Advancements in Polyethylene Oxide (PEO)–Active Filler Composite Polymer Electrolytes for Lithium-Ion Batteries: A Comprehensive Review and Prospects
Abstract
1. Introduction
2. Conventional LIBs
- Charging process
- Oxidation reaction: (Anode) LiMO2 Li1-xMO2 xLi+ xe−
- Reduction reaction: (Cathode) yC xLi+ xe− LixCy
- Discharging process
- Oxidation reaction: (Anode) LixCy yC xLi+ xe−
- Reduction reaction: (Cathode) Li1-xMO2 xLi+ xe− LiMO2
3. Solid-State Electrolytes (SSEs)
4. Improving the Properties of PEO−Based Electrolytes
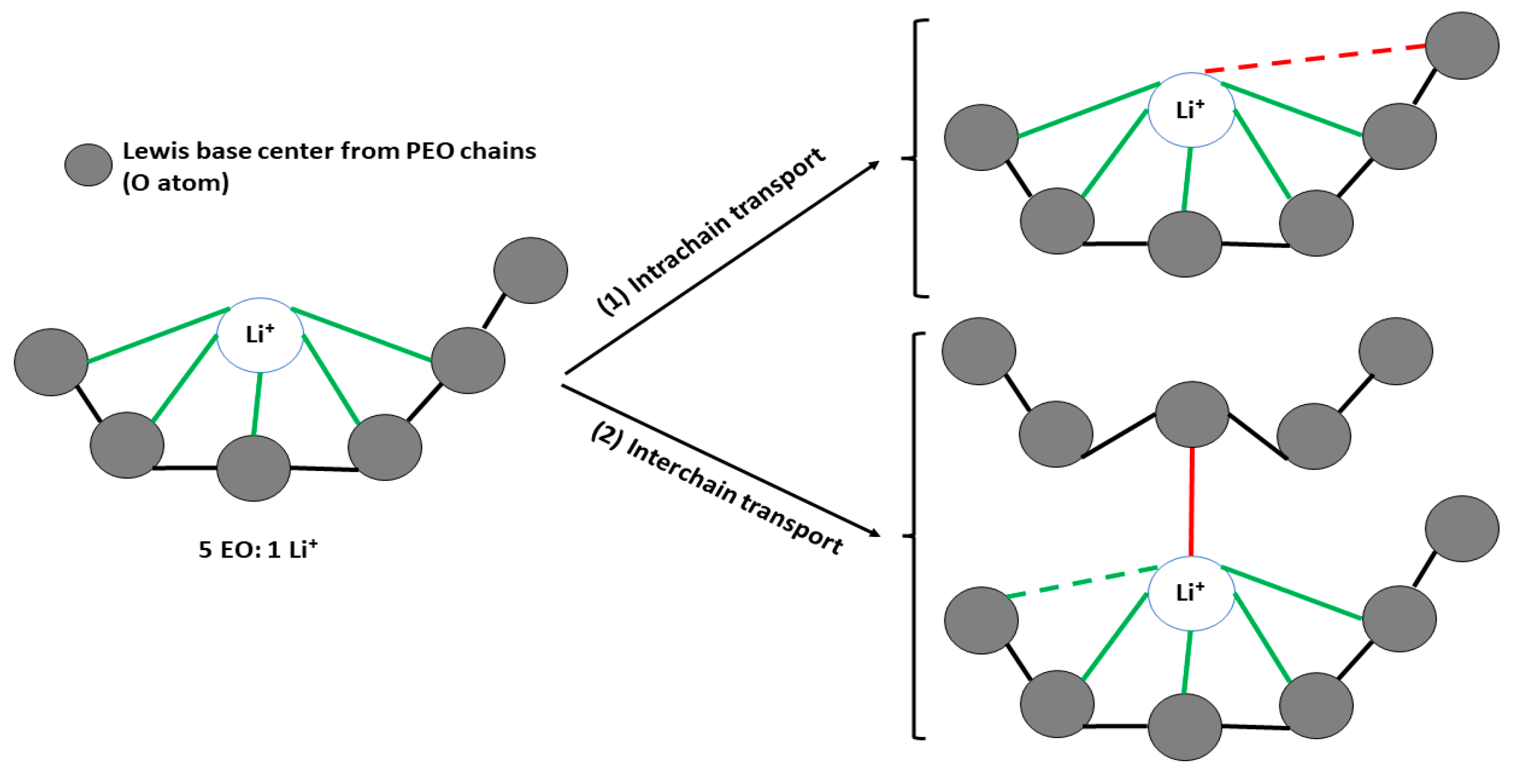
5. Ion Transport Mechanism in PEO-Based CPEs
- High ionic conductivity (10−4 S cm−1 at 25 ) → facilitates Li+ ion transportation.
- Low electronic conductivity (10−12 S cm−1) → avoids the self-discharge of batteries.
- High Li+ transference numbers → effectively contribute to Li+ diffusion, eliminating concentration gradients, suppressing parasitic reactions, and improving battery performance.
- Wide electrochemical window (0−4.5 V vs. Li/Li+) → compatible with the anode and high-voltage cathode.
- Non-flammable, high thermal stability (<5% after 60 min at 90) → avoids thermal runaway.
- High mechanical strength (2 offset yield) → withstands stress, avoids short-circuiting, and suppresses dendrite growth.
- Small pore size (<1 µm) and moderate porosity (40–60).
- Thin form (20–25 µm) → reduces the internal resistance, improves energy utilization, improves power densities, and reduces costs.
- Good processability for larger scales.
- Schottky defect: an equal number of cations and anions are missing from their normal sites, resulting in an equal number of cation and anion vacancies.
- Frenkel defect: the same ions (cations) leave their normal lattice sites and occupy the interstitial sites.
6. Morphology of Active Fillers
7. Characterization Techniques and Performance Evaluation of CPEs
7.1. Determining the Components in CPEs
7.2. Morphological and Structural Study
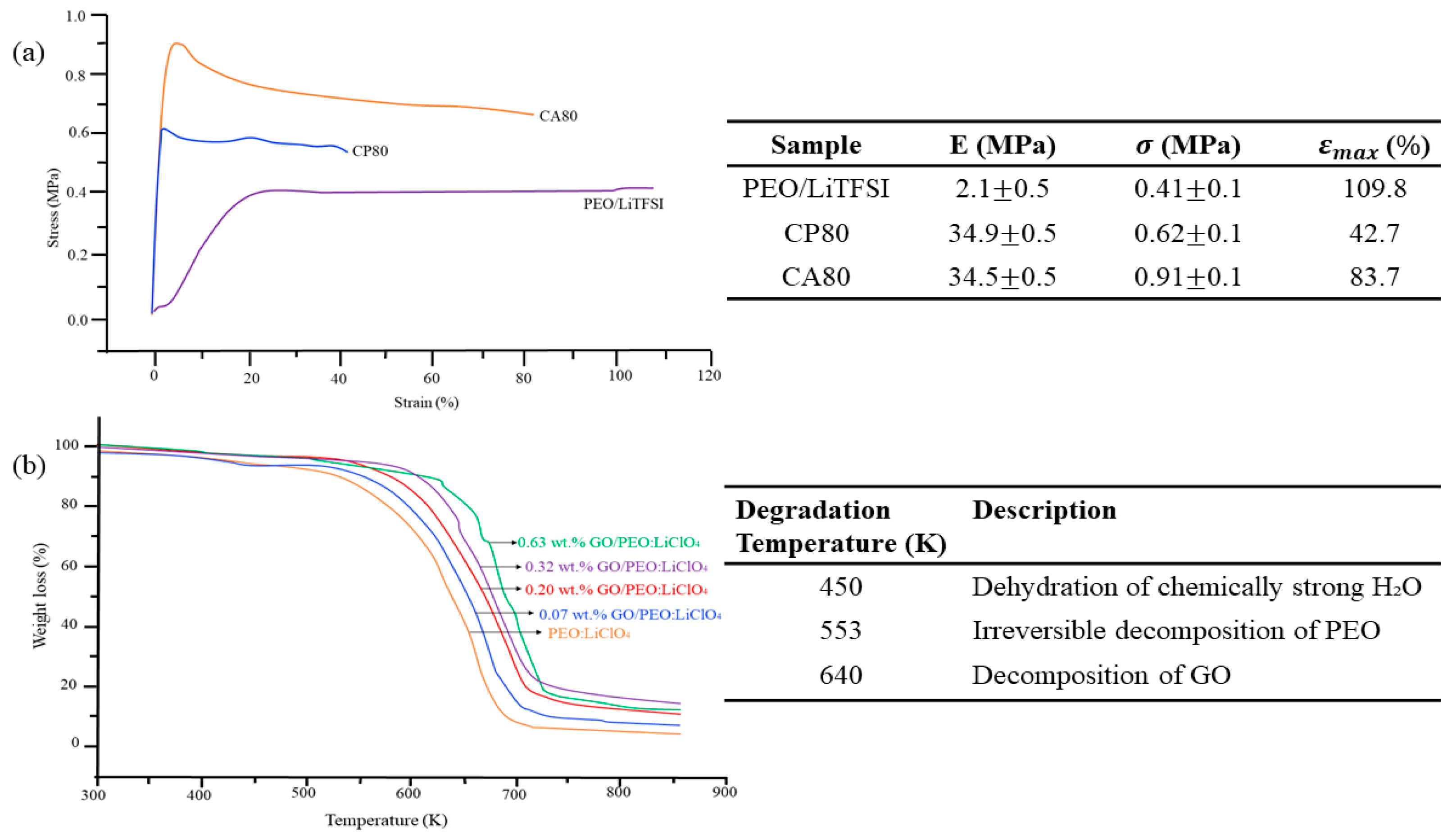
7.3. Stability of CPEs
7.4. Electrochemical Properties
8. Factor Influencing Performance
9. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilanchiev, A.; Nuta, F.; Khan, I.; Khan, H. Urbanization, renewable energy production, and carbon dioxide emission in BSEC member states: Implications for climate change mitigation and every markets. Environ. Sci. Pollut. Res. 2023, 30, 67338–67350. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef]
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of all-solid-state Li-ion batteries: From key technical areas to commercial use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Wang, X. The anode materials for lithium-ion and sodium-ion batteries based on conversion reactions: A review. ChemElectroChem 2023, 10, e202201151. [Google Scholar] [CrossRef]
- Marquina, L.M.; Riveros, L.L.T.; Ccana, V.J.; Muedas-Taipe, G.; Isaacs, M.; Rosa-Tora, A.L. Recent studies on polymer electrolytes containing ionic liquids and their applications in lithium-ion batteries. J. Electroanal. Chem. 2023, 948, 117819. [Google Scholar] [CrossRef]
- Halder, B.; Mohamed, M.G.; Kuo, S.W.; Elumalai, P. Review on composite polymer electrolyte using PVDF-HFP for solid-state lithium-ion battery. Mater. Today Chem. 2024, 36, 101926. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Yang, H.; Zhou, Y.; Shan, Y. A review of composite polymer electrolytes for solid-state lithium-sulfur batteries: Synthesis methods, optimal design, and critical challenges. Chem. Eng. J. 2024, 484, 149433. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The critical role of fillers in composite polymer electrolytes for lithium battery. Nano-Micro Lett. 2023, 15, 74. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, H.; Wu, J. Designing mechanically reinforced filler network for thin and robust composite polymer electrolyte. Batter. Energy 2023, 2, 20230037. [Google Scholar] [CrossRef]
- Gulzar, U.; Goriparti, S.; Miele, E.; Li, T.; Maidecchi, G.; Toma, A.; De Angelis, F.; Capiglia, C.; Zaccaria, R.P. Next generation textiles: From embedded supercapacitors to lithium ion batteries. J. Mater. Chem. A 2016, 43, 16771–16800. [Google Scholar] [CrossRef]
- Hu, Y.; Wayment, L.J.; Haslam, C.; Yang, X.; Lee, S.; Jin, Y.; Zhang, W. Covalent organic framework based lithium-ion battery: Fundamental, design and characterization. EnergyChem 2021, 3, 100048. [Google Scholar] [CrossRef]
- Latest Open Tech from Seed. Available online: https://www.seeedstudio.com/blog/2020/11/25/batteries-lithium-ion-battery-and-lithium-polymer-battery-explained/ (accessed on 1 May 2024).
- Solvionic. Available online: https://solvionic.com/en/electrolytes/5607-1m-lipf6-in-dmc-ec-1-1-vol.html (accessed on 21 August 2024).
- Ramoni, M.O.; Zhang, H.C. End-of-life (EOL) issues and options for electric vehicle batteries. Clean. Technol. Environ. Policy 2013, 15, 881–891. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Li, Z.; Fu, F.; Zhou, X.; Gui, S.; Wei, L.; Yang, H.; Li, H.; Guo, X. Ionic conduction in polymer-based solid electrolytes. Adv. Sci. 2023, 10, 2201718. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Alziyadi, M.O.; Gamal, H.; Hamdy, S. Solid-state lithium-ion battery: The key components enhance the performance and efficiency of anode, cathode, and solid electrolytes. J. Alloys Compd. 2023, 969, 172318. [Google Scholar] [CrossRef]
- Lv, F.; Wang, Z.; Shi, L.; Zhu, J.; Edström, K.; Mindemark, J.; Yuan, S. Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sources 2019, 441, 227175. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Mohanty, S.; Ramadoss, A. Quasi-solid-state electrolytes for pseudocapacitors and batteries. In Smart Supercapacitor; Hussain, C.M., Ahamed, M.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 745–778. [Google Scholar]
- Yang, T.; Wang, C.; Zhang, W.; Xia, Y.; Huang, H.; Gan, Y.; He, X.; Xia, X.; Tao, X.; Zhang, J. A critical review on composite solid electrolytes for lithium batteries: Design strategies and interface engineering. J. Energy Chem. 2023, 84, 189–209. [Google Scholar] [CrossRef]
- Elbinger, L.; Enke, M.; Ziegenbalg, N.; Brendel, J.C.; Schubert, U.S. Beyond lithium-ion batteries: Recent developments in polymer-based electrolytes for alternative metal-ion-batteries. Energy Storage Mater. 2023, 2023, 103063. [Google Scholar] [CrossRef]
- Renoso, D.M.; Frechero, M.A. Strategies for rational design of polymer-based solid electrolytes for advanced lithium energy sorage applications. Energy Storage Mater. 2022, 52, 430–464. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Z.; Cheng, D.; Cheng, H.; Xu, H.; Huang, Y. Molecular regulated polymer electrolytes for solid-state lithium metal batteries: Mechanisms and future prospects. eTransportation 2023, 18, 100264. [Google Scholar] [CrossRef]
- Chen, M.; Yue, Z.; Wu, Y.; Wang, Y.; Li, Y.; Chen, Z. Thermal stble polymer-based solid electrolytes: Design strategies and corresponding stable mechanisms for solid-state Li metal batteries. Sustain. Mater. Technol. 2023, 36, e00587. [Google Scholar]
- Su, X.; Xu, X.P.; Ji, Z.Q.; Wu, J.; Ma, F.; Fan, L.Z. Polyethylene oxide-based composite solid electrolytes for lithium batteries: Current progress, low-temperature and high-voltage limitations, and prospects. Electrochem. Energy Rev. 2024, 7, 2. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; He, D.; Xie, X. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, B.S. Recent progress in poly (ethylene oxide)-based solid-state electrolytes for lithium-ion batteries. Bull. Korean Chem. Soc. 2023, 44, 831–840. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.; Zhen, Y.; Zhao, P.; Wang, X.; Li, L. Effects of lithium salts on PEO-based solid polymer electrolytes and their all-solid-state lithium-ion batteries. Ionics 2022, 28, 2751–2758. [Google Scholar]
- Arya, A.; Sharma, A.L. Polymer Nanocomposite: Synthesis and characterization. In Environmental Nanotechnology Volume 4. Environmental Chemistry for a Sustainable World; Dasgupta, N., Rajan, S., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 32, pp. 265–315. [Google Scholar]
- St-Onge, V.; Cui, M.; Rochon, S.; Daigle, J.C.; Claverie, J.P. Reducing crystallinity in solid polymer electrolytes for lithium-metal batteries via statistical copolymerization. Commun. Mater. 2021, 2, 83. [Google Scholar] [CrossRef]
- Liu, S.; Ao, Z.; Zou, Y.; Li, H.; Chen, N.; Wan, Y.; Lao, Y. Enhanced ionic conductivity of PEO-based solid electrolyte through synergistic dissociation effect. Mater. Lett. 2023, 351, 134981. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Z.; Zhu, P.; Liu, J.; Shang, S. The effects of electrospinning structure on the ion conductivity of PEO-based polymer solid-state electrolytes. Energies 2023, 16, 5819. [Google Scholar] [CrossRef]
- Wang, N.; Wie, Y.; Yu, S.; Zhang, W.; Huang, X.; Fan, B.; Yuan, H.; Tan, Y. A flexible PEO-based polymer electrolyte with cross-linked network for high-voltage all solid-state lithium-ion battery. J. Mater. Sci. Technol. 2024, 183, 206–214. [Google Scholar] [CrossRef]
- Ritter, T.G.; Gonçalves, J.M.; Stoyanov, S.; Ghorbani, A.; Shokuhfar, T.; Shahbazian-Yassar, R. AlTiMgLiO medium entropy oxide additive for PEO-based solid polymer electrolytes in lithium ion batteries. J. Energy Storage 2023, 72, 108491. [Google Scholar] [CrossRef]
- Usta, S.; Celik, M.; Cetinkaya, T. Enhancement of the stability window of PEO for high voltage all-solid-state lithium batteries. J. Power Sources 2023, 580, 233404. [Google Scholar] [CrossRef]
- Li, C.; Yue, H.; Wang, Q.; Yang, S. A high-performance self-reinforced PEO-based blend solid electrolyte membrane for solid-state lithium ion batteries. Russ. J. Electrochem. 2022, 58, 271–283. [Google Scholar]
- Martins, P.M.; Pereira, J.N.; Mndez, S.L.; Costa, C.M. Chapter 17-Synthetic polymer-based membranes for lithium-ion batteries. In Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Ismail, A.F., Salleh, W.N.W., Yusof, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–415. [Google Scholar]
- Nguyen, A.G.; Park, C.J. Insights into tailoring composite solid polymer electrolytes for solid-state lithium batteries. J. Membr. Sci. 2023, 675, 121552. [Google Scholar] [CrossRef]
- Wang, M.; Tian, L.; Cao, Y.; Su, Z.; Zhang, W.; Yi, S.; Zhang, Y.; Niu, B.; Long, D. Surface positive-charged modification of inorganic fillers to optimize lithium ion conductive pathways in composite polymer electrolytes for lithium-metal batteries. J. Colloid. Interface Sci. 2023, 630, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Daems, K.; Yadav, P.; Dermenci, K.B.; Mierlo, J.V.; Berecibar, M. Advances in inorganic, polymer and composite electrolytes: Mechanisms of lithium-ion transport and pathways to enhanced performance. Renew. Sustain. Energy Rev. 2024, 191, 114136. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in solid polymer electrolytes for lithium-ion batteries and beyond. Nano-Micro Small 2022, 18, 2103617. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, L.; Wang, A.; Song, Y.; Wu, Y.; Yang, Y.; He, X. Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: A review. Nano-Micro Lett. 2023, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yu, H.; Hui, W.; Shizhao, X.; Kai, X.; Qingpeng, G. Li1.5Al0.5Ge1.5(PO4)3 based solid composite electrolyte preparation and lithium ion conductive behavior. Chem. J. Chin. Univ. 2016, 37, 306–315. [Google Scholar]
- Gog, H.V.; Huis, M.A.V. Structural and electronic properties of Frenkel and Schottky defects at the MgO {100} Surface: Spin polarization, mid-band gap states, and charge trapping at vacancy sites. J. Phys. Chem. C 2019, 123, 14408–14420. [Google Scholar]
- Shi, Y.; Fan, Z.; Ding, B.; Li, Z.; Lin, Q.; Chen, S.; Dou, H.; Zhang, X. Atomic-scale Al2O3 modified PEO-based composite polymer electrolyte for durable solid-state Li–S batteries. J. Electroanal. Chem. 2021, 881, 114916. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Chen, C.; Feng, X.; Zhang, Z. High-performance polymer electrolyte membrane modified with isocyanate-grafted Ti3+ doped TiO2 nanowires for lithium batteries. Appl. Surf. Sci. 2021, 563, 150248. [Google Scholar] [CrossRef]
- Itoh, T.; Miyamura, Y.; Ichikawa, Y.; Uno, T.; Kubo, M.; Yamamoto, O. Composite polymer electrolytes of poly(ethylene oxide)/BaTiO3/Li salt with hyperbranched polymer. J. Power Sources 2003, 119–121, 403–408. [Google Scholar] [CrossRef]
- Sun, H.Y.; Takeda, Y.; Imanishi, N.; Yamamoto, O.; Sohn, H.J. Ferroelectric materials as a ceramic filler in solid composite polyethylene oxide-based electrolytes. J. Electrochem. Soc. 2000, 147, 2462–2467. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.R.; Kulandainathan, M.A.; Kumar, R.S.; Ferrara, C.; Mustarelli, P.; Stephan, A.M. Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 9948–9954. [Google Scholar] [CrossRef]
- Wu, J.F.; Guo, X. MOF-derived nanoporous multifunctional fillers enhancing the performances of polymer electrolytes for solid-state lithium batteries. J. Mater. Chem. A 2019, 7, 2653–2659. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Shi, J.; Liu, Y.; Zheng, S.; Zou, H.; Chen, Y.; Kuang, W.; Ding, K.; Chen, L.; et al. A 3D interconnected metal-organic framework-derived solid-state electrolyte for dendrite-free lithium metal battery. Energy Storage Mater. 2022, 47, 262–270. [Google Scholar] [CrossRef]
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T.; Guo, X.; Sun, X. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Gopi, S.; Kathiresan, M.; Bose, S.; Gows, E.B.; Nair, J.R.; Angulakshmi, N.; Meligrana, G.; Bella, F.; Gerbaldi, C.; et al. Metal organic framework laden poly(ethylene oxide) based composite electrolytes for all-solid-state Li–S and Li-metal polymer batteries. Electrochim. Acta 2018, 285, 355–364. [Google Scholar] [CrossRef]
- Jamal, H.; Khan, F.; Si, H.R.; Kim, J.H. Enhanced compatibility of a polymer-based electrolyte with Li-metal for stable and dendrite-free all-solid-state Li-metal batteries. J. Mater. Chem. A 2021, 9, 27304–27319. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, Y.; Liu, J. A fast charging/discharging all-solid state lithium ion battery based on PEO-MIL-53(Al)-LiTFSI thin film electrolyte. RSC Adv. 2014, 4, 42278–42284. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Luo, J.; Yuan, H.; Huang, H.; Gan, Y.; Zhang, J.; Xia, Y.; Liang, C.; Zhang, W.; et al. Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance. Nano Lett. 2018, 18, 3104–3112. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.Y.; Yang, S.H.; Ryu, H.M.; Jung, H.Y.; Ban, H.J.; Park, S.J.; Lim, J.S.; Kim, H.S. Fabrication and electrochemical characteristics of NCM based all-solid lithium batteries using nano-grade garnet Al-LLZO powder. J. Ind. Eng. Chem. 2019, 71, 445–451. [Google Scholar] [CrossRef]
- Wan, Z.; Lei, D.; Yan, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.H.; et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Adv. Funct. Mater. 2019, 29, 1805301. [Google Scholar] [CrossRef]
- Li, R.; Guo, S.; Yu, L.; Wang, L.; Wu, D.; Li, Y.; Hu, X. Morphosynthesis of 3D macroporous garnet frameworks and perfusion of polymer-stabilized lithium salts for flexible solid-state hybrid electrolytes. Adv. Mater. Interfaces 2019, 6, 1900200. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, C.H.; Yu, J.H.; Doh, C.H.; Lee, S.M. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 2015, 274, 458–463. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Dai, J.; Gong, A.; Han, X.; Yao, Y.; Wang, C.; Wang, Y.; Chen, Y.; Yan, C.; et al. Flexible, solidstate, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl. Acad. Sci. USA 2016, 113, 7094–7099. [Google Scholar] [CrossRef]
- Zagórski, J.; del Amo, J.M.L.; Cordill, M.J.; Aguesse, F.; Buannic, L.; Llordés, A. Garnet-polymer composite electrolytes: New insights on local Li-ion dynamics and electrodeposition stability with Li metal anodes. ACS Appl. Energy Mater. 2019, 2, 1734–1746. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.W.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zhang, X.Q.; Cheng, X.B.; Zhang, R.; Xu, R.; Chen, P.Y.; Peng, H.J.; Huang, J.Q.; Zhang, Q. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl. Acad. Sci. USA 2017, 114, 11069–11074. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Zhai, H.; Xu, P.; Ning, M.; Cheng, Q.; Mandal, J.; Yang, Y. A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett. 2017, 17, 3182–3187. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Ferrara, C.; Berbenni, V.; Anselmi-Tamburini, U.; Mustarelli, P.; Tealdi, C. NASICON-type polymer-in-ceramic composite electrolytes for lithium batteries. Phys. Chem. Chem. Phys. 2019, 21, 6142–6149. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liang, Y.; Wang, C.; Fan, L.Z. Composite polymer electrolyte with three-dimensional ion transport channels constructed by NaCl template for solid-state lithium metal batteries. Energy Storage Mater. 2022, 45, 1212–1219. [Google Scholar] [CrossRef]
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J. Energy Storage 2019, 26, 100947. [Google Scholar] [CrossRef]
- Li, A.; Liao, X.; Zhang, H.; Shi, L.; Wang, P.; Cheng, Q.; Borovilas, J.; Li, Z.; Huang, W.; Fu, Z.; et al. Nacre-inspired composite electrolytes for load-bearing solid-state lithium-metal batteries. Adv. Mater. 2020, 32, 1905517. [Google Scholar] [CrossRef]
- Cheng, J.; Hou, G.; Sun, Q.; Liang, Z.; Xu, X.; Guo, J.; Dai, L.; Li, D.; Nie, X.; Zeng, Z.; et al. Cold-pressing PEO/LAGP composite electrolyte for integrated all-solid-state lithium metal battery. Solid. State Ion. 2020, 345, 115156. [Google Scholar] [CrossRef]
- Lee, J.; Howell, T.; Rottmayer, M.; Boeckl, J.; Huang, H. Freestanding PEO/LiTFSI/LAGP composite electrolyte membranes for applications to flexible solid-state lithium-based batteries. J. Electrochem. Soc. 2019, 166, A416–A422. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef]
- Xu, H.; Chien, P.H.; Shi, J.; Li, Y.; Wu, N.; Liu, Y.; Hu, Y.Y.; Goodenough, J.B. High-performance all-solid-state batteries enabled by salt bonding to perovskite in poly(ethylene oxide). Proc. Natl. Acad. Sci. USA 2019, 116, 18815–18821. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhang, J.; Zhou, X.; Zhao, F.; Shi, Y.; Goodenough, J.B.; Yu, G. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem. Int. Ed. 2018, 57, 2096–2100. [Google Scholar] [CrossRef]
- Liu, K.; Wu, M.; Wei, L.; Lin, Y.; Zhao, T. A composite solid electrolyte with a framework of vertically aligned perovskite for all-solid-state Li-metal batteries. J. Membr. Sci. 2020, 610, 118265. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Sun, J.; Wu, M.; Zhao, T. Polyoxyethylene (PEO)|PEO-perovskite|PEO composite electrolyte for all solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 2019, 11, 46930–46937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Yan, C.; Dirican, M.; Zhu, J.; Zang, J.; Selvan, R.K.; Chung, C.C.; Jia, H.; Kiyak, Y.; Wu, N.; et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid state lithium batteries. J. Mater. Chem. A 2018, 6, 4279–4285. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Li, L.; Shen, Y.; Nan, C.W. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 24791–24798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Yan, C.; Zhu, J.; Zang, J.; Li, Y.; Jia, H.; Dong, X.; Du, Z.; Zhang, C.; Wu, N.; et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries. Energy Storage Mater. 2019, 17, 220–225. [Google Scholar] [CrossRef]
- Yi, J.; Zhou, D.; Liang, Y.; Liu, H.; Ni, H.; Fa, L.Z. Enabling high performance all-solid-state lithium batteries with high ionicconductive sulfide-based composite solid electrolyte and ex situ artificial SEI film. J. Energy Chem. 2021, 58, 17–24. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, C.; Lou, J.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. Poly(ethylene oxide) reinforced Li6PS5Cl composite solid electrolyte for all-solid-state lithium battery: Enhanced electrochemical performance, mechanical property and interfacial stability. J. Power Sources 2019, 412, 78–85. [Google Scholar] [CrossRef]
- Simon, F.J.; Hanauer, M.; Richter, F.H.; Janek, J. Interphase formation of PEO20:LiTFSI-Li6PS5Cl composite electrolytes with lithium metal. ACS Appl. Mater. Interfaces 2020, 12, 11713–11723. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Fan, A.; Liu, X.; Wang, H.; Ma, W.; Zhu, L.; Zhang, X. A high energy and power all-solid-state lithium battery enabled by modified sulfide electrolyte film. J. Power Sources 2021, 485, 229325. [Google Scholar] [CrossRef]
- Li, M.; Frerichs, J.E.; Kolek, M.; Sun, W.; Zhou, D.; Huang, C.J.; Hwang, B.J.; Hansen, M.R.; Winter, M.; Bieker, P. Solid state lithium-sulfur battery enabled by Thio-LiSICON/polymer composite electrolyte and sulfurized polyacrylonitrile cathode. Adv. Funct. Mater. 2020, 14, 1910123. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, P.; Liu, H.; Hu, Y.Y. Interface-enabled ion conduction in Li10GeP2S12-poly(ethylene oxide) hybrid electrolytes. ACS Appl. Energy Mater. 2019, 2, 1452–1459. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Wang, H.; Yan, H.; Gong, Z.; Yang, Y. Poly(ethylene oxide)-Li10SnP2S12 composite polymer electrolyte enables high-performance all-solid-state lithium sulfur battery. ACS Appl. Mater. Interfaces 2019, 25, 22745–22753. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, L.; Li, J.X.; Wu, H.F.; Shao, W.W.; Wang, P.Q.; Liu, M.Q.; Zhang, G.; Jing, M.-X. Insights into the effects of different inorganic fillers on the electrochemical performances of polymer solid electrolytes. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 671, 131704. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Liu, M.; Guan, X.; Liu, H.; Ma, X.; Wu, Q.; Ge, S.; Zhang, H.; Xu, J. Composite solid electrolytes containing single-ion lithium polymer grafted garnet for dendrite-free, long-life all-solid-state lithium metal batteries. Chem. Eng. J. 2022, 445, 136436. [Google Scholar] [CrossRef]
- Song, X.; Zhang, T.H.; Fan, R.Z.; Biao, J.; Huang, S.H.; Travaš-Sejdić, J.; Gao, W.; Cao, P. A composite solid-state electrolyte of high ionic-conductivity garnet-type Li6.5La3Zr1.5Ta0.1Nb0.4O12 filler in PEO matrix. Solid State Ion. 2023, 403, 116410. [Google Scholar] [CrossRef]
- Han, Q.; Wang, S.; Ren, W.; Zhang, F.; Tang, Z.; Wang, J.; Wang, H. Redox-active ferrocene upgrading PEO electrolyte for durable all-solid-state lithium-metal batteries. J. Power Sources 2023, 581, 233459. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Ma, H.; Peng, L.; Huang, B.; Zhang, P.; Zhao, J. Enhancing Li+ transport kinetics of PEO-based polymer electrolyte with mesoporous silica-derived fillers for lithium-ion batteries. Solid State Ion. 2020, 354, 115412. [Google Scholar] [CrossRef]
- Duan, T.; Cheng, H.; Liu, Y.; Sun, Q.; Nie, W.; Lu, X.; Dong, P.; Song, M.K. A multifunctional Janus layer for LLZTO/PEO composite electrolyte with enhanced interfacial stability in solid-state lithium metal batteries. Energy Storage Mater. 2024, 65, 103091. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Xia, E.; Wu, Y.; Li, Z. PEO/Li2ZrO3 composite electrolyte for solid-state rechargeable lithium battery. J. Energy Storage 2023, 65, 107283. [Google Scholar] [CrossRef]
- Cheng, J.; Hou, G.; Chen, Q.; Li, D.; Li, K.; Yuan, Q.; Wang, J.; Ci, L. Sheet-like garnet structure design for upgrading PEO-based electrolyte. Chem. Eng. J. 2022, 429, 132343. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, H.; Wang, Q.; He, Y.; Hua, Y.C.; Li, C.; Bai, J. In-doped Li7La3Zr2O12 nanofibers enhanced electrochemical properties and conductivity of PEO-based composite electrolyte in all-solid-state lithium battery. J. Energy Storage 2024, 76, 109784. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhao, F.; Zhou, X.; Ding, Y.; Yu, G. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater. 2018, 15, 46–52. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Liao, C.; Gong, W.; Yang, M.; Li, R.K.Y.; Tjong, S.C.; Lu, Z. Enhanced electrochemical performance of solid PEO/LiClO4 electrolytes with a 3D porous Li6.28La3Zr2Al0.24O12 network. Comp. Sci. Technol. 2019, 184, 107863. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Choi, H.; Lim, D.H.; Kim, J.K.; Matic, A.; Jacobsson, P.; Nah, C.; Ahn, J.H. Electrochemical characterization of poly (vinylidene fluoride-co-hexafluoro propylene) based electrospun gel polymer electrolytes incorporating room temperature ionic liquids as green electrolytes for lithium batteries. Solid State Ion. 2014, 262, 77–82. [Google Scholar] [CrossRef]
- Shubha, N.; Prasanth, R.; Hoon, H.H.; Srinivasan, M. Dual phase polymer gel electrolyte based on non-woven poly (vinylidenefluoride-co-hexafluoropropylene)-layered clay nanocomposite fibrous membranes for lithium ion batteries. Mater. Res. Bull. 2013, 48, 526–537. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, N.; Cao, Q.; Jing, B.; Wang, X.; Wang, Q.; Kuang, H. A novel electrospun PVDF/PMMA gel polymer electrolyte with in situ TiO2 for Li-ion batteries. Solid State Ion. 2013, 249–250, 93–95. [Google Scholar] [CrossRef]
- Yang, C.; Jia, Z.; Guan, Z.; Wang, L. Polyvinylidene fluoride membrane by novel electrospinning system for separator of Li-ion batteries. J. Power Sources 2009, 189, 716–720. [Google Scholar] [CrossRef]
- Wu, Y.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Electrochemical studies on electrospun Li(Li1/3Ti5/3)O4 grains as an anode for Li-ion batteries. Electrochim. Acta 2012, 67, 33–40. [Google Scholar] [CrossRef]
- Jeong, H.S.; Choi, E.S.; Lee, S.Y.; Kim, J.H. Evaporation-induced, close-packed silica nanoparticle-embedded nonwoven composite separator membranes for high-voltage/high-rate lithium-ion batteries: Advantageous effect of highly percolated, electrolyte-philic microporous architecture. J. Membr. Sci. 2012, 415–416, 513–519. [Google Scholar] [CrossRef]
- Zhao, E.; Guo, Y.; Liu, Y.; Xu, G. Nanostructured zeolitic imidazolate framework-67 reinforced poly (ethylene oxide) composite electrolytes for all solid state lithium ion batteries. Appl. Surf. Sci. 2022, 573, 151489. [Google Scholar] [CrossRef]
- Li, C.; Xue, P.; Chen, L.; Liu, J.; Wang, Z. Reducing the crystallinity of PEO-based composite electrolyte for high performance lithium batteries. Composites Part. B 2022, 234, 109729. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, M.; Hu, Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolyte. Angew. Chem. 2016, 128, 12726–12730. [Google Scholar] [CrossRef]
- Fu, J.; Li, Z.; Zhou, X.; Guo, X. Ion transport in composite polymer electrolytes. Mater. Adv. 2022, 3, 3809–3819. [Google Scholar] [CrossRef]
- He, K.; Chen, C.; Fan, R.; Liu, C.; Liao, C.; Xu, Y.; Tang, J.; Li, R.K.Y. Polyethylene oxide/granet-type Li6.4La3Zr1.4Nb0.6O12 composite electrolytes with improved electrochemical performance for solid state lithium rechargeable batteries. Compos. Sci. Technol. 2019, 175, 28–34. [Google Scholar] [CrossRef]
- Song, Y.-W.; Heo, K.; Lee, J.; Hwang, D.; Kim, M.-Y.; Kim, S.J.; Kim, J.; Lim, J. Lithium-ion transport in inorganic active fillers used in PEO-based composite solid electrolyte sheets. RSC Adv. 2021, 11, 31855. [Google Scholar] [CrossRef]
- Golozar, M.; Paolella, A.; Demers, H.; Savoie, S.; Girard, G.; Delaporte, N.; Gauvin, R.; Guerfi, A.; Lorrmann, H.; Zaghib, K. Direct observation of lithium metal dendrites with ceramic solid electrolyte. Sci. Rep. 2020, 10, 18410. [Google Scholar] [CrossRef]
- Rong, Y.; Lu, Z.; Jin, C.; Xu, Y.; Peng, L.; Shi, R.; Gu, T.; Lu, C.; Yang, R. Tailoring of Li/LATP-PEO interface via a functional organic layer for high-performance solid lithium metal batteries. ACS Sustain. Chem. Eng. 2023, 11, 785–795. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Dong, L.; Wang, Z.; Li, L.; Shangguan, E.; Li, J.; Gao, S. Composite poly (ethylene oxide)-based solid electrolyte with consecutive and fast ion transport channels constructed by upper-dimensional MIL-53(Al) nanofibers. J. Colloid. Interface Sci. 2024, 657, 632–643. [Google Scholar] [CrossRef]
- Ghorbanzade, P.; Accordo, G.; Gomez, K.; Aranguren, P.L.; Devaraj, S.; Costa, C.M.; Mendez, S.L.; del Amo, J.M.L. Influence of the LLZO-PEO interface on the micro- and macro-scale properties of composite polymer electrolytes for solid-state batteries. Mater. Today Energy 2023, 38, 101448. [Google Scholar] [CrossRef]
- Khan, M.S.; Shakoor, A. Ionic conductance, thermal and morphological behavior of PEO-graphene oxide-salts composites. J. Chem. 2015, 2015, 695930. [Google Scholar] [CrossRef]
- Wei, L.; Xu, X.; Jiang, S.; Xi, K.; Zhang, L.; Lan, Y.; Yin, J.; Wu, H.; Gao, Y. Zeolitic imidazolate framework upgrading polyethylene oxide composite electrolyte for high-energy solid-state lithium batteries. J. Colloid. Interface Sci. 2023, 630, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ma, K.; Wang, H.; Chen, J.; Zheng, Z.; Zhang, J. Enhancing Li+ transfer efficiency and strength of PEO-based composite solid electrolyte for lon stable cycling of all-solid-state lithium metal batteries. Compos. Commun. 2024, 50, 102013. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, X.; Wu, X.; Wu, H.; Liu, X.; Yue, G.; Yang, Y.; Peng, D.L. Yolk-shell ZnO-C microspheres with enhanced electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 2014, 125, 659–665. [Google Scholar] [CrossRef]
- Zhong, Z.; Cao, Q.; Jing, B.; Wang, X.; Li, X.; Deng, H. Electrospun PVdF-PVC nanofibrous polymer electrolytes for polymer lithium-ion batteries. Mater. Sci. Eng. B 2012, 177, 86–91. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Electrospun trilayer polymeric membranes as separator for lithium-ion batteries. Electrochim. Acta 2014, 127, 167–172. [Google Scholar] [CrossRef]
- Aravindan, V.; Kumar, P.S.; Sundaramurthy, J.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Electrospun NiO nanofibers as high performance anode material for Li-ion batteries. J. Power Sources 2013, 227, 284–290. [Google Scholar] [CrossRef]
- Prasanth, R.; Shubha, N.; Hong, H.H.; Srinivasan, M. Effect of nano-clay on ionic conductivity and electrochemical properties of poly (vinylidene fluoride) based nanocomposite porous polymer membranes and their application as polymer electrolyte in lithium ion batteries. Eur. Polym. J. 2013, 49, 307–318. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]








| Year | ISEs | SPEs |
|---|---|---|
| 1800s | First introduction of PbF2, Ag2S, yttrium oxide (Y2O3) −doped zirconium dioxide (ZrO2), silver iodide (Agl) | NA |
| 1960s | Introduction of beta−aluminum oxide (β−Al2O3) −based ISEs Introduction of sodium sulfide (Na−S) batteries with Na+−β−Al2O3 | NA |
| 1970s | Discovery of Na superionic conductor (NASICON) and lithium superionic conductor (LISICON) −based ISEs Introduction of hydride−type ISEs | Discovery of PEO SPE and NASICON
|
| 1980s | NA | Introduction of sodium nickel chloride (Na−NiCl2) batteries with molten sodium tetrachloroaluminate (NaAlCl4)/Na+−β−Al2O3 SSE
|
| 1990s | Non−crystalline lithium oxonitridophosphate (LiPON), sulfides, (anti) perovskite, and garnet for ISEs | The first thin−film battery with the development of LiPON |
| 2000s | NA | Revival of interest in Li metal |
| 2010s | NA | Development of Bolloré Bluecar using a Li−polymer battery |
| Low Mw PEO (Ideal) | Increment of Mw | High Mw PEO |
|---|---|---|
| Low Tg | Increase Tg | High Tg |
| High amorphous proportion | Reduce the amorphous region | Semi-crystalline |
| Good ionic conductivity | Reduce ionic conductivity | Limited ionic conductivity at room temperature |
| Low thermal stability | Increase viscosity | - |
| Low mechanical strength | Increase mechanical strength | Regularity of PEO chains destroyed by the following:
|
| Filler Type | Lithium Salt | 10−4 S cm−1) | 1 EW (V) | Transference Number (t+) | Discharge Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| Inert Filler: Ceramic Oxide-Type | ||||||
| Aluminum oxide (Al2O3) | LiTFSI | 9.60 (25 °C) | 5.00 | 0.81 | 640 (0.1 C) | [53] |
| Ti3+-doped TiO2 | LiTFSI | 1.00 (30 °C) | 5.50 | 0.36 | 151 (60 °C, 0.1 C) | [54] |
| Inert Filler: Ferroelectric-Type | ||||||
| Barium titanate (BaTiO3) | LiTFSI 2 LiClO4 | 1.30 (30 °C) 12.0 (70 °C) | 4.00 - | - 0.37 | - | [55] [56] |
| Lithium niobate (LiNbO3) | LiCF3SO3 | 2.00 (85 °C) | - | 0.52 | - | [56] |
| Inert Filler: Porous-Type | ||||||
| Aluminum benzene tricarboxylate (Al-BTC) | LiTFSI | 10.0 (30 °C) | >3.8 | 0.55 | - | [57] |
| Zirconium benzene dicarboxylate (UiO-66) | LiTFSI LiTFSI | 13.0 (30 °C) 29.0 (60 °C) | 4.50 4.30 | 0.35 0.52 | ~151 (60 °C, 0.5 C) - | [58] [59] |
| Vinyl-functionalized MOF nanoparticles (UIO-66-NH2) | LiTFSI | 63.0 (60 °C) | 4.97 | 0.72 | 141.2 (0.1 C) | [60] |
| Aluminum terephthalate (Al-TPA) | LiTFSI | 1.00 (60 °C) | >3.0 | - | 130 (0.1 C) | [61] |
| Aluminosilicate zeolite (SSZ-13) | LiTFSI | 170.0 (60 °C) | 4.65 | 0.84 | 156.63 | [62] |
| MIL-53 (Al) | LiFSI | 34.0 (120 °C) | 5.10 | 0.34 | 103.5 (120 °C, 10 C) | [63] |
| Inert Filler: Mineral-Type | ||||||
| Kaolinite (Al2Si2O5(OH)4) | LiTFSI | 1.10 (25 °C) | 6.35 | 0.4 | 919 (0.3 C) | [64] |
| Suanite (Mg2B2O5) | LiTFSI | 1.50 (40 °C) | 4.75 | 0.44 | 150 (50 °C, 0.2 C) | [65] |
| Active Filler: Garnet-Type | ||||||
| Li6.25Al0.25La3Zr2O12 (LLZO) Li7La3Zr2O12 (LLZO) | 2 LiClO4 LiTFSI | 3.00 (24 °C) 2.40 (25 °C) | 5.00 6.00 | - - | 122 (70 °C, 0.1 C) 158.8 (60 °C, 0.5 C) | [66] [67] |
| Li7La3Zr2O12 (LLZO) | LiTFSI 2LiCIO4 | 0.90 (25 °C) 4.40 (50 °C) | 5.50 6.00 | - - | 170 (1 C) 166 (55 °C, 0.02 C) | [68] [69] |
| Li6.4La3Zr2Al0.2O12 (LLZO) | LiTFSI | 2.50 (30 °C) | 6.00 | - | - | [70] |
| Li6.55Ga0.15La3Zr2O12 (LLZO) | LiTFSI | 4.50 (70 °C) | - | - | - | [71] |
| Li6.75La3Zr1.75Ta0.25O12 (LLZTO) | 2 LiClO4 LiTFSI | 5.00 (25 °C) 0.11 (25 °C) | - 5.50 | - - | 120 (0.3 C) 155 (60 °C, 0.1 C) | [72] [73] |
| Li6.4La3Zr1.4Ta0.6O12 (LLZTO) | LiTFSI | 2.10 (30 °C) | 4.75 | - | 153.3 (60 °C, 0.05 C) | [74] |
| Active Filler: NASICON-Type | ||||||
| Li1+xAlxTi2−x (PO4)3 (LATP) | 2 LiClO4 | 0.52 (25 °C) | 4.80 | - | - | [75] |
| Li1.3Al0.3Ti1.7(PO4)3 (LATP) | LiTFSI LiTFSI | 0.40 (25 °C) 7.47 (60 °C) | - 5.10 | - - | - 152.8 (1 C) | [76] [77] |
| Li1.5Al0.5Ge1.5(PO4)3 (LAGP) | LiTFSI LiTFSI LiTFSI | 1.67 (20 °C) 1.25 (25 °C) 0.44 (25 °C) | 5.00 >3.8 5.10 | - - - | - 143.6 (0.5 C) - | [78] [79] [80] |
| Li1.4Al0.4Ge1.6(PO4)3 (LAGP) | LiTFSI LiTFSI | 1.72 (25 °C) 0.90 (30 °C) | - 5.12 | - - | - 160.8 (50 °C) | [81] [82] |
| Active Filler: Perovskite-Type | ||||||
| LSTZ | LiTFSI | 0.54 (25 °C) | 5.20 | - | 119 | [83] |
| Li0.35La0.55TiO3 (LLTO) | LiTFSI | 0.88 (25 °C) | 5.10 | - | - | [84] |
| Li0.33La0.557TiO3 (LLTO) | LiTFSI LiTFSI LiTFSI | 1.30 (25 °C) 1.60 (25 °C) 2.40 (25 °C) | > 3.8 4.70 5.00 | - - - | 144.6 (60 °C, 1 C) 135 (60 °C, 2 C) - | [85] [86] [87] |
| Li0.3La0.557TiO3(LLTO) | LiTFSI LiTFSI | 1.80 (25 °C) 2.30 (25 °C) | 4.50 - | - - | - 384 (30 °C, 0.2 C) | [88] [89] |
| Active Filler: Sulfide-Type | ||||||
| Li6PS5Cl (LPSC) | LiTFSI - LiTFSI | 11.0 (25 °C) 10.0 (80 °C) 36.0 (80 °C) | 4.90 >4.0 - | - - - | 135.8 (0.05 C) 110.2 (60 °C) - | [90] [91] [92] |
| Li6PS5Cl/SiO2 (LPSC) | 2 LiClO4 | 30.0 (25 °C) | > 4.2 | - | 134.3 (60 °C, 1 C) | [93] |
| Li10GeP2S12 (LGPS) | LiTFSI LiTFSI | 1.80 (25 °C) 2.20 (25 °C) | >3.0 - | - - | 588 (0.1 C) - | [94] [95] |
| Li10SnP2S12 (LSPS) | LiTFSI | 1.70 (50 °C) | 5.00 | - | - | [96] |
| Characterization Techniques | Purpose |
|---|---|
| Fourier-transform infrared spectroscopy (FTIR) | Identify functional groups and chemical bonding |
| X-ray diffraction (XRD) | Determine crystal structure and phase composition |
| Differential scanning calorimetry (DSC) | Measure thermal properties and phase transitions |
| Scanning electron microscopy (SEM) | Observe morphology and surface, cross-sectional structure |
| Transmission electron microscopy (TEM) | Examine fine structural details |
| Nuclear magnetic resonance (NMR) | Study local molecular environment and dynamics |
| Electrochemical impedance spectroscopy (EIS) | Assess ionic conductivity and charge transport |
| Atomic force microscopy (AFM) | Analyze surface topology at the nanoscale |
| Raman spectroscopy | Probe vibrational modes and molecular interactions |
| Tensile tenting | Evaluate mechanical properties |
| X-ray photoelectron spectroscopy (XPS) | Analyze surface chemistry and elemental composition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junoh, H.; Awang, N.; Zakria, H.S.; Zainuddin, N.A.S.; Nordin, N.A.H.M.; Suhaimin, N.S.; Enoki, T.; Uno, T.; Kubo, M. Advancements in Polyethylene Oxide (PEO)–Active Filler Composite Polymer Electrolytes for Lithium-Ion Batteries: A Comprehensive Review and Prospects. Materials 2024, 17, 4344. https://doi.org/10.3390/ma17174344
Junoh H, Awang N, Zakria HS, Zainuddin NAS, Nordin NAHM, Suhaimin NS, Enoki T, Uno T, Kubo M. Advancements in Polyethylene Oxide (PEO)–Active Filler Composite Polymer Electrolytes for Lithium-Ion Batteries: A Comprehensive Review and Prospects. Materials. 2024; 17(17):4344. https://doi.org/10.3390/ma17174344
Chicago/Turabian StyleJunoh, Hazlina, Nuha Awang, Hazirah Syahirah Zakria, Nurul Amira Shazwani Zainuddin, Nik Abdul Hadi Md Nordin, Nuor Sariyan Suhaimin, Tomoya Enoki, Takahiro Uno, and Masataka Kubo. 2024. "Advancements in Polyethylene Oxide (PEO)–Active Filler Composite Polymer Electrolytes for Lithium-Ion Batteries: A Comprehensive Review and Prospects" Materials 17, no. 17: 4344. https://doi.org/10.3390/ma17174344
APA StyleJunoh, H., Awang, N., Zakria, H. S., Zainuddin, N. A. S., Nordin, N. A. H. M., Suhaimin, N. S., Enoki, T., Uno, T., & Kubo, M. (2024). Advancements in Polyethylene Oxide (PEO)–Active Filler Composite Polymer Electrolytes for Lithium-Ion Batteries: A Comprehensive Review and Prospects. Materials, 17(17), 4344. https://doi.org/10.3390/ma17174344







