Comparative Study for Propranolol Adsorption on the Biochars from Different Agricultural Solid Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solutions and Materials
2.2. Biochar Characterization
2.3. Batch Adsorption Experiments
2.4. Sorption Mathematical Model
3. Results and Discussion
3.1. Characterization of Biochars
3.1.1. SEM Micrograph
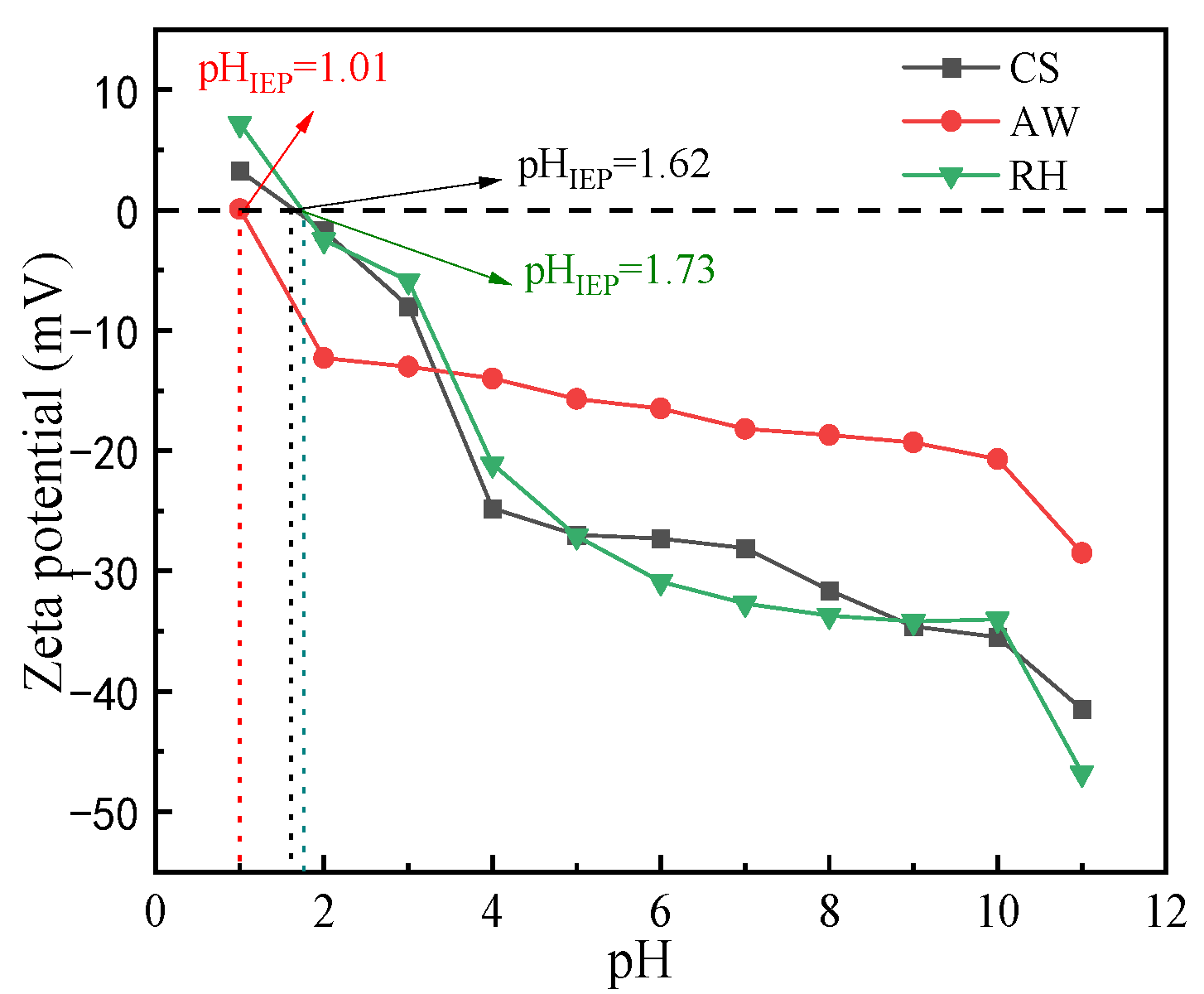
3.1.2. Zeta Potential Analysis
3.2. Effects of Environmental Factors on Adsorption
3.2.1. Effect of pH
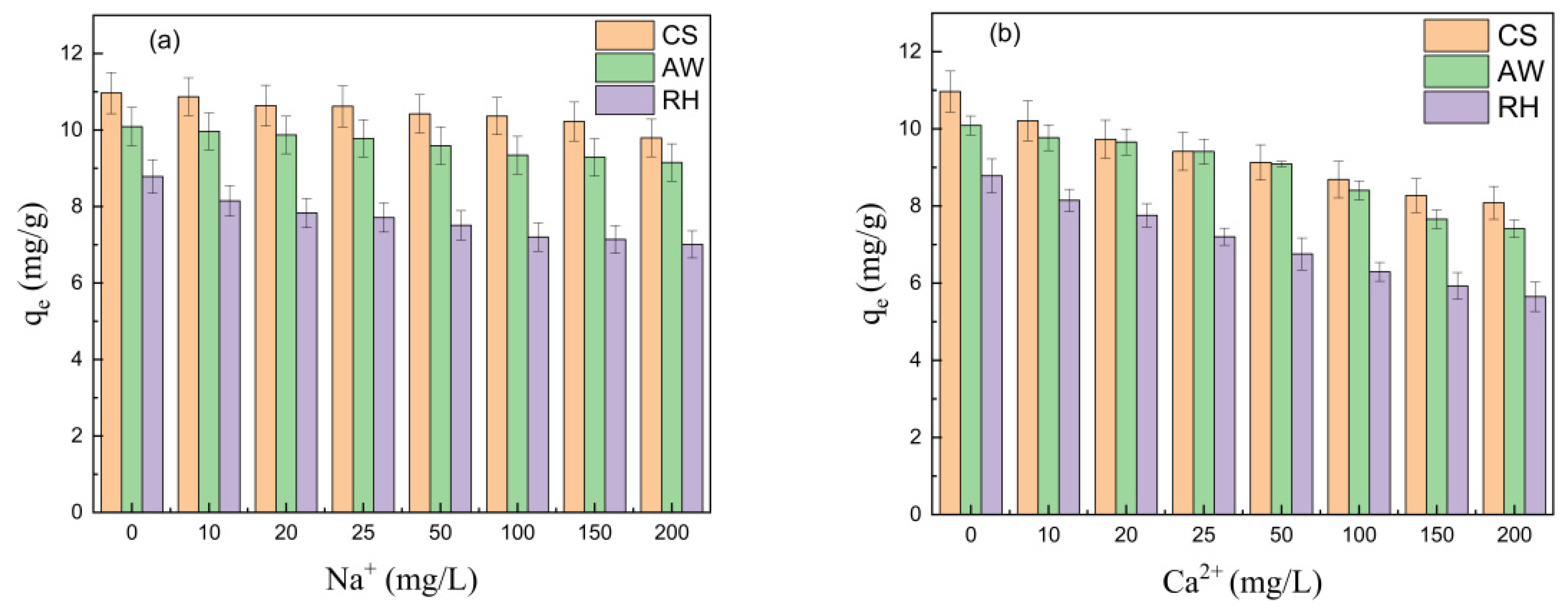
3.2.2. Effect of the Coexisting Ions
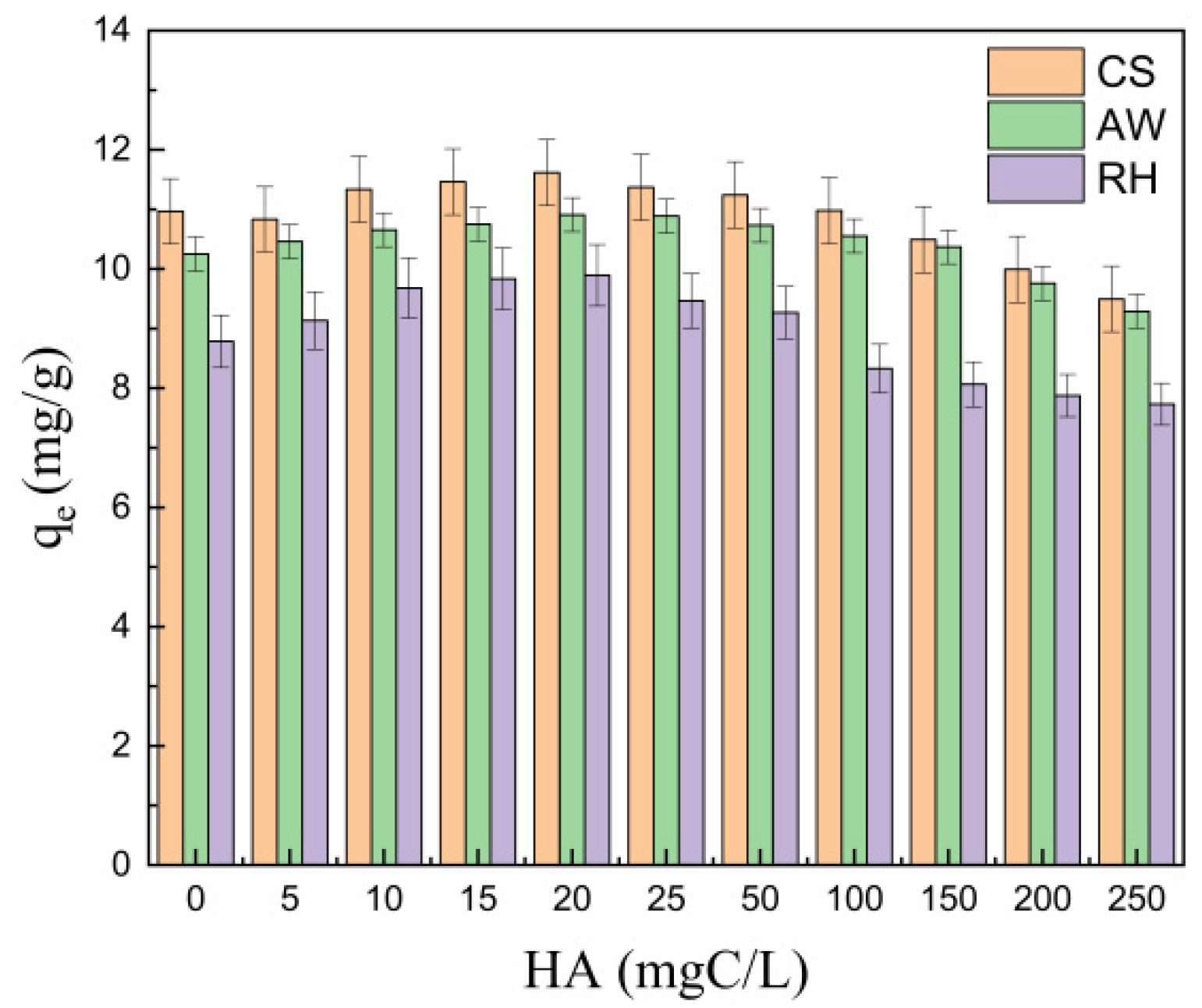
3.2.3. Effect of Humic Acid
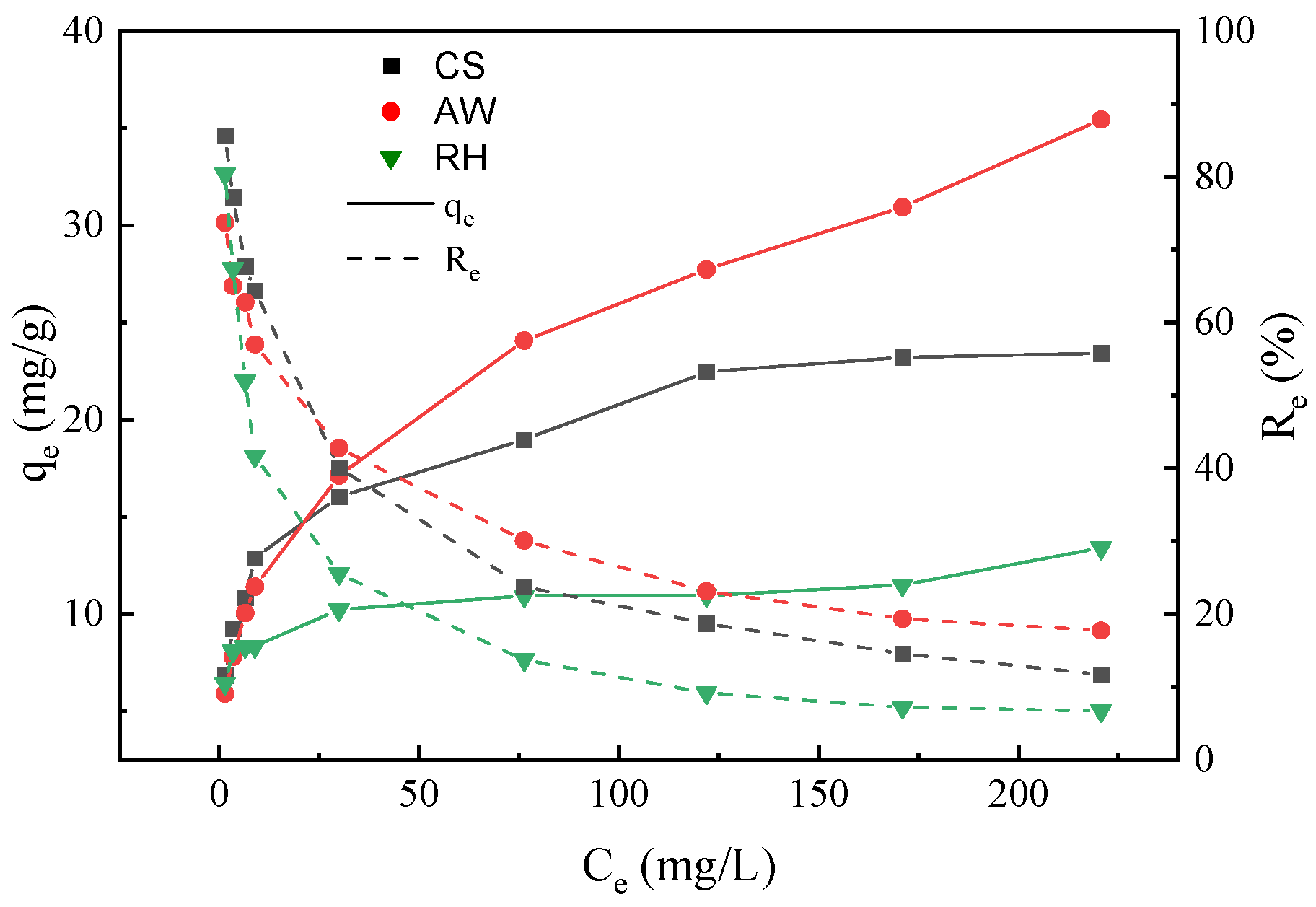
3.3. Sorption Isotherms
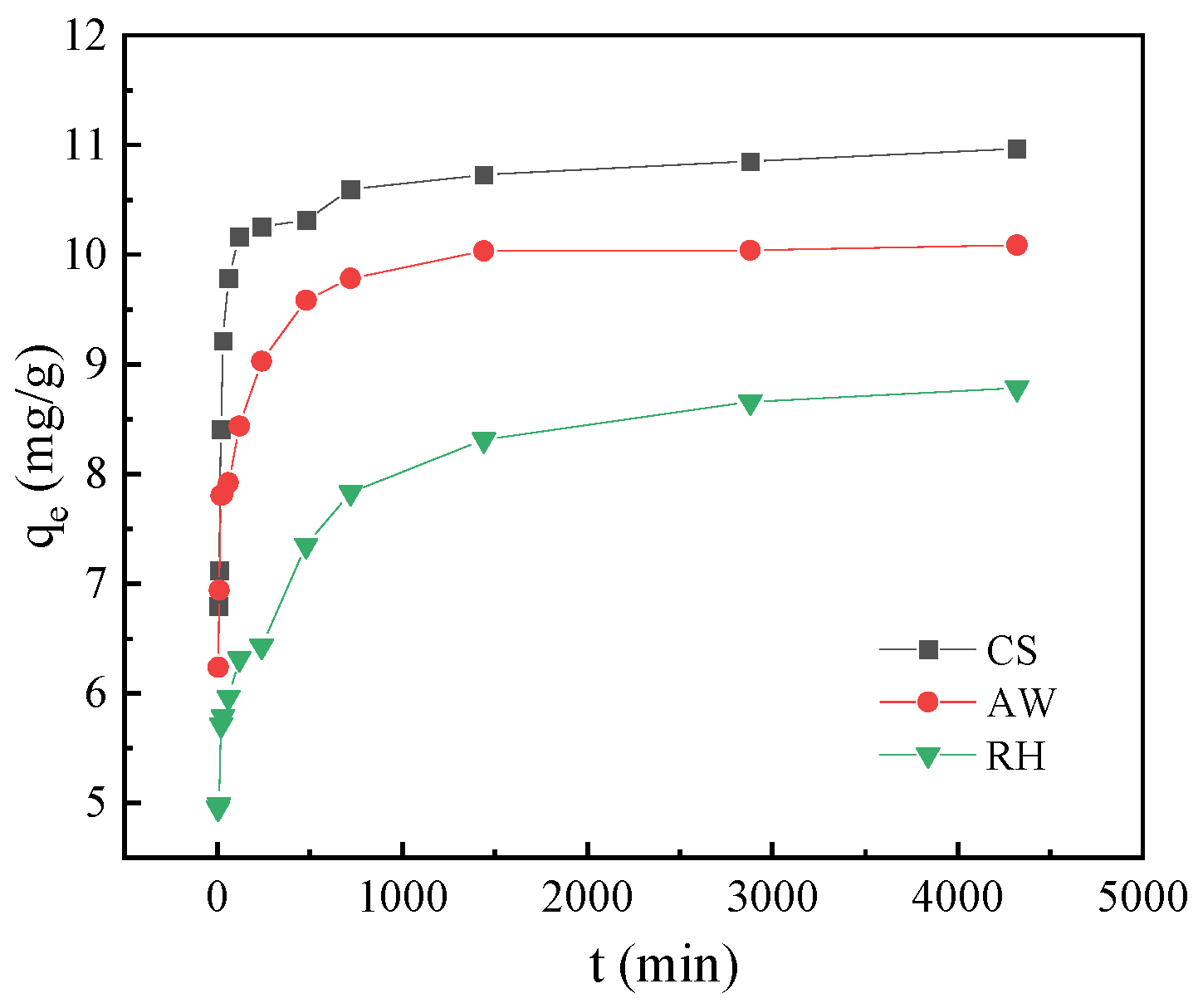
3.4. Adsorption Kinetics
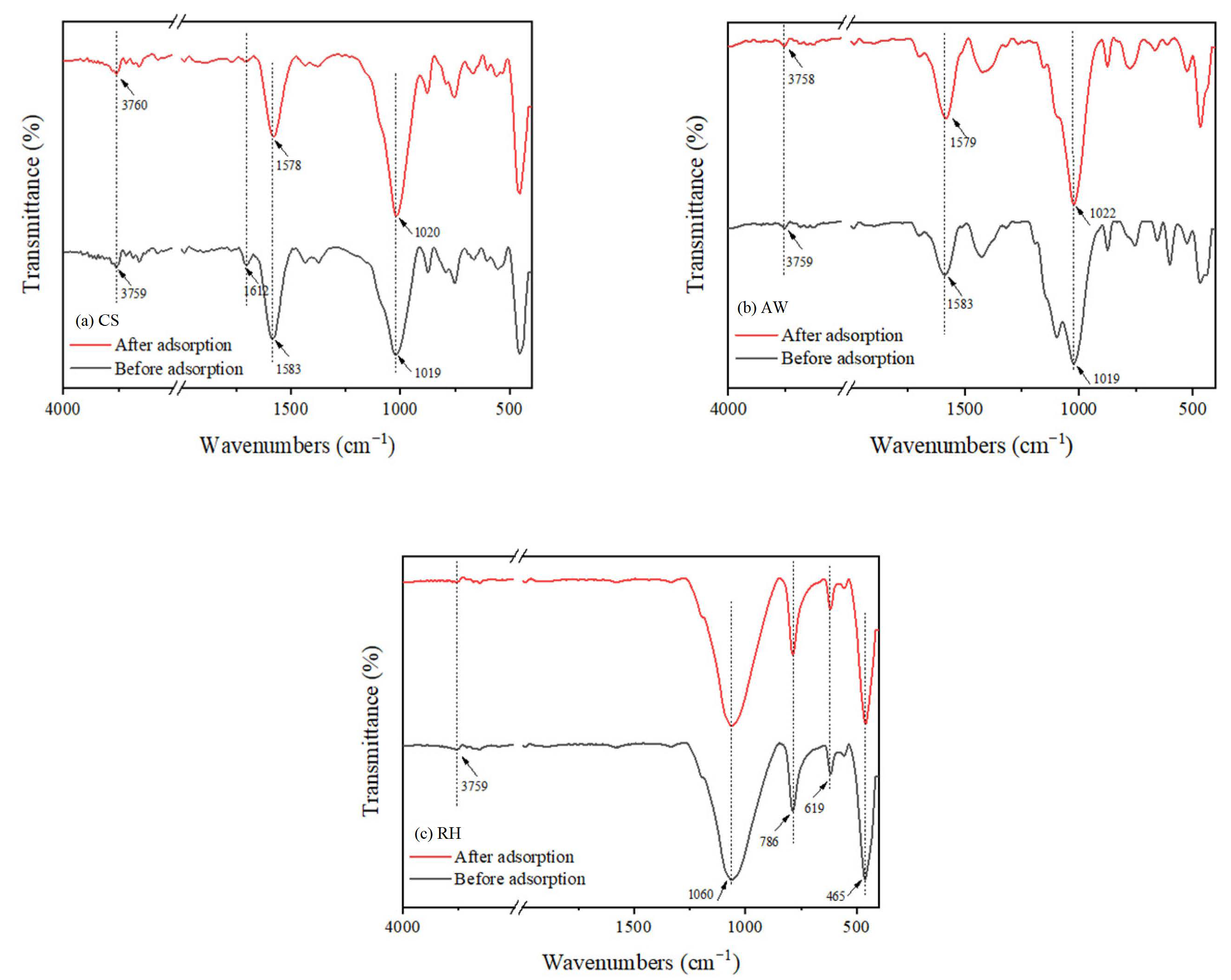
3.5. Possible Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, M.; Sheng, Q.; Sui, Q.; Lu, H. beta-blockers in the environment: Distribution, transformation, and ecotoxicity. Environ. Pollut. 2020, 266, 115269. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.D.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. LC-MS analysis of basic pharmaceuticals (beta-blockers and anti-ulcer agents) in wastewater and surface water. TrAC Trends Anal. Chem. 2007, 26, 581–594. [Google Scholar] [CrossRef]
- Sidhu, J.P.; Ahmed, W.; Gernjak, W.; Aryal, R.; McCarthy, D.; Palmer, A. Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Sci. Total Environ. 2013, 463, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ. Monit. Assess. 2014, 186, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Chen, D.; Zhang, J.; Wu, Y.; Sun, C. Determination of 76 pharmaceutical drugs by liquid chromatography–tandem mass spectrometry in slaughterhouse wastewater. J. Chromatogr. A 2009, 1216, 8312–8318. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Khan, I.A.; Foran, C.M.; Schlenk, D. Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent. Environ. Pollut. 2003, 121, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of beta-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Aravindakumar, C.T.; Aravind, U.K. Removal of beta blockers using polyelectrolyte monolayered membrane and its antifouling performance. J. Ind. Eng. Chem. 2020, 87, 222–233. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- De Oliveira, L.L.D.; Antunes, S.C.; Gonçalves, F.; Rocha, O.; Nunes, B. Acute and chronic ecotoxicological effects of three pharmaceuticals drugs on cladoceran Daphnia magna. Drug Chem. Toxicol. 2016, 39, 13–21. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Kim, H.Y.; Kim, S.D. Multi-generational effects of propranolol on Daphnia magna at different environmental concentrations. Environ. Pollut. 2015, 206, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Bolan, N. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 163968. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Rawash, E.S.A.; Lima, E.C. Magnetic activated carbon nanocomposite from Nigella sativa L. waste (MNSA) for the removal of Coomassie brilliant blue dye from aqueous solution: Statistical design of experiments for optimization of the adsorption conditions. J. Adv. Res. 2019, 17, 55–63. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Jing, F.; Guan, J.; Tang, W.; Chen, J. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Wang, C.C.; Cheng, J.H.; Zhang, C.L.; Yao, J.J. Removal of Cr (VI) by biochar derived from six kinds of garden wastes: Isotherms and kinetics. Materials 2021, 14, 3243. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lei, G.; Quan, S.; Zhang, L.; Wang, B.; Hu, H. The Removal of Cr (VI) from Aqueous Solutions with Corn Stalk Biochar. Int. J. Environ. Res. Public Health 2022, 19, 14188. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic press: Cambridge, MA, USA, 2013. [Google Scholar]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhang, C. Enhanced heavy metals sorption by modified biochars derived from pig manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Kozłowski, M.; Wąsowicz, J.; Pęczek, E.; Białowiec, A. Nitrogen Removal from Landfill Leachate Using Biochar Derived from Wheat Straw. Materials 2024, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, P.; Zhou, H.; Zhao, H.; Yang, B.; Xiong, J. Preparation of Lanthanum-Modified Tea Waste Biochar and Its Adsorption Performance on Fluoride in Water. Materials 2024, 17, 766. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Wang, F.; Sun, H. Sorption mechanisms of polar and apolar organic contaminants onto biochars. Environ. Chem. 2016, 35, 1134–1141. [Google Scholar]
- de Azevedo, C.F.; Rodrigues, D.L.C.; Silveira, L.L. Comprehensive adsorption and spectroscopic studies on the interaction of magnetic biochar from black wattle sawdust with beta-blocker metoprolol. Bioresour. Technol. 2023, 388, 129708. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Nazir, G.; Heo, K.; Hussain, S.; Ikram, M.; Akhter, Z.; Fouda, A.M. A focused review on lignocellulosic biomass-derived porous carbons for effective pharmaceuticals removal: Current trends, challenges and future prospects. Sep. Purif. Technol. 2023, 330, 125356. [Google Scholar] [CrossRef]
- Gui, X.; Song, B.; Chen, M.; Xu, X.; Ren, Z.; Li, X.; Cao, X. Soil colloids affect the aggregation and stability of biochar colloids. Sci. Total Environ. 2021, 771, 145414. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y. Surface-bound humic acid increased propranolol sorption on Fe3O4/attapulgite magnetic nanoparticles. Nanomaterials 2020, 10, 205. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, G.; Ni, X.; Wang, B.; Sun, H.; Yu, Y.; Yin, X. Effect of different aging treatments on the transport of nano-biochar in saturated porous media. Chemosphere 2023, 323, 138272. [Google Scholar] [CrossRef]
- Wang, F.; Sun, H.; Ren, X.; Zhang, K. Sorption of naphthalene and its hydroxyl substitutes onto biochars in single-solute and bi-solute systems with propranolol as the co-solute. Chem. Eng. J. 2017, 326, 281–291. [Google Scholar] [CrossRef]
- Park, C.M.; Han, J.; Chu, K.H.; Al-Hamadani, Y.A.; Her, N.; Heo, J.; Yoon, Y. Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: Experiment and modeling. J. Ind. Eng. Chem. 2017, 48, 186–193. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, K.; Yang, F. Effects of biochar on transport and retention of phosphorus in porous media: Laboratory test and modeling. Environ. Pollut 2022, 297, 118788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van Zwieten, L.; Wang, H.; Wang, L.; Li, R.; Qu, J.; Zhang, Y. Sorption of Pb (II) onto biochar is enhanced through co-sorption of dissolved organic matter. Sci. Total Environ. 2022, 825, 153686. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Tsang, D.C. Enhanced adsorption of Cu (II) and Zn (II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Aghoghovwia, M.P.; Hardie, A.G.; Rozanov, A.B. Characterisation, adsorption and desorption of ammonium and nitrate of biochar derived from different feedstocks. Environ. Technol. 2022, 43, 774–787. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Gao, B.; Yan, Y.; Han, L. Properties of the plant-and manure-derived biochars and their sorption of dibutyl phthalate and phenanthrene. Sci. Rep. 2014, 4, 5295. [Google Scholar] [CrossRef]



| Biochar | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | KL (L·mg−1) | R2 | 1/n | KF | R2 | |
| CS | 19.05 ± 1.84 | 0.35092 ± 0.04752 | 0.90948 | 0.23946 ± 0.01184 | 6.86386 ± 0.30173 | 0.98318 |
| AW | 25.71 ± 4.08 | 0.10182 ± 0.01025 | 0.94468 | 0.40083 ± 0.01106 | 4.23113 ± 0.17391 | 0.99470 |
| RH | 10.79 ± 0.58 | 0.68576 ± 0.15549 | 0.80707 | 0.12881 ± 0.01135 | 6.15836 ± 0.27084 | 0.94844 |
| Parameters | CS | AW | RH | |
|---|---|---|---|---|
| pseudo-first-order kinetics | K1 (min−1) | 0.00306 ± 0.00032 | 0.00375 ± 0.00032 | 0.00316 ± 0.00015 |
| qe (mg·g−1) | 1.79029 ± 0.45953 | 1.95652 ± 0.48531 | 3.27297 ± 0.35299 | |
| R2 | 0.89438 | 0.93051 | 0.97805 | |
| pseudo-second-order kinetics | K2 (g·mg−1·min−1) | 0.00689 ± 0.00328 | 0.00649 ± 0.00179 | 0.00245 ± 0.00106 |
| qe (mg·g−1) | 10.95410 ± 0.30463 | 10.11122 ± 0.21616 | 8.80980 ± 0.07435 | |
| R2 | 0.99993 | 0.99996 | 0.99930 |
| Biochars | CS | AW | RH | |||
|---|---|---|---|---|---|---|
| Before or After Adsorption | Before | After | Before | After | Before | After |
| Specific surface area (m2·g−1) | 18.07 | 8.41 | 3.00 | 3.13 | 26.42 | 5.09 |
| Total pore volume (cm3·g−1) | 0.0313 | 0.0213 | 0.0115 | 0.0215 | 0.0264 | 0.0127 |
| Average pore size (nm) | 6.83 | 9.31 | 13.56 | 19.75 | 4.28 | 9.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Che, Q.; Chen, D.; Cao, H.; Deng, Y. Comparative Study for Propranolol Adsorption on the Biochars from Different Agricultural Solid Wastes. Materials 2024, 17, 2793. https://doi.org/10.3390/ma17122793
Nie W, Che Q, Chen D, Cao H, Deng Y. Comparative Study for Propranolol Adsorption on the Biochars from Different Agricultural Solid Wastes. Materials. 2024; 17(12):2793. https://doi.org/10.3390/ma17122793
Chicago/Turabian StyleNie, Wenjie, Qianqian Che, Danni Chen, Hongyu Cao, and Yuehua Deng. 2024. "Comparative Study for Propranolol Adsorption on the Biochars from Different Agricultural Solid Wastes" Materials 17, no. 12: 2793. https://doi.org/10.3390/ma17122793
APA StyleNie, W., Che, Q., Chen, D., Cao, H., & Deng, Y. (2024). Comparative Study for Propranolol Adsorption on the Biochars from Different Agricultural Solid Wastes. Materials, 17(12), 2793. https://doi.org/10.3390/ma17122793





