after exposure to fire: A review. Constr. Build. Mater. 2013, 45, 208–223. [Google Scholar] [CrossRef]
- Xu, Y.; Wong, Y.; Poon, C.; Anson, M. Influence of PFA on cracking of concrete and cement paste after exposure to high temperatures. Cem. Concr. Res. 2003, 33, 2009–2016. [Google Scholar] [CrossRef]
- Hertz, K. Danish investigations on silica fume concretes at elevated temperatures. ACI Mater. J. 1992, 89, 345–347. [Google Scholar]
- Abdul, A.S.M.; Shehu, I.A. Performance evaluation of concrete containing high volume palm oil fuel ash exposed to elevated temperature. Constr. Build. Mater. 2015, 76, 214–220. [Google Scholar]
- Al-Akhras, N.M.; Al-Akhras, K.M.; Attom, M.F. Performance of olive waste ash concrete exposed to elevated temperatures. Fire Saf. J. 2009, 44, 370–375. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Yuan, G. Effects of elevated temperatures on properties of concrete containing ground granulated blast furnace slag as cementitious material. Constr. Build. Mater. 2012, 35, 687–692. [Google Scholar] [CrossRef]
- Íñiguez-Sánchez, C.A. Análisis de la Solución de los Poros en Pastas de Cemento Pórtland Ordinario Parcialmente Reemplazado con Desecho Geotérmico. Master’s Thesis, UANL, San Nicolás de los Garza, Nuevo León, Mexico, 2008. [Google Scholar]
- Gómez-Zamorano, L.Y. Geothermal Waste as a Replacement Material of Portland Cement Pastes. Doctoral’s Thesis, Cinvestav Unidad Saltillo, Mayo, Coahuila, Mexico, 2004. [Google Scholar]
- Vargas, J.L.; Gómez, L.Y. Estudio de Cementos Compósitos Parcialmente Reemplazados con Ceniza Volante, Escoria Granulada de Alto Horno y Desecho Geotérmico. Master’s Thesis, UANL, San Nicolás de los Garza, Nuevo León, Mexico, 2009. [Google Scholar]
- Gómez-Zamorano, L.Y.; Escalante-García, J.I. Effect of curing temperature on the none vaporable water in Portland cement blended with geothermal silica waste. Cem. Concr. Compos. 2010, 32, 603. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Li, Q. Hydration Properties of Ground Granulated Blast-Furnace Slag (GGBS) Under Different Hydration Environments. Mater. Sci. 2017, 23, 70–77. [Google Scholar] [CrossRef]
- Castellote, M.; Alonso, C.; Andrade, C.; Turrillas, X.; Campo, J. Composition and microstructural changes of cement pastes upon heating, as studied by neutron diffraction. Cem. Concr. Res. 2004, 34, 1633–1644. [Google Scholar] [CrossRef]
- Ingham, J.P. Application of petrographic examination techniques to the assessment of fire-damaged concrete and masonry structures. Mater. Charact. 2009, 60, 700–709. [Google Scholar] [CrossRef]
- Georgali, B.; Tsakiridis, P.E. Microstructure of fire-damaged concrete. A case study. Cem. Concr. Compos. 2005, 27, 255–259. [Google Scholar] [CrossRef]
- Stanislaw, F.; Maciej, S. Analysis of the development of cluster cracks caused by elevated temperatures in cement paste. Constr. Build. Mater. 2015, 83, 223–229. [Google Scholar]
- Alarcon, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Saint-Pierre, F.; Philibert, A.; Giroux, B.; Rivard, P. Concrete quality designation based on ultrasonic pulse velocity. Constr. Build. Mater. 2016, 125, 1022–1027. [Google Scholar] [CrossRef]
- Nadeem, A.; Menon, S.; Yiu Lo, T. The performance of fly ash and metakaolin concrete at elevated temperatures. Constr. Build. Mater. 2014, 62, 67–76. [Google Scholar] [CrossRef]
- Ismail, M.; Ismail, M.E.; Muhammad, B. Influence of elevated temperatures on physical and compressive strength properties of concrete containing palm oil fuel ash. Constr. Build. Mater. 2011, 25, 2358–2364. [Google Scholar] [CrossRef]
- Sancak, E.; Sari, D.Y.; Simsek, O. Effects of elevated temperature on compressive strength and weight loss of the light-weight concrete with silica fume and superplasticizer. Cem. Concr. Compos. 2008, 30, 715–721. [Google Scholar] [CrossRef]
- Akoz, F.; Yuzer, N.; Koral, S. The influence of high temperature on the physical and mechanical properties of ordinary portland cement and silica fume mortar. Turk. Chamb. Civ. Eng. 1995, 6, 287–292. [Google Scholar]
- Ghandehari, M.; Behnood, A.; Khanzadi, M. Residual mechanical properties of high-strength concretes after exposure to elevated temperatures. J. Mater. Civ. Eng. 2010, 22, 59–64. [Google Scholar] [CrossRef]
- Topcu, I.B.; Demir, A. Effect of fire and elevated temperature on reinforced concrete structures. Bull. Chamb. Civ. Eng. Eskiseh. Branch 2002, 16, 34–36. [Google Scholar]
- Tanyildizi, H. Variance analysis of crack characteristics of structural lightweight concrete containing silica fume exposed to high temperatures. Constr. Build. Mater. 2013, 47, 1154–1159. [Google Scholar] [CrossRef]
- Poon, C.S.; Azhar, S.; Anson, M.; Wong, Y.L. Comparison of the strength and durability performance of normal- and high-strength pozzolanic concretes at elevated temperatures. Cem. Concr. Res. 2001, 31, 1291–1300. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high temperature environment. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Fares, H.; Remond, S.; Noumowe, A.; Cousture, A. High temperature behavior of self-consolidating concrete, micro-structure and physicochemical properties. Cem. Concr. Res. 2010, 40, 488–496. [Google Scholar] [CrossRef]
- Akca, A.; Zihnioglu, N. High performance concrete under elevated temperatures. Constr. Build. Mater. 2013, 44, 317–328. [Google Scholar] [CrossRef]
- Fall, M.; Samb, S.S. Effect of high temperature on strength and microstructural properties of cemented paste backfill. Fire Saf. J. 2009, 44, 642–651. [Google Scholar] [CrossRef]
- Ergun, A.; Kurklu, G.; Serhat Baspinar, M.; Mansour, M. The effect of cement dosage on mechanical properties of concrete exposed to high temperatures. Fire Saf. J. 2013, 55, 160–167. [Google Scholar] [CrossRef]
- Peng, G.; Huang, Z. Change in microstructure of hardened cement paste subjected to elevated temperatures. Constr. Build. Mater. 2008, 22, 593–599. [Google Scholar] [CrossRef]




| Compound | Compound Portland Cement (CPC) | Silica Fume (SF) | Geothermal Nano-Silica Waste (GNSW) |
|---|---|---|---|
| (wt.%) | |||
| SiO2 | 18.51 | 95.89 | 98.36 |
| Al2O3 | 4.46 | 0.42 | 0.09 |
| Fe2O3 | 2.61 | 1.22 | 0.04 |
| CaO | 67.45 | 0.61 | 0.45 |
| MgO | 1.27 | 0.42 | - |
| SO3 | 3.26 | 0.45 | 0.03 |
| Na2O | 0.36 | 0.17 | 0.32 |
| K2O | 0.87 | 0.82 | 0.23 |
| Cl− | - | - | 0.06 |
| LOI | 0.79 | 0.29 | 0.31 |
| Density (g/cm3) | 3.03 | 2.20 | 2.04 |
| BET Area (m2/g) | 0.88 | 24.66 | 8.56 |
| Materials | Sample Code Partial Replacements (wt.%) | |||
|---|---|---|---|---|
| C100 | C-GNSW20 | C-GNSW30 | C-SF30 | |
| Portland cement | 14.86 | 11.89 | 10.40 | 10.40 |
| SCM (GNSW or SF) | 0 | 2.97 | 4.46 | 4.46 |
| Coarse aggregate | 42.46 | 42.46 | 42.46 | 42.46 |
| Fine aggregate | 35.03 | 35.03 | 35.03 | 35.03 |
| Water | 7.43 | 7.43 | 7.43 | 7.43 |
| Superplasticizer | 0.22 | 0.22 | 0.22 | 0.22 |
| Total | 100 | 100 | 100 | 100 |
| Temperature Range | Chemical Transformation in Concrete |
|---|---|
| 20–80 °C | Water slow elimination contained in the capillary pores |
| 80–90 °C | Ettringite (Aft) and monsulfate (AFm) decomposition |
| 80–100 °C | Water loss contained in the capillary pores |
| 100–200 °C | Chemical water elimination (absorbed and interlaminar) |
| 200–350 °C | CSH gel decomposition into αC2SH |
| 400–450 °C | (CaOH)2 decomposition into CaO and H2O |
| 570–600 °C | αSiO2 transformation into βSiO2 |
| 650–800 °C | CaCO3 decomposition into CaO and CO2 |
| 800–1200 °C | Dehydrated phases melting |
| >1200 °C | Aggregates melting |
| Physical Properties | Sample Code Partial Replacements (wt.%) | |||
|---|---|---|---|---|
| C100 | C-GNSW20 | C-GNSW30 | C-SF30 | |
| Workabilty (mm) | 150 | 90 | 75 | 75 |
| Open porosity (%) | 18.46 | 21.47 | 21.76 | 21.54 |
| Compressive strength at 28 days (MPa) | 23.03 | 25.42 | 28.23 | 33.33 |
| Compressive strength at 365 days (MPa) | 27.37 | 29.19 | 32.25 | 35.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Perales, J.F.; Alonso-Alonso, M.C.; Vázquez-Rodríguez, F.J.; Guzmán-Hernández, A.M.; Gómez-Zamorano, L.Y.; Rodríguez-Castellanos, E.A.; Puente-Ornelas, R. Geothermal Nano-SiO2 Waste as a Supplementary Cementitious Material for Concrete Exposed at High Critical Temperatures. Materials 2024, 17, 4381. https://doi.org/10.3390/ma17174381
López-Perales JF, Alonso-Alonso MC, Vázquez-Rodríguez FJ, Guzmán-Hernández AM, Gómez-Zamorano LY, Rodríguez-Castellanos EA, Puente-Ornelas R. Geothermal Nano-SiO2 Waste as a Supplementary Cementitious Material for Concrete Exposed at High Critical Temperatures. Materials. 2024; 17(17):4381. https://doi.org/10.3390/ma17174381
Chicago/Turabian StyleLópez-Perales, Jesús Fernando, María Cruz Alonso-Alonso, Francisco Javier Vázquez-Rodríguez, Ana María Guzmán-Hernández, Lauren Yolanda Gómez-Zamorano, Edén Amaral Rodríguez-Castellanos, and Rodrigo Puente-Ornelas. 2024. "Geothermal Nano-SiO2 Waste as a Supplementary Cementitious Material for Concrete Exposed at High Critical Temperatures" Materials 17, no. 17: 4381. https://doi.org/10.3390/ma17174381
APA StyleLópez-Perales, J. F., Alonso-Alonso, M. C., Vázquez-Rodríguez, F. J., Guzmán-Hernández, A. M., Gómez-Zamorano, L. Y., Rodríguez-Castellanos, E. A., & Puente-Ornelas, R. (2024). Geothermal Nano-SiO2 Waste as a Supplementary Cementitious Material for Concrete Exposed at High Critical Temperatures. Materials, 17(17), 4381. https://doi.org/10.3390/ma17174381










