Synthesis and Properties of Size-Adjustable CsPbBr3 Nanosheets for Potential Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
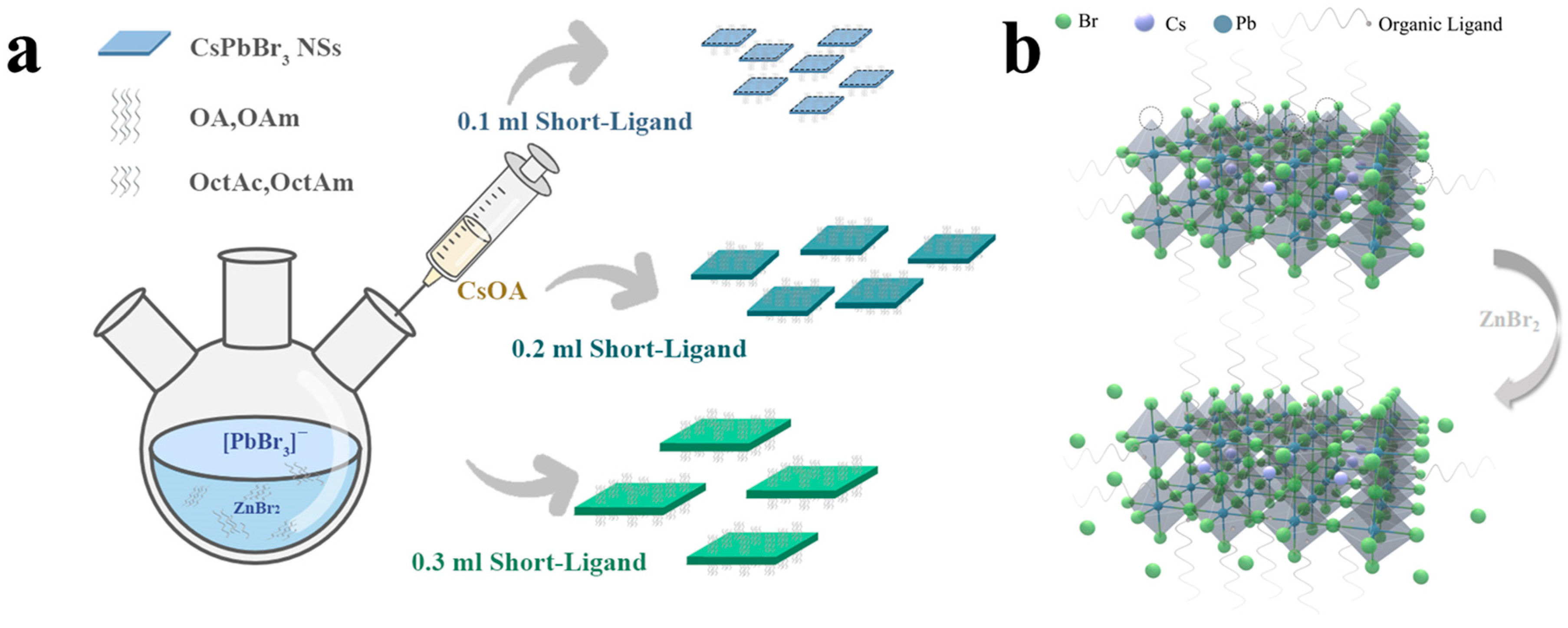
2.2.1. Preparation of Cs–Oleate Precursors
2.2.2. Synthesis of CsPbBr3 NSs
2.2.3. Isolation and Purification of Crystals
2.3. Characterization of Materials
2.3.1. Transmission Electron Microscopy (TEM) Characterization
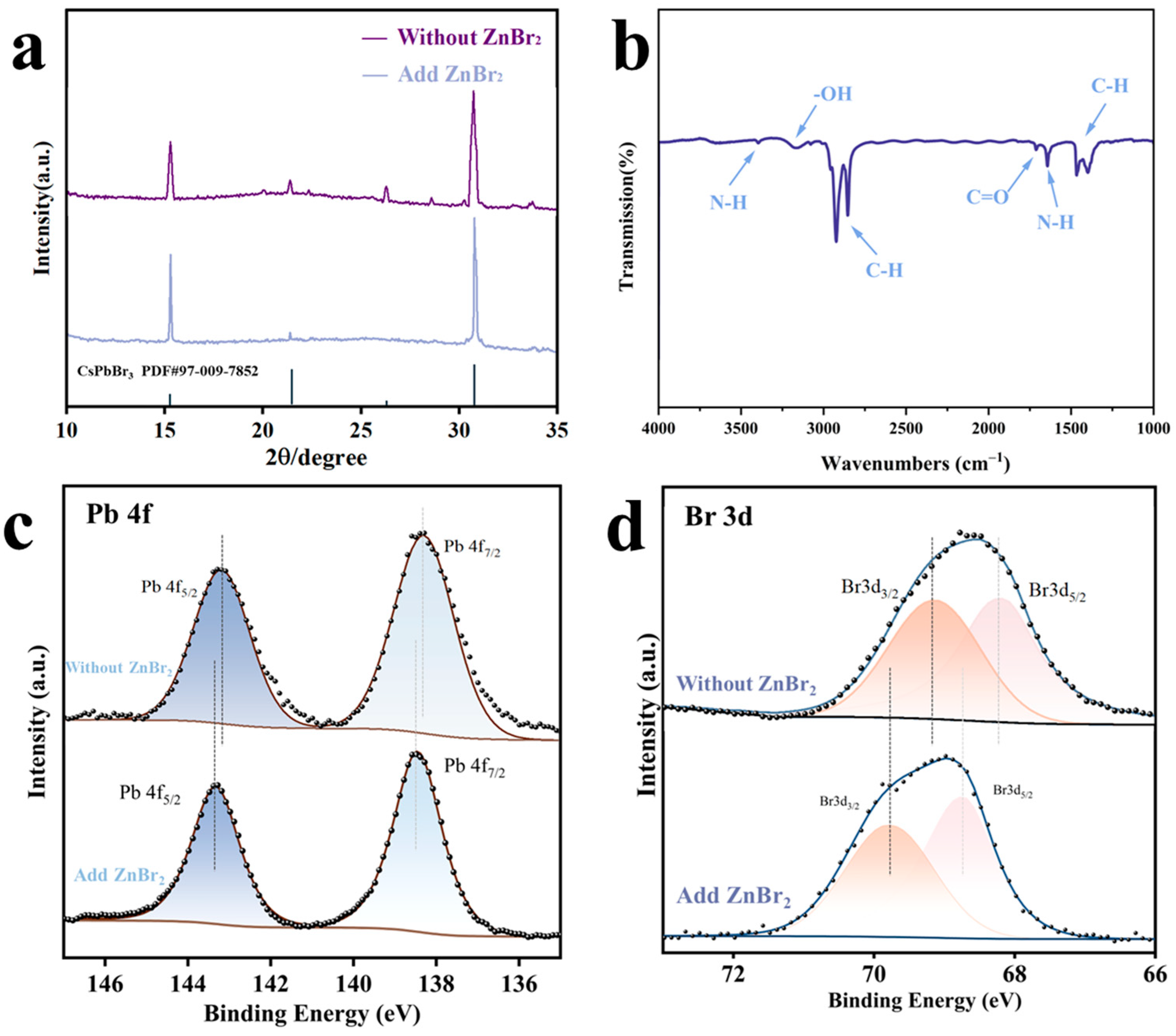
2.3.2. X-ray Diffraction (XRD) Characterization
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy Characterization
2.3.4. High-Resolution Transmission Electron Microscopy (HRTEM) Characterization
2.3.5. X-ray Photoelectron Spectroscopy (XPS) Characterization
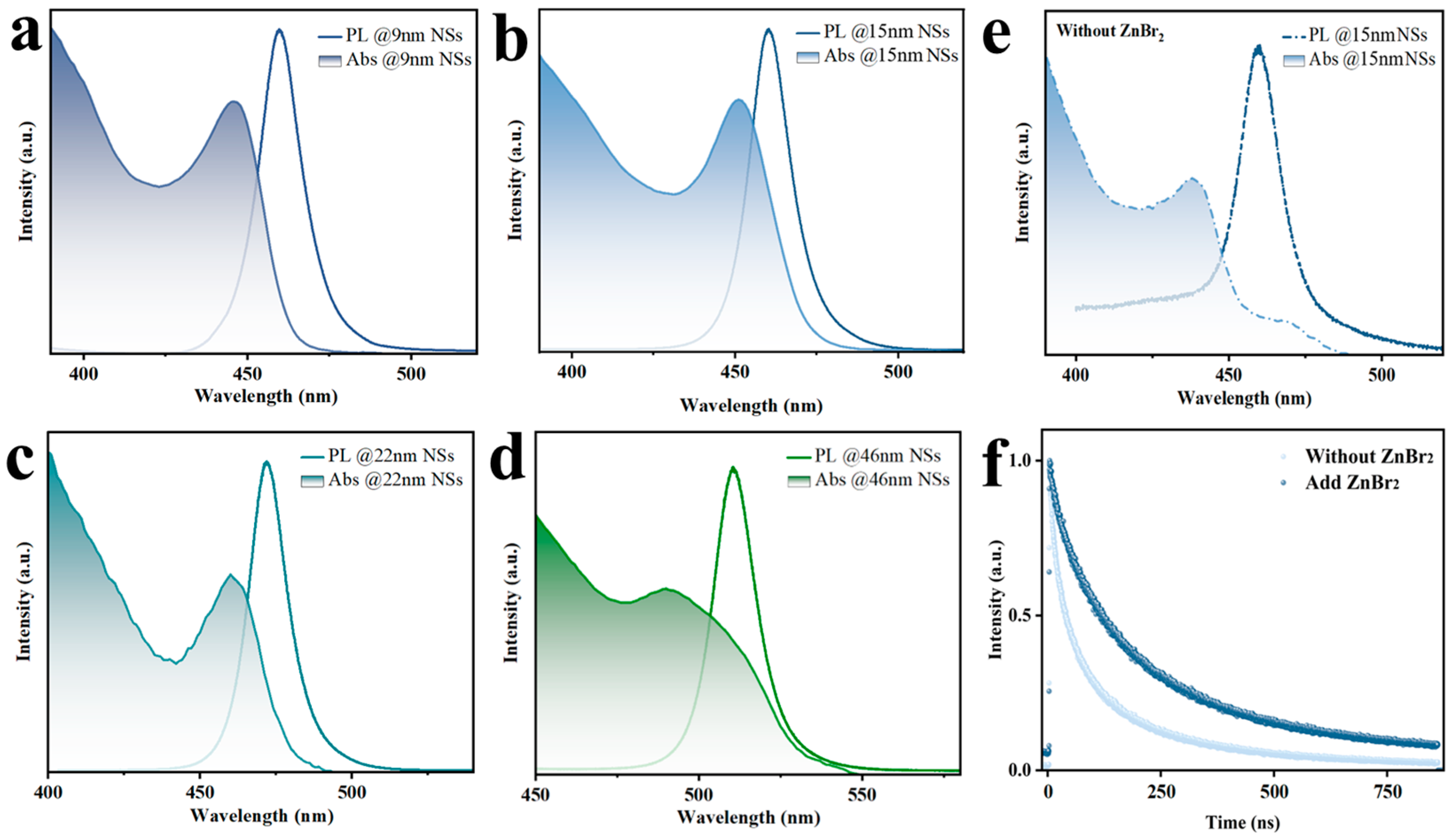
2.3.6. Fluorescence Spectrum Measurements
2.3.7. Thermogravimetric Analysis (TGA) Measurements
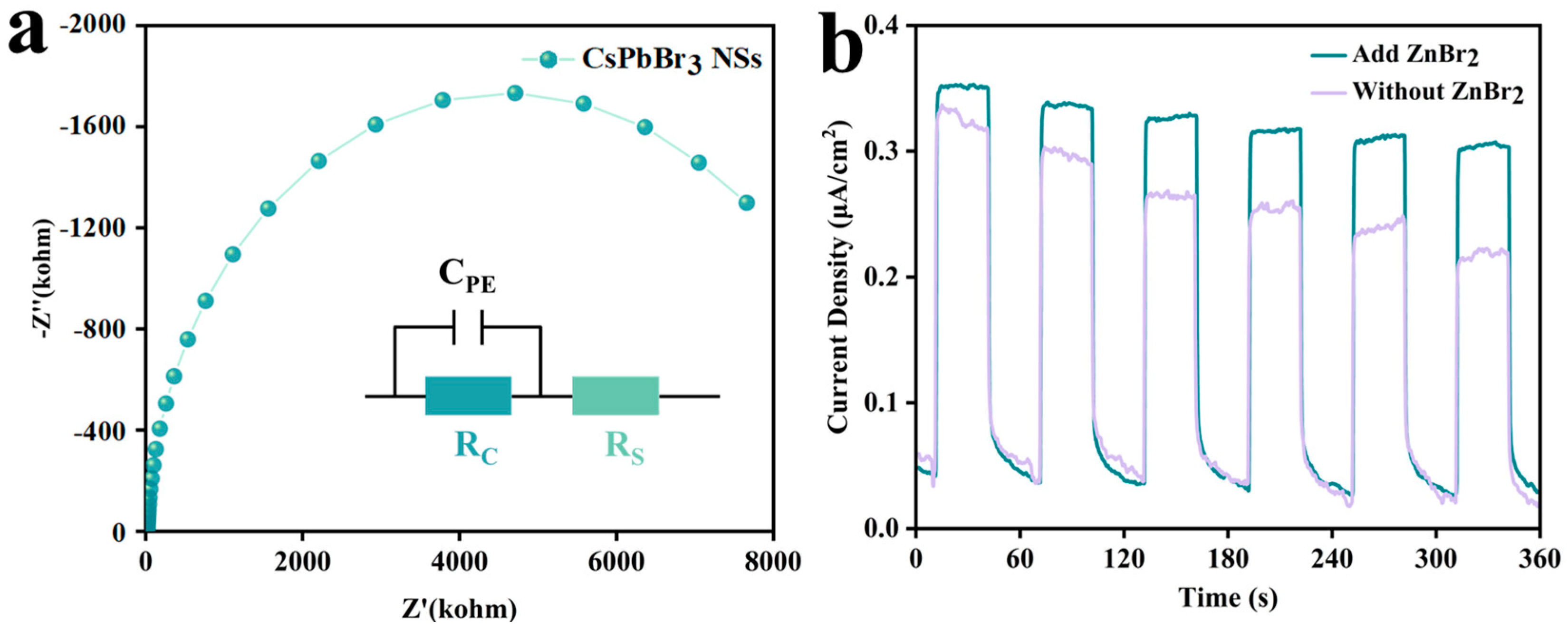
2.3.8. Photoelectrochemistry/Electrochemistry Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enomoto, K.; Oizumi, R.; Aizawa, N.; Chiba, T.; Pu, Y.J. Energy Transfer from Blue-Emitting CsPbBr3 Perovskite Nanocrystals to Green-Emitting CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. C 2021, 125, 19368–19373. [Google Scholar] [CrossRef]
- Goyal, A.; Andrioti, E.; Tang, Y.; Zhao, Q.; Zheng, K.; Newell, K.D.; Schall, P. Mechanochemical Synthesis of Stable, Quantum-Confined CsPbBr3 Perovskite Nanocrystals with Blue-Green Emission and High PLQY. J. Phys. Mater. 2022, 5, 024005. [Google Scholar] [CrossRef]
- Shi, S.S.; Wang, Y.; Zeng, S.Y.; Cui, Y.; Xiao, Y. Surface Regulation of CsPbBr3 Quantum Dots for Standard Blue-Emission with Boosted PLQY. Adv. Opt. Mater. 2020, 8, 2000167. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.J.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide-Based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Boussoufi, F.; Pousthomis, M.; Kuntzmann, A.; D’Amico, M.; Patriarche, G.; Dubertret, B. Spray-Drying Polymer Encapsulation of CsPbBr3 Perovskite Nanocrystals with Enhanced Photostability for LED Downconverters. ACS Appl. Nano Mater. 2021, 4, 7502–7512. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Hathaway, E.; Paulette, H.; Coffer, J.L.; Lin, Y.K.; Cui, J.B. Two-Dimensional Quantum-Confined CsPbBr3 in Silicene for LED Applications. ACS Appl. Nano Mater. 2023, 6, 4028–4033. [Google Scholar] [CrossRef]
- Peng, M.; Sun, S.B.; Xu, B.; Deng, Z.T. Polymer-Encapsulated Halide Perovskite Color Converters to Overcome Blue Overshoot and Cyan Gap of White Light-Emitting Diodes. Adv. Funct. Mater. 2023, 33, 7. [Google Scholar] [CrossRef]
- Chen, S.X.; Lin, J.D.; Zheng, S.; Zheng, Y.H.; Chen, D.Q. Efficient and Stable Perovskite White Light-Emitting Diodes for Backlit Display. Adv. Funct. Mater. 2023, 33, 2300583. [Google Scholar] [CrossRef]
- Fu, Y.P.; Zhu, H.M.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.Y.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Fu, Y.P.; Meng, F.; Wu, X.X.; Gong, Z.Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Fan, H.G.; Liu, X.Y.; Cao, J.; Liu, H.L.; Li, X.; Yang, L.L.; Wei, M.B. 3D ZnO Hollow Spheres-Dispersed CsPbBr3 Quantum Dots S-Scheme Heterojunctions for High-Efficient CO2 Photoreduction. J. Alloys Compd. 2023, 945, 169197. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Yang, M.Z.; Chen, H.Y.; Liao, J.F.; Wang, X.D.; Kuang, D.B. Enhanced Solar-Driven Gaseous CO2 Conversion by CsPbBr3 Nanocrystal/Pd Nanosheet Schottky-Junction Photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089. [Google Scholar] [CrossRef]
- Liu, W.L.; Liu, J.F.; Wang, X.Q.; He, J.Z.; Li, Y.Q.; Liu, Y. Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties. Catalysts 2023, 13, 1048. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.K.; Deng, H.; Qiao, K.K.; Farooq, U.; Ishaq, M.; Yi, F.; Liu, H.; Tang, J.; Song, H.S. Low-Dimensional Halide Perovskites and Their Advanced Optoelectronic Applications. Nano-Micro Lett. 2017, 9, 36. [Google Scholar] [CrossRef]
- Qiu, T.; Hu, Y.Q.; Xu, F.; Yan, Z.; Bai, F.; Jia, G.H.; Zhang, S.F. Recent Advances in One-Dimensional Halide Perovskites for Optoelectronic Applications. Nanoscale 2018, 10, 20963–20989. [Google Scholar] [CrossRef]
- Hu, H.L.; Qin, M.C.; Fong, P.W.K.; Ren, Z.W.; Wan, X.J.; Singh, M.; Su, C.J.; Jeng, U.S.; Li, L.; Zhu, J.J.; et al. Perovskite Quantum Wells Formation Mechanism for Stable Efficient Perovskite Photovoltaics-A Real-Time Phase-Transition Study. Adv. Mater. 2021, 33, e2006238. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, X.Z.; Liang, W.Q.; Li, Y.; Ma, Z.Z.; Wang, M.; Wang, Y.; Wu, D.; Chen, X.; Yang, D.W.; et al. Room-Temperature Synthesis of Blue-Emissive Zero-Dimensional Cesium Indium Halide Quantum Dots for Temperature-Stable Down-Conversion White Light-Emitting Diodes with a Half-Lifetime of 186 h. Mater. Horizons 2021, 8, 3432–3442. [Google Scholar] [CrossRef]
- Cao, J.J.; Yan, C.; Luo, C.; Li, W.; Zeng, X.K.; Xu, Z.; Fu, X.H.; Wang, Q.; Chu, X.; Huang, H.C.; et al. Cryogenic-Temperature Thermodynamically Suppressed and Strongly Confined CsPbBr3 Quantum Dots for Deeply Blue Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2100300. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.D.; Alivisatos, A.P. Highly Luminescent Colloidal Nanoplates of Perovskite Cesium Lead Halide and Their Oriented Assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhou, H.; Jiang, Z.Y.; Wang, X.; Yuan, S.P.; Lan, J.Y.; Fu, Y.P.; Zhang, X.H.; Zheng, W.H.; Wang, X.X.; et al. Direct Vapor Growth of Perovskite CsPbBr3 Nanoplate Electroluminescence Devices. ACS Nano 2017, 11, 9869–9876. [Google Scholar] [CrossRef]
- Shamsi, J.; Dang, Z.Y.; Bianchini, P.; Canale, C.; Di Stasio, F.; Brescia, R.; Prato, M.; Manna, L. Colloidal Synthesis of Quantum Confined Single Crystal CsPbBr3 Nanosheets with Lateral Size Control up to the Micrometer Range. J. Am. Chem. Soc. 2016, 138, 7240–7243. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Motti, S.G.; Kandada, A.R.S.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F.; et al. Solution Synthesis Approach to Colloidal Cesium Lead Halide Perovskite Nanoplatelets with Monolayer-Level Thickness Control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.B.; Askerka, M.; Quan, L.N.; Gong, X.W.; Zhao, W.J.; Zhao, J.X.; Tan, H.R.; Long, G.K.; Gao, L.; et al. Color-Stable Highly Luminescent Sky-Blue Perovskite Light-Emitting Diodes. Nat. Commun. 2018, 9, 36. [Google Scholar] [CrossRef]
- Bohn, B.J.; Tong, Y.; Gramlich, M.; Lai, M.L.; Döblinger, M.; Wang, K.; Hoye, R.L.Z.; Müller-Buschbaum, P.; Stranks, S.D.; Urban, A.S.; et al. Boosting Tunable Blue Luminescence of Halide Perovskite Nanoplatelets through Postsynthetic Surface Trap Repair. Nano Lett. 2018, 18, 5231–5238. [Google Scholar] [CrossRef]
- Udayabhaskararao, T.; Kazes, M.; Houben, L.; Lin, H.; Oron, D. Nucleation, Growth, and Structural Transformations of Perovskite Nanocrystals. Chem. Mater. 2017, 29, 1302–1308. [Google Scholar] [CrossRef]
- Liu, Y.; Siron, M.; Lu, D.L.; Yang, J.J.; dos Reis, R.; Cui, F.; Gao, M.Y.; Lai, M.L.; Lin, J.; Kong, Q.; et al. Self-Assembly of Two-Dimensional Perovskite Nanosheet Building Blocks into Ordered Ruddlesden-Popper Perovskite Phase. J. Am. Chem. Soc. 2019, 141, 13028–13032. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Guan, W.H.; Xu, Y.F.; Wang, X.C.; Wu, L.Z.; Chen, M.; Zhong, Q.X.; Xu, Y.; Li, Y.Y.; Sham, T.K.; et al. Construction of Single-Atom Platinum Catalysts Enabled by CsPbBr3 Nanocrystals. ACS Nano 2021, 15, 13129–13139. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.B.; Liang, R.; Ge, M.Z.; Sun, G.X.; Zhang, Y.; Xing, G.C. Surface Passivation Using 2D Perovskites toward Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef] [PubMed]
- Ehrler, B.; Hutter, E.M. Routes toward Long-Term Stability of Mixed-Halide Perovskites. Matter 2020, 2, 800–802. [Google Scholar] [CrossRef]
- Oranskaia, A.; Yin, J.; Bakr, O.M.; Brédas, J.L.; Mohammed, O.F. Halogen Migration in Hybrid Perovskites: The Organic Cation Matters. J. Phys. Chem. Lett. 2018, 9, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.; Van Driessche, I.; Kovalenko, M.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.T.; Zhang, R.; Liu, X.K.; Zhang, W.H.; Zhang, J.B.; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]

| Material Type | Application Areas | Advantages | Disadvantages |
|---|---|---|---|
| Perovskites | Electrocatalysis/photocatalysis | Efficient photocatalysis, adjustable bandgap | Poor stability, potential toxicity |
| Metals | Electrocatalysis | High conductivity, good activity | Prone to corrosion, expensive |
| Metal oxides | Electrocatalysis | Good stability and activity | High cost, high overpotentials needed |
| MXenes | Electrocatalysis/photocatalysis | High conductivity, excellent mechanics | Poor oxidative stability, dispersibility |
| MOFs | Electrocatalysis | High surface area, diverse functions | Structural instability, complex fabrication |
| COFs | Electrocatalysis | Designable, precise reaction control | Fabrication difficulty, water instability |
| Graphene-based materials | Electrocatalysis/photocatalysis | High stability, suitable for electrodes | Limited activity, requires modifications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Li, H.; Wang, X.; He, J.; Luo, X.; Wang, M.; Liu, J.; Liu, Y. Synthesis and Properties of Size-Adjustable CsPbBr3 Nanosheets for Potential Photocatalysis. Materials 2024, 17, 2563. https://doi.org/10.3390/ma17112563
Liu Q, Li H, Wang X, He J, Luo X, Wang M, Liu J, Liu Y. Synthesis and Properties of Size-Adjustable CsPbBr3 Nanosheets for Potential Photocatalysis. Materials. 2024; 17(11):2563. https://doi.org/10.3390/ma17112563
Chicago/Turabian StyleLiu, Qi, Hang Li, Xiaoqian Wang, Jiazhen He, Xuemin Luo, Mingwei Wang, Jinfeng Liu, and Yong Liu. 2024. "Synthesis and Properties of Size-Adjustable CsPbBr3 Nanosheets for Potential Photocatalysis" Materials 17, no. 11: 2563. https://doi.org/10.3390/ma17112563
APA StyleLiu, Q., Li, H., Wang, X., He, J., Luo, X., Wang, M., Liu, J., & Liu, Y. (2024). Synthesis and Properties of Size-Adjustable CsPbBr3 Nanosheets for Potential Photocatalysis. Materials, 17(11), 2563. https://doi.org/10.3390/ma17112563






