Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of FH and FH-HA Complexes
2.2. Sb(V) Adsorption Experimental Designs
2.3. Isothermal Desorption Experiments
2.4. Transformation Experiments
2.5. Analytical Methods
3. Results and Discussion
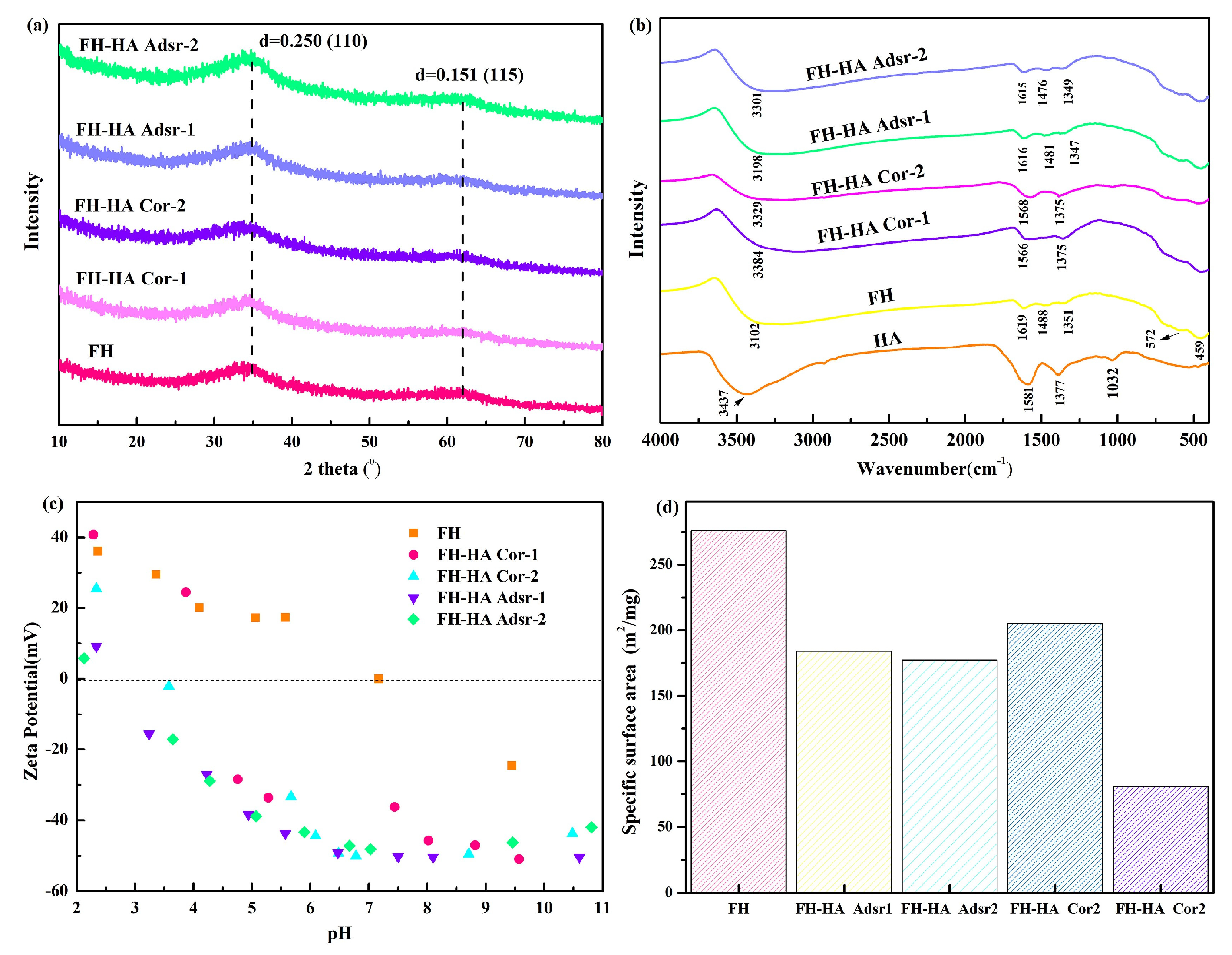
3.1. Characteristics of FH and FH-HA Complexes
3.2. Adsorption Edges
3.3. Adsorption Kinetics
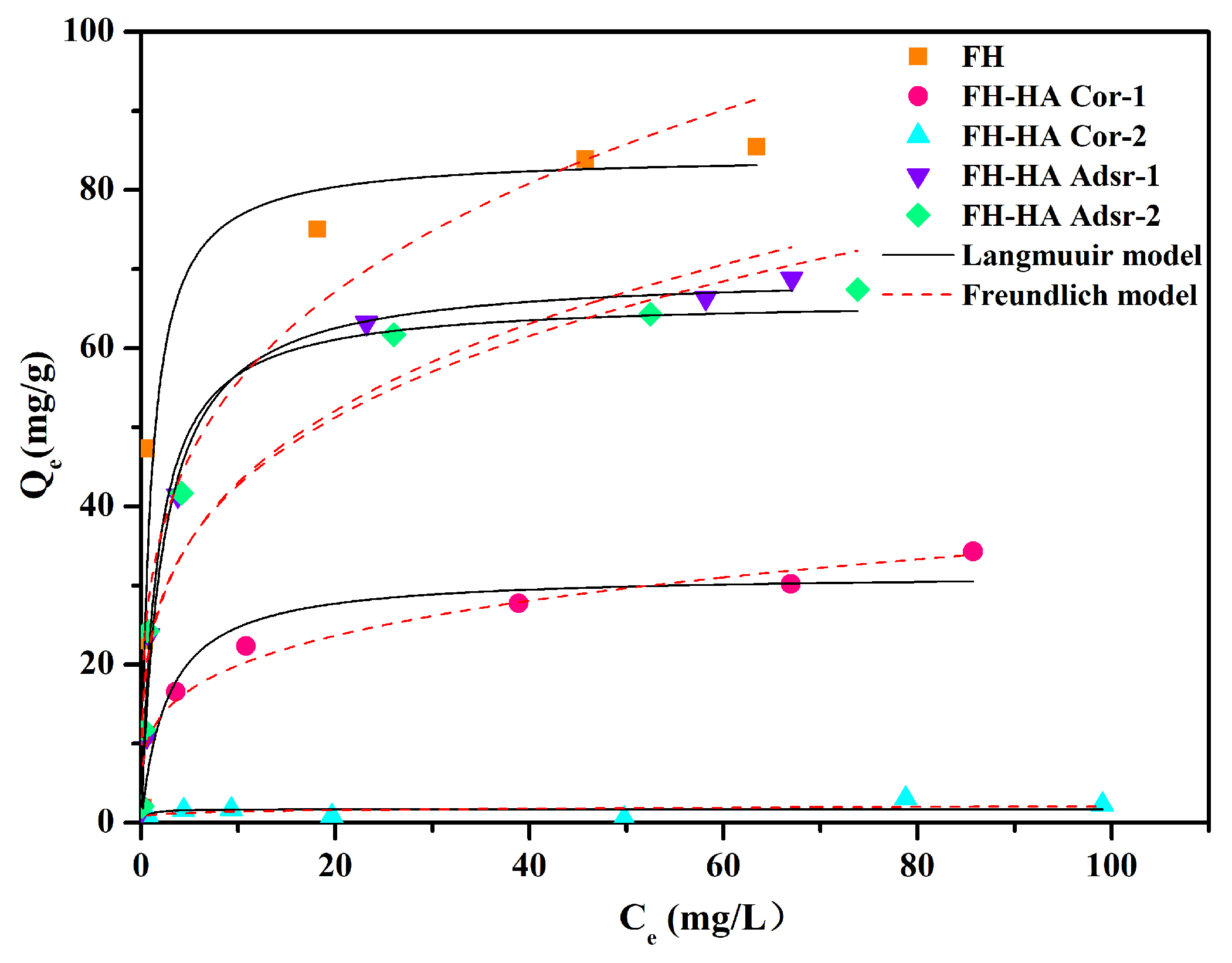
3.4. Adsorption Isotherms
3.5. Desorption Isotherms
3.6. Effect of Transformation on Antimony Adsorption
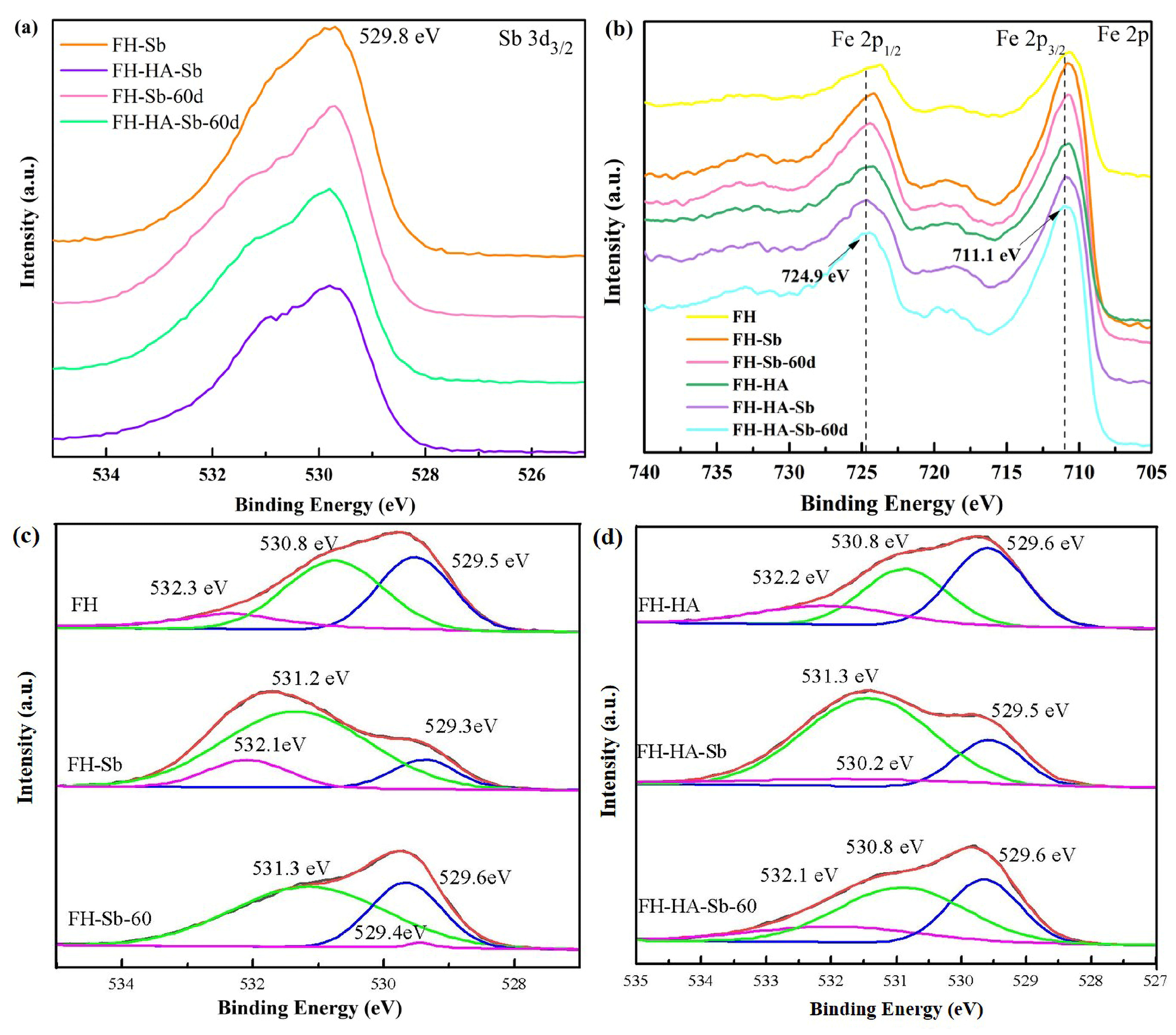
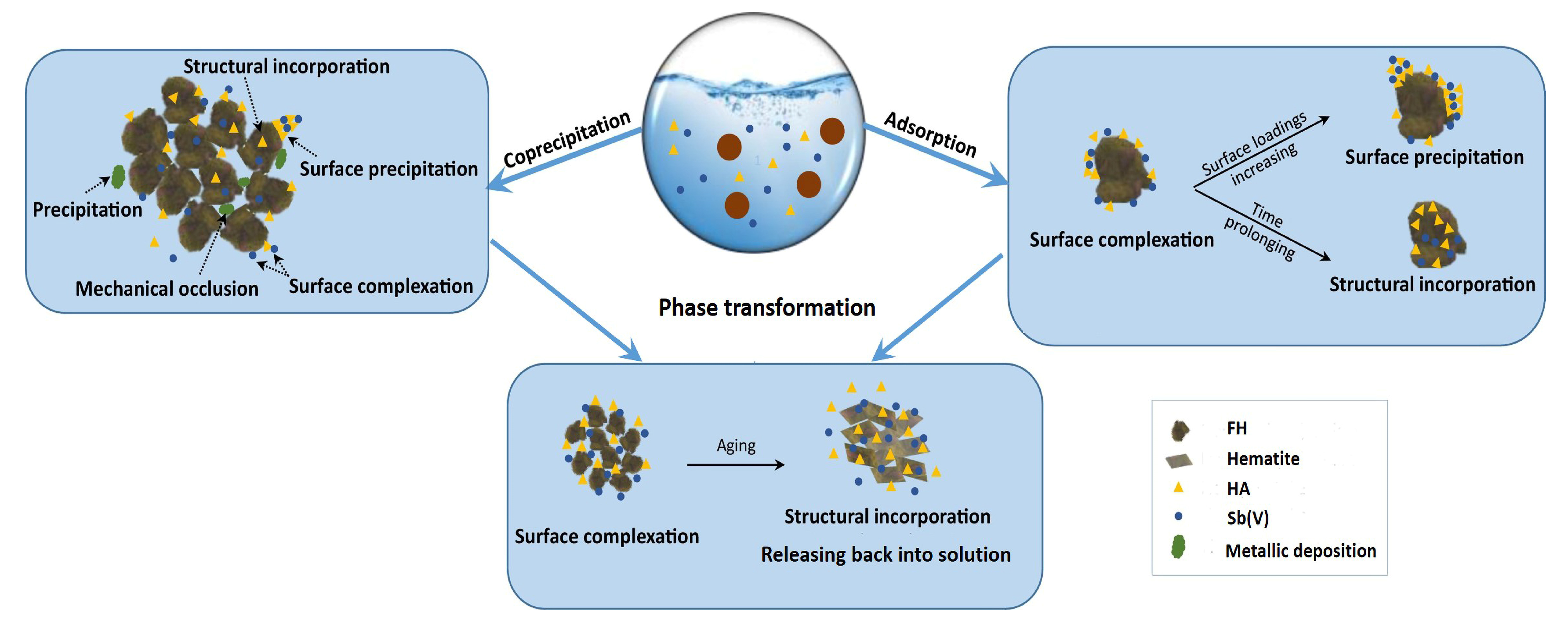
3.7. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Wang, N.; Long, X.; Zhang, C.; Ma, C.; Zhong, Q.; Wang, A.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef]
- Verbeeck, M.; Warrinnier, R.; Gustafsson, J.P.; Thiry, Y.; Smolders, E. Soil organic matter increases antimonate mobility in soil: An Sb(OH)6 sorption and modelling study. Appl. Geochem. 2019, 104, 33–41. [Google Scholar] [CrossRef]
- Chu, J.; Hu, X.; Kong, L.; Wang, N.; Zhang, S.; He, M.; Ouyang, W.; Liu, X.; Lin, C. Dynamic flow and pollution of antimony from polyethylene terephthalate (PET) fibers in China. Sci. Total Environ. 2021, 771, 144643. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bao, S.X.; Zhang, Y.M. Effects of key impurities (Al, Fe, P, Si and Na) on the precipitation process of vanadium in the novel ultrasound-assisted precipitation system. Hydrometallurgy 2024, 224, 106233. [Google Scholar] [CrossRef]
- Arsic, M.; Teasdale, P.R.; Welsh, D.T.; Johnston, S.G.; Burton, E.D.; Hockmann, K.; Bennett, W.W. Diffusive gradients in thin films reveals differences in antimony and arsenic mobility in a contaminated wetland sediment during an oxic-anoxic transition. Environ. Sci. Technol. 2018, 52, 1118–1127. [Google Scholar] [CrossRef]
- Takaya, Y.; Kadokura, M.; Kato, T.; Tokoro, C. Removal mechanisms of arsenite by coprecipitation with ferrihydrite. J. Environ. Chem. Eng. 2021, 9, 105819. [Google Scholar] [CrossRef]
- Qi, P.; Wang, Y.; Zeng, J.; Sui, K.; Zhao, J. Progress in antimony capturing by superior materials: Mechanisms, properties and perspectives. Chem. Eng. J. 2021, 419, 130013. [Google Scholar] [CrossRef]
- Boreiko, C.J.; Hendriks, G.; Derr, R.; Huppert, M.; Rossman, T.G. Mode of action assessment of the genotoxic properties of antimony and its compounds evaluated in the ToxTracker assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2021, 865, 503333. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Bao, S.X.; Chen, B.; Zhang, Y.M.; Ren, L.Y.; Xin, C.F.; Ding, W.; Yang, S.Y.; Zhang, W.C. A comprehensive review on the ultrasound-enhanced leaching recovery of valuable metals: Applications, mechanisms and prospects. Ultrason. Sonochem. 2023, 98, 106525. [Google Scholar] [CrossRef]
- Burton, E.D.; Hockmann, K.; Karimian, N.; Johnston, S.G. Antimony mobility in reducing environments: The effect of microbial iron (III)-reduction and associated secondary mineralization. Geochim. Cosmochim. Acta 2019, 245, 278–289. [Google Scholar] [CrossRef]
- Pallud, C.; Masue-Slowey, Y.; Fendorf, S. Aggregate-scale spatial heterogeneity in reductive transformation of ferrihydrite resulting from coupled biogeochemical and physical processes. Geochim. Cosmochim. Acta 2010, 74, 2811–2825. [Google Scholar] [CrossRef]
- Mao, W.J.; Wang, D.J.; Wu, P.; Zhu, J.; Liao, P.; Lai, K.D.; Ding, Z.H.; Zhang, Y.Q.; He, Z.X.; Zheng, R.Y.; et al. Co-transport of ferrihydrite–organic matter colloids with Sb (V) in saturated porous media: Implications for antimony mobility. Environ. Sci. Nano 2024, 11, 1462–1476. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.X.; Zhang, Y.M.; Xiao, J.H. Efficient selective extraction of scandium from red mud. Miner. Process. Extr. Metall. Rev. 2023, 44, 304–312. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Antimony and arsenic partitioning during Fe2+-induced transformation of jarosite under acidic conditions. Chemosphere 2018, 195, 515–523. [Google Scholar] [CrossRef]
- Namayandeh, A.; Zhang, W.; Watson, S.K.; Borkiewicz, O.J.; Bompoti, N.M.; Chrysochoou, M.; Penn, R.L.; Michel, F.M. Goethite and Hematite Nucleation and Growth from Ferrihydrite: Effects of Oxyanion Surface Complexes. Environ. Sci. Technol. 2024, 58, 5952–5962. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Johnston, S.G.; Planer-Friedrich, B. Coupling of arsenic mobility to sulfur transformations during microbial sulfate reduction in the presence and absence of humic acid. Chem. Geol. 2013, 343, 12–24. [Google Scholar] [CrossRef]
- Karimian, N.; Burton, E.D.; Johnston, S.G.; Hockmann, K.; Choppala, G. Humic acid impacts antimony partitioning and speciation during iron (II)-induced ferrihydrite transformation. Sci. Total Environ. 2019, 683, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, Y.; Li, W.; Zhan, X.H.; Wang, H.; Shi, C.C.; Sun, Y.P.; Shi, H.X. Removal of Sb (V) from wastewater via siliceous ferrihydrite: Interactions among ferrihydrite, coprecipitated Si, and adsorbed Sb (V). Chemosphere 2022, 291, 133043. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From Classic to Cutting-Edge Solutions: A Comprehensive Review of Materials and Methods for Heavy Metal Removal from Water Bodies. Desalination Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Sharma, P.; Rolle, M.; Kocar, B.; Fendorf, S.; Kappler, A. Influence of natural organic matter on As transport and retention. Environ. Sci. Technol. 2011, 45, 546–553. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Shi, L.; Gong, X. Release of adsorbed copper and carbon during Fe (II) catalytic conversion of ferrihydrite-humic acid coprecipitation under acidic condition: Mechanism and properties. J. Environ. Chem. Eng. 2023, 11, 109519. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, S.; Liu, F.; Liang, Y.; Shi, Z. Effects of humic acid and fulvic acid on the sequestration of copper and carbon during the iron oxide transformation. Chem. Eng. J. 2020, 383, 123194. [Google Scholar] [CrossRef]
- Du, H.; Huang, Q.; Lei, M.; Tie, B. Sorption of Pb (II) by nanosized ferrihydrite organo-mineral composites formed by adsorption versus coprecipitation. ACS Earth Space Chem. 2018, 2, 556–564. [Google Scholar] [CrossRef]
- Sun, Y.C.; Bai, D.X.; Lu, L.; Li, Z.Y.; Zhang, B.; Liu, Y.F.; Zhuang, L.L.; Yang, T.; Chen, T. The potential of ferrihydrite-synthetic humic-like acid composite to remove metal ions from contaminated water: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130771. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A.; Mitchell, D.R.G. Reductive transformation of iron and sulfur in schwertmannite-rich accumulations associated with acidified coastal lowlands. Geochim. Cosmochim. Acta 2007, 71, 4456–4473. [Google Scholar] [CrossRef]
- Hockmann, K.; Karimian, N.; Schlagenhauff, S.; Planer-Friedrich, B.; Burton, E.D. Impact of antimony (V) on iron (II)-catalyzed ferrihydrite transformation pathways: A novel mineral switch for feroxyhyte formation. Environ. Sci. Technol. 2021, 55, 4954–4963. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Byler, D.M.; Gerasimowicz, W.V.; Susi, H.; Schnitzer, M. FT-IR spectra of soil constituents: Fulvic acid and fulvic acid complex with ferric ions. Appl. Spectrosc. 1987, 41, 1428–1430. [Google Scholar] [CrossRef]
- Dreier, L.B.; Bonn, M.; Backus, E.H. Hydration and orientation of carbonyl groups in oppositely charged lipid monolayers on water. J. Phys. Chem. B 2019, 123, 1085–1089. [Google Scholar] [CrossRef]
- Wang, H.; Tsang, Y.F.; Wang, Y.N.; Sun, Y.J.; Zhang, D.Y.; Pan, X.L. Adsorption capacities of poorly crystalline Fe minerals for antimonate and arsenate removal from water: Adsorption properties and effects of environmental and chemical conditions. Clean Technol. Environ. Policy 2018, 20, 2169–2179. [Google Scholar] [CrossRef]
- Su, W.; Kumar, N.; Krayev, A.; Chaigneau, M. In situ topographical chemical and electrical imaging of carboxyl graphene oxide at the nanoscale. Nat. Commun. 2018, 9, 2891. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, Z.; Shi, M.; Yao, L.; Fan, T.; Wang, Z. Effect of humic acid on the stabilization of cadmium in soil by coprecipitating with ferrihydrite. Environ. Sci. Pollut. Res. 2019, 26, 27330–27337. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.C.; Hiemstra, T. Surface area of ferrihydrite consistently related to primary surface charge, ion pair formation, and specific ion adsorption. Chem. Geol. 2020, 532, 119304. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Zheng, Y.; Yin, M.; Wang, J.; Bolan, N.; Fan, F.; Yun, Z.; Zhou, C.; Yin, H. The fate of Sb (V) and As (V) during the aging of ferrihydrite. Chem. Eng. J. 2024, 479, 147671. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.S.; Bollinger, J.C.; Chao, H.P. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Gai, S.; Liu, B.; Lan, Y.; Han, L.; Hu, Y.; Dongye, G.; Cheng, K.; Liu, Z.; Yang, F. Artificial humic acid coated ferrihydrite strengthens the adsorption of phosphate and increases soil phosphate retention. Sci. Total Environ. 2024, 915, 169870. [Google Scholar] [CrossRef]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; de Souza, A.A.U.; de Souza, S.M.d.A.G.U.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Song, C.; Shen, Z.; Wang, T.; Yang, K.; Miao, H.; Yang, J.; Wang, J.; Xu, X. Novel insight into the competitive adsorption behaviors of As (V), Sb (V), and P (V) on {1 1 0} facets of Goethite: Existing form and coordination structure affinity. Chem. Eng. J. 2024, 479, 147677. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Li, P.; Li, G.; Jiang, T. Comparative study on the adsorption interactions of humic acid onto natural magnetite, hematite and quartz: Effect of initial HA concentration. Powder Technol. 2014, 251, 1–8. [Google Scholar] [CrossRef]
- Hui, W.; Jun, Z.; Fu, Q.L.; Xiong, J.W.; Can, H.; Hu, H.Q.; Violante, A. Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 2015, 25, 405–414. [Google Scholar]
- Yu, G.; Fu, F.; Ye, C.; Tang, B. Behaviors and fate of adsorbed Cr (VI) during Fe (II)-induced transformation of ferrihydrite-humic acid co-precipitates. J. Hazard. Mater. 2020, 392, 122272. [Google Scholar] [CrossRef]
- Ye, C.; Ariya, P.A.; Fu, F.; Yu, G.; Tang, B. Influence of Al (III) and Sb (V) on the transformation of ferrihydrite nanoparticles: Interaction among ferrihydrite, coprecipitated Al (III) and Sb (V). J. Hazard. Mater. 2021, 408, 124423. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Zhou, J.S.; Lou, Z.M.; Zhou, X.R.; Liu, Y.L.; Li, Y.Z.; Baig, S.A.; Xu, X.H. Removal of Sb (V) from aqueous solutions using Fe-Mn binary oxides: The influence of iron oxides forms and the role of manganese oxides. Chem. Eng. J. 2018, 354, 577–588. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Takahashi, Y.; Terada, Y.; Sakata, M. Antimony (V) incorporation into synthetic ferrihydrite, goethite, and natural iron oxyhydroxides. Environ. Sci. Technol. 2010, 44, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Bao, S.X.; Zhang, Y.M.; Ren, L.Y.; Xin, C.F.; Chen, B.; Liu, B.; Xiao, J.H.; Hou, X.C. Stepwise Recycling of Valuable Metals from Spent Lithium-ion Batteries Based on In-situ Thermal Reduction and Ultrasonic-assisted Water Leaching. Green Chem. 2023, 25, 6652–6665. [Google Scholar] [CrossRef]
- Rong, Q.; Nong, X.; Zhang, C.; Zhong, K.; Zhao, H. Immobilization mechanism of antimony by applying zirconium-manganese oxide in soil. Sci. Total Environ. 2022, 823, 153435. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ma, X.M.; Zhou, H.B.; Ma, J.X.; Ding, Y.X.; He, D. Chelated heavy metals removal by in-situ formed Fe (II) and Fe (III) iron (oxy) hydroxides: Mechanism and performance. J. Environ. Chem. Eng. 2023, 11, 110531. [Google Scholar] [CrossRef]
- Bao, Y.; Lai, J.; Wang, Y.; Fang, Z.; Su, Y.; Alessi, D.S.; Bolan, N.S.; Wu, X.; Zhang, Y.; Jiang, X. Effect of fulvic acid co-precipitation on biosynthesis of Fe (III) hydroxysulfate and its adsorption of lead. Environ. Pollut. 2022, 295, 118669. [Google Scholar] [CrossRef]
- Senn, A.C.; Kaegi, R.; Hug, S.J.; Hering, J.G.; Mangold, S.; Voegelin, A. Composition and structure of Fe(III)-precipitates formed by Fe (II) oxidation in water at near-neutral pH: Interdependent effects of phosphate, silicate and Ca. Geochim. Cosmochim. Acta 2015, 162, 220–246. [Google Scholar] [CrossRef]
- Rahnemaie, R.; Hiemstra, T.; van Riemsdijk, W.H. Inner-and outer-sphere complexation of ions at the goethite–solution interface. J. Colloid Interface Sci. 2006, 297, 379–388. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Xiang, H.; Wu, J.; Yan, X.; Zhang, W.; Lin, Z.; Chai, L. Unveiling the crucial role of iron mineral phase transformation in antimony (V) elimination from natural water. Eco-Environ. Health 2023, 2, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Namayandeh, A.; Borkiewicz, O.J.; Bompoti, N.M.; Chrysochoou, M.; Michel, F.M. Oxyanion surface complexes control the kinetics and pathway of ferrihydrite transformation to goethite and hematite. Environ. Sci. Technol. 2022, 56, 15672–15684. [Google Scholar] [CrossRef]
- Lin, W.; Peng, L.; Li, H.; Xiao, T.; Wang, J.; Wang, N.; Zhang, X.; Zhang, H. Antimony (V) behavior during the Fe (II)-induced transformation of Sb (V)-bearing natural multicomponent secondary iron mineral under acidic conditions. Sci. Total Environ. 2024, 912, 169592. [Google Scholar] [CrossRef]
- Shi, M.Q.; Min, X.B.; Ke, Y.; Lin, Z.; Yang, Z.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.H.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr) oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Z.; Zhi, L.; Zhou, S. Binding of Hg to preformed ferrihydrite-humic acid composites synthesized via co-precipitation and adsorption with different morphologies. Ecotoxicol. Environ. Saf. 2020, 204, 111097. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Bao, S.X.; Zhang, Y.M.; Xin, C.F.; Chen, B.; Li, J.; Liu, B.; Xia, Y.F.; Hou, X.C.; Xu, K.H. Sustainable regeneration of high-performance cathode materials from spent lithium-ion batteries through magnetic separation and coprecipitation. J. Clean. Prod. 2024, 438, 140798. [Google Scholar] [CrossRef]

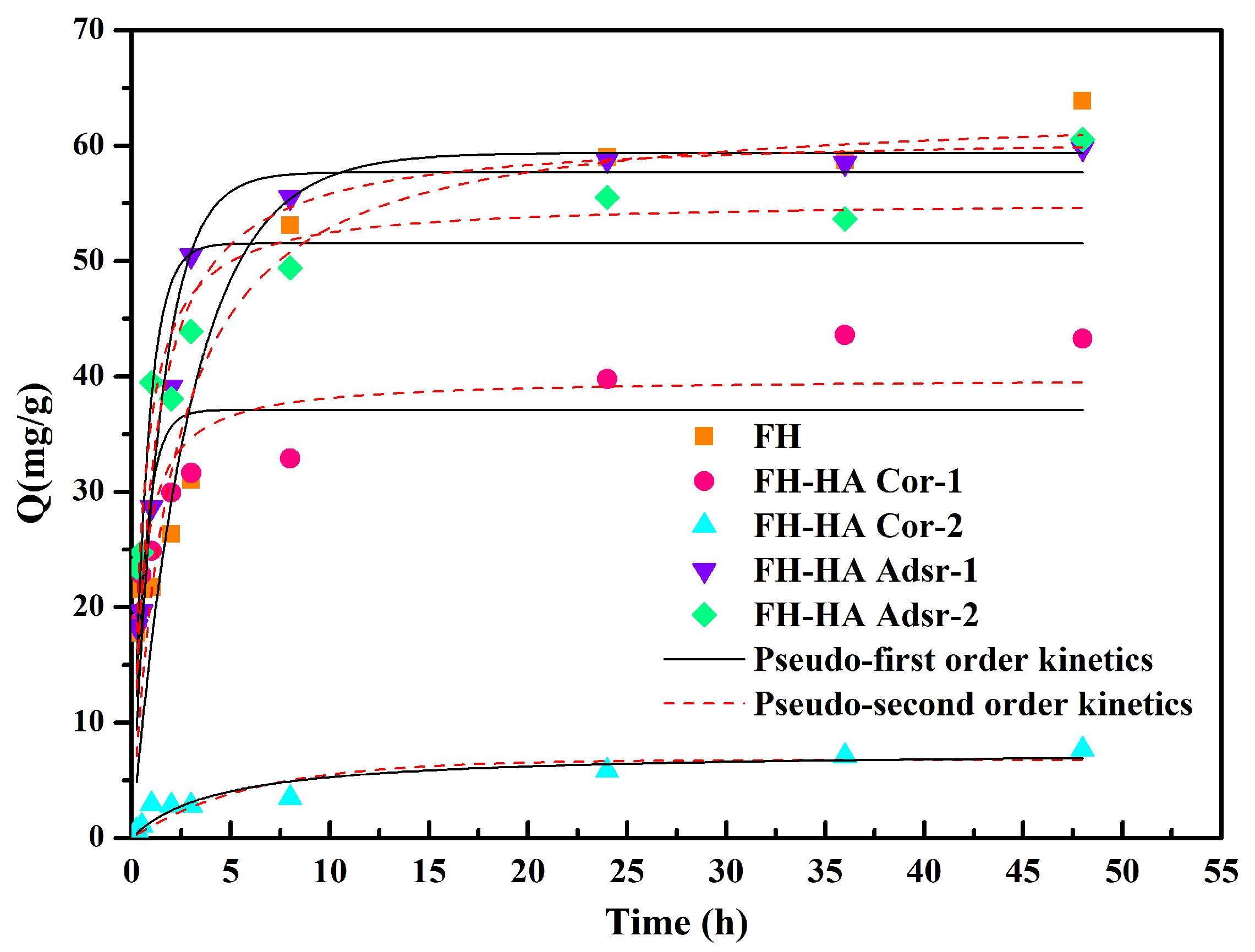


| Adsorbent | PFO Kinetics | PSO Kinetics | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | K1 | R2 | qe (mg/g) | K2 | R2 | |
| FH | 59.36 | 0.34 | 0.853 | 63.45 | 0.0079 | 0.900 |
| FH-HA Cor-1 | 37.09 | 1.62 | 0.640 | 39.84 | 0.056 | 0.843 |
| FH-HA Cor-2 | 6.77 | 0.16 | 0.824 | 7.53 | 0.031 | 0.884 |
| FH-HA Adsr-1 | 57.70 | 0.71 | 0.950 | 61.03 | 0.018 | 0.973 |
| FH-HA Adsr-2 | 51.55 | 1.34 | 0.760 | 55.20 | 0.035 | 0.903 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q (mg/g) | K (L/mg) | R2 | Kf (mg1−n·Ln/g) | n | R2 | |
| FH | 84.45 | 0.99 | 0.903 | 30.07 | 3.73 | 0.892 |
| FH-HA Cor-1 | 31.47 | 0.37 | 0.948 | 11.27 | 4.05 | 0.896 |
| FH-HA Cor-2 | 1.73 | 1.95 | 0.862 | 0.944 | 5.84 | 0.755 |
| FH-HA Adsr-1 | 69.55 | 0.44 | 0.989 | 23.26 | 3.79 | 0.932 |
| FH-HA Adsr-2 | 66.17 | 0.60 | 0.984 | 22.79 | 3.62 | 0.926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Bao, S.; Zhang, Y.; Chen, B.; Wang, Z. Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes. Materials 2024, 17, 4172. https://doi.org/10.3390/ma17174172
Ding W, Bao S, Zhang Y, Chen B, Wang Z. Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes. Materials. 2024; 17(17):4172. https://doi.org/10.3390/ma17174172
Chicago/Turabian StyleDing, Wei, Shenxu Bao, Yimin Zhang, Bo Chen, and Zhanhao Wang. 2024. "Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes" Materials 17, no. 17: 4172. https://doi.org/10.3390/ma17174172
APA StyleDing, W., Bao, S., Zhang, Y., Chen, B., & Wang, Z. (2024). Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes. Materials, 17(17), 4172. https://doi.org/10.3390/ma17174172








