Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Anodizing Conditions of Ti G4
2.3. FE-SEM Measurements
2.4. ATR-FTIR Measurements
2.5. In Vitro Corrosion Resistance in Artificial Saliva Solution
2.6. Gentamicin Sulfate Loading and Release from Oxide Nantubes on Ti G4
3. Results and Discussion
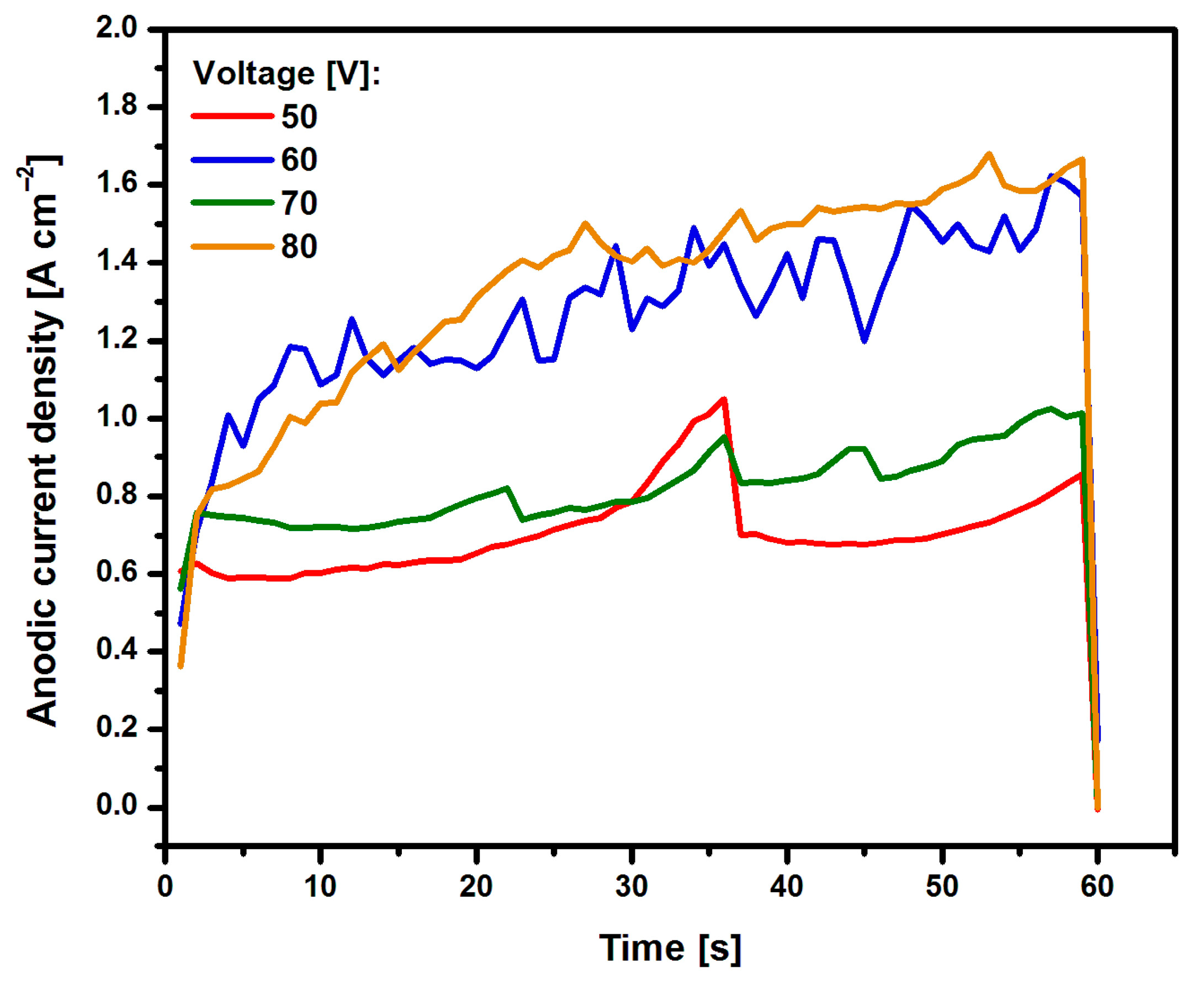
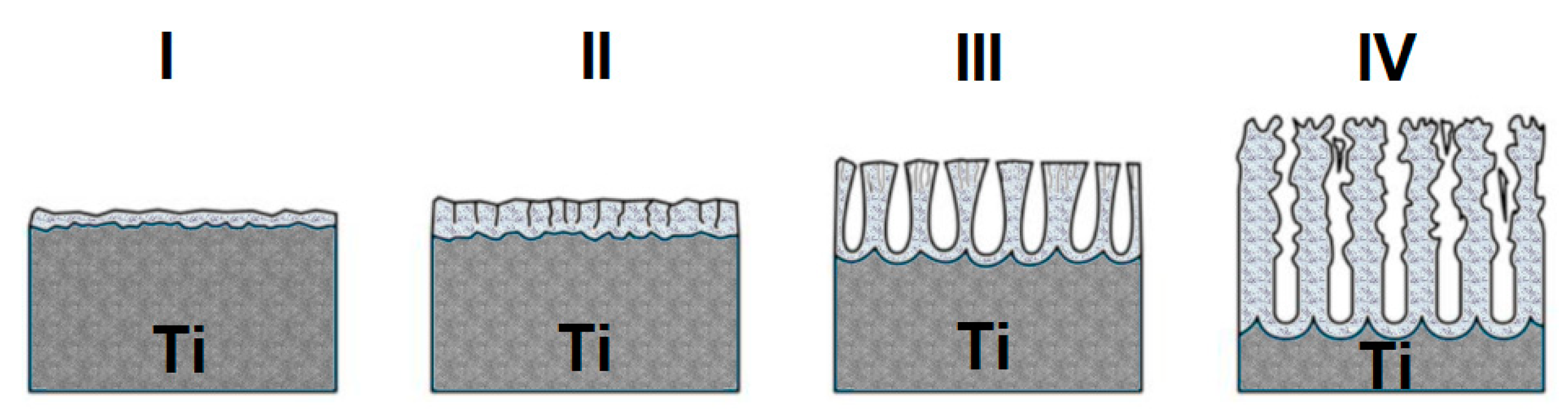
3.1. Formation of Oxide Nanotubes on Ti G4
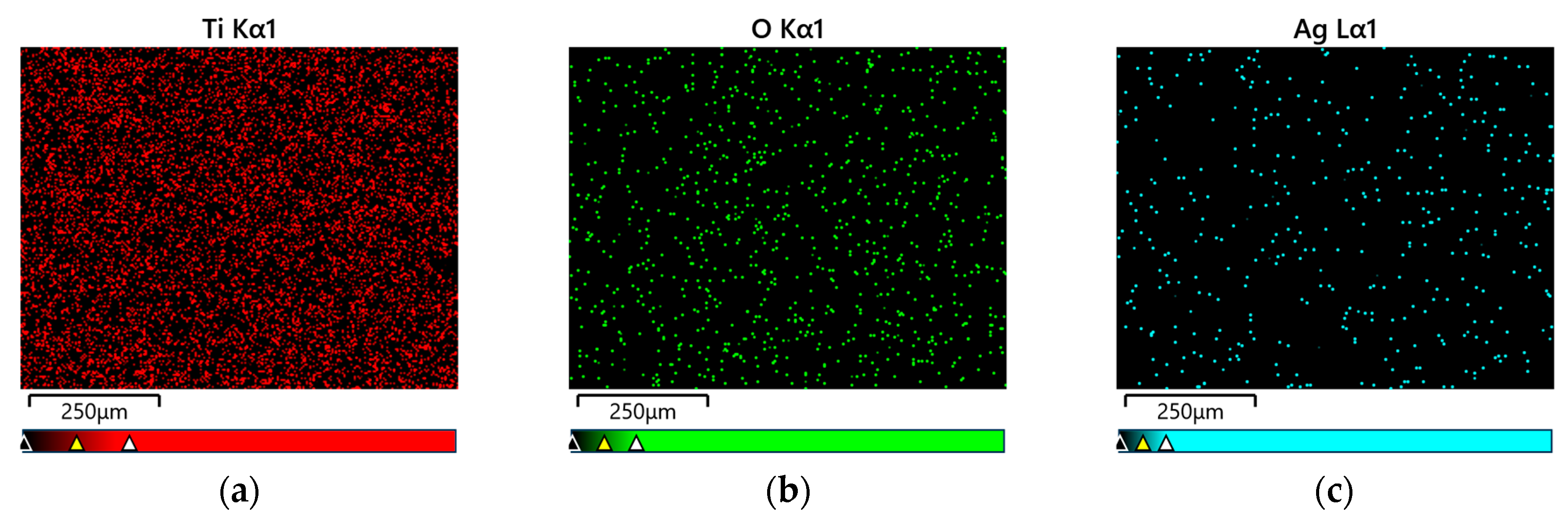
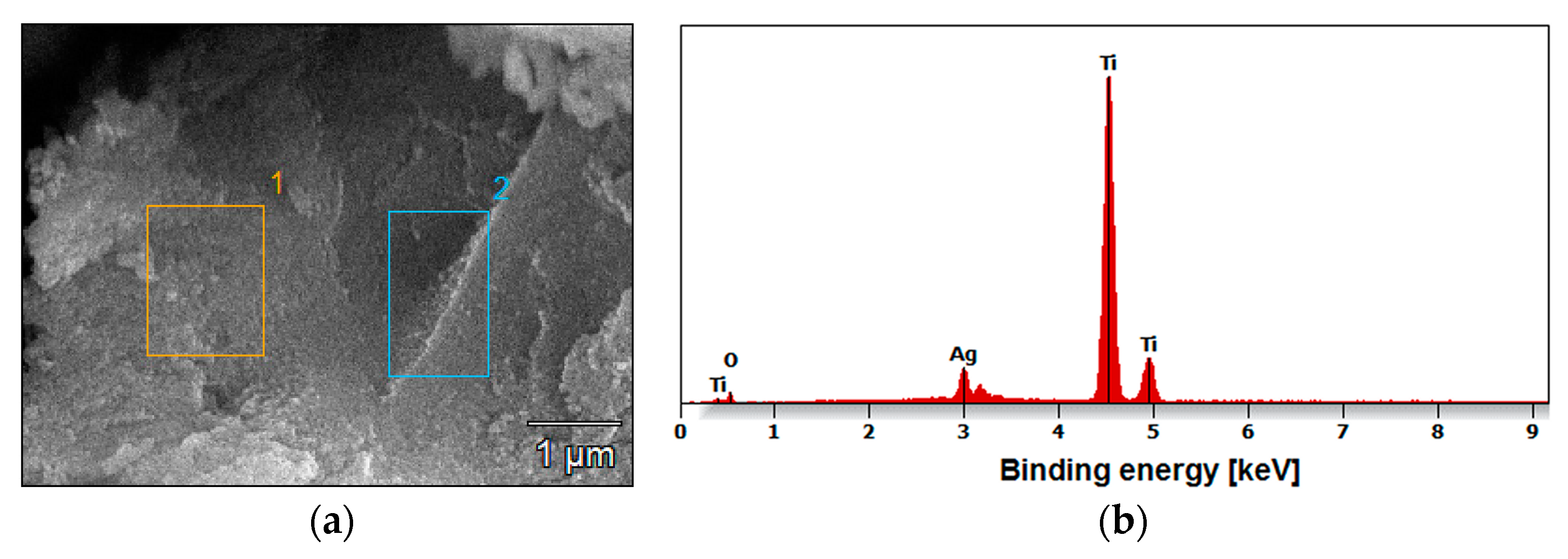
3.2. FE-SEM/EDS Characterization of Oxide Nanutubes on TiG4
3.3. Assessment of In Vitro Corrosion Resistance in Artificial Saliva Solution
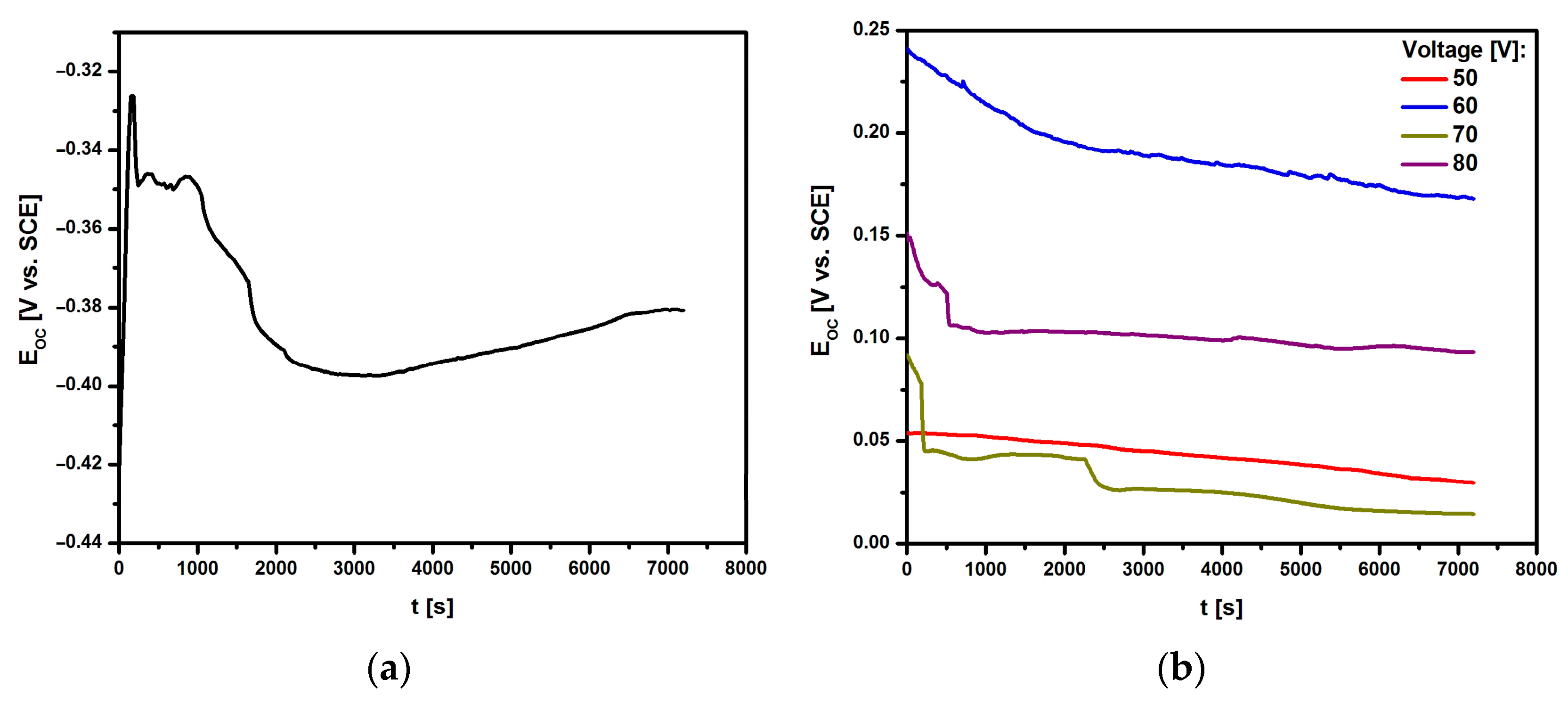
3.3.1. Open Circuit Potential Study
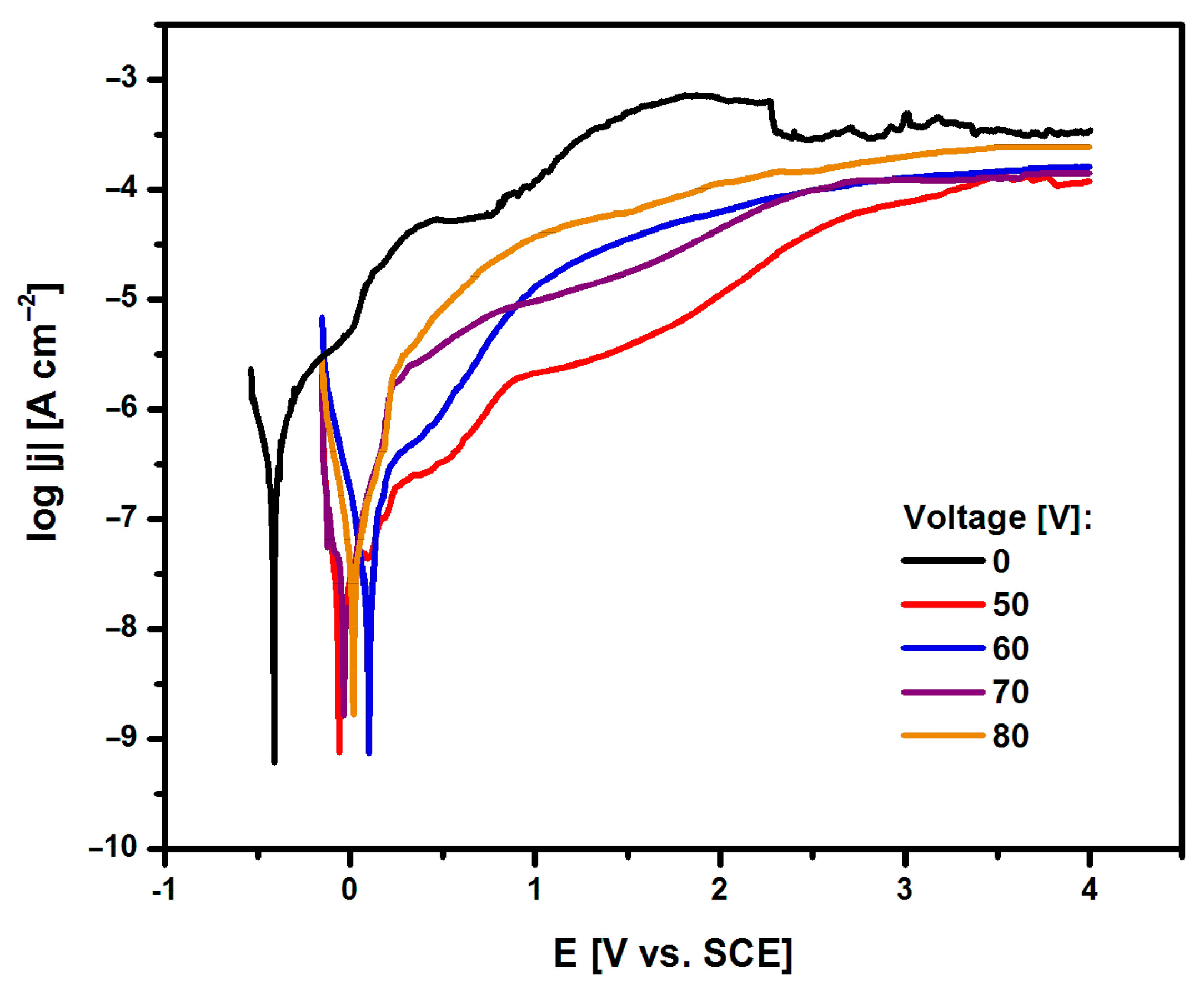
3.3.2. Anodic Polarization Curves Study
3.4. Assessment of Oxide Nanotube Layers on Ti G4 as Drug Carriers
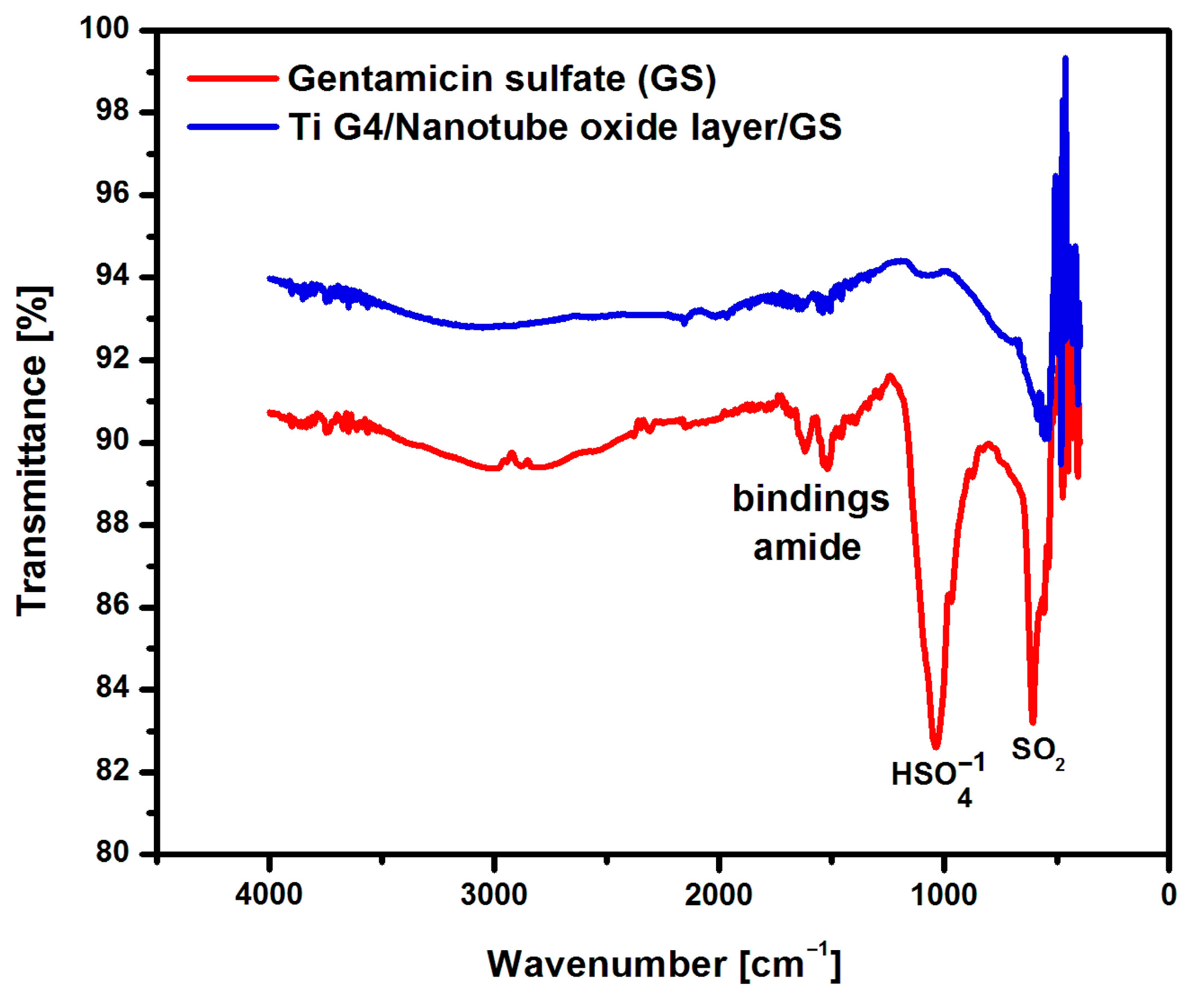
3.4.1. ATR-FTIR Characterization of Anodized Ti G4 after GS Implementation
3.4.2. Release Kinetics of Gentamicin Sulfate from Oxide Nanotube Layers on Ti G4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in Titanium Dental Implants/Prostheses—A Review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A., Jr. Biomaterial Properties of Titanium in Dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Batool, S.A.; Salman Maqbool, M.; Javed, M.A.; Niaz, A.; Rehman, M.A.U. A Review on the Fabrication and Characterization of Titania Nanotubes Obtained via Electrochemical Anodization. Surfaces 2022, 5, 456–480. [Google Scholar] [CrossRef]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-Organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.B.; Thirunavukkarasu, G.K.; Liapun, V.; Makarov, H.; Gregor, M.; Roch, T.; Plecenik, T.; Hensel, K.; Sihor, M.; Monfort, O.; et al. Fluoride-Free Synthesis of Anodic TiO2 Nanotube Layers: A Promising Environmentally Friendly Method for Efficient Photocatalysts. Nanoscale 2022, 14, 11703–11709. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Grimes, C.A. Formation of Vertically Oriented TiO2 Nanotube Arrays Using a Fluoride Free HCl Aqueous Electrolyte. J. Phys. Chem. C 2007, 111, 13028–13032. [Google Scholar] [CrossRef]
- Allam, N.; Shankar, K.; Grimes, C. Photoelectrochemical and Water Photoelectrolysis Properties of Ordered TiO2 Nanotubes Fabricated by Ti Anodization in Fluoride-Free HCl Electrolytes. J. Mater. Chem. 2008, 18, 2341–2348. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lai, C.W.; Lockman, Z. Extremely Fast Growth Rate of TiO2 Nanotube Arrays in Electrochemical Bath Containing H2O2. J. Electrochem. Soc. 2011, 158, C397. [Google Scholar] [CrossRef]
- Abramo, F.; Luca, F.; Passalacqua, R.; Centi, G.; Giorgianni, G.; Perathoner, S.; Abate, S. Electrocatalytic Production of Glycolic Acid via Oxalic Acid Reduction on Titania Debris Supported on a TiO2 Nanotube Array. J. Energy Chem. 2021, 68, 669–678. [Google Scholar] [CrossRef]
- Moseke, C.; Lehmann, C.; Schmitz, T.; Reinert, F.; Groll, J.; Gbureck, U. Nanostructuring of Refractory Metal Surfaces by Electrochemical Oxidation: Nb and the Binary Systems Ti-Ta and Nb-Ta. Curr. Nanosci. 2013, 9, 132–138. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, J.; Dan, Y.; Sun, M.; Gong, T.; Li, X.; Zhu, X. Growth of Porous Anodic TiO2 in Silver Nitrate Solution without Fluoride: Evidence against the Field-Assisted Dissolution Reactions of Fluoride Ions. Electrochem. Commun. 2021, 126, 107022. [Google Scholar] [CrossRef]
- Wtulich, M.; Szkoda, M.; Gajowiec, G.; Jurak, K.; Trykowski, G.; Lisowska-Oleksiak, A. Hydrothermal Modification of TiO2 Nanotubes in Water and Alkali Metal Electrolytes (LiNO3, NaNO3, KNO3)—Direct Evidence for Photocatalytic Activity Enhancement. Electrochim. Acta 2022, 426, 140802. [Google Scholar] [CrossRef]
- Nandal, S.; Ghalaut, P.; Shekhawat, H.; Nagar, P. Osseointegration in Dental Implants: A Literature Review. Med. Sci. 2014, 4, 411–413. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Baradaran, S.; Amiri, A.; Saber-Samandari, S.; Khanahmadi, S.; Zeimaran, E.; Basirun, W.J.; Kalaiselvam, K.; et al. Simultaneous Enhanced Antibacterial and Osteoblast Cytocompatibility Performance of Ti6Al7Nb Implant by Nano-Silver/Graphene Oxide Decorated Mixed Oxide Nanotube Composite. Surf. Coat. Technol. 2019, 360, 181–195. [Google Scholar] [CrossRef]
- Cheng, L.; Naibijiang, N.; Hasenbai, A.; Dong, H.; He, H. Bacteriostatic Effects of Nanometer Silver Disinfectant on the Biofilms in Dental Unit Water Lines. J. Dent. Sci. 2021, 16, 327–332. [Google Scholar] [CrossRef]
- Smith, M.V. Textbook of Rabbit Medicine, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; pp. 100–137. [Google Scholar] [CrossRef]
- ISO 5832-2:2018-08; Implants for Surgery–Metallic Materials–Part 2: Unalloyed Titanium. ISO: Geneve, Switzerland, 2018.
- ASTM F67-13; Standard Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700). ASTM: West Conshohocken, PA, USA, 2017.
- ISO 10271:2021-02; Dentistry–Corrosion Test Methods for Metallic Materials. ISO: Geneva, Switzerland, 2021.
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical formation of self-organized nanotubular oxide layers on Ti13Zr13Nb alloy for biomedical applications. Acta Phys. Pol. A 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Song, Y.; Jia, H.; Yu, H.; Xiao, X.; Yang, X. Oxygen Bubble Mould Effect: Serrated Nanopore Formation and Porous Alumina Growth. Monatshefte Für Chem. Chem. Mon. 2008, 139, 999–1003. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering Biocompatible Implant Surfaces: Part I: Materials and Surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef]
- Hang, R.; Zhao, F.; Yao, X.; Tang, B.; Chu, P.K. Self-Assembled Anodization of NiTi Alloys for Biomedical Applications. Appl. Surf. Sci. 2020, 517, 146118. [Google Scholar] [CrossRef]
- Wang, K.; Liu, G.; Hoivik, N.; Johannessen, E.; Jakobsen, H. Electrochemical Engineering of Hollow Nanoarchitectures: Pulse/Step Anodization (Si, Al, Ti) and Their Applications. Chem. Soc. Rev. 2014, 43, 1476–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-F.; Song, Y.; Liu, L.; Wang, C.-Y.; Zheng, J.; Jia, H.-B.; Wang, X.-L. Electronic Currents and the Formation of Nanopores in Porous Anodic Alumina. Nanotechnology 2009, 20, 475303. [Google Scholar] [CrossRef] [PubMed]
- Albella, J.M.; Montero, I.; Martinez-Duart, J.M. A Theory of Avalanche Breakdown during Anodic Oxidation. Electrochim. Acta 1987, 32, 255–258. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Janusas, G.; Palevicius, A.; Vilkauskas, A. Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method. Sensors 2020, 20, 3833. [Google Scholar] [CrossRef] [PubMed]
- Zaraska, L.; Brudzisz, A.; Wierzbicka, E.; Sulka, G.D. The Effect of Electrolyte Change on the Morphology and Degree of Nanopore Order of Porous Alumina Formed by Two-Step Anodization. Electrochim. Acta 2016, 198, 259–267. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Thick Self-Organized Porous Zirconium Oxide Formed in H2SO4/NH4F Electrolytes. Electrochem. Commun. 2004, 6, 1131–1134. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.; Hossain, A.; Ahmed, M.S.; Rana, M.M.; Sultana, S. Synthesis and Characterization of Alocasia Indica Infused Silver Nanoparticles for Dental Implant Applications. Chem. Phys. Impact 2023, 6, 100239. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.L.; Tang, J.; Chu, C.H. Developing Biocompatible Silver Nanoparticles Using Epigallocatechin Gallate for Dental Use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential Genotoxicity Mechanisms of Silver Nanoparticles and Silver Ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef]
- Wassilkowska, A.; Czaplicka, A.; Zielina, M.; Bielski, A. An Analysis of the Elemental Composition of Micro-Samples Using EDS Technique. Czas. Tech. 2014, 18, 133–148. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of Autoclaving Time on Corrosion Resistance of Sandblasted Ti G4 in Artificial Saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Ratajczak, P.; Łosiewicz, B. Long-Term Assessment of the In Vitro Corrosion Resistance of Biomimetic ACP Coatings Electrodeposited from an Acetate Bath. J. Funct. Biomater. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Smołka, A.; Dercz, G.; Rodak, K.; Łosiewicz, B. Evaluation of corrosion resistance of nanotubular oxide layers on the Ti13Zr13Nb alloy in physiological saline solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Łosiewicz, B.; Rak, J.; Simka, W. The influence of passivation type on corrosion resistance of Ti15Mo alloy in simulated body fluids. Arch. Metall. Mater. 2015, 60, 2687–2693. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.; Ramteke, P. Fabrication and Assessment of Gentamicin Loaded Electrospun Nanofibrous Scaffolds as a Quick Wound Healing Dressing Material. Curr. Nanosci. 2015, 11, 222–228. [Google Scholar] [CrossRef]
- Visan, A.; Popescu-Pelin, G.; Gherasim, O.; Grumezescu, V.; Socol, M.; Zgura, I.; Florica, C.; Popescu, R.C.; Savu, D.; Holban, A.; et al. Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite. Polymers 2019, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Lai, Y.-K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2016, 12, 151–165. [Google Scholar] [CrossRef]



| Voltage (V) | Element (wt.%) | ||
|---|---|---|---|
| O | Ti | Ag | |
| 50 | 35.8(2) | 62.8(4) | 1.4(2) |
| 60 | 38.1(1) | 61.5(2) | 1.4(1) |
| 70 | 25.0(7) | 73.0(3) | 1.9(1) |
| 80 | 30.9(2) | 66.9(1) | 2.2(5) |
| Voltage (V) | Amount of Gentamicin Sulfate Loaded (mg) |
|---|---|
| 50 | 200(16) |
| 60 | 175(14) |
| 70 | 147(12) |
| 80 | 116(9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osak, P.; Skwarek, S.; Łukowiec, D.; Przeliorz, G.; Łosiewicz, B. Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials 2024, 17, 3753. https://doi.org/10.3390/ma17153753
Osak P, Skwarek S, Łukowiec D, Przeliorz G, Łosiewicz B. Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials. 2024; 17(15):3753. https://doi.org/10.3390/ma17153753
Chicago/Turabian StyleOsak, Patrycja, Sandra Skwarek, Dariusz Łukowiec, Grzegorz Przeliorz, and Bożena Łosiewicz. 2024. "Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems" Materials 17, no. 15: 3753. https://doi.org/10.3390/ma17153753
APA StyleOsak, P., Skwarek, S., Łukowiec, D., Przeliorz, G., & Łosiewicz, B. (2024). Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials, 17(15), 3753. https://doi.org/10.3390/ma17153753






