Polyurethane Composites Recycling with Styrene–Acrylonitrile and Calcium Carbonate Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycolysis Reaction Process
2.3. Extraction and Purification Processes
2.4. Polyurethane Synthesis
2.5. Characterization Techniques
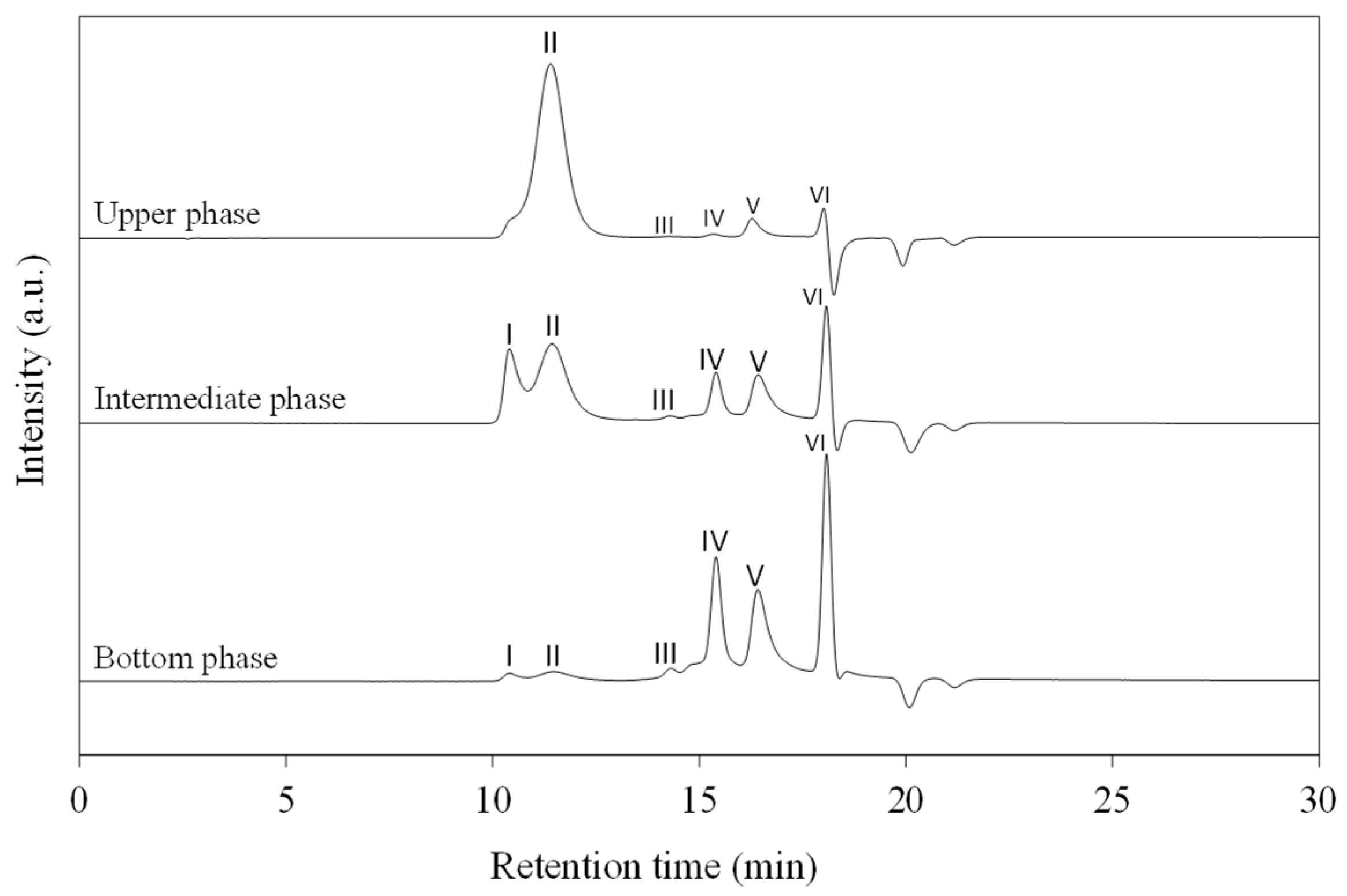
2.5.1. Molecular Weight and Product Composition Determination by Gel Permeation Chromatography (GPC)
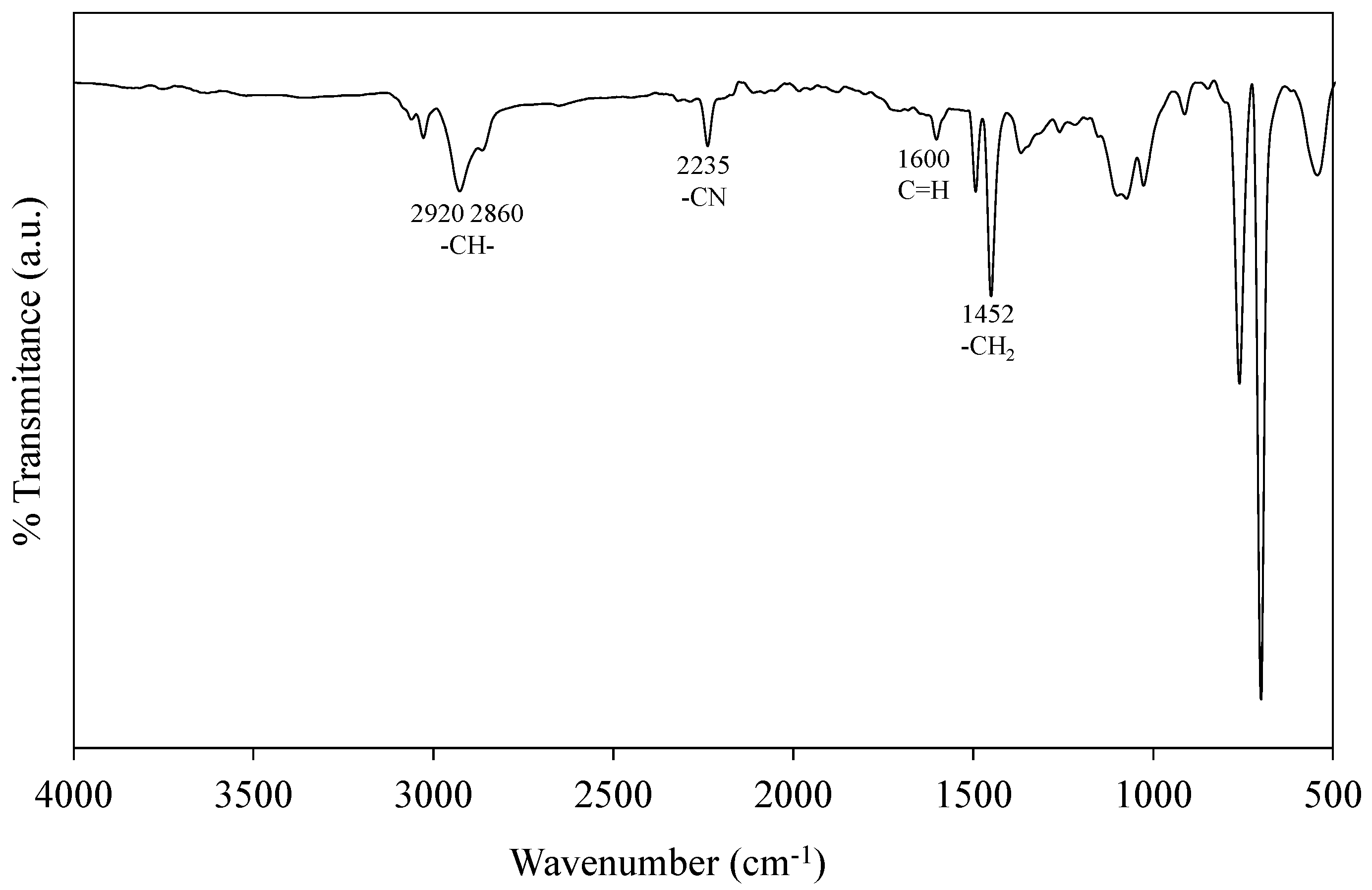
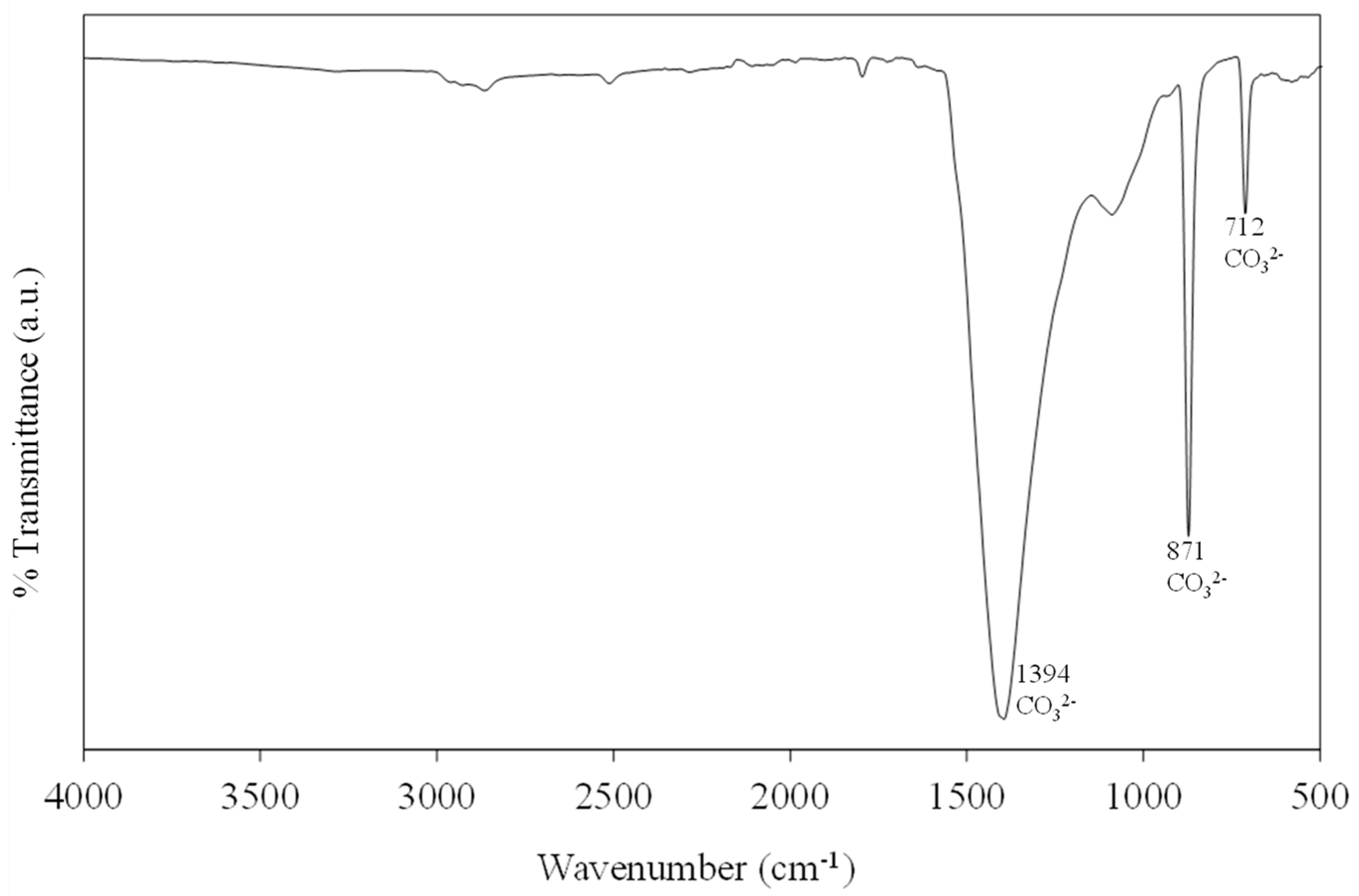
2.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.3. Measurement of Hydroxyl Index (iOH)
2.5.4. Viscosity
2.5.5. Water Content
2.5.6. X-ray Diffraction (XRD)
2.5.7. Foams Characterization
3. Results
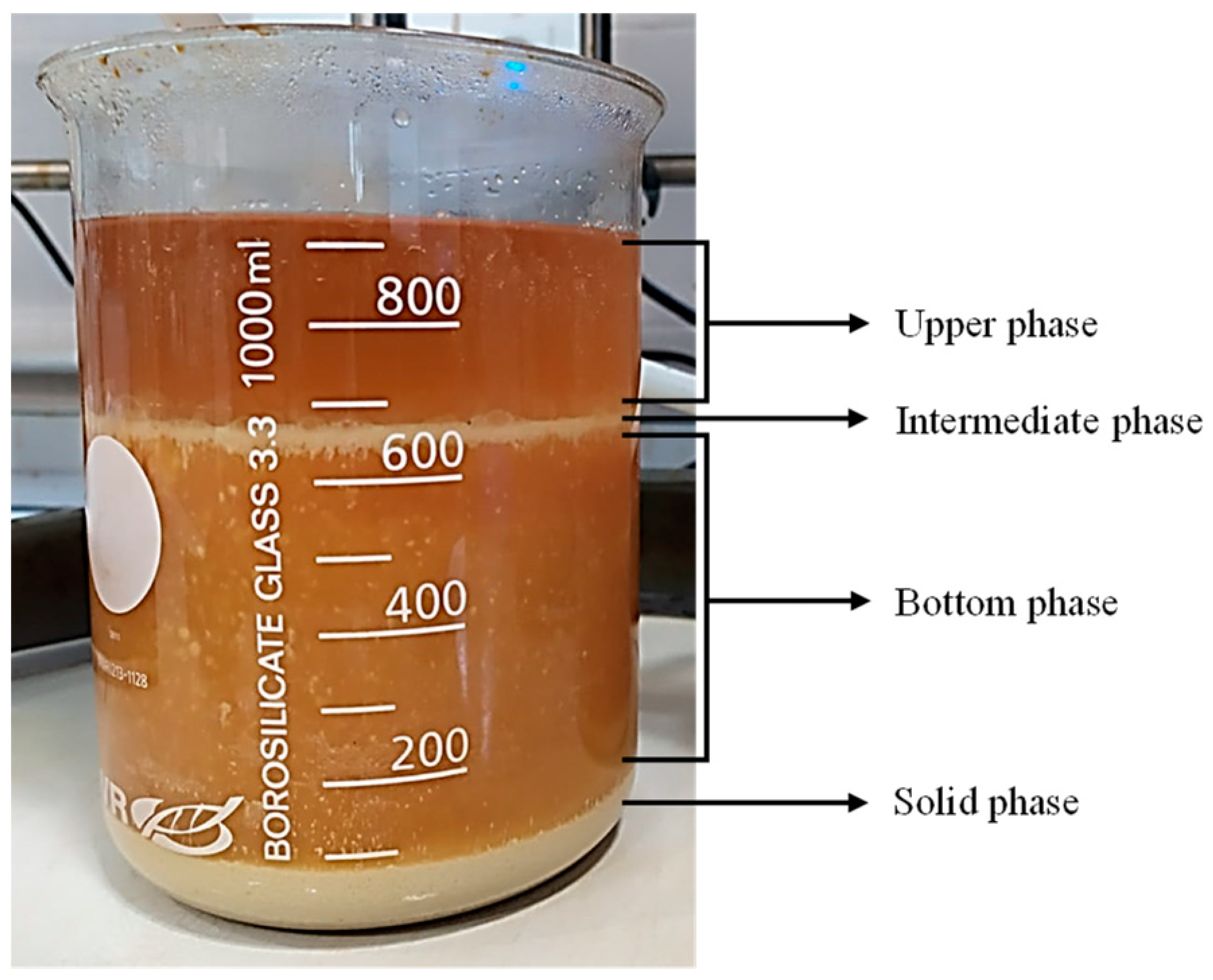
3.1. Glycolysis Process of Flexible PU Foams Containing CaCO3 and SAN as Fillers
3.2. Purification and Characterization of Recovered Polyol
3.3. Synthesis and Characterization of Flexible Polyurethane Foams Using the Recovered Polyol
3.4. Purification and Characterization of the Recovered Styrene–Acrylonitrile Copolymer
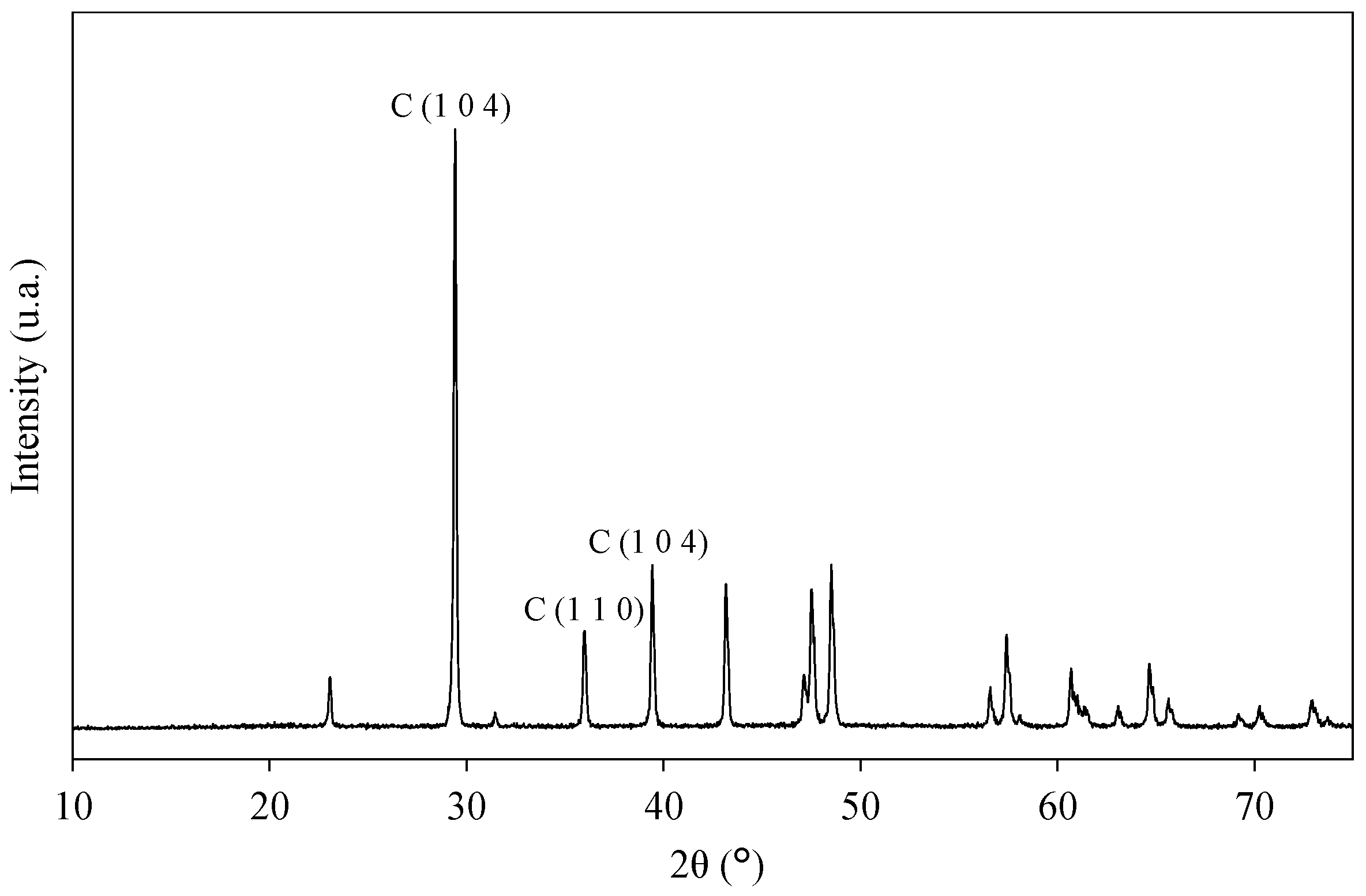
3.5. Purification and Characterization of Recovered Calcium Carbonate
3.6. Synthesis and Characterization of the Synthesized Foams Using Recovered Fillers
4. Conclusions
- -
- The use of DABCO as a catalyst and DEG as the glycolysis agent allowed us to achieve the glycolysis of flexible polyurethane foam waste composites in the split phase, recovering the styrene–acrylonitrile and calcium carbonate contained as fillers.
- -
- The use of DABCO as a catalyst allowed us to reduce the catalyst concentration and the mass ratio of polyurethane to glycol while maintaining the split phase to 0.1% and 1:1, respectively.
- -
- The recovered polyol presented higher purity than those reported in previous works, after which the purification of the upper phase was obtained with an approximate purity of 99% by GPC and a hydroxyl value of 48 mg KOH/g.
- -
- The applicability of the raffinate recovered polyol for the synthesis of polyurethane foams was demonstrated, but percentages from 50% by weight led to foams with a closed-cell structure and therefore with higher air resistance values.
- -
- The closed-cell structure was corrected by using a blend of TDI65 and TDI80 isocyanates, finding an optimized ratio of 70 and 30%, respectively.
- -
- Foams with up to 70% recovered polyol with all properties within the specification were synthesized using the optimized isocyanate blend.
- -
- Styrene–acrylonitrile and calcium carbonate, recovered from foam waste, were proved to be suitable for the synthesis of new polyurethane foams, with properties quite similar to the reference one for the case of CaCO3. However, in both cases, the foams presented a closed-cell structure, which could be corrected by the addition of TDI65 to TDI80.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, L. Market Volume of Polyurethane Worldwide from 2015 to 2025, with a Forecast for 2022 to 2029. 2022. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 21 May 2024).
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe, Plastics—The Facts 2021. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 21 May 2024).
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Gutiérrez, C.; Rodríguez, J.F. Sustainable polyurethanes: Chemical recycling to get it. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Javni, I.; Song, K.; Lin, J.; Petrovic, Z.S. Structure and properties of flexible polyurethane foams with nano- and micro-fillers. J. Cell. Plast. 2011, 47, 357–372. [Google Scholar] [CrossRef]
- Trzebiatowska, P.J.; Echart, A.S.; Correas, T.C.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coatings 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Hou, X.; Feng, G. La-Doped Sm2Zr2O7/PU-Coated Leather Composites with Enhanced Mechanical Properties and Highly Efficient Photocatalytic Performance. Materials 2024, 17, 1575. [Google Scholar] [CrossRef] [PubMed]
- Karamikamkar, S.; Abidli, A.; Tafreshi, O.A.; Ghaffari-Mosanenzadeh, S.; Buahom, P.; Naguib, H.E.; Park, C.B. Nanocomposite Aerogel Network Featuring High Surface Area and Superinsulation Properties. Chem. Mater. 2024, 36, 642–656. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Properties of rigid polyurethane-polyisocyanurate foams modified with the selected fillers. J. Appl. Polym. Sci. 2010, 115, 2460–2469. [Google Scholar] [CrossRef]
- Choe, H.; Lee, J.H.; Kim, J.H. Polyurethane composite foams including CaCO3 fillers for enhanced sound absorption and compression properties. Compos. Sci. Technol. 2020, 194, 108153. [Google Scholar] [CrossRef]
- Izarra, I.; Borreguero, A.M.; Garrido, I.; Rodriguez, J.F.; Carmona, M. Comparison of flexible polyurethane foams properties from different polymer polyether polyols. Polym. Test. 2021, 100, 107268. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Smithers Rapra Publishing: London, UK, 2005. [Google Scholar]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer Through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 22. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Molero, C.; Rodríguez, J.F. Novel polyol initiator from polyurethane recycling residue. J. Mater. Cycles Waste Manag. 2014, 16, 525–553. [Google Scholar] [CrossRef]
- Simón, D.; García, M.; de Lucas, A.; Borreguero, A.; Rodríguez, J. Glycolysis of flexible polyurethane wastes using stannous octoate as the catalyst: Study on the influence of reaction parameters. Polym. Degrad. Stab. 2013, 98, 144–149. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Glycolysis of flexible polyurethane wastes containing polymeric polyols. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- del Amo, J.; Borreguero, A.M.; Ramos, M.J.; Rodríguez, J.F. Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers 2021, 13, 1418. [Google Scholar] [CrossRef]
- AOCS Cd 13-60; AOCS Official Method, Official Methods and Recommended Practices of the American Oils Chemists Society. American Oil Chemists’ Society: Champaign, IL, USA, 2009.
- ASTM D4672-12; Standard Test Method for Polyurethane Raw Materials: Determination of Water Content of Polyols. ASTM: West Conshohocken, PA, USA, 2022.
- ISO 845; Cellular Plastics and Rubbers—Determination of Apparent Density. ISO: Geneve, Switzerland, 2006.
- ISO 9237; Determination of Permeability of Frabrics to Air. ISO: Geneve, Switzerland, 1995.
- ISO 3386/1; Polymeric Materials, Cellular Flexible—Determination of Stress-Strain Characteristics in Compression—Part 1: Low-Density Materials. ISO: Geneve, Switzerland, 2010.
- ISO 1856A; Flexible Cellular Polymeric Materials—Determination of Compression Set. ISO: Geneve, Switzerland, 2018.
- Bhabhe, M.D.; Athawale, V.D. Gel permeation chromatographic method for monitoring the transesterification reaction in a two-step chemoenzymatic synthesis of urethane oil based on vegetable oils. J. Chromatogr. A 1995, 718, 299–304. [Google Scholar] [CrossRef]
- Libretexst, Infrared Spectroscopy Absorption Table, Sonoma State University Saves Students Hard Cash with the Libretexts. 2013. Available online: https://chem.libretexts.org/ (accessed on 21 May 2024).
- Yasunaga, K.; Neff, R.A.; Zhang, X.D.; Macosko, C.W. Study of Cell Opening in Flexible Polyurethane Foam. J. Cell. Plast. 1996, 32, 427–448. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. ChemSusChem 2020, 13, 3835–3843. [Google Scholar] [CrossRef]
- Aneja, A.; Wilkes, G.L.; Rightor, E.G. Study of slabstock flexible polyurethane foams based on varied toluene diisocyanate isomer ratios. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 258–268. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhang, X.; Huang, H.; Chang, J.; Chen, H. A recycling model of excess toluene diisocyanate isomers in the preparation of polyurethane prepolymer. J. Appl. Polym. Sci. 2013, 127, 2176–2183. [Google Scholar] [CrossRef]
- Ono, K.; Erhard, A. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Brereton, G.; Emanuel, R.M., Jr.; Lomax, R.; Pennington, K.; Ryan, T.; Tebbe, H.; Timm, M.; Ware, P.; Winkler, K.; Yuan, T.; et al. Wussow, Polyurethanes. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2019; pp. 1–76. [Google Scholar] [CrossRef]
- Al-Harthi, M.; Sardashti, A.; Soares, J.B.; Simon, L.C. Atom transfer radical polymerization (ATRP) of styrene and acrylonitrile with monofunctional and bifunctional initiators. Polymer 2007, 48, 1954–1961. [Google Scholar] [CrossRef]
- Zhou, G.-T.; Yu, J.C.; Wang, X.-C.; Zhang, L.-Z. Sonochemical synthesis of aragonite-type calcium carbonate with different morphologies. New J. Chem. 2004, 28, 1027–1031. [Google Scholar] [CrossRef]






| Reaction Conditions | Reaction Recipe | ||
|---|---|---|---|
| Ratio PU: glycolysis agent | 1: 1 | Polyurethane foam | 450 g |
| Catalyst concentration | 0.1 wt% | data | |
| Reaction temperature | 200 °C | Catalyst (DABCO) | 0.45 g |
| Stirrer type | Rushton | ||
| Stirring speed | 300 rpm | Diethylene glycol | 450 g |
| Feeding time | 1 h | ||
| Reaction time | 3 h | ||
| Compound | p/p w |
|---|---|
| Polyol | 100 |
| Water | 4.22 |
| Surfactant | 1.2 |
| Amine catalyst | 0.22 |
| Metal catalyst | 0.32 |
| Isocyanate TDI80 | 53.77 |
| Index | 110 |
| Concentration (wt%) | Molecular Weight (g/mol) | ||||
|---|---|---|---|---|---|
| Upper Phase | Intermediate Phase | Bottom Phase | |||
| SAN | Peak I | 0 | 21 | 2.5 | 70,000 |
| Polyol | Peak II | 89.6 | 39 | 5.2 | 3500 |
| Reaction by-products | Peak III | 5.9 | 25 | 57.1 | 700 |
| Peak IV | 475 | ||||
| Peak V | 320 | ||||
| DEG | Peak VI | 4.5 | 15 | 35.2 | 106 |
| Composition (wt%) | SAN | 0 |
| Polyol | 99.1 | |
| Reaction by-products | 0.9 | |
| DEG | 0 | |
| Hydroxyl number (mg KOH/g) | 48.4 ± 0.5 | |
| Water content (wt%) | 0.07 | |
| Viscosity (Pa·s) | 1.5 | |
| TDI80 (%) | TDI65 (%) | Air Resistance (cm H2O) |
|---|---|---|
| 100 | 0 | 85 |
| 90 | 10 | 84.5 |
| 50 | 50 | 85.5 |
| 30 | 70 | 10 |
| 0 | 100 | 6.8 |
| Recovered Polyol (%) | 0 * | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|
| Blow-off time (s) | 86 | 87 | 86 | 84 | 85 | 85 | 82 |
| Density (kg/m3) | 24.7 | 25.4 | 25.1 | 25.5 | 25.8 | 26.4 | 24.5 |
| Air resistance (cm H2O) min | 6.2 | 4.9 | 5.1 | 5.0 | 8.1 | 6.8 | 9.4 |
| Air resistance (cm H2O) max | 6.7 | 5.2 | 5.5 | 6.7 | 8.5 | 7.0 | 10.0 |
| CLD hardness 25% (kPa) | 3.4 | 3.5 | 3.8 | 4.1 | 4.2 | 4.4 | 4.1 |
| CLD hardness 40% (kPa) | 3.9 | 4.2 | 4.3 | 4.8 | 4.9 | 5.0 | 4.7 |
| CLD hardness 60% (kPa) | 5.9 | 6.8 | 7.0 | 7.9 | 8.0 | 8.3 | 7.8 |
| Ball rebound (%) | 43 | 37 | 37 | 36 | 33 | 34 | 34 |
| Compression set 50% (%) | 2.1 | 1.9 | |||||
| Compression set 75% (%) | 4.1 | 4.6 | |||||
| WCS 50 °C 95% RH (%) | 7.0 | 9.6 | |||||
| Tensile strength (kPa) | 88 | 73 | |||||
| Elongation at break (%) | 139 | 91 | |||||
| Tear resistance (N/cm) | 3.9 | 3.6 |
| Conventional polyol | 75 | 89 |
| Conventional SAN polymer polyol | 25 | - |
| Recovered SAN | 0 | 11 |
| Isocyanate TDI80 | 100% | 100% |
| Blow-off time (s) | 82 | 85 |
| Density (kg/m3) | 24.9 | 24.6 |
| Air resistance (cm H2O) min | 26.1 | 85.5 |
| Air resistance (cm H2O) max | 49.7 | 85.8 |
| CLD hardness 25% (kPa) | 5 | 3.8 |
| CLD hardness 40% (kPa) | 5.8 | 4.5 |
| CLD hardness 60% (kPa) | 9.1 | 7.3 |
| Compression set 50% (%) | 3 | 3.4 |
| Ball rebound (%) | 34 | 20 |
| Conventional polyol | 86 | 86 |
| Commercial CaCO3 | 14 | 0 |
| Recovered CaCO3 | 0 | 14 |
| Isocyanate TDI80 | 100% | 100% |
| Blow-off time (s) | 80 | 88 |
| Density (kg/m3) | 24.2 | 24.3 |
| Air resistance (cm H2O) min | 33 | 85.4 |
| Air resistance (cm H2O) max | 40.5 | 85.6 |
| CLD hardness 25% (kPa) | 4.3 | 4.3 |
| CLD hardness 40% (kPa) | 4.9 | 4.9 |
| CLD hardness 60% (kPa) | 7.9 | 7.8 |
| Compression set 50% (%) | 3.2 | 3.2 |
| Ball rebound (%) | 34 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Amo, J.; Iswar, S.; Vanbergen, T.; Borreguero, A.M.; De Vos, S.D.E.; Verlent, I.; Willems, J.; Rodriguez Romero, J.F. Polyurethane Composites Recycling with Styrene–Acrylonitrile and Calcium Carbonate Recovery. Materials 2024, 17, 2844. https://doi.org/10.3390/ma17122844
del Amo J, Iswar S, Vanbergen T, Borreguero AM, De Vos SDE, Verlent I, Willems J, Rodriguez Romero JF. Polyurethane Composites Recycling with Styrene–Acrylonitrile and Calcium Carbonate Recovery. Materials. 2024; 17(12):2844. https://doi.org/10.3390/ma17122844
Chicago/Turabian Styledel Amo, Jesús, Subramaniam Iswar, Thomas Vanbergen, Ana Maria Borreguero, Simon Dirk E. De Vos, Isabel Verlent, Jan Willems, and Juan Francisco Rodriguez Romero. 2024. "Polyurethane Composites Recycling with Styrene–Acrylonitrile and Calcium Carbonate Recovery" Materials 17, no. 12: 2844. https://doi.org/10.3390/ma17122844
APA Styledel Amo, J., Iswar, S., Vanbergen, T., Borreguero, A. M., De Vos, S. D. E., Verlent, I., Willems, J., & Rodriguez Romero, J. F. (2024). Polyurethane Composites Recycling with Styrene–Acrylonitrile and Calcium Carbonate Recovery. Materials, 17(12), 2844. https://doi.org/10.3390/ma17122844









