Strongly Fluorescent Blue-Emitting La2O3: Bi3+ Phosphor for Latent Fingerprint Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis and Characterization
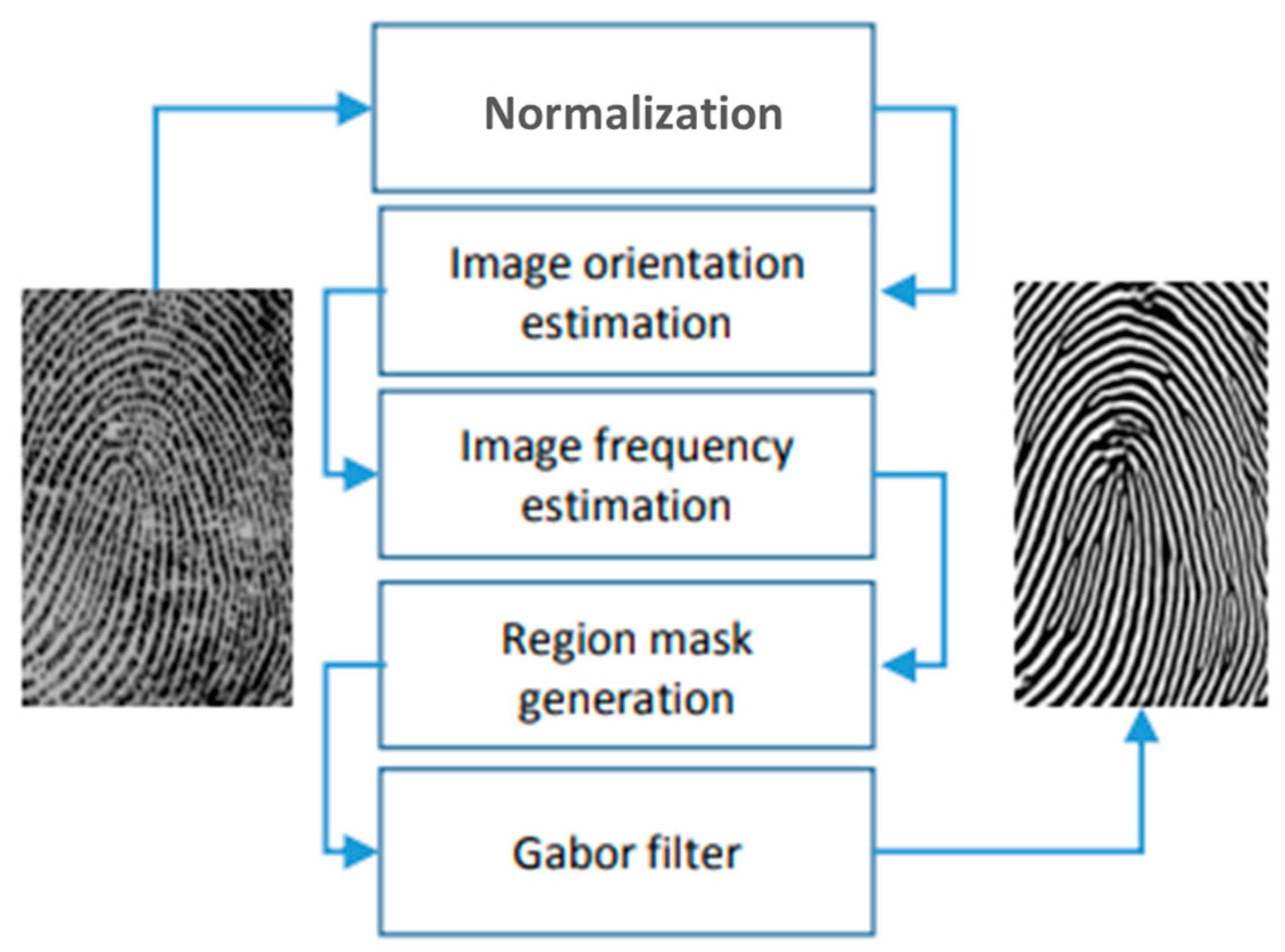
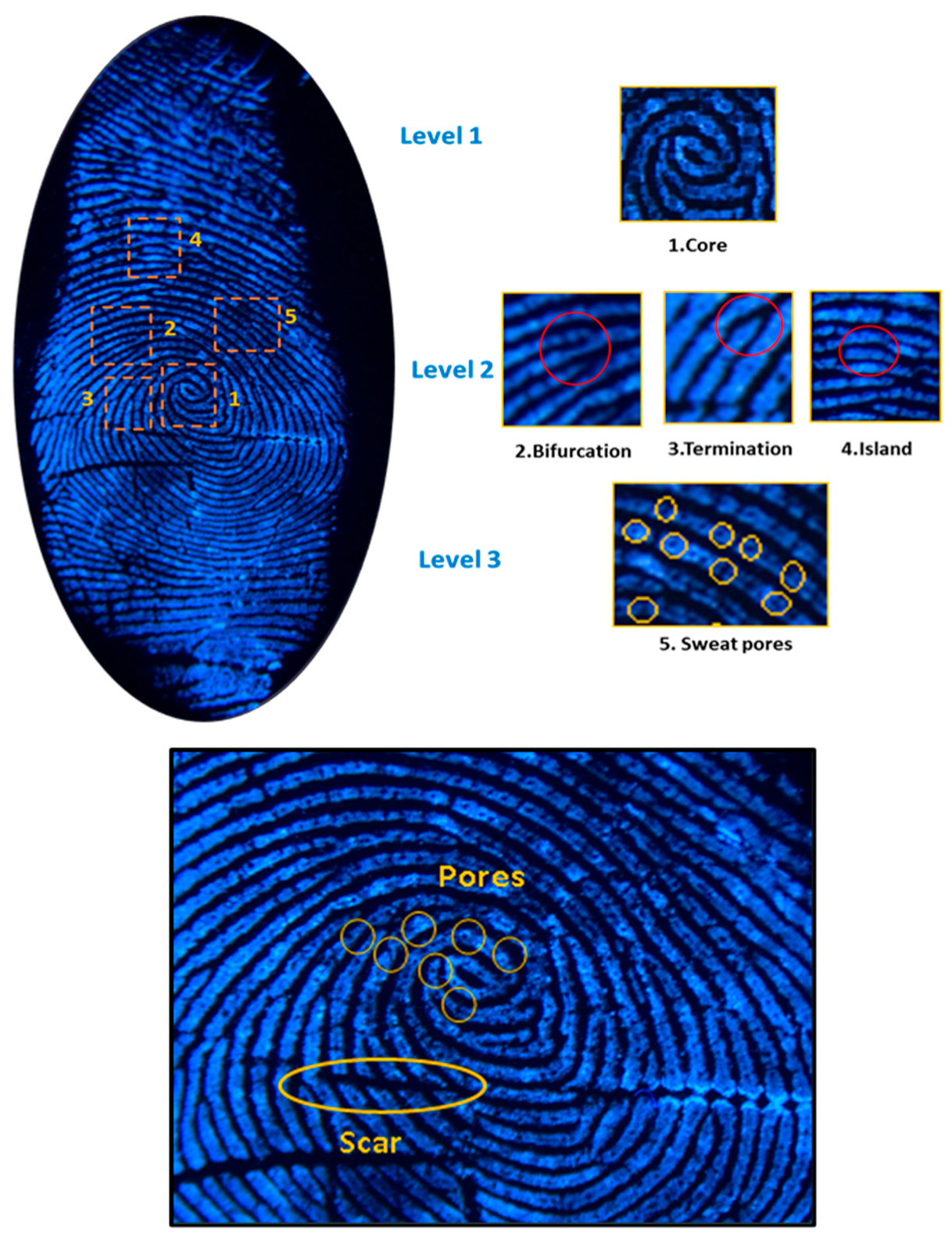
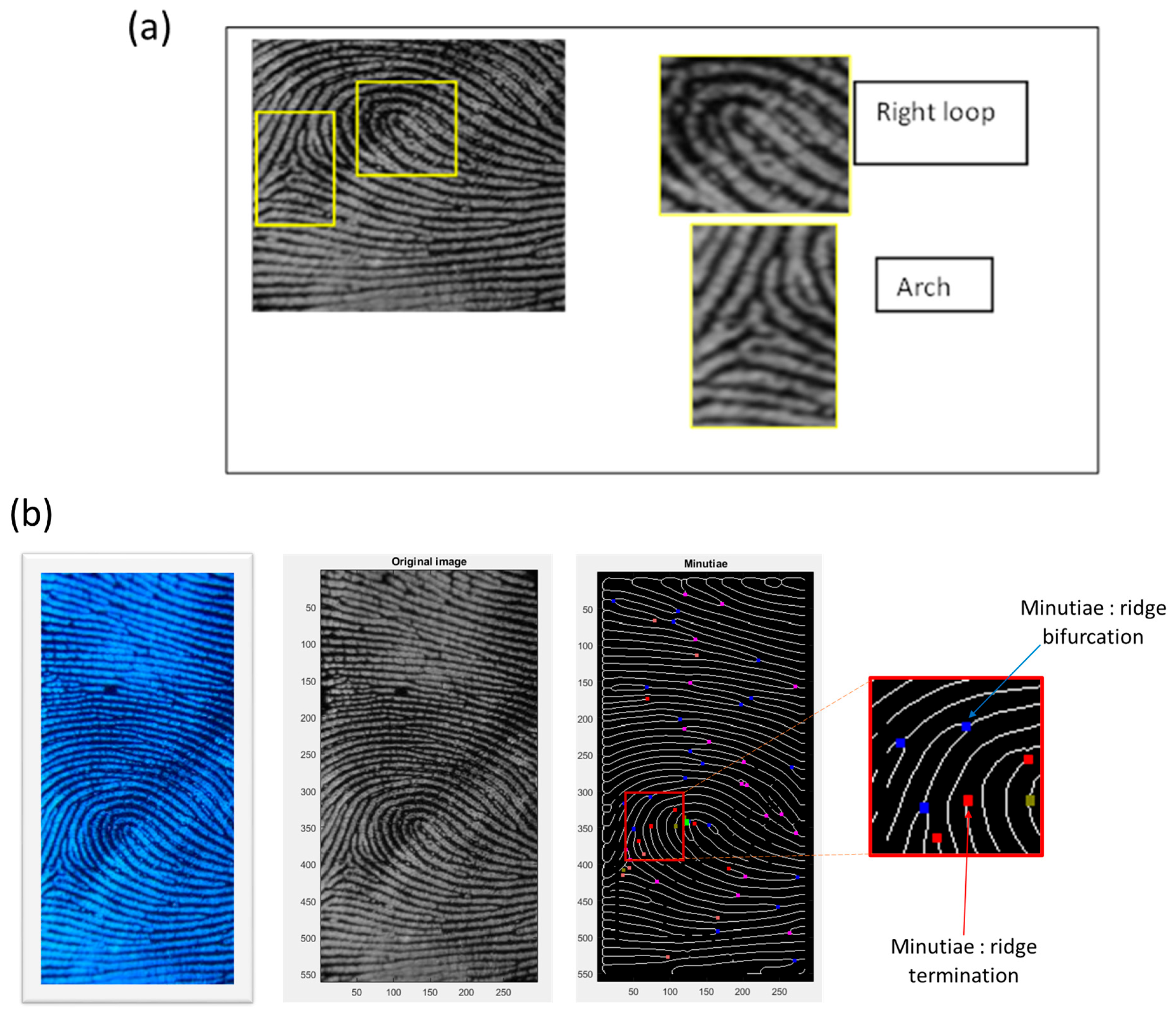
2.2. Latent Fingerprint Feature Extraction
- -
- Level 1: This level consists of coarse features like arches, loops and whorls.
- -
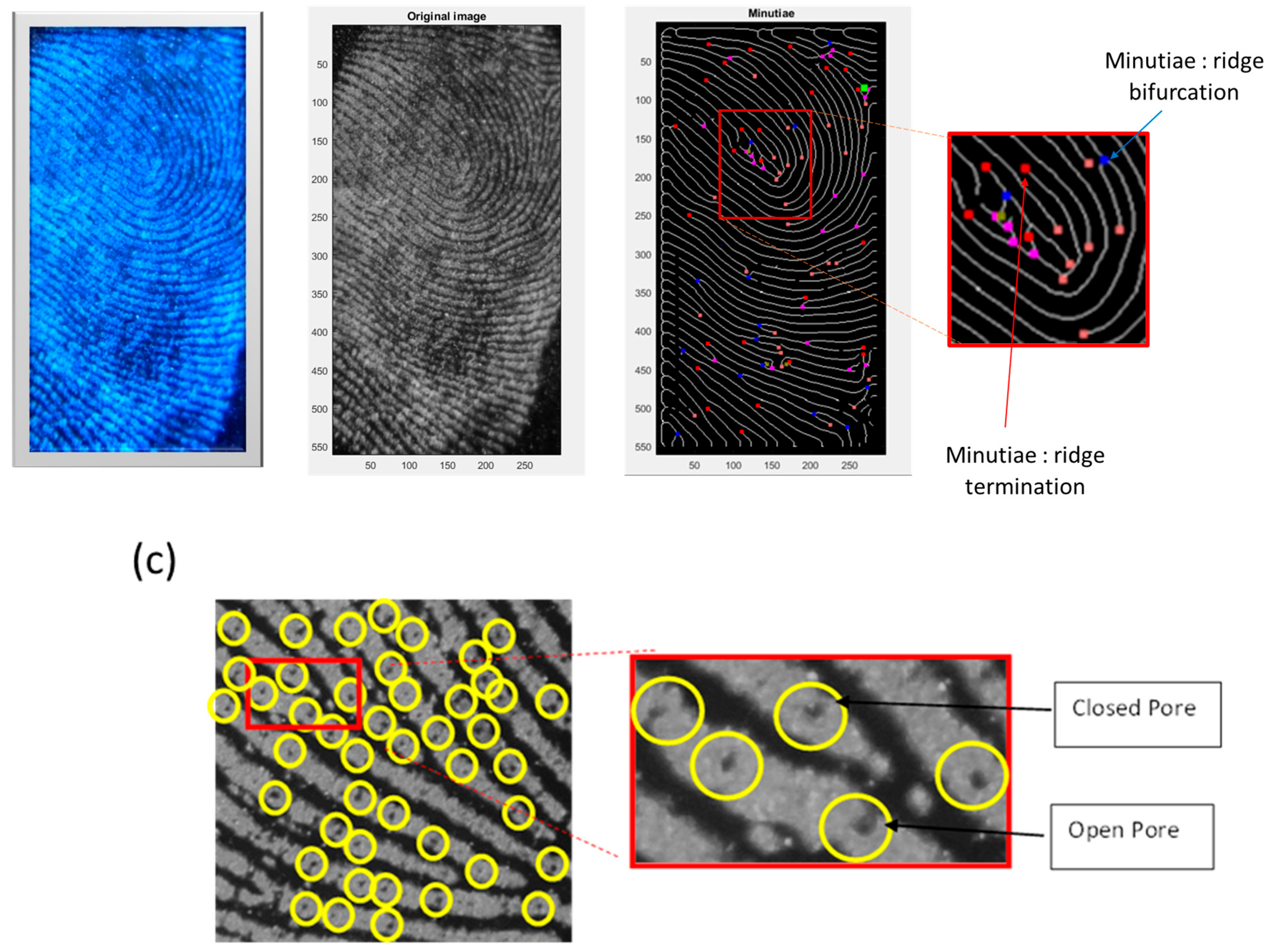
- Level 2: This level involves identifying irregularities, known as minutiae, on capillary lines. These minutiae are then categorized into four types: terminations, bifurcations, islands and lakes.
- -
- Level 3: This level involves microscopic details, including pores.
- Preprocessing: This involves enhancing the image through a series of five main steps aimed at improving image quality for more effective feature extraction (Figure 1). The process begins with image normalization, followed by orienting the peaks of the fingerprint and estimating the frequency. Subsequently, a segmentation step is applied to identify the peak regions in a fingerprint image, creating a mask to delineate the region and normalizing image intensity values. Ultimately, oriented Gabor filters are utilized.
- Feature extraction: The process of extracting minutiae features commences with an enhanced grayscale image. Following image binarization, a morphological thinning technique is applied to reduce ridge structures to a single pixel width, creating what is referred to as the skeleton. Subsequently, every pixel of the thinned binary image is analyzed to identify minutiae locations.
3. Results
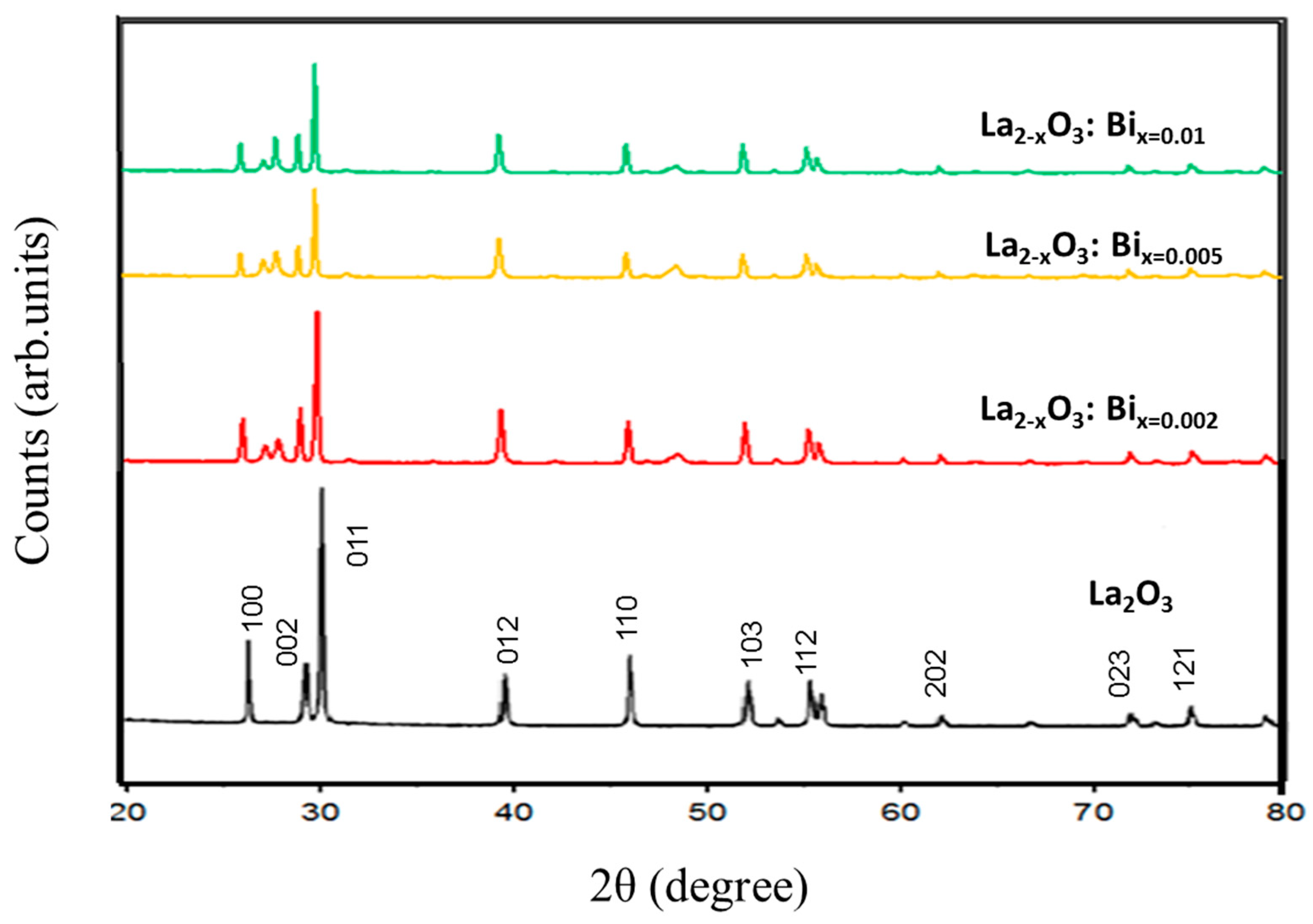
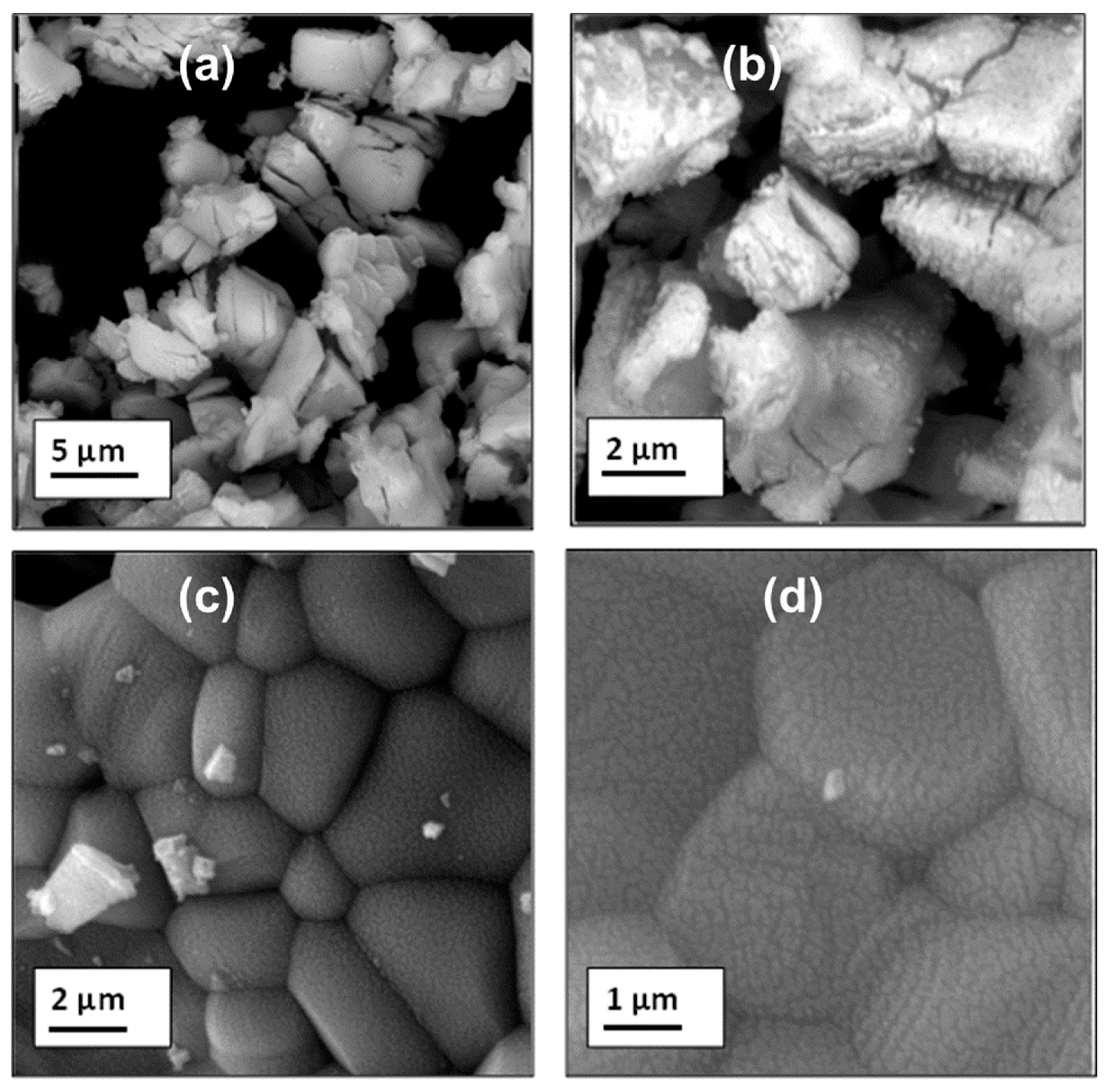
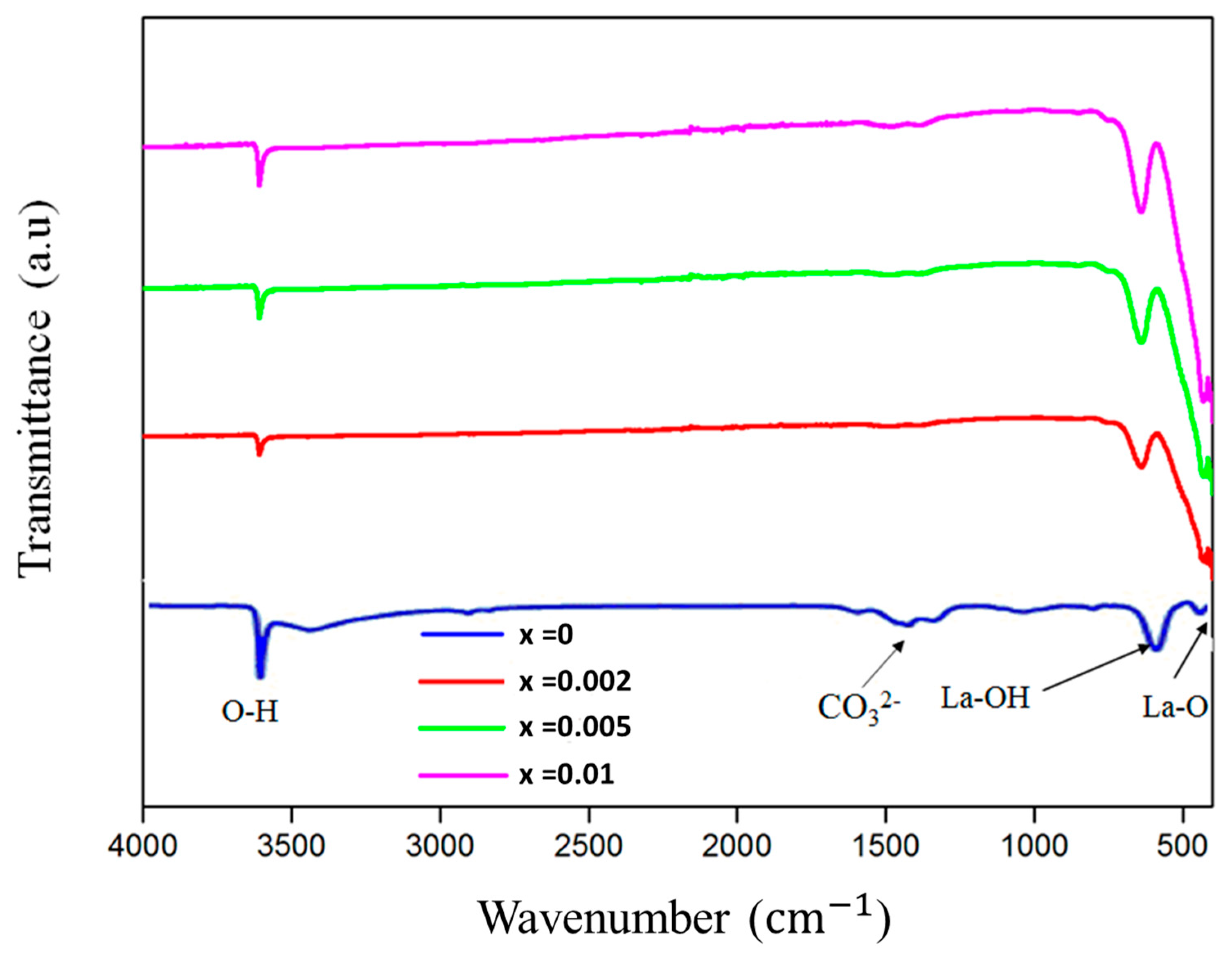
3.1. Structure and Morphology: XRD, SEM and EDX Studies
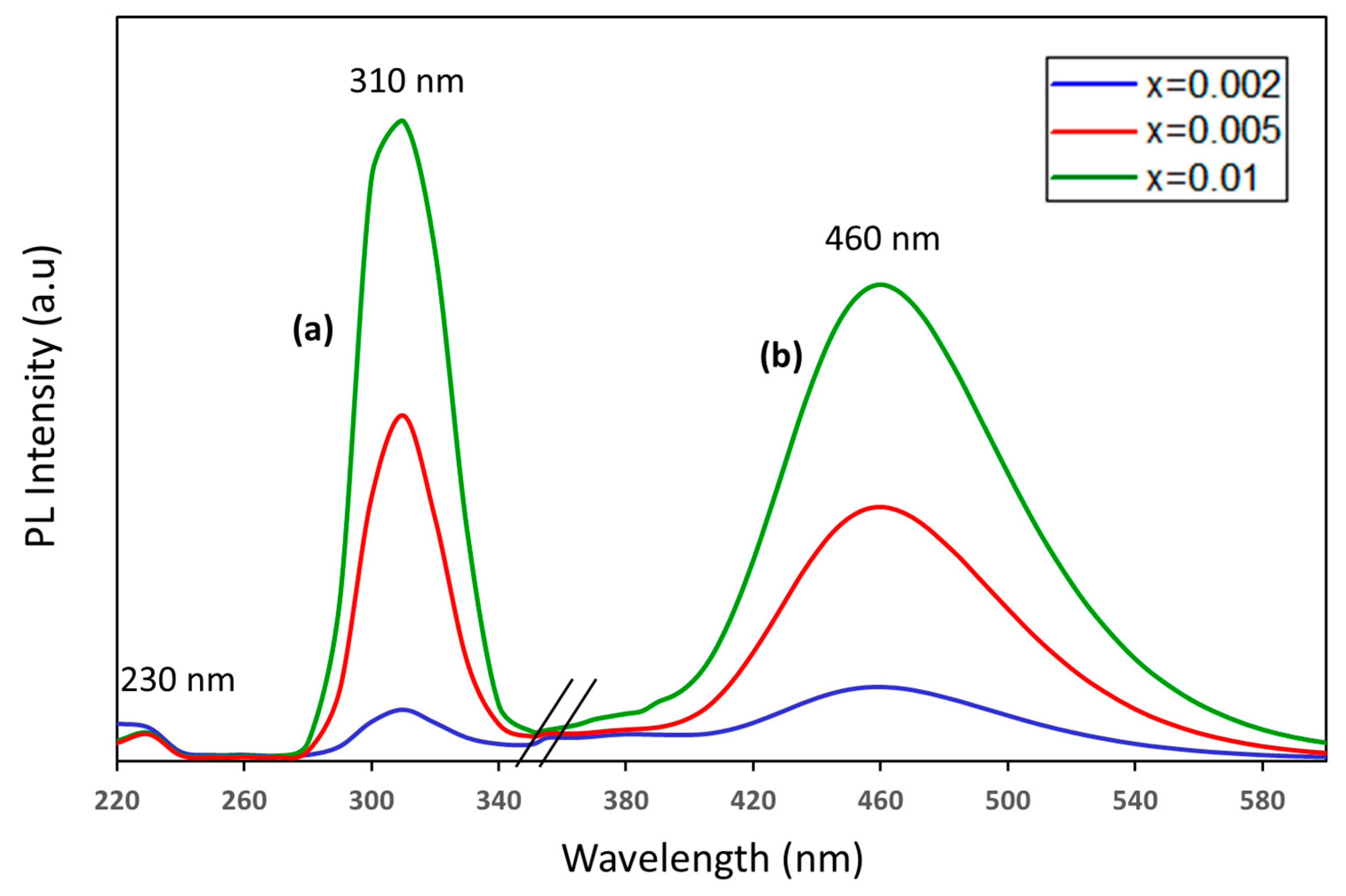
3.2. Photoluminescence (PL) Properties
3.3. CIE Chromaticity
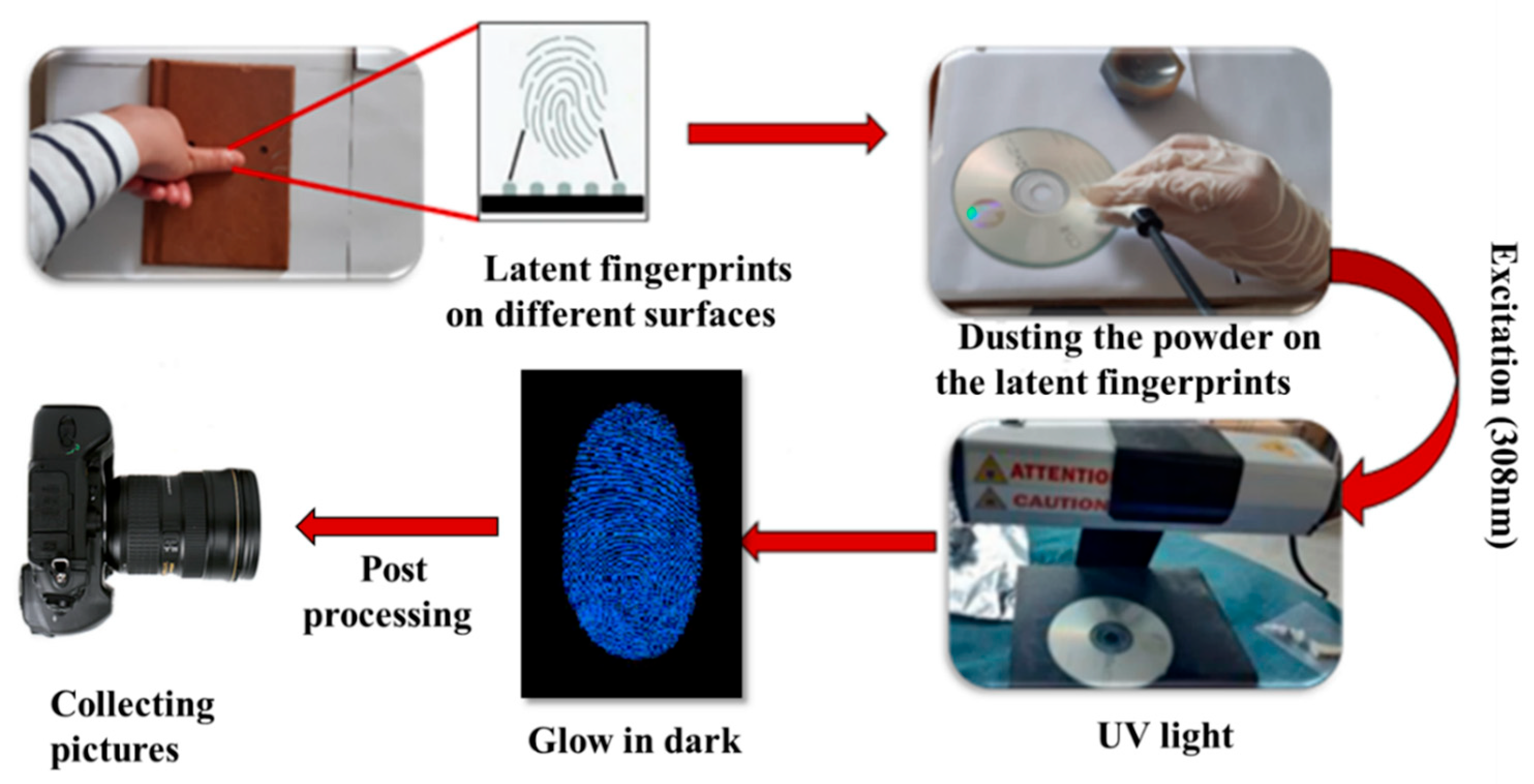
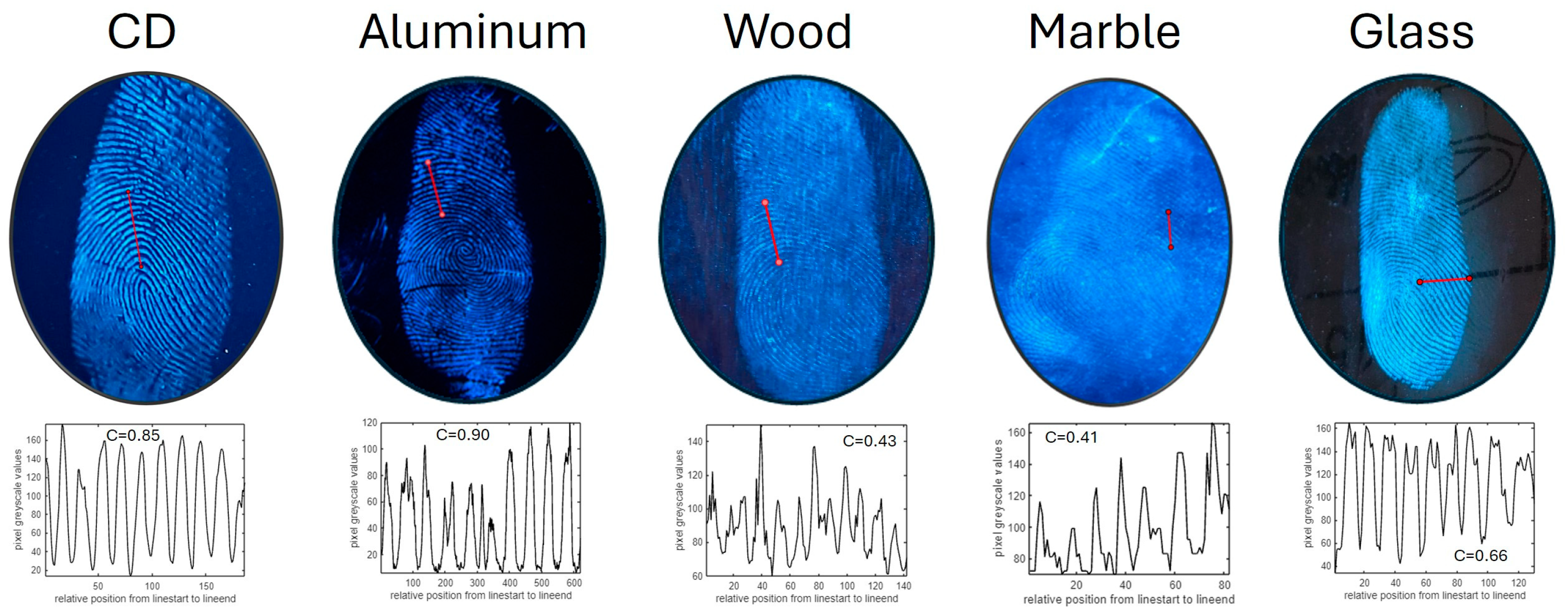
3.4. Latent Fingerprint Detection Using the Powder Dusting Method
3.5. Automatic Minutiae Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Feng, J.; Kuo, C.-C.J. Deep convolutional neural network for latent fingerprint enhancement. Signal Process. Image Commun. 2018, 60, 52–63. [Google Scholar] [CrossRef]
- Cao, K.; Jain, A.K. Automated latent fingerprint recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2018, 41, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.; Lulu, L.; Song, Q. Rapid Visualization of Latent Fingerprints with Color-Tunable Solid Fluorescent Carbon Dots. Part. Part. Syst. Charact. 2018, 35, 1700387. [Google Scholar] [CrossRef]
- Basavaraj, R.B.; Darshan, G.P.; Prasad, B.D.; Sharma, S.C.; Nagabhushana, H. Rapid visualization of latent fingerprints using novel CaSiO3:Sm3+ nanophosphors fabricated via ultrasound route. J. Rare Earths 2019, 37, 32–44. [Google Scholar] [CrossRef]
- Marappa, B.; Rudresha, M.S.; Basavaraj, R.B.; Darshan, G.P.; Daruka Prasad, B.; Sharma, S.C.; Sivakumari, S.; Amudha, P.; Nagabhushana, H. EGCG assisted Y2O3:Eu3+ nanopowders with 3D micro-architecture assemblies useful for latent figerprint recognition and anti-counterfeiting applications. Sens. Actuators B Chem. 2018, 264, 426–439. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Basavaraj, R.B.; Darshan, G.P.; Sharma, S.C.; Nagabhushana, H. Pivotal role of fluxes in BaTiO3:Eu3+ nano probes for visualization of latent fingerprints on multifaceted substrates and anti-counterfeiting applications. Microchem. J. 2019, 145, 226–234. [Google Scholar] [CrossRef]
- Singla, N.; Kaur, M.; Sofat, S. Automated Latent Fingerprint Identification System: A Review. Forensic Sci. Int. 2020, 309, 110187. [Google Scholar] [CrossRef]
- Kolhatkar, G.; Parisien, C.; Ruediger, A.; Muehlethaler, C. Latent Fingermark Imaging by Single-Metal Deposition of Gold Nanoparticles and Surface Enhanced Raman Spectroscopy. Front. Chem. 2019, 7, 440–448. [Google Scholar] [CrossRef]
- Basavaraj, R.B.; Nagabhushana, H.; Darshan, G.P.; Daruka Prasad, B.; Rahul, M.; Sharma, S.C.; Sudaramani, R.; Archana, K.V. Red and green emitting CTAB assisted CdSiO3:Tb3+/Eu3+ nanopowders as fluorescent labeling agents used in forensic and display applications. Dye. Pigment. 2017, 147, 364–377. [Google Scholar] [CrossRef]
- Cantu, A.A. Silver physical developers for the visualization of latent prints on paper. Forensic Sci. Rev. 2001, 13, 29–64. [Google Scholar]
- Schnetz, B.; Margot, P. Technical note: Latent fingermarks, colloidal gold and multimetal deposition(MMD): Optimisation of the method. Forensic Sci. Int. 2001, 118, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Brini, L.; Bennour, I.; Toncelli, A.; Maalej, R.; Abdelhedi, M. Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications. Materials 2022, 15, 2423. [Google Scholar] [CrossRef]
- Chávez, D.; Garcia, C.R.; Oliva, J.; Diaz-Torres, L.A. A review of phosphorescent and fluorescent phosphors for fingerprint detection. Ceram. Int. 2021, 47, 10–41. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldajani, K.M.; AlHazaa, A.N.; Albrithen, H.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Lian, J.; Meng, F.; Wang, W.; Zhang, Z. Recent Trends in Fluorescent Organic Materials for Latent Fingerprint Imaging. Front. Chem. 2020, 8, 594864. [Google Scholar] [CrossRef]
- Wang, M.; Mi, C.C.; Wang, W.X.; Liu, C.H.; Wu, Y.F.; Xu, Z.R.; Mao, C.B.; Xu, S.K. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4: Yb, Er upconversion nanoparticles. ACS Nano 2009, 3, 1580–1586. [Google Scholar] [CrossRef]
- Ang, L.Y.; Lim, M.E.; Ong, L.C.; Zhang, Y. Applications of upconversion nanoparticles in imaging, detection and therapy. Nanomedicine 2011, 6, 1273–1288. [Google Scholar] [CrossRef]
- Wang, M. Latent fingermarks light up: Facile development of latent fingermarks using NIR-responsive upconversion fluorescent nanocrystals. RSC Adv. 2016, 6, 36264–36268. [Google Scholar] [CrossRef]
- Lundberg, P.; Lindh, E.M.; Tang, S.; Edman, L. Toward efficient and metal-free emissive devices: A solution-processed host-guest light-emitting electrochemical cell featuring thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 2017, 9, 28810–28816. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yu, A.; Zhu, Y.; Yang, M.; Mao, C. Fluorescent nanomaterials for the development of latent fingerprints in forensic sciences. Adv. Funct. Mater. 2017, 27, 1606243–1606259. [Google Scholar] [CrossRef]
- Brini, L.; Douiri, H.; Abid, M.; Toncelli, A.; Qasymeh, M.; Maalej, R.; Abdelhedi, M. Potential of Y2Sn2O7:Eu3+, Dy3+ Inoganic Nanophosphors in Latent Fingermark Detection. Crystals 2024, 14, 300. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, W.; Zhuo, Y.; Yan, C.; Wen, J.; Brgoch, J. Understanding the blue-emitting orthoborate phosphor NaBaBO3: Ce3+ through experiment and computation. J. Mater. Chem. C 2019, 7, 654–662. [Google Scholar] [CrossRef]
- Guss, P.; Foster, M.E.; Wong, B.M.; Doty, F.P.; Shah, K.; Squillante, M.R.; Shirwadkar, U.; Hawrami, R.; Tower, J.; Yuan, D. Results for aliovalent doping of CeBr3 with Ce2+. J. Appl. Phys. 2014, 115, 034908. [Google Scholar] [CrossRef]
- Wei, C.; Fan, J.; Gong, H. Structural, thermodynamic, and mechanical properties of bulk La and A-La2O3. J. Alloys Compd. 2015, 618, 615–622. [Google Scholar] [CrossRef]
- Pisecny, P.; Husekova, K.; Frohlich, K.; Harmatha, L.; Soltys, J.; Machajdik, D.; Espinos, J.P.; Jergel, M.; Jakabovic, J. Growth of lanthanum oxide films for application as a gate dielectric in CMOS technology. Mater. Sci. Semicon. Proc. 2004, 7, 231–236. [Google Scholar] [CrossRef]
- Mangla, O.; Srivastava, A.; Malhotra, Y.; Ostrikov, K. Lanthanum oxide nanostructured films synthesized using hot dense and extremely non-equilibrium plasma for nanoelectronic device applications. J. Mater. Sci. 2014, 49, 1594–1605. [Google Scholar] [CrossRef]
- Sasidharan, M.; Gunawardhana, N.; Inoue, M.; Yusa, S.-I.; Yoshio, M.; Nakashima, K. La2O3 hollow nanospheres for high performance lithium-ion rechargeable batteries. Chem. Commun. 2012, 48, 3200–3202. [Google Scholar] [CrossRef]
- Gangwar, B.P.; Palakollu, V.; Singh, A.; Kanvah, S.; Sharma, S. Combustion synthesized La2O3 and La (OH)3: Recyclable catalytic activity towards Knoevenagel and Hantzsch reactions. RSC Adv. 2014, 4, 55407–55416. [Google Scholar] [CrossRef]
- Xu, Z.; Bian, S.; Wang, J.; Liu, T.; Wang, L.; Gao, Y. Preparation and luminescence of La2O3:Ln3+ (Ln3+ = Eu3+, Tb3+, Dy3+, Sm3+, Er3+, Ho3+, Tm3+, Yb3+/Er3+, Yb3+/Ho3+) microspheres. RSC Adv. 2013, 3, 1410–1419. [Google Scholar] [CrossRef]
- Dey, R.; Pandey, A.; Rai, V.K. The Er3+- Yb3+ codoped La2O3 phosphor in fingerprint detection and optical heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 508–513. [Google Scholar] [CrossRef]
- Pushpa, N.; Kokila, M.K.; Shivaramu, N.J. Luminescence properties of La2O3:Eu3+ nanophosphor prepared by sol–gel method. Nucl. Instrum. Methods Phys. Res. B 2016, 379, 69–72. [Google Scholar] [CrossRef]
- Reddy, P.V.S.S.S.N.; Babu, D.N.; Kishore, G.M.M.; Naveen, S.V.; Ramana, B.V.; Srinivas, B.; Naveen, B.V.; Rao, K.R.; Ch, S.K. Polyol derived Bi3+ and Er3+ co-doped lanthanum oxide nanophosphors for display & LED applications. Mater. Today Proc. 2019, 18, 2118–2122. [Google Scholar] [CrossRef]
- Catauro, M.; Ciprioti, S. Vecchio.Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Mura, S.; Ludmerczki, R.; Stagi, L.; Garroni, S.; Carbonaro, C.M.; Ricci, P.C.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Integrating sol-gel and carbon dots chemistry for the fabrication of fluorescent hybrid organic-inorganic films. Sci. Rep. 2020, 10, 4770. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Haddada, L.R.; Amara, N.E.B. Score-Level Fusion of Fingerprint, Face and Iris based on Choquet Integral. In Proceedings of the 2019 5th International Conference on Nanotechnology for Instrumentation and Measurement (NanofIM), Sfax, Tunisia, 30–31 October 2019. [Google Scholar] [CrossRef]
- Gouiaa, M.; Bennour, I.; Haddada, L.R.; Toncelli, A.; Xu, J.; Mbarek, A.; Moscardini, A.; Amara, N.E.B.; Maalej, R. Spectroscopic characterization of Er, Yb: Y2Ti2O7 phosphor for latent fingerprint detection. Phys. B Condens. Matter 2020, 582, 412009. [Google Scholar] [CrossRef]
- Maalej, N.M.; Qurashi, A.; Bennour, I.; Haddada, L.R.; Shaikh, M.N.; Ilyas, M.; Amara, N.E.B.; Maalej, R.; Gondal, M.A. Green emitting rare earth Gd2O3:Tb3+ nanoparticles for rapid imaging of latent fingerprint. Methods Appl. Fluores 2021, 9, 025002. [Google Scholar] [CrossRef]
- Singh, A.; Palakollu, V.; Pandey, A.; Kanvah, S.; Sharma, S. Green synthesis of 1,4-benzodiazepines over La2O3 and La (OH)3 catalysts: Possibility of Langmuir-Hinshelwood adsorption. RSC Adv. 2016, 6, 103455–103462. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, Y. Synthesis, characterization, shape-preserved transformation, and optical properties of La (OH)3, La2O2CO3, and La2O3 nanorods. J. Alloys Compd. 2011, 509, 396–401. [Google Scholar] [CrossRef]
- Méndez, M.; Cesteros, Y.; Marsal, L.F.; Giguère, A.; Drouin, D.; Salagre, P.; Formentıín, P.; Pallarès, J.; Aguiloó, M.; Dıaz, F.; et al. Effect of Thermal Annealing on the Kinetics of Rehydroxylation of Eu3+: La2O3 Nanocrystals. J. Inorg. Chem. 2012, 5, 16139–16146. [Google Scholar] [CrossRef]
- He, F.; Yang, P.; Wang, D.; Li, C.; Niu, N.; Gai, S.; Zhang, M. Preparation and up-conversion luminescence of hollow La2O3: Ln (Ln = Yb/Er, Yb/Ho) microspheres. Langmuir 2011, 27, 5616–5623. [Google Scholar] [CrossRef] [PubMed]
- Tabaza, W.A.I.; Swart, H.C.; Kroon, R.E. Optical properties of Bi and energy transfer from Bi to Tb in MgAl2O4 phosphor. J. Lumin. 2014, 148, 192–197. [Google Scholar] [CrossRef]
- Bautinaud, P. Revisiting the spectroscopy of the Bi3+ ion in oxide compounds. Inorg. Chem. 2013, 52, 6028–6038. [Google Scholar] [CrossRef]
- Krasnikov, A.; Tsiumra, V.; Vasylechko, L.; Zazubovich, S.; Zhydachevskyy, Y. Photoluminescence origin in Bi3+- doped YVO4, LuVO4, and GdVO4 orthovanadates. J. Lumin. 2019, 212, 52–60. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Comanzo, H.A. The ultraviolet and visible luminescence of Bi3+ in orthorhombic perovskite. Opt. Mater. 2017, 63, 118–121. [Google Scholar] [CrossRef]
- Azman, A.R.; Mahat, N.A.; Wahab, R.A.; Ahmad, W.A.; Huri, M.A.M.; Hamzah, H.H. Relevant Visualization Technologies for Latent Fingerprints on Wet Objects and Its Challenges: A Review. Egypt. J. Forensic Sci. 2019, 9, 23. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douiri, H.; Abid, M.; Rzouga Haddada, L.; Brini, L.; Toncelli, A.; Essoukri Ben Amara, N.; Maalej, R. Strongly Fluorescent Blue-Emitting La2O3: Bi3+ Phosphor for Latent Fingerprint Detection. Materials 2024, 17, 4217. https://doi.org/10.3390/ma17174217
Douiri H, Abid M, Rzouga Haddada L, Brini L, Toncelli A, Essoukri Ben Amara N, Maalej R. Strongly Fluorescent Blue-Emitting La2O3: Bi3+ Phosphor for Latent Fingerprint Detection. Materials. 2024; 17(17):4217. https://doi.org/10.3390/ma17174217
Chicago/Turabian StyleDouiri, Hanen, Marwa Abid, Lamia Rzouga Haddada, Layla Brini, Alessandra Toncelli, Najoua Essoukri Ben Amara, and Ramzi Maalej. 2024. "Strongly Fluorescent Blue-Emitting La2O3: Bi3+ Phosphor for Latent Fingerprint Detection" Materials 17, no. 17: 4217. https://doi.org/10.3390/ma17174217
APA StyleDouiri, H., Abid, M., Rzouga Haddada, L., Brini, L., Toncelli, A., Essoukri Ben Amara, N., & Maalej, R. (2024). Strongly Fluorescent Blue-Emitting La2O3: Bi3+ Phosphor for Latent Fingerprint Detection. Materials, 17(17), 4217. https://doi.org/10.3390/ma17174217








