A Study on the Effect of Pd Layer Thickness on the Properties of Cu-Ag Intermetallic Compounds at the Bonding Interface
Abstract
1. Introduction
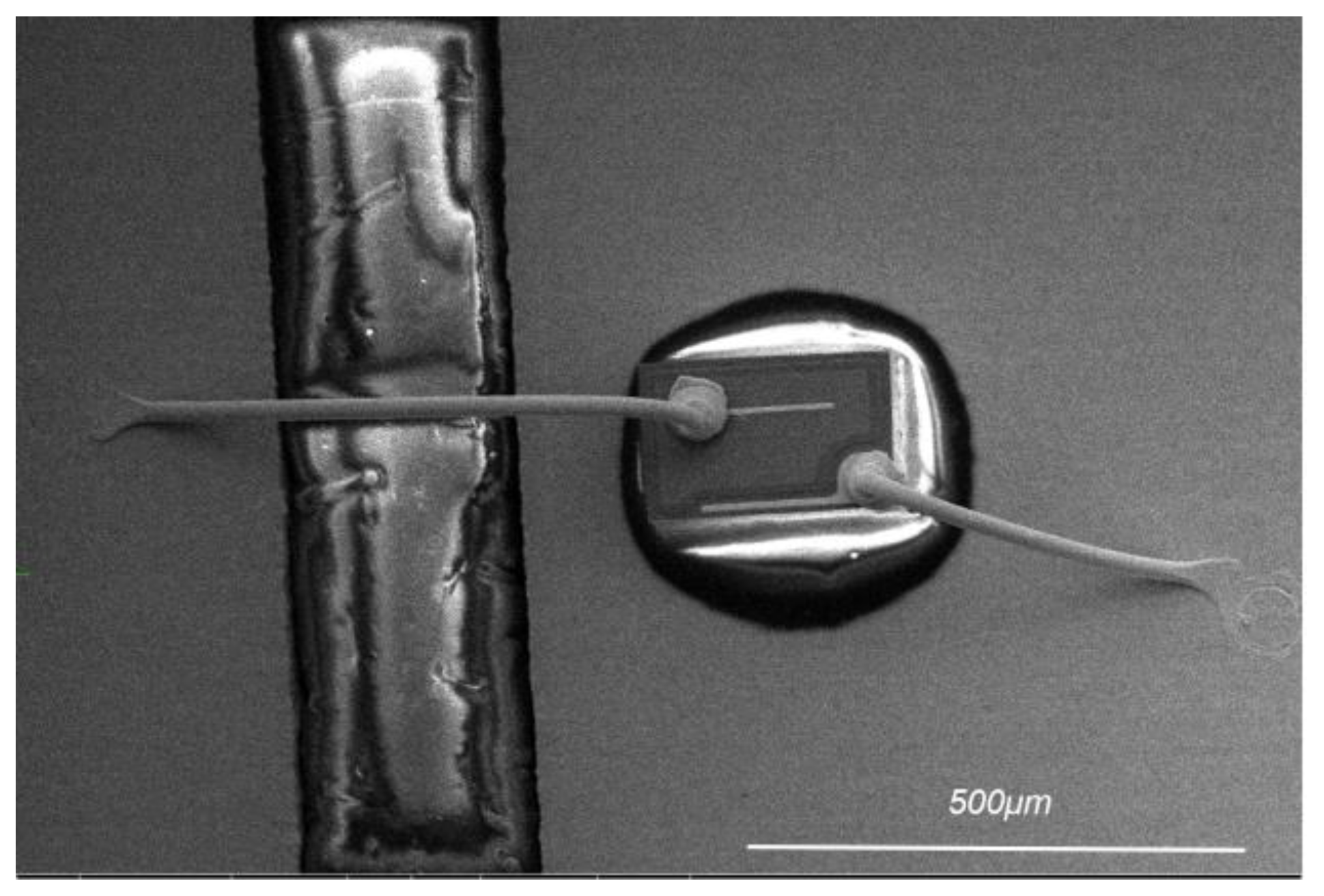
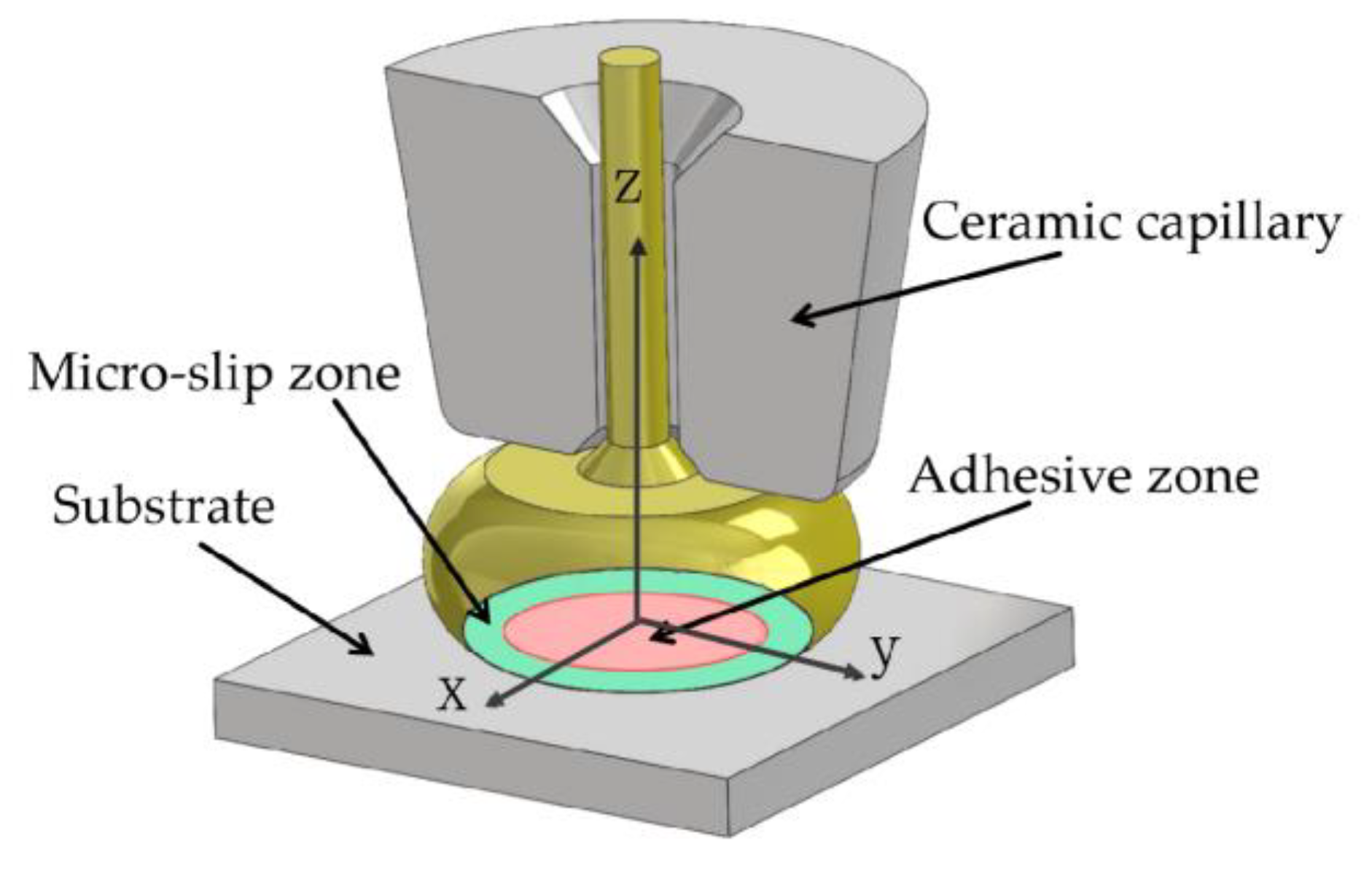
2. Experimental Materials and Methods
3. Results and Discussion
3.1. Study of Pd100 IMCs
3.2. Study of Pd120 IMCs
4. Conclusions
- (1)
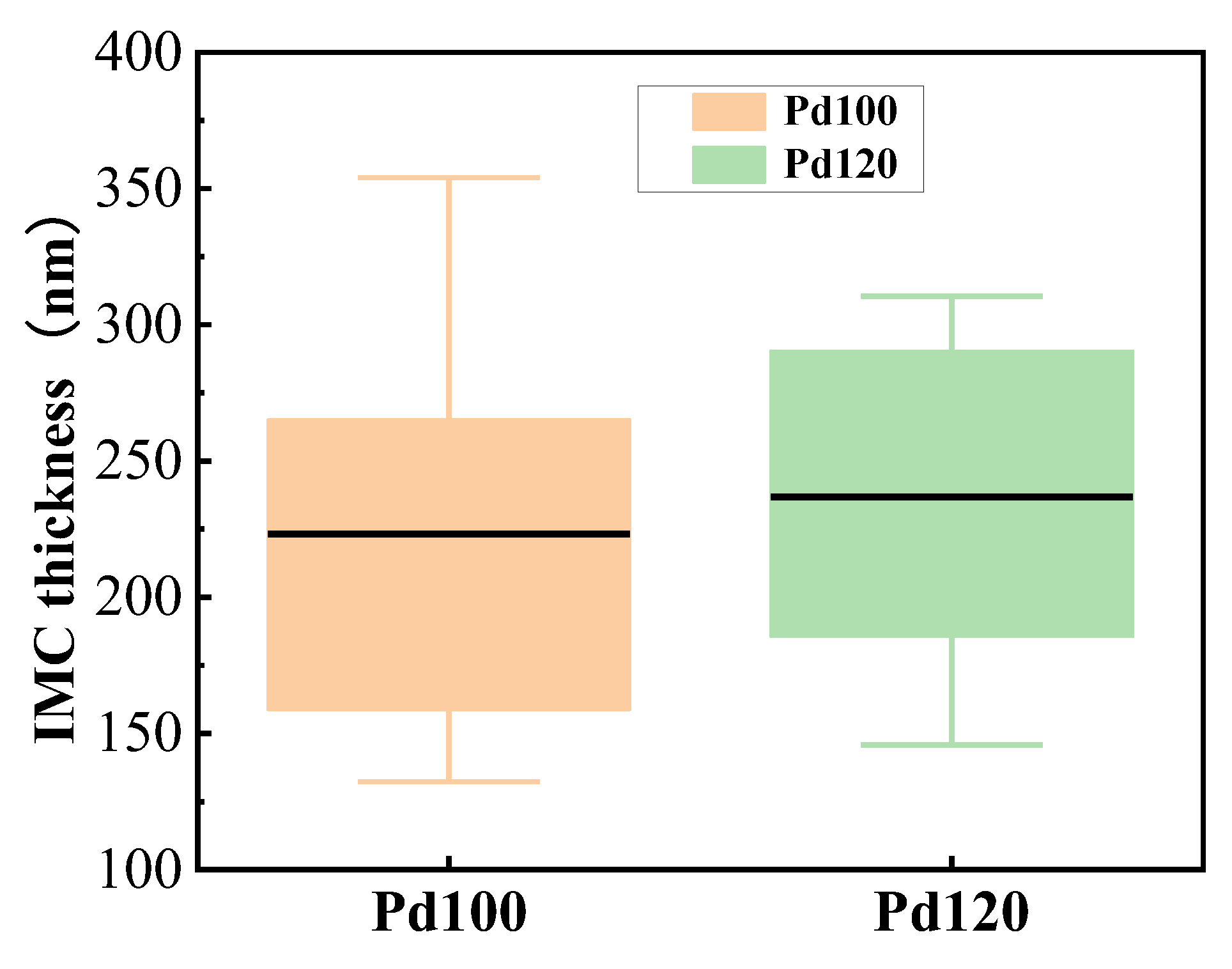
- Compared to Pd120-bonded sample, the Kirkendall effect is more pronounced at the bonding interface of the Pd100-bonded sample, with larger pore and crack sizes. The thickness of the IMC is uneven, and it is mainly composed of CuAg2 and CuAg. In contrast, the bonding interface of the Pd120-bonded sample has almost no microcracks, and the Kirkendall voids are small. The IMCs are uniform and relatively thin, with the main components being CuAg4, CuAg2, and CuAg.
- (2)
- Pd was not detected in all of the IMCs, indicating that Pd is not involved in their formation and growth. The formation sequence of the IMCs begins with Cu atoms diffusing into the Ag layer to form a silver-rich compound, CuAg4, and the diffusion of Cu elements increases to form CuAg2, which finally forms CuAg. The Pd element can significantly weaken the Kirkendall effect and slow down the growth of Cu-Ag IMCs.
- (3)
- IMCs grow rapidly in areas with low Pd content, resulting in pores and microcracks that may cause delamination at the bonding interface, whichwill hinder the diffusion of Cu and Ag atoms, and inhibit the sustained growth of IMCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, G.I.A.; Lambert, A.; Caperton, J.; Asokan, M.; Yi, W.; Chyan, O. Comparative Study of Chloride and Fluoride Induced Aluminum Pad Corrosion in Wire-Bonded Device Packaging Assembly. Corros. Mater. Degrad. 2021, 2, 447–460. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Gao, Y.; Peng, X.; Derby, B. Microstructure and mechanical properties of a high strength Cu-Ni-Si alloy treated by combined aging processes. J. Alloys Compd. 2017, 695, 2413–2423. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; He, X.; Wang, C.; Jiang, L.; Chen, L.-Q.; Xie, J. Dramatically enhanced combination of ultimate tensile Strength and electric conductivity of alloys via machine learning screening. Acta Mater. 2020, 200, 803–810. [Google Scholar] [CrossRef]
- Qin, W.; Anderson, T.; Chang, G. Mechanism to improve the reliability of copper wire bonding with palladium-coating of the wire. Microelectron. Reliab. 2019, 99, 239–244. [Google Scholar] [CrossRef]
- Zhou, H.; Chang, A.; Fan, J.; Cao, J.; An, B.; Xia, J.; Yao, J.; Cui, X.; Zhang, Y. Copper Wire Bonding: A Review. Micromachines 2023, 14, 1612. [Google Scholar] [CrossRef]
- Gan, C.L.; Hashim, U. Evolutions of bonding wires used in semiconductor electronics: Perspective over 25 years. J. Mater. Sci. Mater. Electron. 2015, 26, 4412–4424. [Google Scholar] [CrossRef]
- Breach, C.D. What is the future of bonding wire? Will copper entirely replace gold? Gold Bull. 2010, 43, 150–168. [Google Scholar] [CrossRef]
- Goh, C.S.; Chong, W.L.E.; Lee, T.K.; Breach, C. Corrosion study and intermetallics formation in gold and copper wire bonding in microelectronics packaging. Crystals 2013, 3, 391–404. [Google Scholar] [CrossRef]
- Li, N.M.; Das, D. Shelf life evaluation method for electronic and other components using a physics of failure (PoF) approach. In Proceedings of the Machinery Failure Prevention Technology (MFPT) Conference, Virginia Beach, VA, USA, 16–18 May 2017. [Google Scholar]
- Han, M.; Wang, M.; Zhang, L.; Yan, B.; Li, J.; Song, M.; Mathew, V. Copper wire bond pad/IMC interfacial layer crack study during HTSL (high temperature storage life) test. In Proceedings of the 2016 IEEE 18th Electronics Packaging Technology Conference (EPTC), Singapore, 30 November–3 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 797–800. [Google Scholar]
- Arjmand, E.; Agyakwa, P.A.; Corfield, M.R.; Li, J.; Mouawad, B.; Johnson, C.M. A thermal cycling reliability study of ultrasonically bonded copper wires. Microelectron. Reliab. 2016, 59, 126–133. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Z.; Chen, Q.; Zhou, J.; Du, M.; Kim, S.; Chae, J.; Chung, M. Reliability of copper wire bonding in humidity environment. In Proceedings of the 2011 IEEE 13th Electronics Packaging Technology Conference (EPTC 2011), Singapore, 7–9 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 53–58. [Google Scholar]
- Gan, C.L.; Classe, F.C.; Chan, B.L.; Hashim, U. Evolution and investigation of copper and gold ball bonds in extended reliability stressing. Gold Bull. 2014, 47, 141–151. [Google Scholar] [CrossRef]
- Gan, C.L.; Ng, E.K.; Chan, B.L.; Hashim, U.; Classe, F.C. Technical barriers and development of cu wire bonding in nanoelectronics device packaging. J. Nanomater. 2012, 2012, 17305. [Google Scholar] [CrossRef]
- Uno, T.; Yamada, T. Improving humidity bond reliability of copper bonding wires. In Proceedings of the Electronic Components & Technology Conference, Las Vegas, NV, USA, 1–4 June 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Osenbach, J.; Wang, B.Q.; Emerich, S.; Delucca, J.; Meng, D. Corrosion of the Cu/Al interface in Cu-Wire-bonded integrated cir-cuits. In Proceedings of the Electronic Components & Technology Conference, Las Vegas, NV, USA, 28–31 May 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Qin, W.; Anderson, H.; Anderson, T.; Chang, G.; Barrientos, D. Corrosion Mechanisms of Cu Wire Bonding on Al Pads. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2018; pp. 1446–1454. [Google Scholar]
- Yang, H.; Cao, K.; Lu, J.; Lu, Y. Effects of Pd surface coating on the strength and fracture behavior of Cu micro bonding wires. Met. Mater. Trans. A 2019, 50, 3013–3018. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, Y.-C.; Shih, Y.-T.; Chen, S.-C.; Tsai, C.-H.; Wang, S.-C.; Chuang, T.-H. Evaluation of corrosion resistance of Ag-alloy bonding wires for electronic packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 8, 146–153. [Google Scholar] [CrossRef]
- Ho, C.; Hsieh, W.; Lee, P.; Huang, Y.; Kuo, T. High-temperature stability of Au/Pd/Cu and Au/Pd (P)/Cu surface finishes. Appl. Surf. Sci. 2017, 434, 1353–1360. [Google Scholar] [CrossRef]
- Chen, K.-J.; Hung, F.-Y.; Chang, C.-Y. A study of the sulfidation behavior on palladium-coated copper wire with a flash-gold layer (PCA) after wire bonding. Electronics 2019, 8, 792. [Google Scholar] [CrossRef]
- Abe, H.; Dong, C.K.; Yamamoto, T.; Yagihashi, T.; Iwasaki, T. Cu wire and Pd-Cu wire package reliability and molding com-pounds. In Proceedings of the Electronic Components & Technology Conference, San Diego, CA, USA, 29 May–1 June 2012; IEEE: Piscataway, NJ, USA, 2012. [Google Scholar]
- Lim, A.B.Y.; Chang, A.C.K.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Ultra-fine pitch palladi-um-coated copper wire bonding: Effect of bonding parameters. Microelectron. Reliab. 2014, 54, 2555–2563. [Google Scholar] [CrossRef]
- Cheng, P.Y.; Lai, P.Y.; Ye, Z.J.; Hsieh, C.L.; Ye, J.M. Effect of Pd distribution on Pd-plated Cu wire using different electronic flame off settings. J. Mater. Sci. Mater. Electron. 2017, 28, 4613–4618. [Google Scholar] [CrossRef]
- Boettcher, T.; Rother, M.; Liedtke, S.; Ullrich, M.; Klengel, R. On the intermetallic corrosion of Cu-Al wire bonds. In Proceedings of the Electronics Packaging Technology Conference, Singapore, 8–10 December 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Chen, C.Y.; Hwang, W.S. Effect of Annealing on the Interfacial Structure of Aluminum-Copper Joints. Mater. Trans. 2007, 48, 1938–1947. [Google Scholar] [CrossRef]
- Lim, A.B.; Long, X.; Shen, L.; Chen, X.; Ramanujan, R.; Gan, C.L.; Chen, Z. Effect of palladium on the mechanical properties of Cu–Al intermetallic compounds. J. Alloys Compd. 2015, 628, 107–112. [Google Scholar] [CrossRef]
- Tavassoli, S.; Abbasi, M.; Tahavvori, R. Controlling of IMCs layers formation sequence, bond strength and electrical resistance in AlCu bimetal compound casting process. Mater. Des. 2016, 108, 343–353. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.; Chen, Z.; Wei, J.; Sivakumar, M. Effect of bonding duration and substrate temperature in copper ball bonding on aluminium pads: A TEM study of interfacial evolution. Microelectron. Reliab. 2011, 51, 113–118. [Google Scholar] [CrossRef]
- Lim, A.B.Y.; Neo, W.J.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Evaluation of the corrosion performance of Cu–Al intermetallic compounds and the effect of Pd addition. Microelectron. Reliab. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- Park, H.-W.; Lee, S.-J.; Cho, D.-C.; Lee, S.-H.; Kim, J.-K.; Lee, J.-H.; Jung, S.-K.; Nam, H.-S.; Hsu, P.; Low, S.; et al. Behavior of Au and Pd and the effects of these metals on IMCs in Pd-Au-coated copper wire. Microelectron. Reliab. 2018, 91, 283–290. [Google Scholar] [CrossRef]
- Drozdov, M.; Gur, G.; Atzmon, Z.; Kaplan, W.D. Detailed investigation of ultrasonic Al–Cu wire-bonds: II. Microstructural evolution during annealing. J. Mater. Sci. 2008, 43, 6038–6048. [Google Scholar] [CrossRef]
- Hang, C.; Wang, C.; Mayer, M.; Tian, Y.; Zhou, Y.; Wang, H. Growth behavior of Cu/Al intermetallic compounds and cracks in copper ball bonds during isothermal aging. Microelectron. Reliab. 2008, 48, 416–424. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z. A re-examination of the mechanism of thermosonic copper ball bonding on aluminium metallization pads. Scr. Mater. 2009, 61, 165–168. [Google Scholar] [CrossRef]
- Sung, P.H.; Chen, T.C. Performance of Cu–Ag Thin Films as Diffusion Barrier Layer. Coatings 2020, 10, 1087. [Google Scholar] [CrossRef]
- Mousavi, Z.; Pourabdoli, M. Physical and chemical properties of Ag–Cu composite electrical contacts prepared by cold-press and sintering of silver-coated copper powder. Mater. Chem. Phys. 2022, 290, 126608. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Zhang, L.; Zhang, Z.; Han, Y.; Hu, X.; Lu, H.; Zhang, S. Forming mechanism and growth of Kirkendall voids of Sn/Cu joints for electronic packaging: A recent review. J. Adv. Join. Process. 2022, 6, 100125. [Google Scholar] [CrossRef]
- Zou, Y.S.; Gan, C.L.; Chung, M.-H.; Takiar, H. A review of interconnect materials used in emerging memory device packaging: First- and second-level interconnect materials. J. Mater. Sci. Mater. Electron. 2021, 32, 27133–27147. [Google Scholar] [CrossRef]
- Rezvani, A.; Mayer, M.; Shah, A.; Zhou, N.; Hong, S.J.; Moon, J.T. Free-air ball formation and deformability with Pd coated Cu wire. In Proceedings of the Electronic Components & Technology Conference, Lake Buena Vista, FL, USA, 31 May–3 June 2011; IEEE: Piscataway, NJ, USA, 2011. [Google Scholar]
- Fan, J.; Yuan, D.; Du, J.; Hou, T.; Wang, F.; Cao, J.; Yang, X.; Zhan, Y. Influence of Pd-Layer Thickness on Bonding Reliability of Pd-Coated Cu Wire. Micromachines 2024, 15, 931. [Google Scholar] [CrossRef] [PubMed]
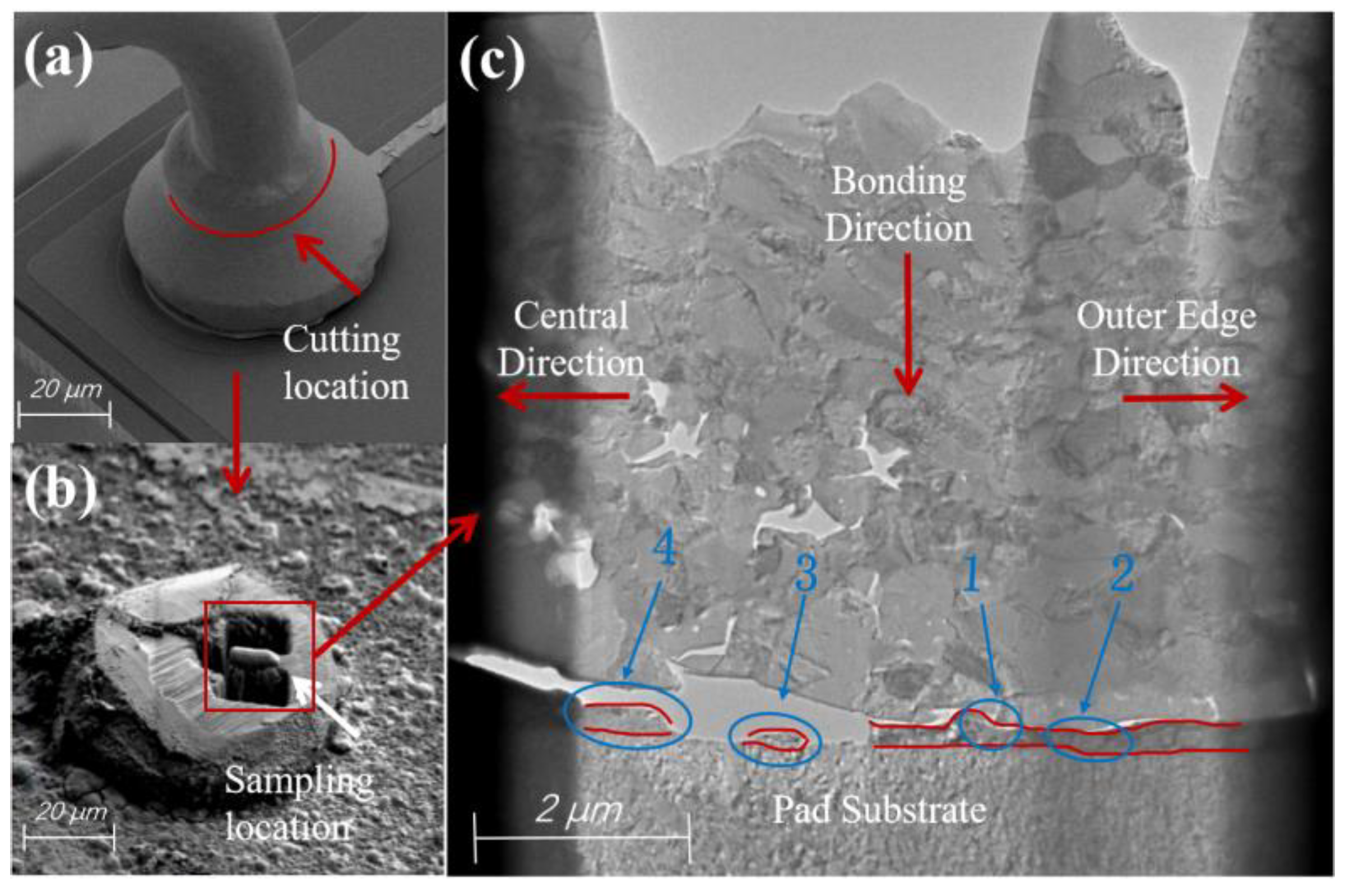
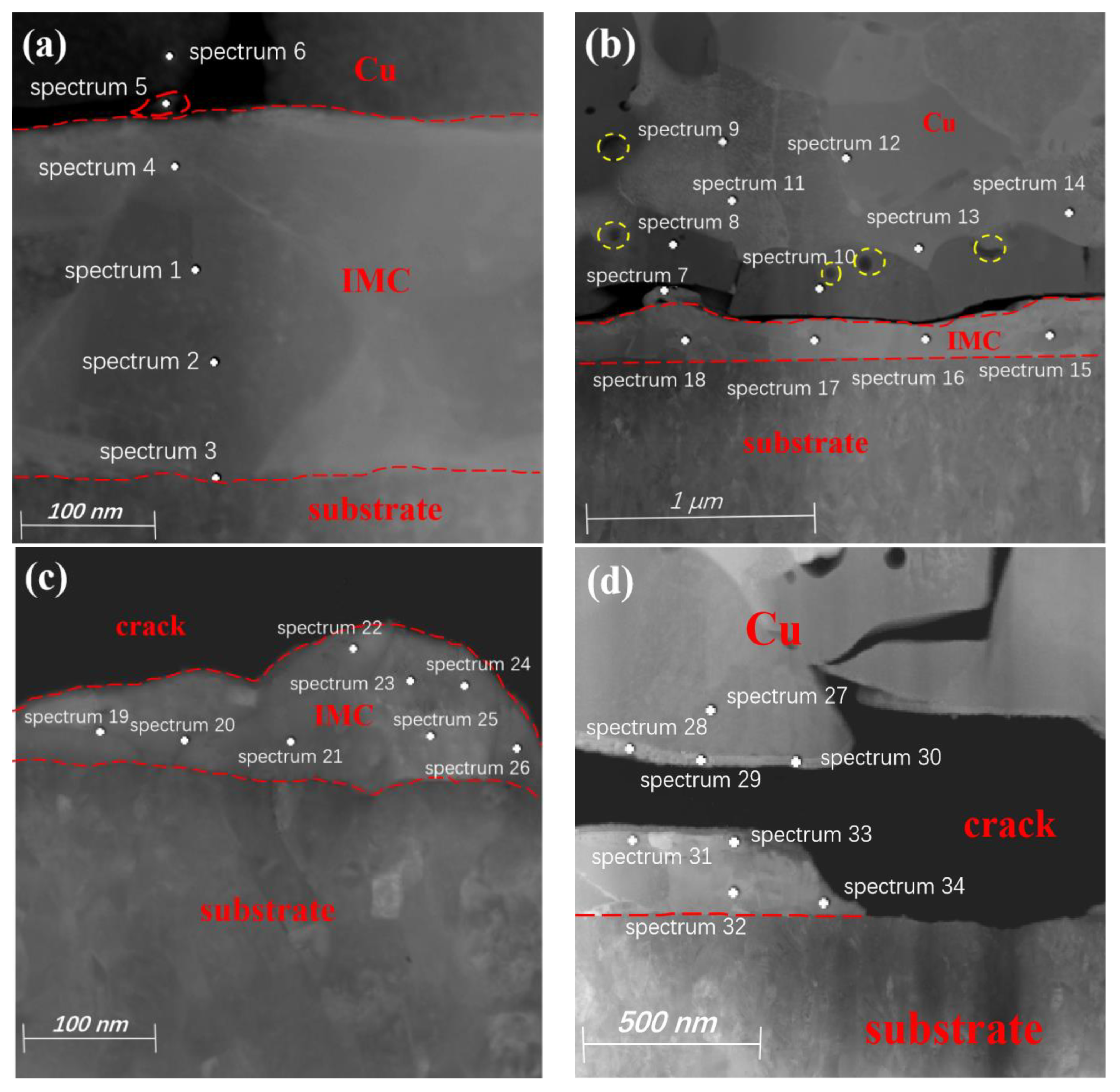
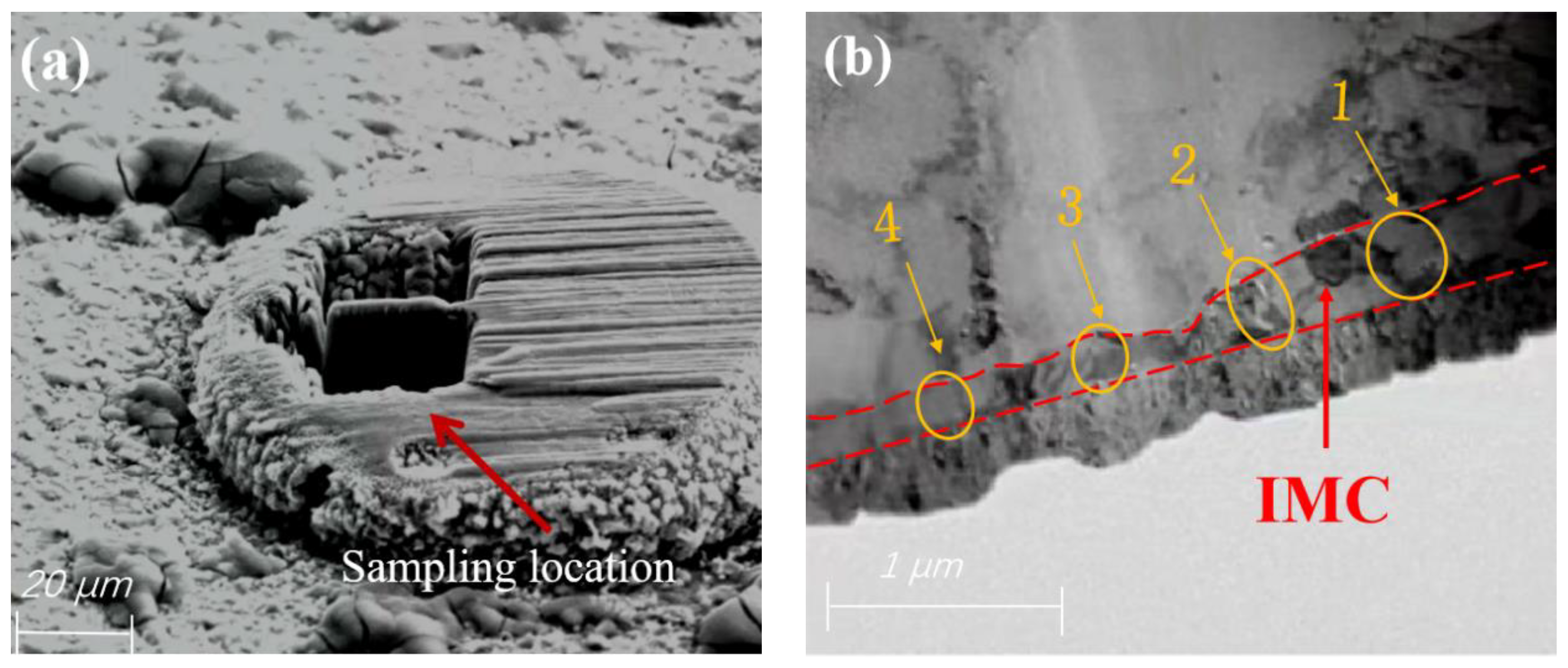
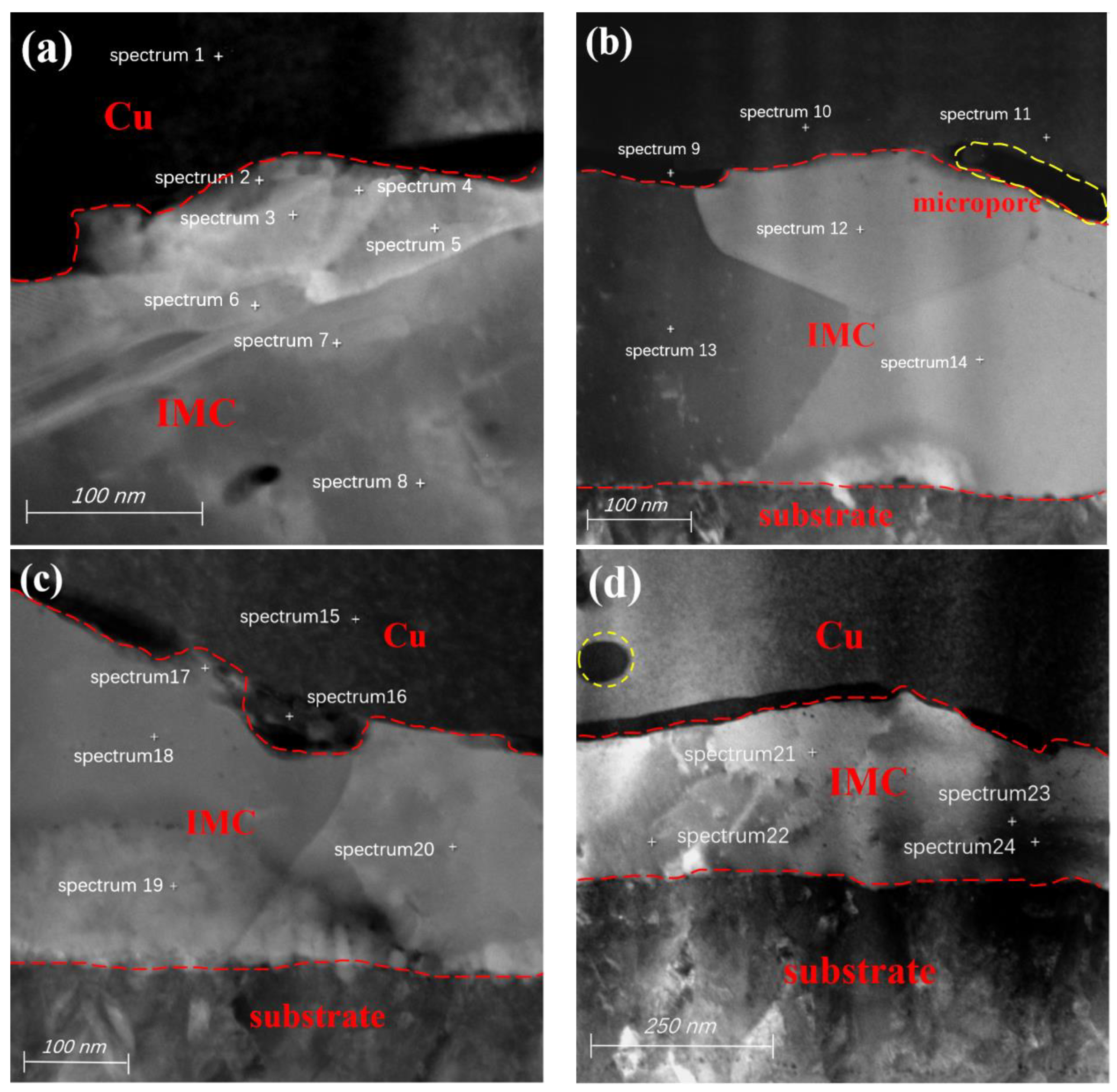
| Detection Position | Cu (at.%) | Ag (at.%) | Pd (at.%) | Detection Position | Cu (at.%) | Ag (at.%) | Pd (at.%) |
|---|---|---|---|---|---|---|---|
| 1 | 32.27 | 67.14 | 0 | 18 | 32.18 | 67.63 | 0 |
| 2 | 32.31 | 67.14 | 0 | 19 | 50.81 | 48.57 | 0 |
| 3 | 32.65 | 65.37 | 0 | 20 | 47.5 | 52.05 | 0 |
| 4 | 32.35 | 67.14 | 0 | 21 | 47.3 | 52.07 | 0 |
| 5 | 53.82 | 45.21 | 0 | 22 | 42.93 | 56.99 | 0 |
| 6 | 98.88 | 0.67 | 0 | 23 | 44.72 | 55.12 | 0 |
| 7 | 93.28 | 5.34 | 0.14 | 24 | 44.99 | 54.52 | 0 |
| 8 | 99.72 | 0.1 | 0.1 | 25 | 47.75 | 51.88 | 0 |
| 9 | 98.37 | 0.51 | 1.04 | 26 | 42.49 | 57.22 | 0 |
| 10 | 99.5 | 0.3 | 0.03 | 27 | 91.29 | 1.66 | 0.03 |
| 11 | 98.3 | 0.47 | 1.17 | 28 | 99.45 | 0.29 | 0.06 |
| 12 | 98.04 | 0.93 | 0.96 | 29 | 94.73 | 2.24 | 0 |
| 13 | 98.05 | 0.47 | 1.27 | 30 | 91.9 | 3.47 | 0 |
| 14 | 98.07 | 0.52 | 1.24 | 31 | 49.46 | 49.95 | 0 |
| 15 | 31.56 | 67.97 | 0 | 32 | 48.53 | 50.86 | 0 |
| 16 | 31.73 | 68.56 | 0 | 33 | 49.14 | 50.01 | 0 |
| 17 | 31.38 | 68.31 | 0 | 34 | 49.34 | 50.04 | 0 |
| Detection Position | Cu (at.%) | Ag (at.%) | Pd (at.%) | Detection Position | Cu (at.%) | Ag (at.%) | Pd (at.%) |
|---|---|---|---|---|---|---|---|
| 1 | 98.07 | 0.71 | 1.22 | 13 | 27.33 | 72.67 | 0 |
| 2 | 43.26 | 56.74 | 0 | 14 | 24.46 | 75.54 | 0 |
| 3 | 19.39 | 80.61 | 0 | 15 | 99.22 | 0.77 | 0.01 |
| 4 | 20.28 | 79.72 | 0 | 16 | 99.34 | 0.65 | 0.01 |
| 5 | 19.74 | 80.26 | 0 | 17 | 79.31 | 20.69 | 0 |
| 6 | 19.01 | 80.99 | 0 | 18 | 18.72 | 81.28 | 0 |
| 7 | 19.26 | 80.74 | 0 | 19 | 18.85 | 81.15 | 0 |
| 8 | 19.68 | 80.32 | 0 | 20 | 18.91 | 81.09 | 0 |
| 9 | 100 | 0 | 0 | 21 | 37.16 | 62.84 | 0 |
| 10 | 99.72 | 0.28 | 0 | 22 | 32.08 | 66.97 | 0 |
| 11 | 99.88 | 0.12 | 0 | 23 | 45.93 | 54.05 | 0 |
| 12 | 25.37 | 74.63 | 0 | 24 | 46.3 | 53.7 | 0 |
| Micropores | IMC Fault | IMC Cracks | Main Components of IMC | |
|---|---|---|---|---|
| Pd100 | More | existence | existence | CuAg2, CuAg |
| Pd120 | Less | Less | Less | CuAg4, CuAg2, CuAg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Yuan, D.; Du, J.; Hou, T.; Wang, F.; Cao, J.; Yang, X.; Zhang, Y. A Study on the Effect of Pd Layer Thickness on the Properties of Cu-Ag Intermetallic Compounds at the Bonding Interface. Materials 2024, 17, 4335. https://doi.org/10.3390/ma17174335
Fan J, Yuan D, Du J, Hou T, Wang F, Cao J, Yang X, Zhang Y. A Study on the Effect of Pd Layer Thickness on the Properties of Cu-Ag Intermetallic Compounds at the Bonding Interface. Materials. 2024; 17(17):4335. https://doi.org/10.3390/ma17174335
Chicago/Turabian StyleFan, Junling, Donglin Yuan, Juan Du, Tao Hou, Furong Wang, Jun Cao, Xuemei Yang, and Yuemin Zhang. 2024. "A Study on the Effect of Pd Layer Thickness on the Properties of Cu-Ag Intermetallic Compounds at the Bonding Interface" Materials 17, no. 17: 4335. https://doi.org/10.3390/ma17174335
APA StyleFan, J., Yuan, D., Du, J., Hou, T., Wang, F., Cao, J., Yang, X., & Zhang, Y. (2024). A Study on the Effect of Pd Layer Thickness on the Properties of Cu-Ag Intermetallic Compounds at the Bonding Interface. Materials, 17(17), 4335. https://doi.org/10.3390/ma17174335






