Characteristics of Circulating Fluidized Bed Combustion (CFBC) Ash as Carbon Dioxide Storage Medium and Development of Construction Materials by Recycling Carbonated Ash
Abstract
1. Introduction
2. Materials and Method
2.1. Raw Materials
2.2. Carbonation of CFBC Fly Ash
2.3. Recycling Experiment with Concrete Admixture
- ;
- ;
- .
2.4. Manufacturing of Foam Concrete
2.5. Physical Properties of Cement Pastes
2.5.1. Density of Cement Pastes
- : mass of sample(g);
- : volume of container.
2.5.2. Flow and Sinking Depth of Cement Paste
2.6. Measurement of Absolute Dry Bulk Density and Compressive Strength of Foamed Concrete
- ;
- .
2.7. Measurement of Thermal Conductivity of Foamed Concrete
- ;
- ;
- ;
- ;
- ;
- .
3. Results and Discussion
3.1. Carbonation of CFBC Ash
- ;
- ;
- ;
- .
3.2. Pilot Test for Carbonation of CFBC Ash
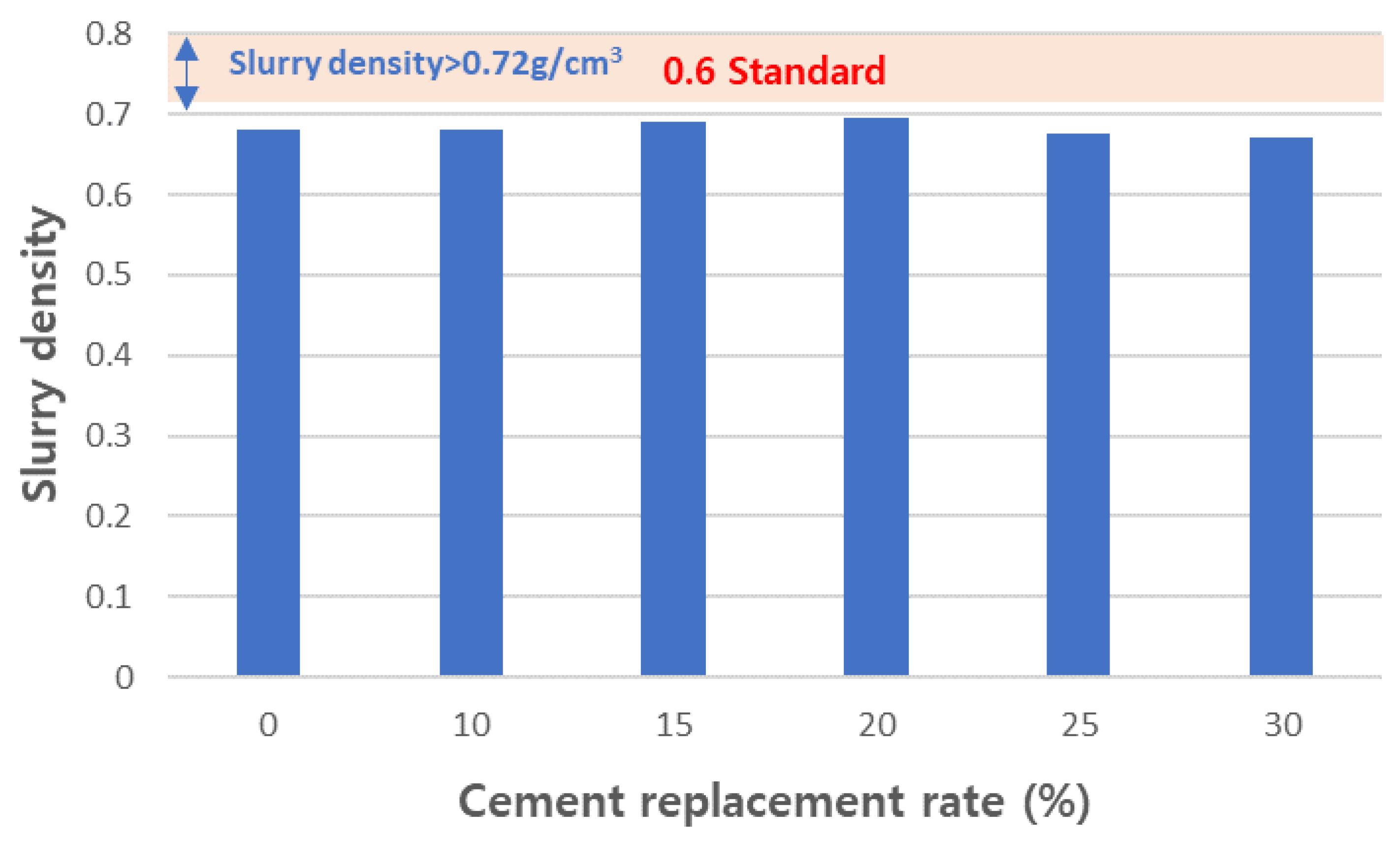
3.3. Evaluation of the Potential of CFBC Ash as a Concrete Admixture
3.4. Foamed Concrete
3.4.1. Physical Properties of Foamed Concrete Paste
3.4.2. Physical Properties of Cured Concrete
4. Conclusions
- In the carbonation experiment of CFBC ash, the saturation concentration was reached under the condition of 700 cc/min and the optimal carbonation speed was obtained and the reaction rate did not increase, even at a higher CO2 flow.
- As the amount of CFBC ash increased, the carbonation time increased linearly. This suggests that the dominant reaction of the mineral carbonation reaction in the slurry was a dissolution of each ion and has characteristics different from the solid–gas system.
- When recycling carbonated CFBC ash as a concrete admixture, we confirmed that up to 20 wt% could be replaced in the blast furnace slag cement system and that the initial strength in the blast furnace slag cement system could be improved.
- As the content of CFBC ash increases, the flow increases, which means that the viscosity decreases. The decrease in viscosity increases the sinking height and worsens the pore structure, so the replacement amount should be limited to 30 wt% or less.
- As the amount of added carbonated CFBC ash increased, the sinking depth increased when foamed concrete was manufactured, so the replacement amount was limited to 30 wt% or less.
- Since a rapid decrease in density and strength occurs when the substitution amount is 30%, the replacement amount should be limited to 25 wt% or less.
- When carbonated CFBC ash was recycled into foamed concrete, it was possible to manufacture foam concrete that satisfied KS F 4039 by substituting up to 25 wt% of OPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CFBC | Circulating Fluidized Bed Combustion |
| PCC | Pulverized Coal Combustion |
| Anhydrous gypsum | CaSO4 |
| XRF | X-ray fluorescence |
| OPC | Ordinary Portland Cement |
References
- Pan, Z.; Ye, J.; Zhou, F.; Tan, Y.; Connell, L.D.; Fan, J. CO2 storage in coal to enhance coalbed methane recovery: A review of field experiments in China. Int. Geol. Rev. 2018, 60, 754–776. [Google Scholar] [CrossRef]
- Hiep, D.T.; Hoffmann, C. A power development planning for Vietnam under the CO2 emission reduction targets. Energy Rep. 2020, 6, 19–24. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2020, 112, 4173–4182. [Google Scholar] [CrossRef]
- Rahmanihanzaki, M.; Hemmati, A. A review of mineral carbonation by alkaline solidwaste. Int. J. Greenh. Gas Control 2022, 121, 103798. [Google Scholar] [CrossRef]
- Han, S.-J.; Im, H.J.; Wee, J.-H. Leaching and indirect mineral carbonation performance of coal fly ash-water solution system. Appl. Energy 2015, 142, 274–282. [Google Scholar] [CrossRef]
- Ouyang, M.; Cao, Y. Utilizations of reaction exothermic heat to compensate the cost of the permanent CO2 sequestration through the geological mineral CO2 carbonation. Energy 2023, 284, 128626. [Google Scholar] [CrossRef]
- Meijssen, M.; Marinello, L.; di Bella, C.; Gasós, A.; Mazzotti, M. Industrial demonstration of indirect mineral carbonation in the cement and concrete sector. J. Environ. Chem. Eng. 2023, 11, 110900. [Google Scholar] [CrossRef]
- Miao, E.; Du, Y.; Zheng, X.; Zhang, X.; Xiong, Z.; Zhao, Y.; Zhang, J. CO2 sequestration by direct mineral carbonation of municipal solid waste incinerator fly ash in ammonium salt solution: Performance evaluation and reaction kinetics. Sep. Purif. Technol. 2023, 309, 123103. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, H.-K. Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash. Minerals 2017, 7, 237. [Google Scholar] [CrossRef]
- Bae, S.J.; Lee, K.G. Carbonation Behavior of Fly Ash with Circulating Fluidized Bed Combustion (CFBC). J. Korean Ceram. Soc. 2015, 52, 154–158. [Google Scholar] [CrossRef]
- Jang, J.G.; Ji, S.; Ahn, J.-W. Utilization of Circulating Fluidized Bed Combustion Ash and Related Specifications for Mine Backfills. J. Korean Inst. Resour. Recycl. 2017, 26, 71–79. [Google Scholar] [CrossRef]
- Basu, P. Combustion of coal in circulating fluidized-bed boilers: A review. Chem. Eng. Sci. 1999, 54, 5547–5557. [Google Scholar] [CrossRef]
- Manz, O. Worldwide production of coal ash and utilization in concrete and other products. Fuel 1997, 76, 691–696. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Jang, J.; Kim, H.; Lee, H. Resistance of coal bottom ash mortar against the coupled deterioration of carbonation and chloride penetration. Mater. Des. 2016, 93, 160–167. [Google Scholar] [CrossRef]
- Meena, A.; Singh, N.; Singh, S. High-volume fly ash Self Consolidating Concrete with coal bottom ash and recycled concrete aggregates: Fresh, mechanical and microstructural properties. J. Build. Eng. 2023, 63, 105447. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Khankhaje, E.; Rezania, S. Assessment of environmental and chemical properties of coal ashes including fly ash and bottom ash, and coal ash concrete. J. Build. Eng. 2022, 49, 104040. [Google Scholar] [CrossRef]
- Hasim, A.M.; Shahid, K.A.; Ariffin, N.F.; Nasrudin, N.N.; Zaimi, M.N.S. Study on mechanical properties of concrete inclusion of high-volume coal bottom ash with the addition of fly ash. Mater. Today Proc. 2022, 51, 1355–1361. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yang, F. A Review on the Application of Circulating Fluidized Bed Fly Ash in Building Materials. Adv. Mater. Sci. Eng. 2022, 7099430. [Google Scholar] [CrossRef]
- Wu, W.; Matalkah, F.; Darsanasiri, A.; Soroushian, P. Fluidized bed combustion coal fly ash: Comparative evaluation for potential use in alkali-activated binders. Int. J. Coal Prep. Util. 2022, 42, 51–66. [Google Scholar] [CrossRef]
- Baek, C.; Seo, J.; Choi, M.; Cho, J.; Ahn, J.; Cho, K. Utilization of CFBC Fly Ash as a Binder to Produce In-Furnace Desulfurization Sorbent. Sustainability 2018, 10, 4854. [Google Scholar] [CrossRef]
- Chi, M. Synthesis and characterization of mortars with circulating fluidized bed combustion fly ash and ground granulated blast-furnace slag. Constr. Build. Mater. 2016, 123, 565–573. [Google Scholar] [CrossRef]
- Wu, R.; Dai, S.; Jian, S.; Huang, J.; Lv, Y.; Li, B.; Azizbek, N. Utilization of the circulating fluidized bed combustion ash in autoclaved aerated concrete: Effect of superplasticizer. Constr. Build. Mater. 2020, 237, 117644. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.-M.; Chung, S.; Ahn, J.-W.; Kim, H.-K. Utilization of circulating fluidized bed combustion ash in producing controlled low-strength materials with cement or sodium carbonate as activator. Constr. Build. Mater. 2018, 159, 642–651. [Google Scholar] [CrossRef]
- Zahedi, M.; Jafari, K.; Rajabipour, F. Properties and durability of concrete containing fluidized bed combustion (FBC) fly ash. Constr. Build. Mater. 2020, 258, 119663. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Chen, X.; Ma, Y.; Zhou, M. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Zhang, Z.; Qian, J.; You, C.; Hu, C. Use of circulating fluidized bed combustion fly ash and slag in autoclaved brick. Constr. Build. Mater. 2012, 35, 109–116. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Gao, J.; Guo, Y.; Cheng, F. Synthesis and characterization of geopolymer prepared from circulating fluidized bed-derived fly ash. Ceram. Int. 2022, 48, 11820–11829. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, H.; Sagawa, T.; Hama, Y. Compressive strength development and durability of an environmental load-reduction material manufactured using circulating fluidized bed ash and blast-furnace slag. Constr. Build. Mater. 2017, 146, 102–113. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Liu, Y.; Hu, Z. Utilization of circulating fluidized bed fly ash for the preparation of foam concrete. Constr. Build. Mater. 2014, 54, 137–146. [Google Scholar] [CrossRef]
- Matsushima, N.; Li, Y.; Nishioka, M.; Sadakata, M.; Qi, H.; Xu, X. Novel Dry-Desulfurization Process Using Ca(OH)2/Fly Ash Sorbent in a Circulating Fluidized Bed. Environ. Sci. Technol. 2004, 38, 6867–6874. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.C.; Rodrigues, S.M.; Carvalho, L.; Duarte, A.C.; Pereira, E.; Römkens, P.F.; Tarelho, L.A. Ashes from fluidized bed combustion of residual forest biomass: Recycling to soil as a viable management option. Environ. Sci. Pollut. Res. 2017, 24, 14770–14781. [Google Scholar] [CrossRef]
- Quddus, S.; Saha, M.; Hasanuzzaman; Sharmin, N.; Bashar, M.S. Low energy synthesis of crystalline mesoporous aluminosilicate consisting of Na-P1 zeolite derived from coal fly ash. Clean. Mater. 2024, 12, 100247. [Google Scholar] [CrossRef]
- Marey, H.; Kozma, G.; Szabó, G. Green concrete materials selection for achieving circular economy in residential buildings using system dynamics. Clean. Mater. 2024, 11, 100221. [Google Scholar] [CrossRef]
- Kravchenko, E.; Lazorenko, G.; Jiang, X.; Leng, Z. Alkali-activated materials made of construction and demolition waste as precursors: A review. Sustain. Mater. Technol. 2024, 39, e00829. [Google Scholar] [CrossRef]
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Wu, H.; Li, C.; Zhang, M.; Yang, H. Critical Review of Ca(OH)2/CaO Thermochemical Energy Storage Materials. Energies 2023, 16, 3019. [Google Scholar] [CrossRef]
- Hanein, T.; Simoni, M.; Woo, C.L.; Provis, J.L.; Kinoshita, H. Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate. Energy Environ. Sci. 2021, 14, 6595–6604. [Google Scholar] [CrossRef]
- Juvekar, V.; Sharma, M. Absorption of CO2 in a suspension of lime. Chem. Eng. Sci. 1973, 28, 825–837. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C188-17; Standard Test Method for Density of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2017.
- KS L ISO 9597:2009; Determination of Setting Time and Soundness of Cements. Korean Standards Association: Seoul, Republic of Korea, 2009.
- ASTM D4380; Standard Test Method for Determining Density of Construction Slurres. ASTM International: West Conshohocken, PA, USA, 2019.
- KS F 2459:2022; Testing Methods for Density, Water Content, Absorption and Compressive Strength of Cellular Concrete. Korean Standards Association: Seoul, Republic of Korea, 2022.
- KS L 9016:2022; Test Methods for Thermal Transmission Properties of Thermal Insulations. Korean Standards Association: Seoul, Republic of Korea, 2022.
- Kingery, W.D.; Uhlmann, D.R.; Bowen, H.K. Introduction to Ceramics; BANDO Publisher: Seoul, Republic of Korea, 1995; pp. 485–504. [Google Scholar]
- Hwang, J.W.; Lee, Y.; Lee, D.H. Morphological cahnge of precipitated calcium carbonate by reaction rate in bubble column reactor. Kor. Chem. Eng. Res. 2009, 47, 727–733. [Google Scholar]
- Wei, Y.; Sun, R.; Su, H.; Xu, H.; Zhang, L.; Huang, D.; Liang, Z.; Hu, Y.; Zhao, L.; Lian, X. Synthesis and characterization of porous CaCO3 microspheres templated by yeast cells and the application as pH value-sensitive anticancer drug carrier. Colloids Surf. B Biointerfaces 2021, 199, 111545. [Google Scholar] [CrossRef]
- Fayaz, M.; Krishnaiah, R.; Raju, K.; Chauhan, M. Experimental study on mechanical properties of concrete using mineral admixtures. Mater. Today Proc. 2023. [CrossRef]
- Jaf, D.K.I.; Abdulrahman, P.I.; Mohammed, A.S.; Kurda, R.; Qaidi, S.M.; Asteris, P.G. Machine learning techniques and multi-scale models to evaluate the impact of silicon dioxide (SiO2) and calcium oxide (CaO) in fly ash on the compressive strength of green concrete. Constr. Build. Mater. 2023, 400, 132604. [Google Scholar] [CrossRef]
- Frýbort, A.; Štulířová, J.; Grošek, J.; Gregerová, M. Changes in the chemical composition of silica fume in the concrete composite system. Case Stud. Constr. Mater. 2023, 18, e01916. [Google Scholar] [CrossRef]
- Babalu, R.; Anil, A.; Sudarshan, K.; Amol, P. Compressive strength, flexural strength, and durability of high-volume fly ash concrete. Innov. Infrastruct. Solut. 2023, 8, 154. [Google Scholar] [CrossRef]
- Chishi, A.K.; Gautam, L. Sustainable use of silica fume in green cement concrete production: A review. Innov. Infrastruct. Solut. 2023, 8, 195. [Google Scholar] [CrossRef]
- Hargis, C.W.; Chen, I.A.; Devenney, M.; Fernandez, M.J.; Gilliam, R.J.; Thatcher, R.P. Calcium Carbonate Cement: A Carbon Capture, Utilization, and Storage (CCUS) Technique. Materials 2021, 14, 2709. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Cosentino, I.; Liendo, F.; Arduino, M.; Restuccia, L.; Bensaid, S.; Deorsola, F.; Ferro, G.A. Nano CaCO3 particles in cement mortars towards developing a circular economy in the cement industry. Procedia Struct. Integr. 2020, 26, 155–165. [Google Scholar] [CrossRef]
- Lee, H.S.; Jong, J.O.; Jeon, M.J.; Kee, S.H.; Han, D.Y. Influence of Rheological Properties of Lightweight Foamed Concrete on Preventing Foam Collapse. J. Kor. Recycl. Constr. Resour. Inst. 2018, 6, 304–310. [Google Scholar] [CrossRef]
- Lee, D.H.; Jun, M.H.; Ko, J.S. Physical Properties and Quality Control of Foamed Concrete with Fly Ash for Cast-in-Site. J. Korea Concr. Inst. 2001, 13, 69–76. [Google Scholar] [CrossRef]
- Lee, J.; Yang, K.; Mun, J. Fluidity and Compressive Strength Properties of Foamed Concrete Mixed with Fly Ash and Ground Granulated Blast Furnace Slag. J. Korea Concr. Inst. 2020, 32, 137–144. [Google Scholar] [CrossRef]
















| ig-Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | MnO | C | S | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFBC ash | 1.05 | 24.73 | 10.33 | 4.95 | 38.35 | 4.54 | 4.03 | 0.84 | 0.51 | 0.13 | 0.00 | 0.32 | 10.15 | 99.94 |
| OPC | 2.45 | 20.03 | 5.40 | 2.87 | 64.35 | 3.42 | 0.35 | 0.54 | 0.30 | 0.11 | 0.11 | - | 99.93 | |
| Slag cement | 0.9 | 29.42 | 11.12 | 1.27 | 51.55 | 4.27 | 0.24 | 0.27 | 0.66 | 0.02 | 0.21 | - | - | 99.93 |
| Carbonation Chemical Reaction in Water | ΔG (Unit: KJmole−1) | Reaction | |
|---|---|---|---|
| CaO (s) + H2O (l) → Ca(OH)2 (s) Ca(OH)2 (s) + H2O (l) → Ca2+ (aq) + 2OH− (aq) + H2O (l) | −57.83 30.42 | R. (1) R. (2) | (I) Ca ionization |
| CO2 (g) + H2O (l) → CO2 (aq) + H2O (l) CO2 (aq) + OH− (aq) → HCO3− (aq) HCO3− (aq) + OH− (aq) → H2O (l) + CO32− (aq) | 8.38 −43.55 −20.92 | R. (3) R. (4) R. (5) | (II) CO2 dissolution and ionization |
| Ca2+ (aq) + CO32− (aq) → CaCO3 (s) | −47.40 | R. (6) | (III) CaCO3 Precipitation |
| (1) Experimental Conditions for Carbonation Reaction According to CO2 Gas Flow Rate Change | ||
| Ca(oh)2 mass | Flow Rate of CO2 Gas | Solid/Liquid Ratio |
| 50 g | 100 cc/min | 1:5 |
| 300 cc/min | ||
| 500 cc/min | ||
| 700 cc/min | ||
| 1000 cc/min | ||
| (2) Conditions for the Carbonation Reaction Experiment According to the Solids Amount Changes | ||
| Solid (CFBC ash/Ca(OH)2) | Flow Rate of CO2 Gas | Solid/Liquid Ratio |
| 100 g/30 g | 700 cc/min | 1:5 |
| 200 g/60 g | ||
| 300 g/90 g | ||
| 400 g/120 g | ||
| Samples | Cement | Ash | Standard Sand | Water |
|---|---|---|---|---|
| Plain (OPC) | 450 ± 2 g | 0 g | 1350 ± 5 g | 225 ± 1 g |
| Plain (Slag Cement) | 450 ± 2 g | 0 g | 1350 ± 5 g | 225 ± 1 g |
| R10 | 405 ± 2 g | 45 ± 0.5 g | 1350 ± 5 g | 225 ± 1 g |
| R20 | 360 ± 2 g | 90 ± 0.5 g | 1350 ± 5 g | 225 ± 1 g |
| No. | W/B (%) | Unit Binder (kg/m3) | Foam Rate (%) | Binder Ratio (%) | Admixture (%) | ||
|---|---|---|---|---|---|---|---|
| Cement | Gypsum | Carbonated CFBC Ash | |||||
| 1 | 25 | 500 | 65.8 | 97 | 3 | 0 | 0.4 |
| 2 | 65.0 | 87 | 10 | ||||
| 3 | 64.5 | 82 | 15 | ||||
| 4 | 64.1 | 77 | 20 | ||||
| 5 | 63.7 | 72 | 25 | ||||
| 6 | 63.2 | 67 | 30 | ||||
| CFBC Ash Mass (g) | Flow Rate of CO2 Gas (cc/min) | Solid: Liquid Ratio |
|---|---|---|
| 300 | 100 | 1:2 |
| 200 | ||
| 300 | ||
| 400 | ||
| 500 |
| No. | Sample Name | Cement Replacement Amount | Concrete Activation | ||
|---|---|---|---|---|---|
| 3 Day | 7 Day | 14 Day | |||
| 1 | Plain (OPC) | 0 | 100 | 100 | 100 |
| 2 | R10 | 10 | 91 | 85 | 88 |
| 3 | R20 | 20 | 100 | 88 | 89 |
| 4 | Plain (slag cement) | 0 | 100 | 100 | 100 |
| 5 | R10 | 10 | 106 | 112 | 99 |
| 6 | R20 | 20 | 106 | 105 | 94 |
| No. | Experiment Item | Unit | Plain (OPC) | Plain (Slag Cement) | R10 | R20 |
|---|---|---|---|---|---|---|
| 1 | Density | g/cm3 | 3.07 | 3.03 | 3.04 | 2.91 |
| 2 | Stability | mm | 0.5 | 0 | 0.5 | 0 |
| 3 | Setting time (beginning) | Min | 222 | 216 | 218 | 230 |
| 4 | Setting time (closing) | Min | 260 | 288 | 275 | 280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wie, Y.M.; Lee, K.G.; Lee, K.H. Characteristics of Circulating Fluidized Bed Combustion (CFBC) Ash as Carbon Dioxide Storage Medium and Development of Construction Materials by Recycling Carbonated Ash. Materials 2024, 17, 4359. https://doi.org/10.3390/ma17174359
Wie YM, Lee KG, Lee KH. Characteristics of Circulating Fluidized Bed Combustion (CFBC) Ash as Carbon Dioxide Storage Medium and Development of Construction Materials by Recycling Carbonated Ash. Materials. 2024; 17(17):4359. https://doi.org/10.3390/ma17174359
Chicago/Turabian StyleWie, Young Min, Ki Gang Lee, and Kang Hoon Lee. 2024. "Characteristics of Circulating Fluidized Bed Combustion (CFBC) Ash as Carbon Dioxide Storage Medium and Development of Construction Materials by Recycling Carbonated Ash" Materials 17, no. 17: 4359. https://doi.org/10.3390/ma17174359
APA StyleWie, Y. M., Lee, K. G., & Lee, K. H. (2024). Characteristics of Circulating Fluidized Bed Combustion (CFBC) Ash as Carbon Dioxide Storage Medium and Development of Construction Materials by Recycling Carbonated Ash. Materials, 17(17), 4359. https://doi.org/10.3390/ma17174359







