An Experimental Study on the Thermal Performance of a Heat Sink Filled with Porous Aluminum Skeleton/Paraffin Composite Phase Change Material
Abstract
1. Introduction
2. Preparation and Experimental Setup
2.1. AS-PCM Heat Sinks with a LED Light Source
2.2. Preparation of AS-PCM
2.3. Experimental Setup
3. Results and Discussion
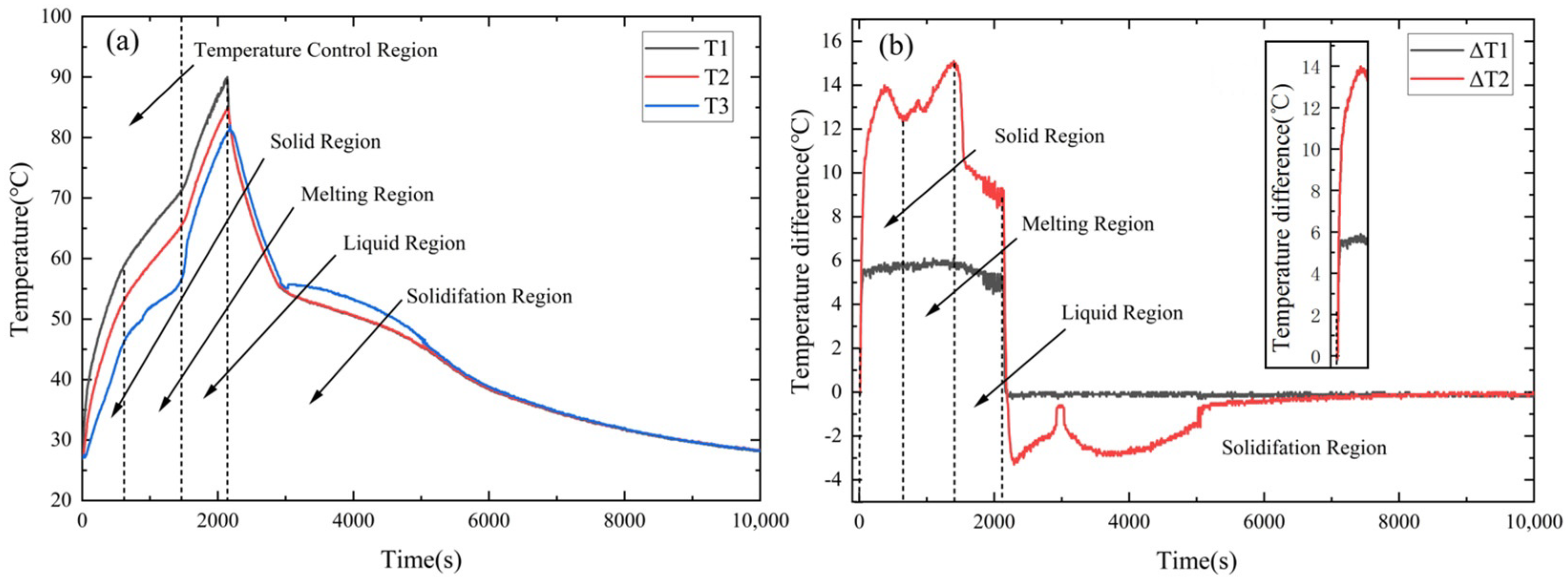
3.1. Temperature Control Performance of a Typical AS-PCM Heat Sink
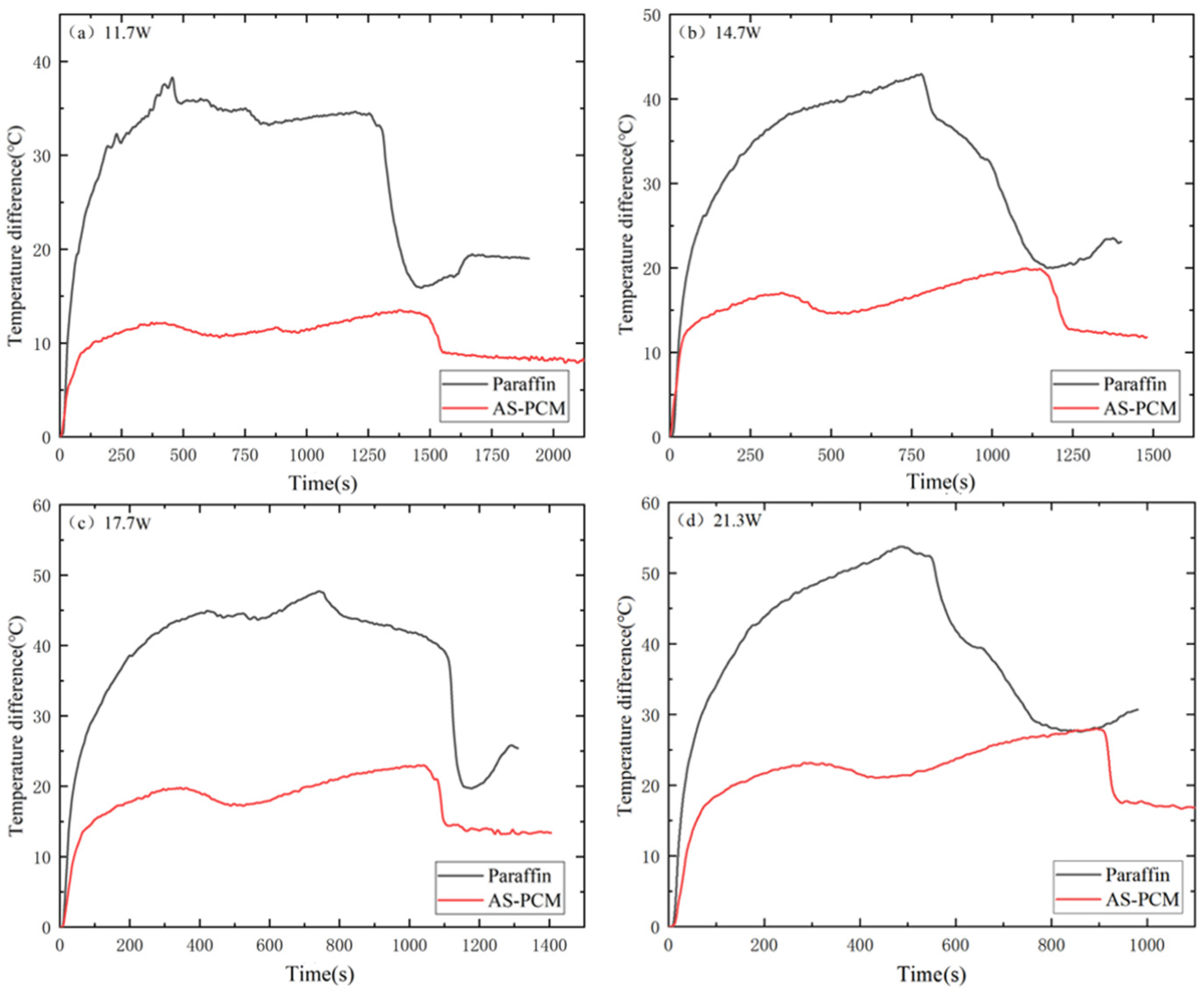
3.2. Influence of Different Heat Sink Types during the Heating Stage
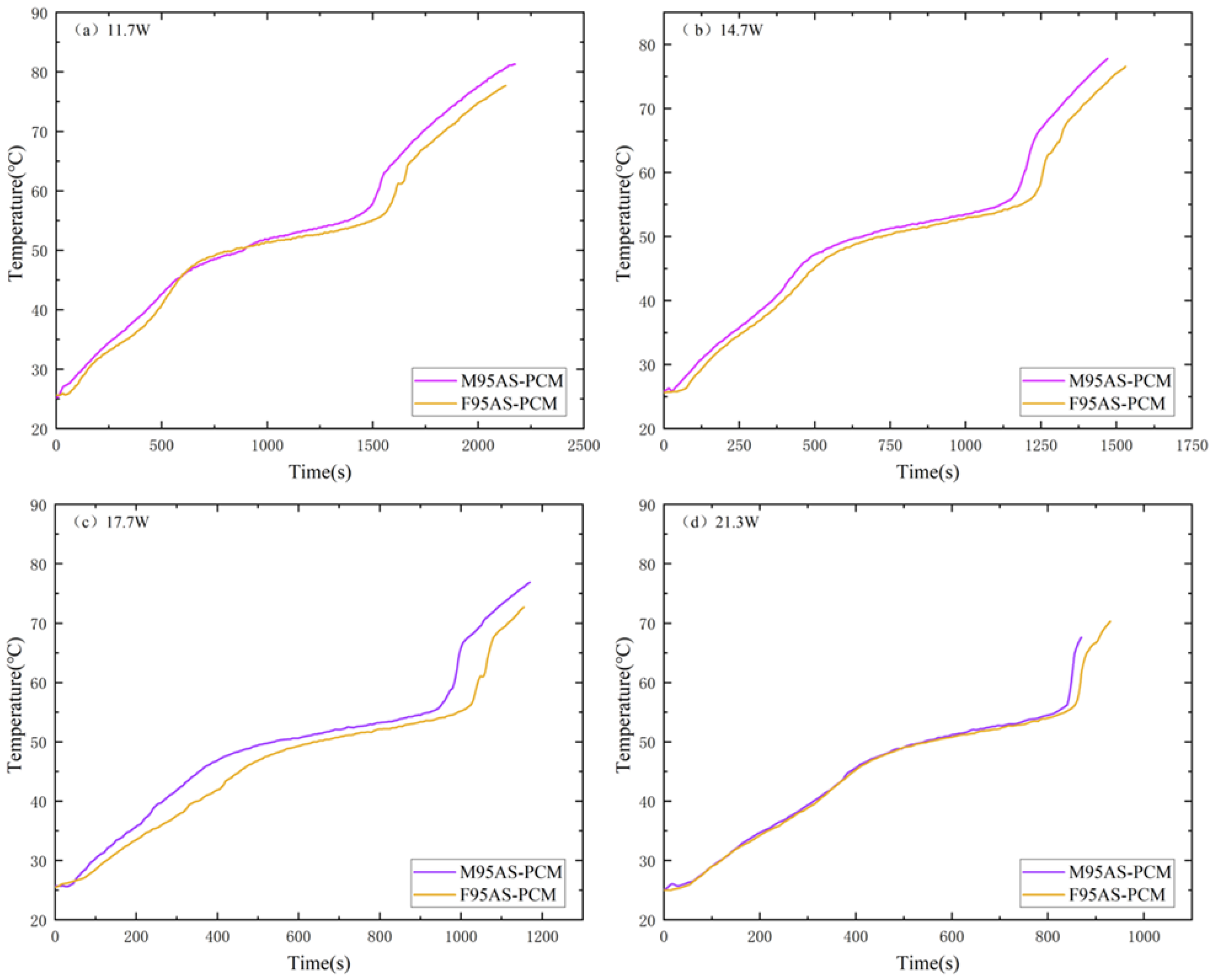
3.3. Influence of Porosity of MAS-PCM during the Heating Stage
3.4. Influence of the Skeleton Structure of AS-PCM during the Heating Stage
3.5. Heat Sink Performance during the Cooling Stage
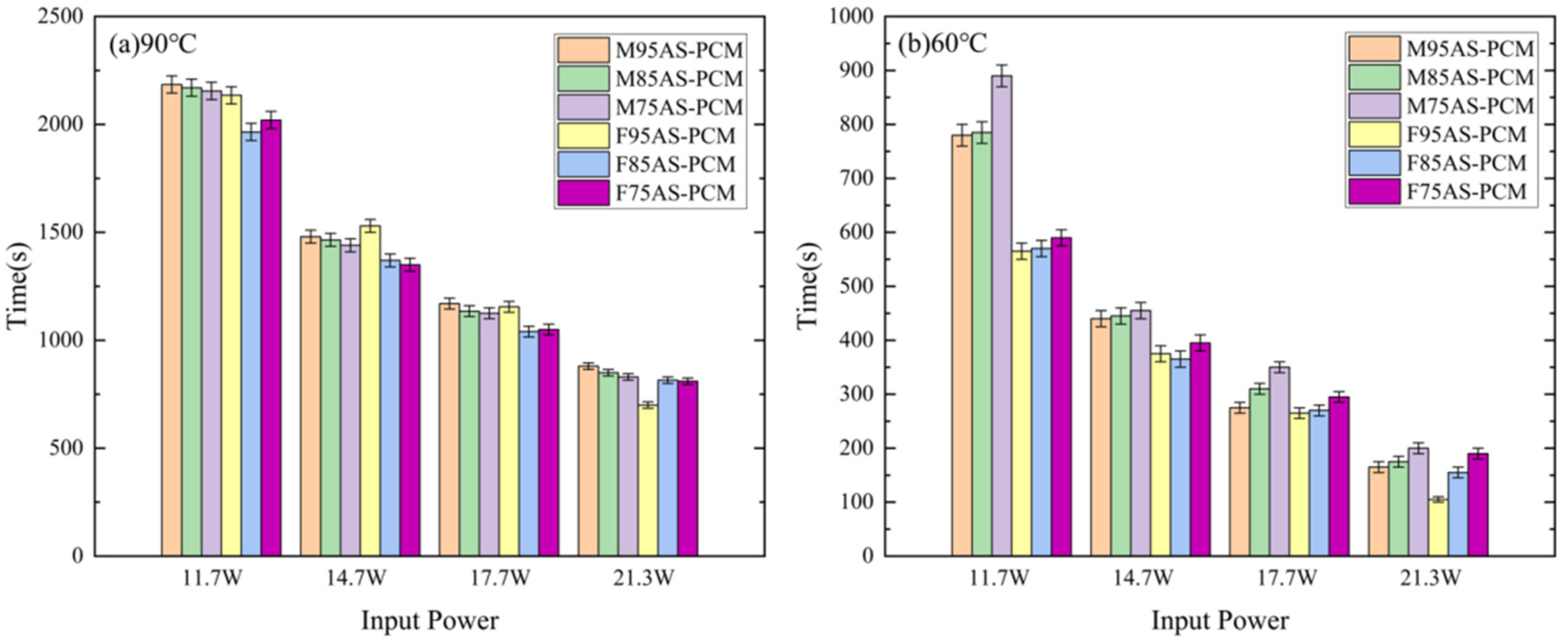
3.6. Influence of Different AS-PCM on Temperature Control Time
3.7. Comparison with Other Reported PCMs
4. Conclusions
- (1)
- A new porous aluminum skeleton was proposed to solve the low thermal conductivity problem of organic PCM. Compared with organic PCM, the thermal conductivity of the new AS-PCM is 32.2~59.6 times higher than that of pure paraffin.
- (2)
- The lower the porosity of the AS, the faster the cooling speed, and the MAS-PCM heat sink enables the LED device to operate at a lower temperature.
- (3)
- At a critical temperature of 90 °C, the temperature control time of M95AS-PCM is longer than that of others. The temperature control time of the M95AS-PCM heat sink is increased by 5.7–20.5% compared with that of the pure paraffin heat sink. At the critical temperature of 60 °C, the temperature control time of the M75AS-PCM heat sink is increased by 5.3–50.8% compared with that of F75AS-PCM. The new AS-PCM is a promising and reliable material for the thermal control of electronic devices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Nomenclature | |||
| Cp | heat capacity (kJ/kg) | AS | porous aluminum skeleton |
| V | volume (m3) | MAS | Mcc porous aluminum skeleton |
| m | mass (kg) | FAS | Fcc porous aluminum skeleton |
| T | temperature | AS-PCM | aluminum skeleton/paraffin composite |
| ΔT | temperature difference | Greek symbols | |
| Tm | paraffin melting temperature | ε | porosity (%) |
| K | thermal conductivity (W/m·K) | ρAl | density of aluminum skeleton |
| dep | wire diameter (m) | ρ | paraffin wax density (kg/m3) |
| mAl | weight of aluminum skeleton (kg) | Δθ | time (s) |
| Abbreviations | ϕsf | specific surface area | |
| PCM | phase change material | ||
References
- Tang, H.; Tang, Y.; Wan, Z.; Li, J.; Yuan, W.; Lu, L.; Li, Y.; Tang, K. Review of applications and developments of ultra-thin micro heat pipes for electronic cooling. Appl. Energy 2018, 223, 383–400. [Google Scholar] [CrossRef]
- Javadi, F.S.; Metselaar, H.S.C.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM), a review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Al-Shamkhee, D.; Al-Aasam, A.B.; Al-Waeli, A.H.A.; Abusaibaa, G.Y.; Moria, H. Passive cooling techniques for ventilation: An updated review. Renew. Energy Environ. Sustain. 2022, 7, 23. [Google Scholar] [CrossRef]
- Kothari, R.; Sahu, S.K.; Kundalwal, S.I.; Sahoo, S. Experimental investigation of the effect of inclination angle on the performance of phase change material based finned heat sink. J. Energy Storage 2021, 37, 102462. [Google Scholar] [CrossRef]
- Kumar, A.; Kothari, R.; Sahu, S.K.; I Kundalwal, S. A comparative study and optimization of phase change material based heat sinks for thermal management of electronic components. J. Energy Storage 2021, 43, 103224. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Zhuang, B.; Zhao, X.; Tang, Y.; Ding, X.; Chen, K. Preparation of novel copper-powder-sintered frame/paraffin form-stable phase change materials with extremely high thermal conductivity. Appl. Energy 2017, 206, 1147–1157. [Google Scholar] [CrossRef]
- Fang, X.; Fan, L.W.; Ding, Q.; Yao, X.-L.; Wu, Y.-Y.; Hou, J.-F.; Wang, X.; Yu, Z.-T.; Cheng, G.-H.; Hu, Y.-C. Thermal energy storage performance of paraffin-based composite phase change materials filled with hexagonal boron nitride nanosheets. Energy Convers. Manag. 2014, 80, 103–109. [Google Scholar] [CrossRef]
- Mahmoud, S.; Tang, A.; Toh, C.; Al-Dadah, R.; Soo, S.L. Experimental investigation of inserts configurations and PCM type on the thermal performance of PCM based heat sinks. Appl. Energy 2013, 112, 1349–1356. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Experimental investigations on phase change material based finned heat sinks for electronic equipment cooling. Int. J. Heat Mass Transf. 2011, 55, 1642–1649. [Google Scholar] [CrossRef]
- Hosseinizadeh, S.F.; Tan, F.L.; Moosania, S.M. Experimental and numerical studies on performance of PCM-based heat sink with different configurations of internal fins. Appl. Therm. Eng. 2011, 31, 3827–3838. [Google Scholar] [CrossRef]
- Kim, S.H.; Heu, C.S.; Mok, J.Y.; Kang, S.-W.; Kim, D.R. Enhanced thermal performance of phase change material-integrated fin-type heat sinks for high power electronics cooling. Int. J. Heat Mass Transf. 2022, 184, 122257. [Google Scholar] [CrossRef]
- Afaynou, I.; Faraji, H.; Choukairy, K.; Arshad, A.; Arıcı, M. Heat transfer enhancement of phase-change materials (PCMs) based thermal management systems for electronic components: A review of recent advances. Int. Commun. Heat Mass Transf. 2023, 143, 106690. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Efficacy of porous foam on discharging of phase change material with inclusion of hybrid nanomaterial. J. Energy Storage 2023, 62, 106925. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Weigand, R.M.; Fleischer, A.S. Temperature-dependent thermal properties of a paraffin phase change material embedded with herringbone style graphite nanofibers. Appl. Energy 2015, 137, 716–725. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Das, M.K.; Rath, P. Application of TCE-PCM based heat sinks for cooling of electronic components: A review. Renew. Sustain. Energy Rev. 2016, 59, 550–582. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M. Experimental investigation on graphene based nanoparticles enhanced phase change materials (GbNePCMs) for thermal management of electronic equipment. J. Energy Storage 2020, 30, 101497. [Google Scholar] [CrossRef]
- Yang, T.; Kang, J.G.; Weisensee, P.B.; Kwon, B.; Braun, P.V.; Miljkovic, N.; King, W.P. A composite phase change material thermal buffer based on porous metal foam and low-melting-temperature metal alloy. Appl. Phys. Lett. 2020, 116, 071901. [Google Scholar] [CrossRef]
- Saghir, M.Z.; Bayomy, A.M. Experimental measurements and numerical computation of nanofluid and microencapsulated phase change material in porous material. Int. J. Energy Res. 2019, 43, 6353–6365. [Google Scholar] [CrossRef]
- Mustaffar, A.; Harvey, A.; Reay, D. Melting of phase change material assisted by expanded metal mesh. Appl. Therm. Eng. 2015, 90, 1052–1060. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Mancin, S.; Diani, A.; Doretti, L.; Hooman, K.; Rossetto, L. Experimental analysis of phase change phenomenon of paraffin waxes embedded in copper foams. Int. J. Therm. Sci. 2015, 90, 79–89. [Google Scholar] [CrossRef]
- Rahmanian-Koushkaki, H.; Rahmanian, S.; Moein-Jahromi, M.; Sopian, K. Performance evaluation of concentrated photovoltaics with phase change materials embedded metal foam-based heat sink using gradient strategy. Int. J. Energy Res. 2022, 46, 12290–12315. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Li, Z.; Tang, Y.; Yu, B.; Yuan, W. Experimental investigation on the thermal performance of a heat sink filled with porous metal fiber sintered felt/paraffin composite phase change material. Appl. Energy 2016, 176, 221–232. [Google Scholar] [CrossRef]
- Li, W.Q.; Qu, Z.G.; Zhang, B.L.; Zhao, K.; Tao, W.Q. Thermal behavior of porous stainless-steel fiber felt saturated with phase change material. Energy 2013, 55, 846–852. [Google Scholar] [CrossRef]
- Ali, H.M.; Saieed, A.; Pao, W.; Ali, M. Copper foam/PCMs based heat sinks: An experimental study for electronic cooling systems. Int. J. Heat Mass Transf. 2018, 127, 381–393. [Google Scholar]
- Lu, T.J.; Stone, H.A.; Ashby, M.F. Heat transfer in open-cell metal foams. Acta Mater. 1998, 46, 3619–3635. [Google Scholar] [CrossRef]
- Gupta, S.; Solanki, S.C. Enhancing the performance of double-slope solar still using nano-enhanced eutectic phase change materials and steel wool fibre as wick material. J. Braz. Soc. Mech. Sci. Eng. 2024, 153, 115–128. [Google Scholar] [CrossRef]
- Lafdi, K.; Mesalhy, O.; Shaikh, S. Experimental study on the influence of foam porosity and pore size on the melting of phase change materials. J. Appl. Phys. 2007, 102, 083549. [Google Scholar] [CrossRef]
- Salma, G.; Souad, H.; Sadok, B. Experimental comparison between different confi gurations of PCM based heat sinks for cooling electronic components. Appl. Therm. Eng. 2015, 87, 454–462. [Google Scholar]
- Kothari, R.; Sahu, S.K.; Kundalwal, S.I.; Mahalkar, P. Thermal performance of phase change material–based heat sink for passive cooling of electronic components: An experimental study. Int. J. Energy Res. 2020, 45, 5939–5963. [Google Scholar] [CrossRef]














| Tm (°C) | K (W/m·K) | ρ (kg/m3) | Cp (kJ/kg) | Heat Storage Capacity (kJ/kg) |
|---|---|---|---|---|
| 56–58 | 0.2 | 880/790 | 173.6 | 2.8 |
| AS-PCM | M75AS-PCM | M85AS-PCM | M95AS-PCM | F75AS-PCM | F85AS-PCM | F95AS-PCM |
|---|---|---|---|---|---|---|
| K (W/m·K) | 11.92 | 7.54 | 6.73 | 11.45 | 7.29 | 6.45 |
| Authors | Configuration | kpcm (W/m·K) | PCM |
|---|---|---|---|
| Present study | AS-PCM | 6.45–11.92 | Paraffin |
| Farid et al. [27] | Graphite-based nanocomposites–PCM | 2.8 | Paraffin |
| Lafdi K. et al. [28] | A luminum foam–PCM | 7.5 | Paraffin |
| Salma Gharbi et al. [29] | Graphite matrix–PCM | 1 | Paraffin |
| Kothari R. et al. [30] | Copper foam–PCM | 10 | Paraffin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Hu, Z.; Chen, Z.; Yang, D.; Huang, W.; Zhang, B. An Experimental Study on the Thermal Performance of a Heat Sink Filled with Porous Aluminum Skeleton/Paraffin Composite Phase Change Material. Materials 2024, 17, 4332. https://doi.org/10.3390/ma17174332
Huang S, Hu Z, Chen Z, Yang D, Huang W, Zhang B. An Experimental Study on the Thermal Performance of a Heat Sink Filled with Porous Aluminum Skeleton/Paraffin Composite Phase Change Material. Materials. 2024; 17(17):4332. https://doi.org/10.3390/ma17174332
Chicago/Turabian StyleHuang, Shufeng, Zhihan Hu, Zhixin Chen, Dayong Yang, Weili Huang, and Bin Zhang. 2024. "An Experimental Study on the Thermal Performance of a Heat Sink Filled with Porous Aluminum Skeleton/Paraffin Composite Phase Change Material" Materials 17, no. 17: 4332. https://doi.org/10.3390/ma17174332
APA StyleHuang, S., Hu, Z., Chen, Z., Yang, D., Huang, W., & Zhang, B. (2024). An Experimental Study on the Thermal Performance of a Heat Sink Filled with Porous Aluminum Skeleton/Paraffin Composite Phase Change Material. Materials, 17(17), 4332. https://doi.org/10.3390/ma17174332






