Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer
Abstract
1. Introduction
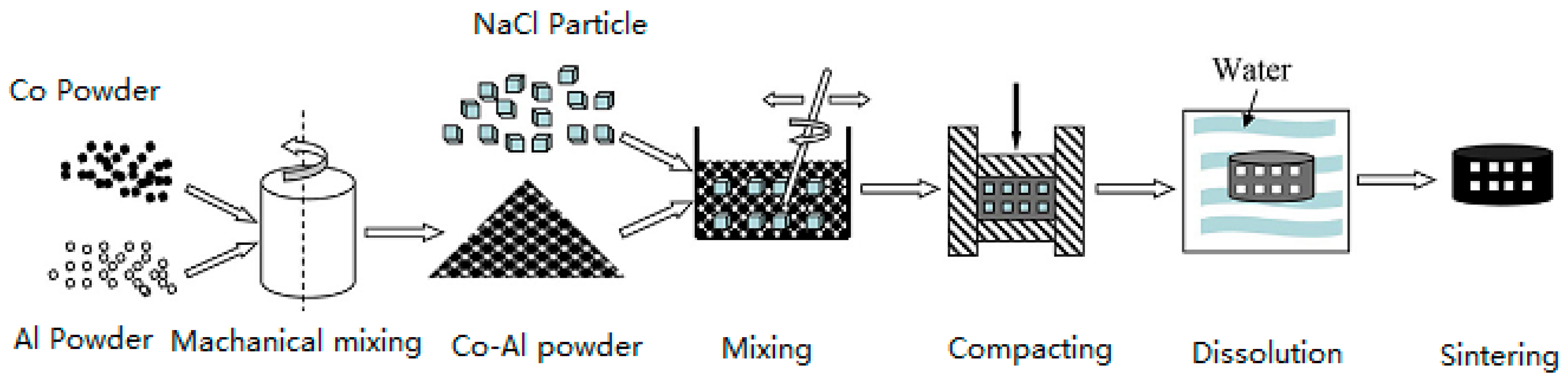
2. Experiments
3. Results and Discussion
3.1. Exothermic Curves of the Co-Al System
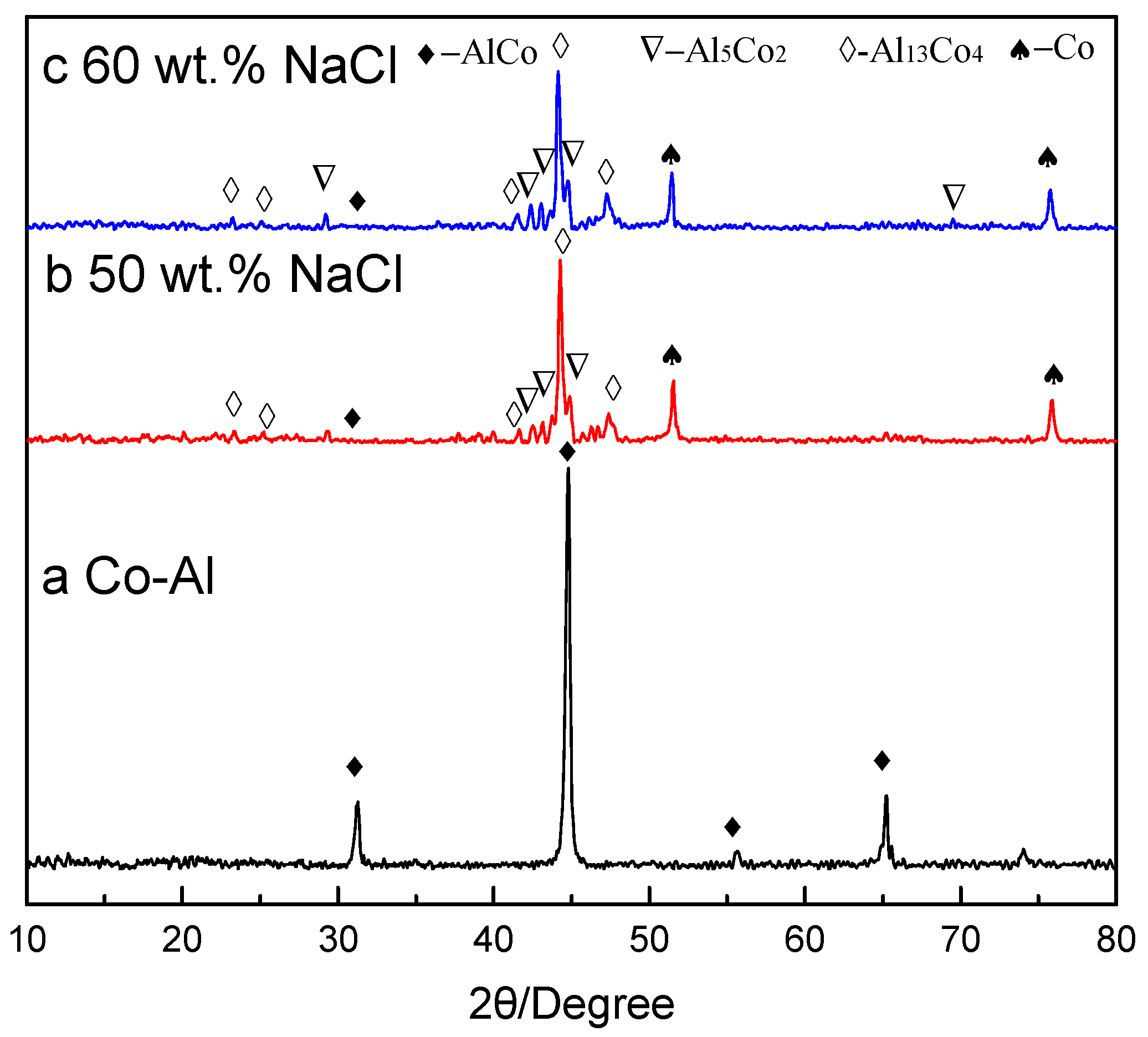
3.2. Analysis of TE Reaction Products
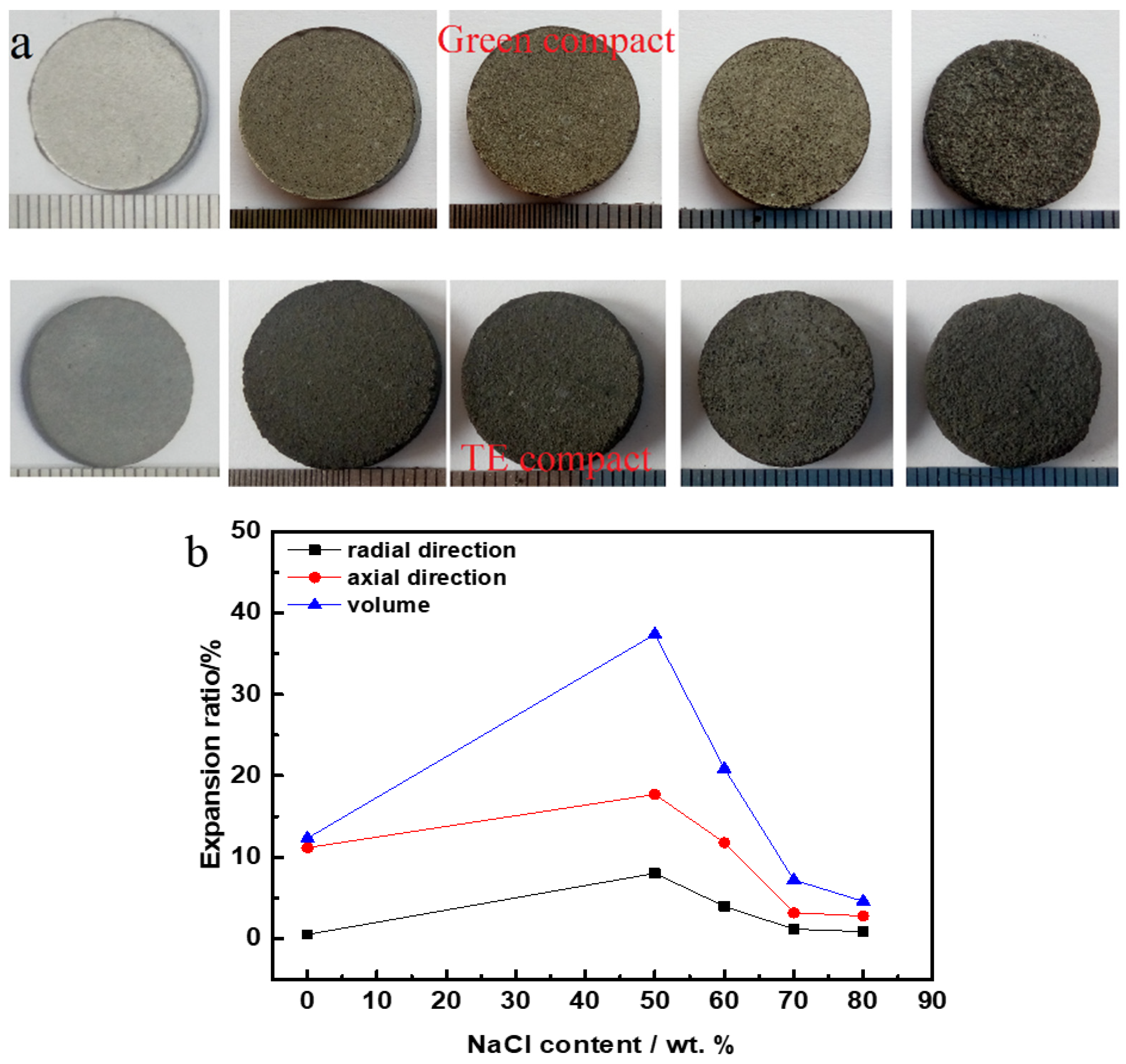
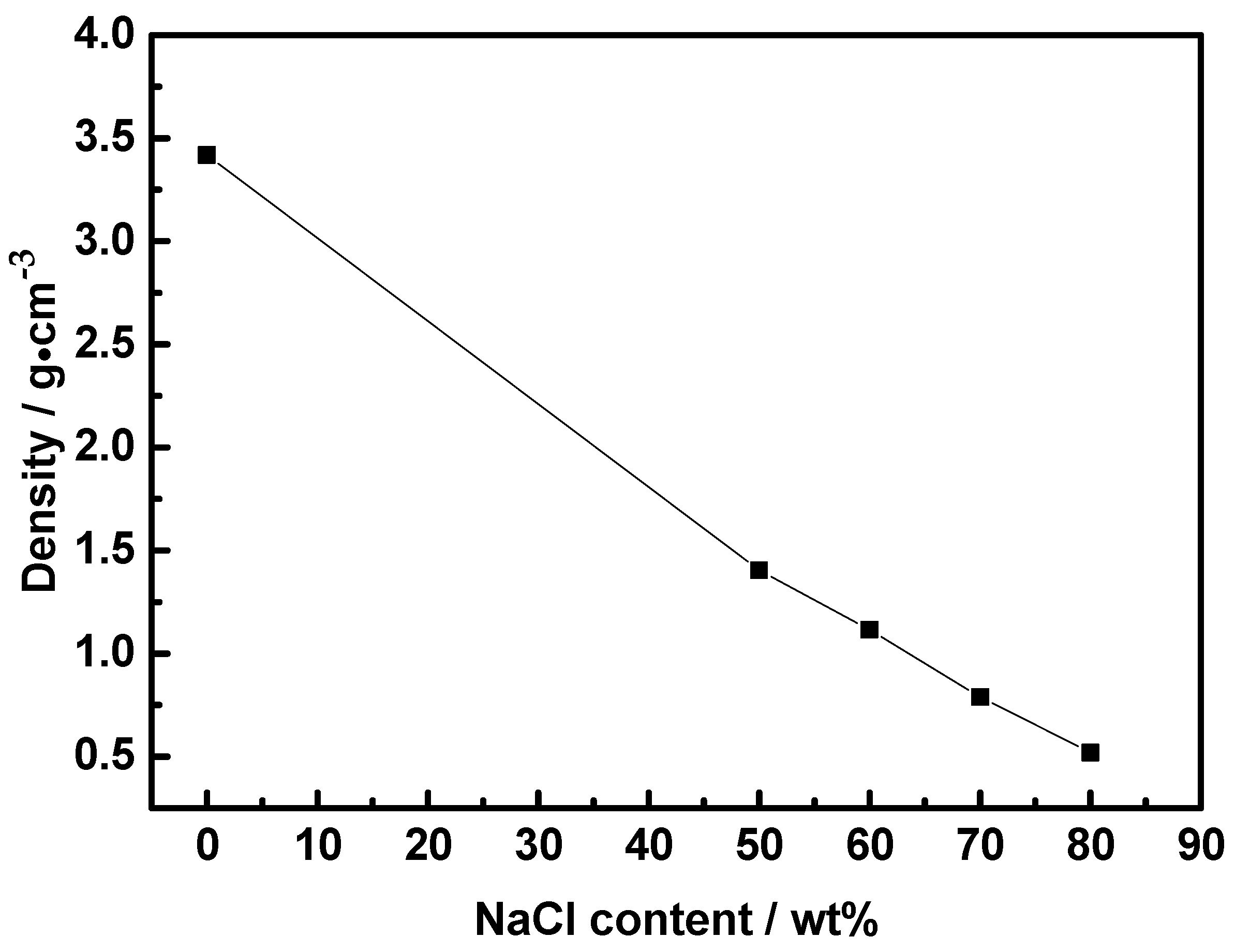
3.3. Analysis of Density and Volume Change
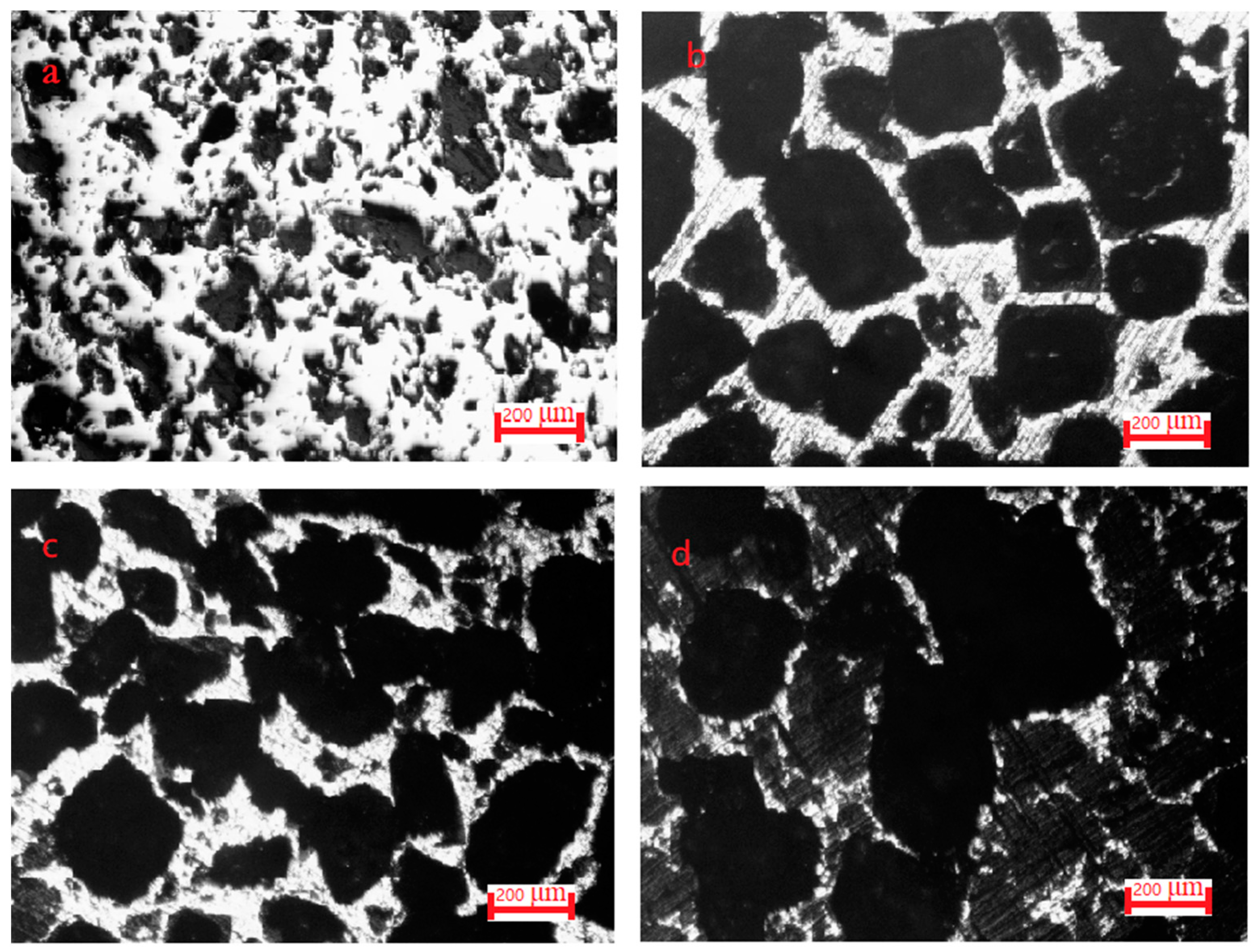
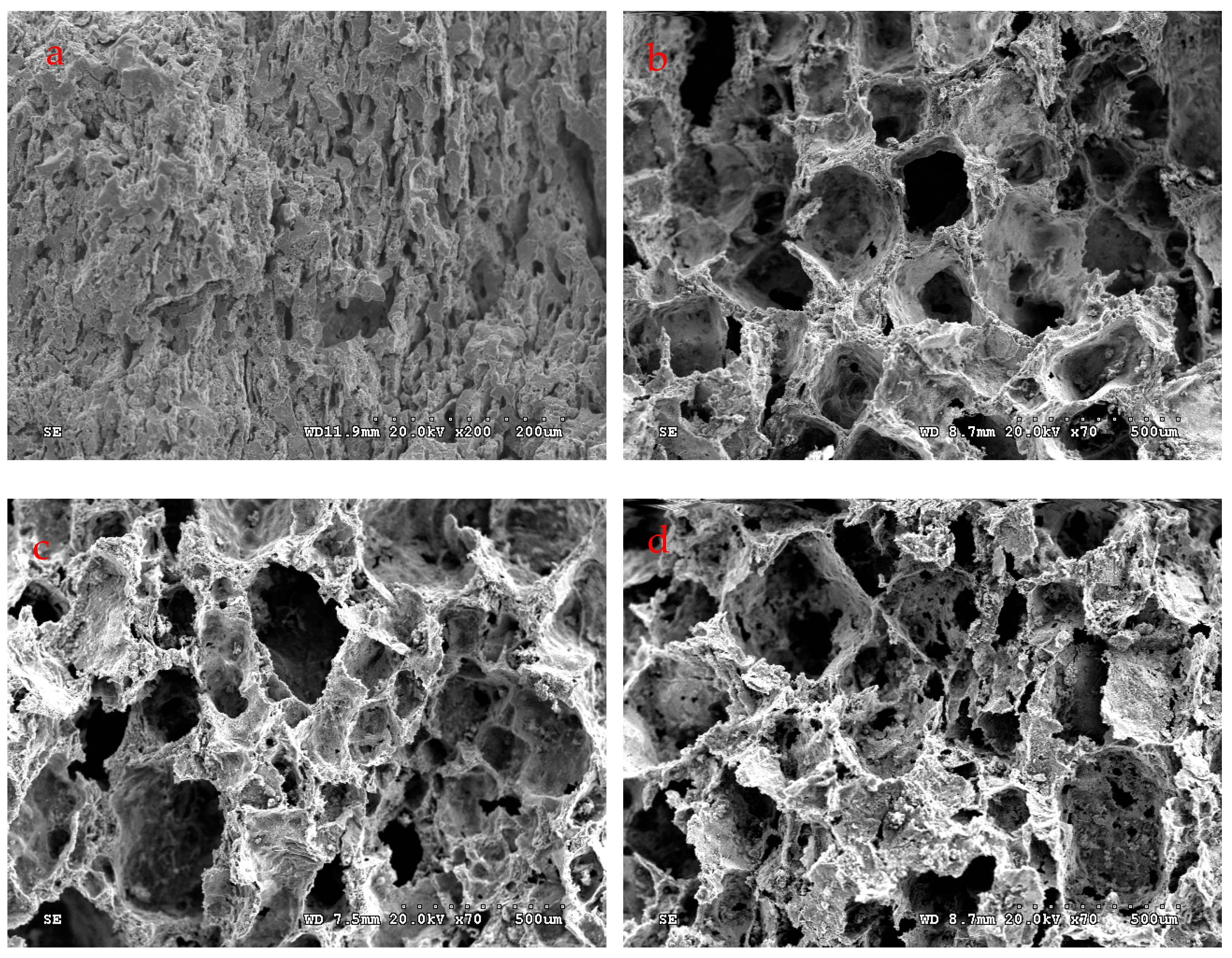
3.4. Microscopic Analysis of Pore Structure
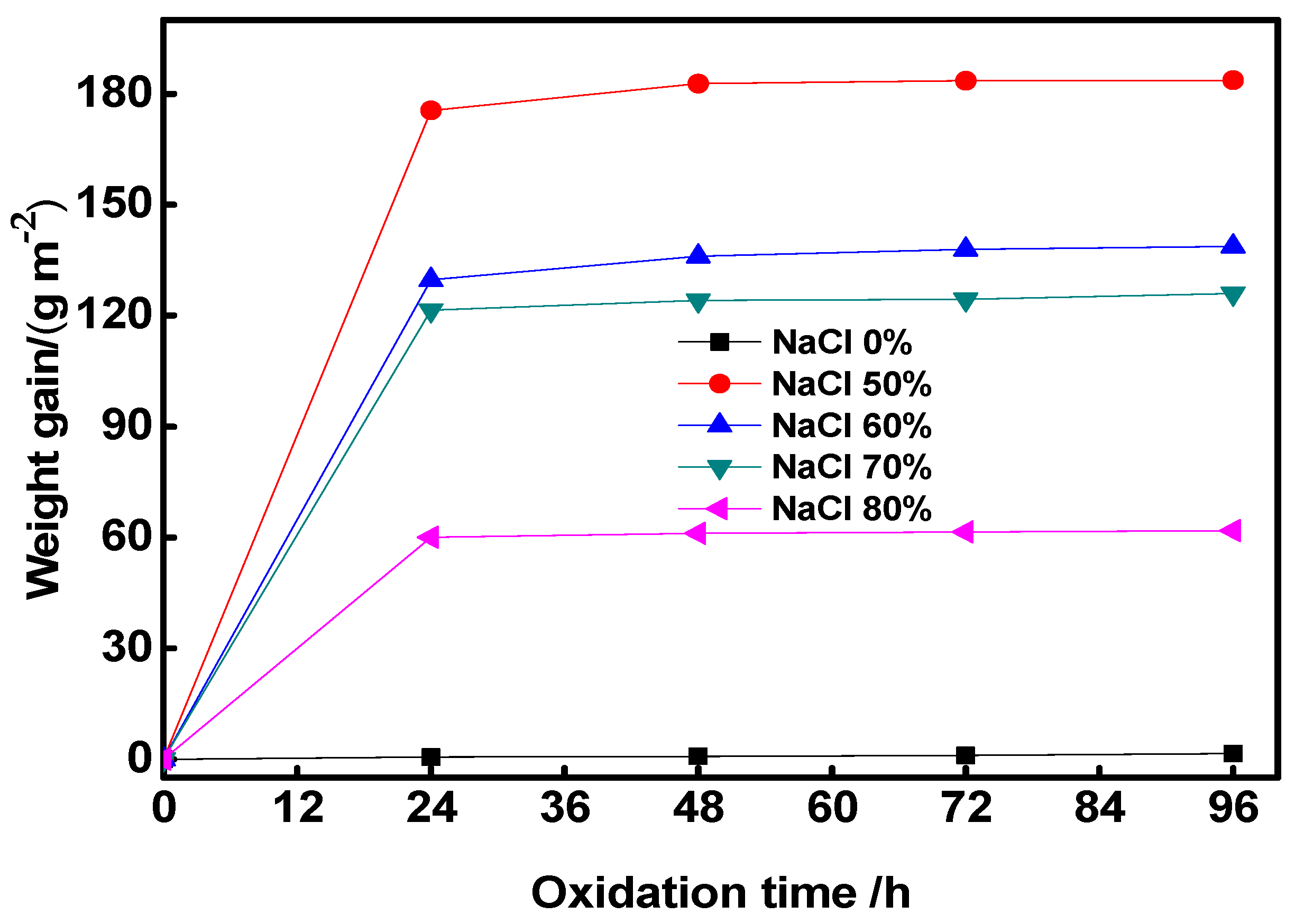
3.5. Analysis of Co-Al Compound Antioxidation Performance
4. Conclusions
- (1)
- High-porosity and low-density Co-Al compounds were manufactured using a high-efficiency and energy-conserving thermal explosion method with a leachable NaCl space retainer. Before sintering, the added NaCl was dissolved out completely.
- (2)
- The Tig value of the Co-Al specimen was near to the melting temperature of Al, while the temperatures of the Co-Al specimens after the added NaCl dissolved out were higher than the melting temperature of Al. But the Tc of the Co-Al specimen was higher than that of the Co-Al specimens after the added NaCl dissolved out.
- (3)
- The final product was solely Co-Al intermetallic for the Co-Al sample, while the final products were Al13Co4 with minor abundant Co, Al5Co2 and Co-Al phases with Co-Al specimens after the added NaCl dissolved out, due to the low Tc.
- (4)
- The maximum opening porosity of the sintered Co-Al compound was 79.5% after 80 wt.% NaCl was added and dissolved out. Porous Co-Al compounds displayed an inherited pore structure, including large pores originating from the dissolution of NaCl and small pores in the Co-Al matrix caused by volume expansion owing to TE reaction.
- (5)
- The specimen without NaCl exhibited the best high-temperature oxidation resistance because it was a single phase and formed a dense oxide film more easily at high temperatures. High-temperature antioxidation of the Co-Al compound after the added NaCl dissolved out was not high as that of the Co-Al specimen because the abundant Co could be oxidized into cobalt oxide, decreasing its oxidation resistance. In the future, we can increase the oxidation resistance of sintered products by increasing the furnace temperature to produce a single Co-Al intermetallic or reducing the residual amount of Co.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; He, Z.L.; He, Y.H.; Qiu, Y.; Wang, Z.H.; Jiang, Y. Porous TiFe2 intermetallic compound fabricated via element powder reactive synthesis. Int. J. Miner. Metall. Mater. 2024, 31, 764–772. [Google Scholar] [CrossRef]
- Xu, X.W.; Shang, Z.C.; Zhang, B.J.; Cai, X.P.; Feng, P.Z. Mechanical and electrochemical properties of porous NiTi intermetallics synthesized via rapid thermal explosion. Intermetallics 2024, 165, 108162. [Google Scholar] [CrossRef]
- Zhang, H.B.; Ma, J.L.; Gao, Z.C.; Guo, F.; Xu, S.H.; Hou, G.Y.; Zheng, G.Q. Study on stability of mechanical properties for porous Fe-Cr-Al alloys after long-term aging. Materials 2022, 15, 3718. [Google Scholar] [CrossRef]
- Sienkiewicz, J.; Kuroda, S.; Molak, R.M.; Murakami, H.; Araki, H.; Takamori, S.; Kurzydłowski, K.J. Fabrication of TiAl intermetallic phase by heat treatment of warm sprayed metal precursors. Intermetallics 2014, 49, 57–64. [Google Scholar] [CrossRef]
- Yang, F.; Tane, M.; Lin, J.P.; Song, Y.H.; Nakajima, H. Pore formation and compressive deformation in porous TiAl-Nb alloys containing directional pores. Mater. Des. 2013, 49, 755–760. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Zhang, Y.; Wu, Q.L.; Shang, Z.C.; Yu, Y.; Zhang, X.X.; Feng, P.Z. High-temperature oxidation behavior and pore structure of porous TiAl3 intermetallics at 650 °C to 900 °C. J. Mater. Res. Technol. 2023, 22, 2398–2408. [Google Scholar] [CrossRef]
- Kobashi, M.; Miyake, S.; Kanetake, N. Hierarchical open cellular porous TiAl manufactured by space holder process. Intermetallics 2013, 42, 32–34. [Google Scholar] [CrossRef]
- Hao, G.L.; Wang, H.; Li, X.Y. Novel double pore structures of TiAl produced by powder metallurgy processing. Mater. Lett. 2015, 142, 11–14. [Google Scholar] [CrossRef]
- Ye, S.L.; Hao, H.L.; Mo, W.; Yu, K.P.; Liu, L.T.; Deng, C.J.; Yu, P. Effects of cold compacting pressure on the expansion behavior of Ti-48Al during sintering. J. Alloys Compd. 2016, 673, 399–404. [Google Scholar] [CrossRef]
- Li, K.Y.; Zhang, T.C.; Zhu, Y.Z. Pore channel formation of porous TiAl3 intermetallics prepared by thermal explosion with space holder process. J. Mater. Res. Technol. 2023, 32, 3716–3728. [Google Scholar] [CrossRef]
- Li, K.Y.; Zhang, T.C.; Zhu, Y.Z. Reaction behavior of porous TiAl3 intermetallics fabricated by thermal explosion with different particle sizes. Materials 2021, 14, 7417. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Zhang, Y.; Yu, Y.; Xu, C.Y. One-step sintering of porous TiAl3 intermetallics using TiH2 as raw material in thermal explosion reaction. Mater. Lett. 2022, 318, 132177. [Google Scholar] [CrossRef]
- Xu, Z.G.; Wang, W.J.; Wang, H.; Yang, C.; Shen, Q.; Wang, C.B. Fabrication of a porous Fe-Al intermetallic alloy with ultrahigh open porosity. Adv. Eng. Mater. 2023, 25, 2201835. [Google Scholar] [CrossRef]
- Zhang, H.B.; Yu, H.; Ma, J.L.; Wang, L.F.; Xu, S.H.; Zhang, G.Q. Improving oxidation resistance of porous FeAl-based intermetallics with high boron/yttrium alloying. Trans. Nonferrous Met. Soc. China 2022, 32, 2620–2633. [Google Scholar] [CrossRef]
- Maznoy, A.; Kirdyashkin, A.; Kitler, C.; Solovyev, A. Combustion synthesis and characterization of porous Ni-Al materials for metal-supported solid oxide fuel cells application. J. Alloys Compd. 2017, 697, 114–123. [Google Scholar] [CrossRef]
- Fursenko, R.; Maznoy, A.; Odintsov, E.; Kirdyashkin, A.; Minaev, S.; Sudarchan, K. Temperature and radiative characteristics of cylindrical porous Ni-Al burners. Int. J. Heat Mass Tran. 2016, 98, 277–284. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, X.P.; Jiao, X.Y.; Xu, C.Y.; Niu, J.N.; Feng, P.Z. Oxidation resistance at 900 °C of porous Ni-Al-Cr intermetallics synthesized via rapid thermal explosion reaction. J. Alloys Compd. 2022, 906, 164374. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, F.; Li, S.S.; Sha, J.B. High-temperature oxidation behavior of novel Co-Al-W-Ta-B-(Mo, Hf, Nb) alloys with a coherent γ/γ’-dominant microstructure. Prog. Nat. Sci. Mater. Int. 2016, 26, 600–612. [Google Scholar] [CrossRef]
- Zhu, L.L.; Wei, C.D.; Qi, H.Y.; Jiang, L.; Jin, Z.P.; Zhao, J.C. Experimental investigation of phase equilibria in the Co-rich part of the Co-Al-X (X = W, Mo, Nb, Ni, Ta) ternary systems using diffusion multiples. J. Alloys Compd. 2017, 691, 110–118. [Google Scholar] [CrossRef]
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic hydrogenation of biomass-derived organics: A review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, Z.; Xiao, Z. An efficient support-free nanoporous Co-catalyst for reverse water-gas shift reaction. Catalysts 2019, 9, 423. [Google Scholar] [CrossRef]
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef]
- Xu, C.Y.; Cai, X.P.; Jiao, X.Y.; Vu, K.L.; Shang, Z.C.; Feng, P.Z. Near-net forming of porous Co-Al by isothermal treatment: Phase formation sequence and diffusion kinetics. Mater. Charact. 2023, 199, 112785. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yeh, C.C. Preparation of CoAl intermetallic compound by combustion synthesis in self-propagating mode. J. Alloys Compd. 2005, 388, 241–249. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Karantzalis, A.E.; Sioulas, D. Microstructure and corrosion performance of Al-32%Co alloys. Corros. Sci. 2012, 63, 193–209. [Google Scholar] [CrossRef]
- Kang, X.Q.; Qiao, L.; Zhang, H.F.; Wang, J.Z.; Feng, P.Z. Microstructure and properties of Co-Al porous intermetallics fabricated by thermal explosion reaction. High Temp. Mater. Proc. 2021, 40, 141–150. [Google Scholar] [CrossRef]
- Shang, Z.C.; Cai, X.P.; Wang, H.; Pahlevani, F.; Zheng, Y.; Yu, Y.; Zhang, B.J.; Feng, P.Z. High temperature anti-oxidation and filtration behavior of micro/nano-scale porous CoAl intermetallic synthesized via rapid thermal explosion. Corros. Sci. 2023, 219, 111216. [Google Scholar] [CrossRef]
- Shinobu, H.; Sawao, H.; Tomoki, H.; Yuji, I. Fabrication of unidirectional porous spinel (MgAl2O4) from porous alumina using a template method. Ceram. Int. 2013, 39, 2077–2081. [Google Scholar]
- Yang, S.H.; Kim, W.Y.; Kim, M.S. Fabrication of unidirectional porous TiAl-Mn intermetallic compounds by reactive sintering using extrude powder mixtures. Intermetallics 2003, 11, 849–855. [Google Scholar] [CrossRef]
- Gaillard, C.; Despois, J.F.; Mortensen, A. Processing of NaCl powders of controlled size and shape for the microstructural tailoring of aluminium foams. Mater. Sci. Eng. A 2004, 374, 250–262. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Wang, X.H.; Kang, X.Q.; Feng, P.Z.; Zhang, L.Q.; Wang, J.Z.; Akhtar, F. Hierarchical porous TiAl3 intermetallics synthesized by thermal explosion with a leachable space-holder material. Mater. Lett. 2016, 181, 261–264. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, X.Y.; Feng, P.Z.; Wang, X.H.; Liu, Z.S.; Akhtar, F. Highly porous open cellular TiAl-based intermetallics fabricated by thermal explosion with space holder process. Intermetallics 2016, 68, 95–100. [Google Scholar] [CrossRef]
- Shi, Q.L.; Qin, B.T.; Feng, P.Z.; Ran, H.S.; Song, B.B.; Wang, J.Z.; Ge, Y. Synthesis, microstructure and properties of Ti-Al porous intermetallic compounds prepared by a thermal explosion reaction. RSC Adv. 2015, 5, 46339–46347. [Google Scholar] [CrossRef]
- Bertolino, N.; Monagheddu, M.; Tacca, A.; Giuliani, P.; Zanotti, C.; Tamurini, U.A. Ignition mechanism in combustion synthesis of Ti-Al and Ti-Ni systems. Intermetallics 2003, 11, 41–49. [Google Scholar] [CrossRef]
- Liang, Y.F.; Yang, F.; Zhang, L.Q.; Lin, J.P.; Shang, S.L.; Liu, Z.K. Reaction behavior and pore formation mechanism of TiAl-Nb porous alloys prepared by element powder metallurgy. Intermetallics 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Šulhánek, P.; Drienovský, M.; Černičková, I.; Ďuriška, L.; Skaudžius, R.; Gerhátová, Ž.; Palcut, M. Oxidation of Al-Co Alloys at High Temperatures. Materials 2020, 13, 3152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhou, D.; Qiao, L.; Feng, P.; Kang, X.; Yang, C. Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer. Materials 2024, 17, 4380. https://doi.org/10.3390/ma17174380
Yu Y, Zhou D, Qiao L, Feng P, Kang X, Yang C. Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer. Materials. 2024; 17(17):4380. https://doi.org/10.3390/ma17174380
Chicago/Turabian StyleYu, Yonghao, Dapeng Zhou, Lei Qiao, Peizhong Feng, Xueqin Kang, and Chunmin Yang. 2024. "Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer" Materials 17, no. 17: 4380. https://doi.org/10.3390/ma17174380
APA StyleYu, Y., Zhou, D., Qiao, L., Feng, P., Kang, X., & Yang, C. (2024). Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer. Materials, 17(17), 4380. https://doi.org/10.3390/ma17174380






