Recent Advances in Positive Photoresists: Mechanisms and Fabrication
Abstract
1. Introduction
2. Working Mechanism and Synthesis Methods
2.1. Non-Chemically Amplified Resists
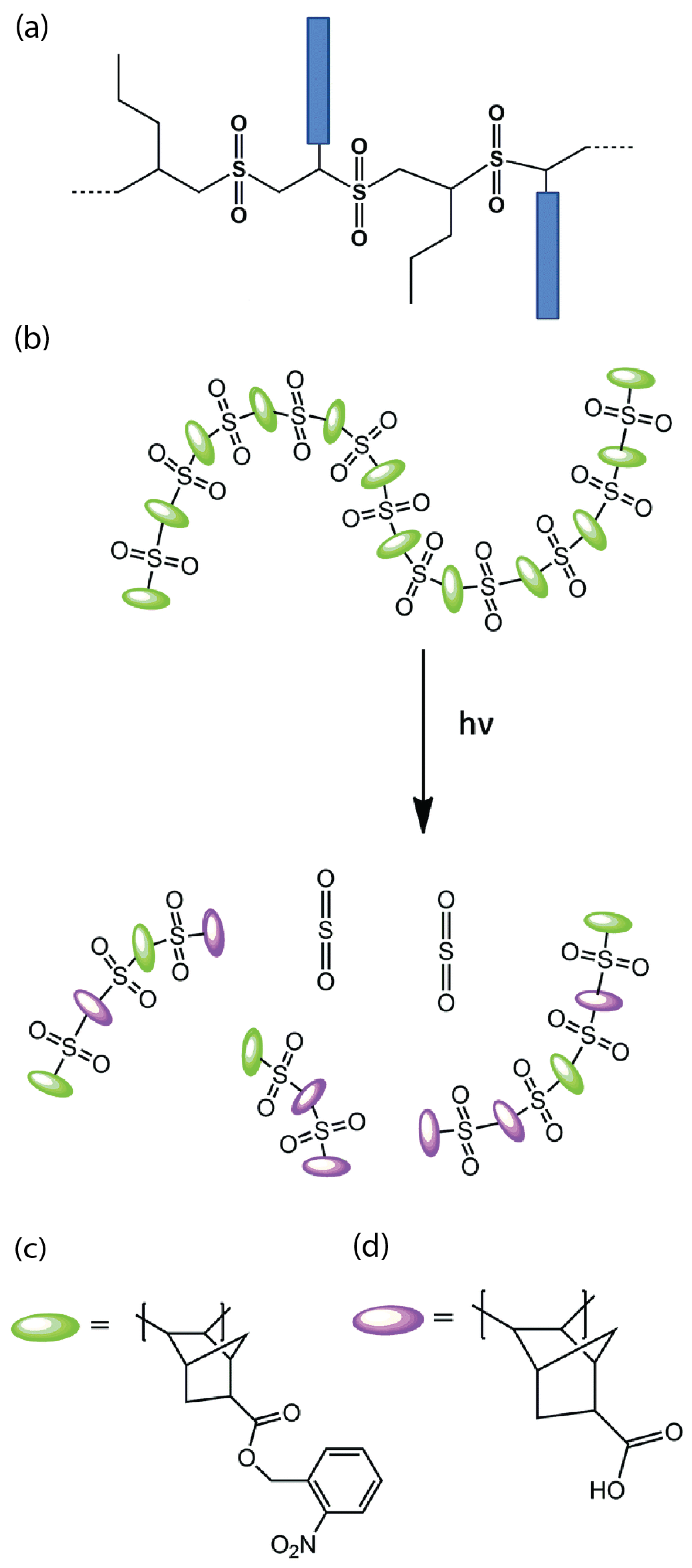
2.1.1. Non-Chemically Amplified Polymeric Photoresist
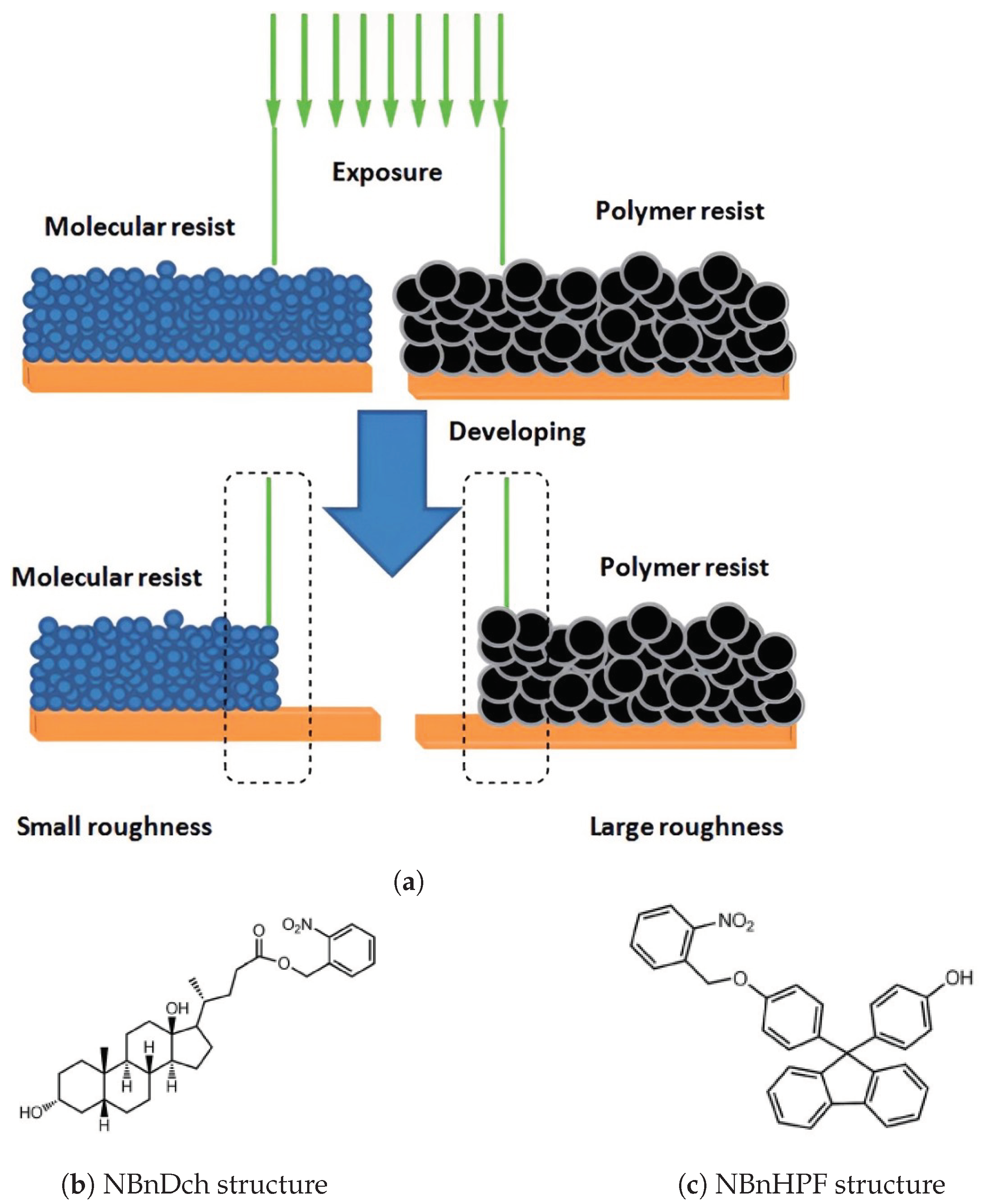
2.1.2. Non-Chemically Amplified Molecular Photoresist
2.2. Organic Photoresists
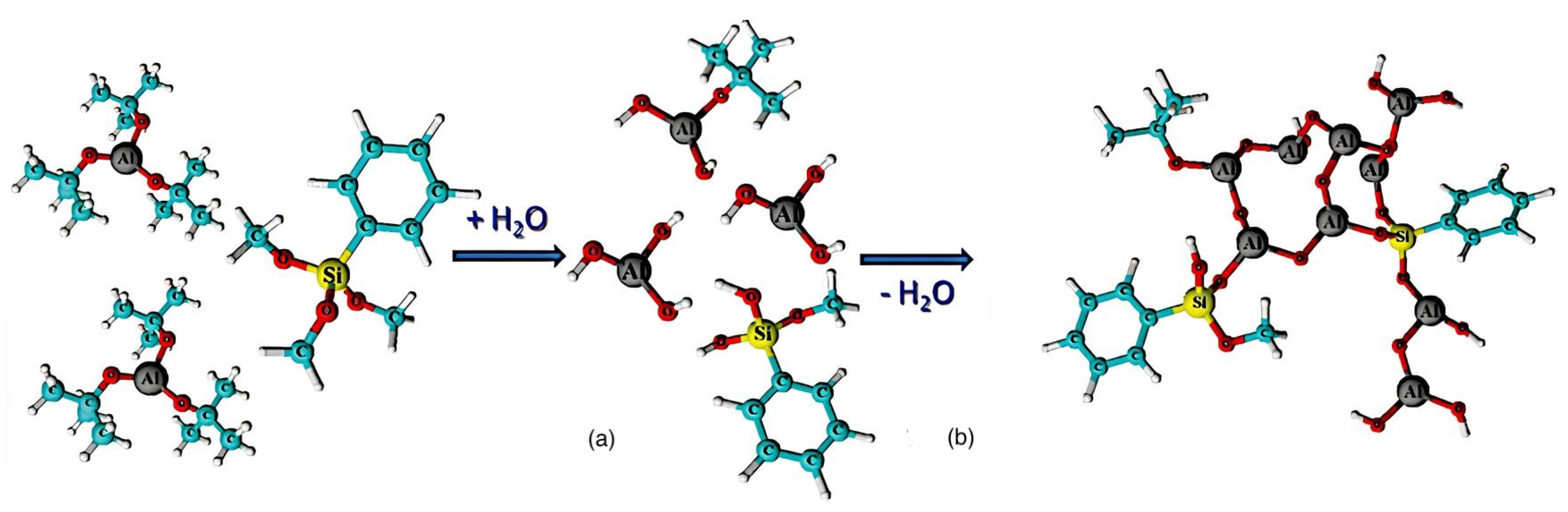
2.3. Inorganic–Organic Hybrid Photoresists
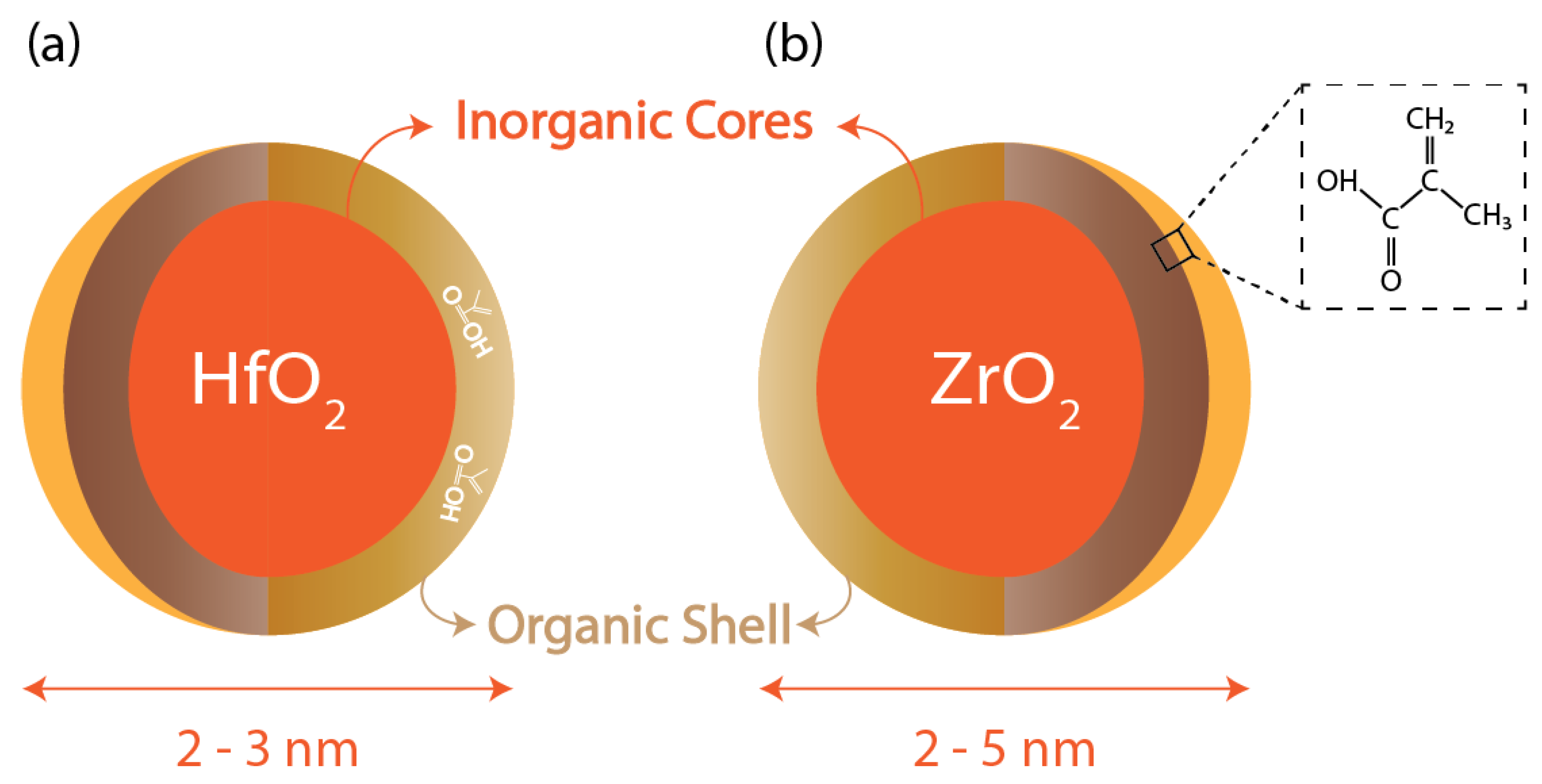
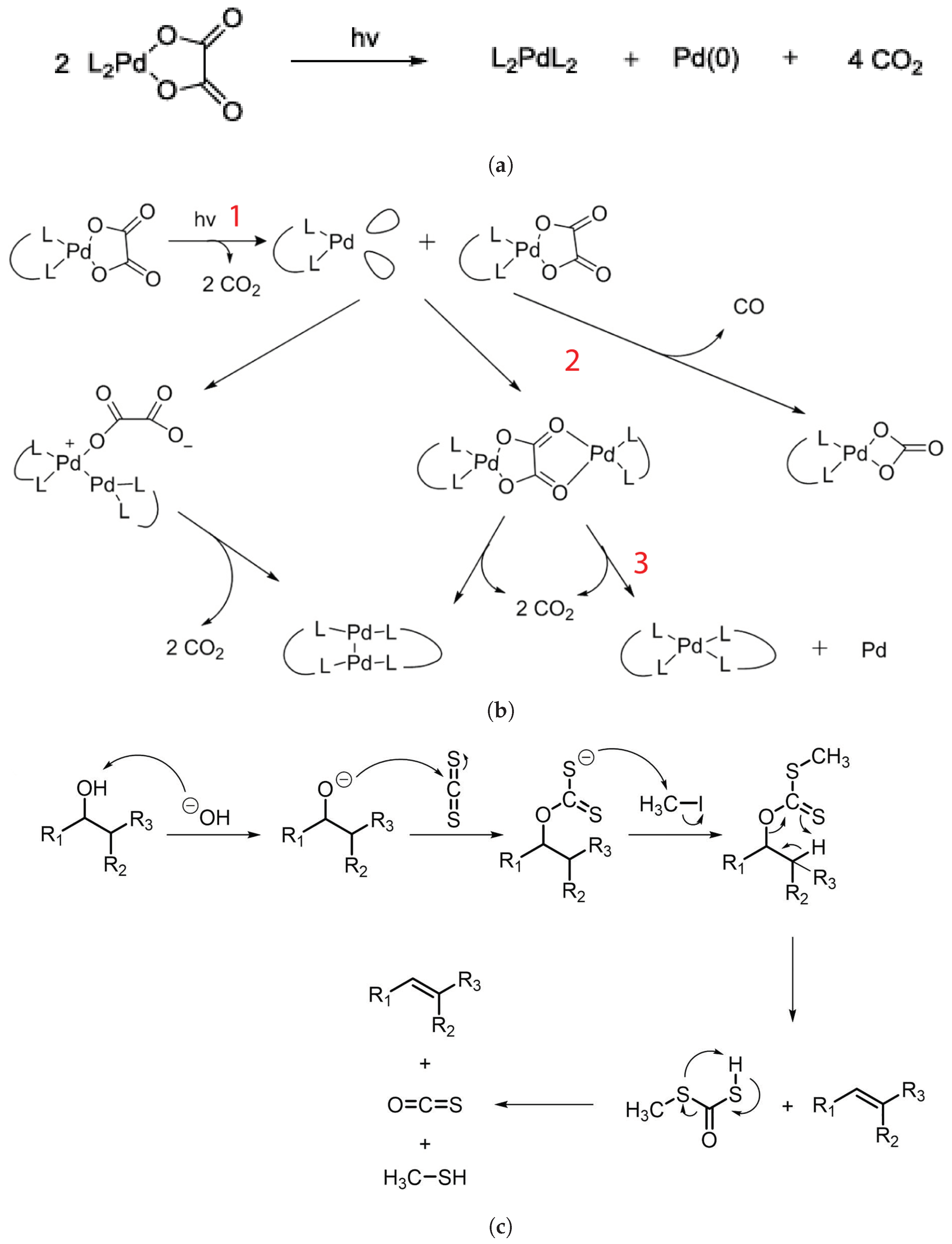
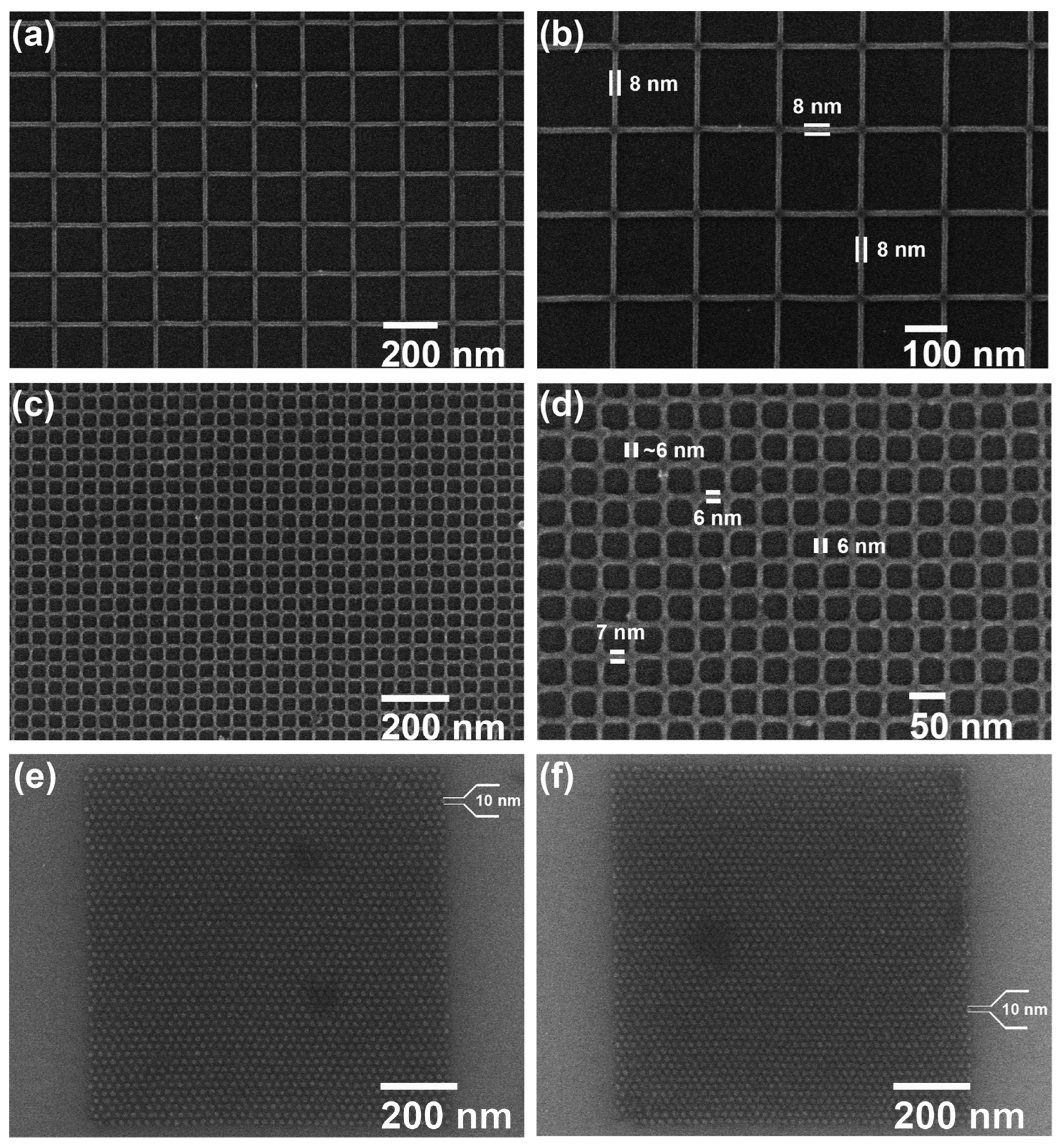
2.3.1. Metal Nanoparticles
- Ligand exchange/surface alternation: In this process, carboxylic acid groups replace the surface ligands of the nanoparticles dissolved in an acetate solution after going through heating, precipitation, and washing using acetone steps to get rid of free acid molecules. The product is added to an organic solution to get the desired “hybrid resist”.
- Controlled hydrolysis: Hafnium isopropoxide (C12H28HfO4) undergoes hydrolysis at an elevated temperature for several hours to give HfO2 after going through processes such as precipitation, centrifugation, washing, and drying under vacuum. This nanoparticle powder is then added to an organic substance to form the hybrid resist.
2.3.2. Low Molecular Weight Complexes
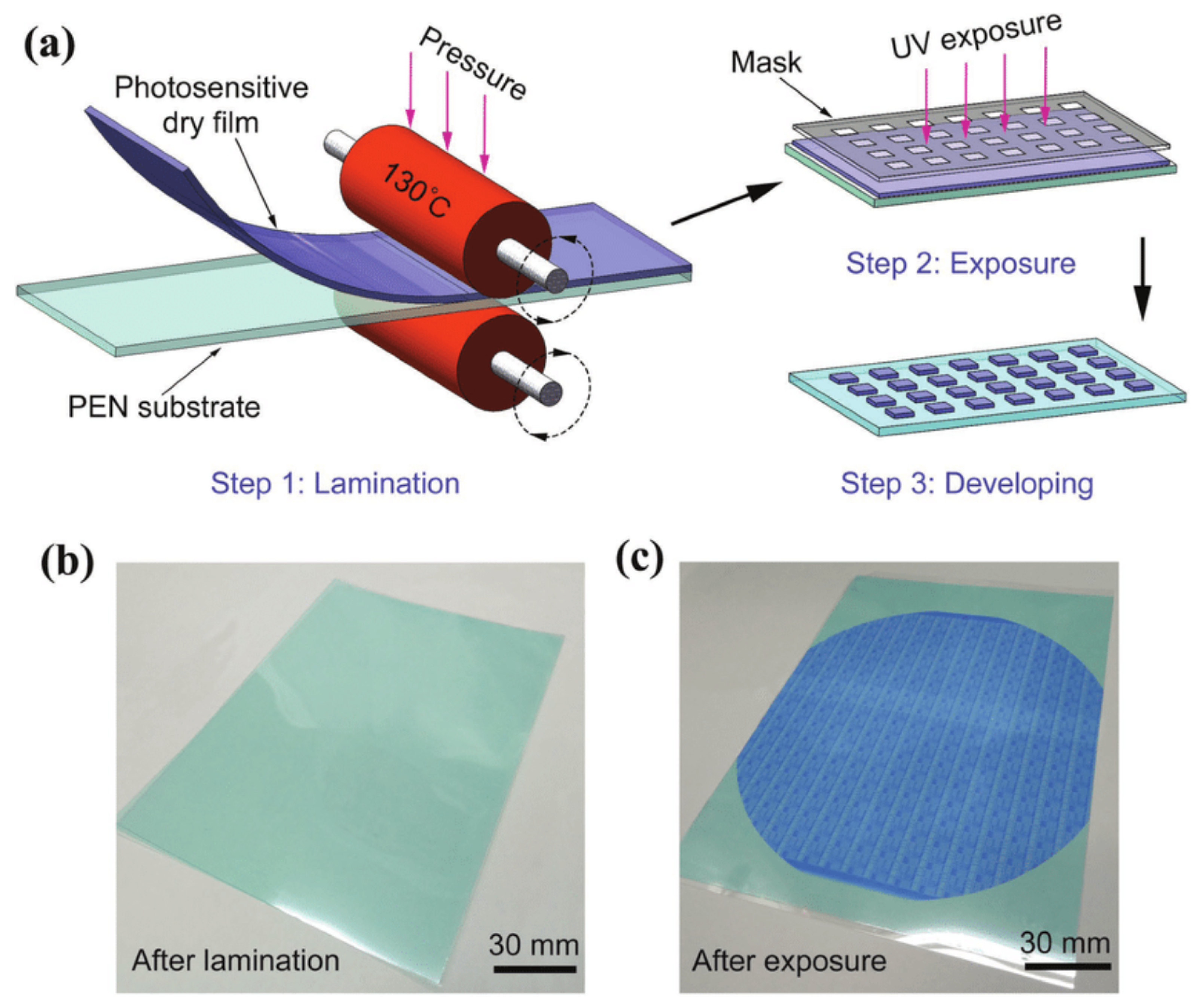
2.4. Dry Film Photoresists
3. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEMS | Micro-Electromechanical Systems |
| DUV | Deep Ultraviolet |
| EUV | Extreme Ultraviolet |
| CARs | Chemically Amplified Resists |
| n-CARs | Non-Chemically Amplified Resists |
| LER | Line Edge Roughness |
| LWR | Line Width Roughness |
| PMMA | Poly(methyl methacrylate) |
| DNQ | Diazonaphthoquinone |
| DFP | Dry Film Photoresist |
| PCB | Printed Circuit Board |
References
- Coorporation, I. Moore’s Law. 2023. Available online: https://www.intel.com/content/www/us/en/newsroom/resources/moores-law.html (accessed on 3 March 2024).
- Sharma, E.; Rathi, R.; Misharwal, J.; Sinhmar, B.; Kumari, S.; Dalal, J.; Kumar, A. Evolution in Lithography Techniques: Microlithography to Nanolithography. Nanomaterials 2022, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pradeep, C.P.; Sharma, S.K.; Reddy, P.G.; Pal, S.P.; Gonsalves, K.E. Recent advances in non-chemically amplified photoresists for next generation IC technology. RSC Adv. 2016, 6, 74462–74481. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.; Choi, S.; Yoon, H.J. Organometallic and coordinative photoresist materials for EUV lithography and related photolytic mechanisms. Coord. Chem. Rev. 2023, 493, 215307. [Google Scholar] [CrossRef]
- Manouras, T.; Argitis, P. High Sensitivity Resists for EUV Lithography: A Review of Material Design Strategies and Performance Results. Nanomaterials 2020, 10, 1593. [Google Scholar] [CrossRef]
- Ober, C.K.; Käfer, F.; Yuan, C. Recent developments in photoresists for extreme-ultraviolet lithography. Polymer 2023, 280, 126020. [Google Scholar] [CrossRef]
- Schmid, G.M.; Stewart, M.D.; Wang, C.Y.; Vogt, B.D.; Prabhu, V.M.; Lin, E.K.; Willson, C.G. Resolution limitations in chemically amplified photoresist systems. In Proceedings of the Advances in Resist Technology and Processing XXI; Sturtevant, J.L., Ed.; Society of Photo-Optical Instrumentation Engineers (SPIE) Conference Series; SPIE: San Jose, CA, USA, 2004; Volume 5376, pp. 333–342. [Google Scholar] [CrossRef]
- Sortland, M.; Re, R.D.; Passarelli, J.; Hotalen, J.; Vockenhuber, M.; Ekinci, Y.; Neisser, M.; Freedman, D.A.; Brainard, R.L. Positive-tone EUV resists: Complexes of platinum and palladium. Spie Proc. 2015, 9422, 697–705. [Google Scholar] [CrossRef]
- Shioya, T.; Maruyama, K.; Kimura, T. Novel Material Development for EUV Resist towards sub-20nm half pitch. J. Photopolym. Sci. Technol. 2011, 24, 199–204. [Google Scholar] [CrossRef]
- Brainard, R.L.; Trefonas, P.; Lammers, J.H.; Cutler, C.A.; Mackevich, J.F.; Trefonas, A.; Robertson, S.A. Shot noise, LER, and quantum efficiency of EUV photoresists. In Proceedings Volume 5374, Emerging Lithographic Technologies VIII; SPIE: San Jose, CA, USA, 2004; pp. 74–85. [Google Scholar] [CrossRef]
- Ashby, P.D.; Olynick, D.L.; Ogletree, D.F.; Naulleau, P.P. Resist Materials for Extreme Ultraviolet Lithography: Toward Low-Cost Single-Digit-Nanometer Patterning. Adv. Mater. 2015, 27, 5813–5819. [Google Scholar] [CrossRef]
- Belete, Z.T.; Bisschop, P.D.; Welling, U.; Erdmann, A. Stochastic simulation and calibration of organometallic photoresists for extreme ultraviolet lithography. J. Micro/Nanopatterning Mater. Metrol. 2021, 20, 014801. [Google Scholar] [CrossRef]
- Ogletree, D.F. Chapter 2—Molecular excitation and relaxation of extreme ultraviolet lithography photoresists. In Materials and Processes for Next Generation Lithography; Robinson, A., Lawson, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 11, pp. 91–113. [Google Scholar] [CrossRef]
- Yu, M.; Xu, H.; Kosma, V.; Odent, J.; Kasahara, K.; Giannelis, E.; Ober, C. Positive Tone Nanoparticle Photoresists: New Insight on the Patterning Mechanism. J. Photopolym. Sci. Technol. 2016, 29, 509–512. [Google Scholar] [CrossRef]
- Sharma, S.K.; Pal, S.P.; Reddy, P.G.; Kumar, P.; Ghosh, S.; Gonsalves, K.E. Design and development of low activation energy based nonchemically amplified resists (n-CARs) for next generation EUV lithography. Microelectron. Eng. 2016, 164, 115–122. [Google Scholar] [CrossRef]
- Bangalore Prakash, P.; Blackwell, J.; Theofanis, P.; Escobedo, F. Atomistic Modeling Approach for Predicting Association of Photoacid Generators in Extreme Ultraviolet Polymeric Photoresists. Chem. Mater. 2023, 35, 9050–9063. [Google Scholar] [CrossRef]
- Lawrie, K.J.; Blakey, I.; Blinco, J.P.; Cheng, H.H.; Gronheid, R.; Jack, K.S.; Pollentier, I.; Leeson, M.J.; Younkin, T.R.; Whittaker, A.K. Chain scission resists for extreme ultraviolet lithography based on high performance polysulfone-containing polymers. J. Mater. Chem. 2011, 21, 5629–5637. [Google Scholar] [CrossRef]
- Cho, I.; Kim, H.s.; Hong, J.Y.; Oh, H.K.; Kim, S. Reduction of Line Width and Edge Roughness by Resist Reflow Process for Extreme Ultra-Violet Lithography. J. Korean Phys. Soc. Korean Phys. Soc. 2010, 56, 1767–1771. [Google Scholar] [CrossRef]
- Yasin, S.; Hasko, D.; Ahmed, H. Fabrication of <5 nm width lines in poly(methylmethacrylate) resist using a water:isopropyl alcohol developer and ultrasonically-assisted development. Appl. Phys. Lett. 2001, 78, 2760–2762. [Google Scholar] [CrossRef]
- Gilbert, B.C.; King, D.M.; Thomas, C.B. Radical reactions of carbohydrates. Part 2. An electron spin resonance study of the oxidation of D-glucose and related compounds with the hydroxyl radical. J. Chem. Soc. Perkin Trans. 1981, 2, 1186–1199. [Google Scholar] [CrossRef]
- Chen, L.; Goh, Y.K.; Cheng, H.H.; Smith, B.W.; Xie, P.; Montgomery, W.; Whittaker, A.K.; Blakey, I. Aqueous developable dual switching photoresists for nanolithography. J. Polym. Sci. Part Polym. Chem. 2012, 50, 4255–4265. [Google Scholar] [CrossRef]
- Chang, S.W.; Ayothi, R.; Bratton, D.; Yang, D.; Felix, N.; Cao, H.B.; Deng, H.; Ober, C.K. Sub-50 nm feature sizes using positive tone molecular glass resists for EUV lithography. J. Mater. Chem. 2006, 16, 1470–1474. [Google Scholar] [CrossRef]
- Bratton, D.; Yang, D.; Dai, J.; Ober, C. Recent progress in high resolution lithography. Polym. Adv. Technol. 2006, 17, 94–103. [Google Scholar] [CrossRef]
- Luo, C.; Xu, C.; Lv, L.; Li, H.; Huang, X.; Liu, W. Review of recent advances in inorganic photoresists. RSC Adv. 2020, 10, 7066–7084. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Ueda, M. A new positive-type photoresist based on mono-substituted hydroquinone calix[8]arene and diazonaphthoquinone. J. Mater. Chem. 1999, 9, 697–702. [Google Scholar] [CrossRef]
- Süptitz, P.L.; Thompson, F.; Willson, C.G.; Bowden, M.J. (Eds.) Introduction to Microlithography, 2nd ed.; American Chemical Society: Washington, DC, USA, 1994; IBBN 8–8412–2848–5. [Google Scholar] [CrossRef]
- Cairns, T.; Eglinton, G. Hydrogen Bonding in Phenols. Nature 1962, 196, 535–537. [Google Scholar] [CrossRef]
- Tiwale, N.; Subramanian, A.; Kisslinger, K.; Lu, M.; Kim, J.; Stein, A.; Nam, C.Y. Advancing next generation nanolithography with infiltration synthesis of hybrid nanocomposite resists. J. Mater. Chem. 2019, 7, 8889–8899. [Google Scholar] [CrossRef]
- Troian-Gautier, L.; Mattiuzzi, A.; Reinaud, O.; Lagrost, C.; Jabin, I. Use of calixarenes bearing diazonium groups for the development of robust monolayers with unique tailored properties. Org. Biomol. Chem. 2020, 18, 3624–3637. [Google Scholar] [CrossRef]
- Fallica, R.; Haitjema, J.; Wu, L.; Ortega, S.C.; Brouwer, A.M.; Ekinci, Y. Absorption coefficient of metal-containing photoresists in the extreme ultraviolet. J. Micro/Nanolithogr. Mems Moems 2018, 17, 023505. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. Organometallic Compounds: An Opportunity for Chemical Biology? ChemBioChem 2012, 13, 1232–1252. [Google Scholar] [CrossRef]
- Subramanian, A.; Tiwale, N.; Lee, W.I.; Kisslinger, K.; Lu, M.; Stein, A.; Kim, J.; Nam, C.Y. Vapor-Phase Infiltrated Organic–Inorganic Positive-Tone Hybrid Photoresist for Extreme UV Lithography. Adv. Mater. Interfaces 2023, 10, 2300420. [Google Scholar] [CrossRef]
- Elias, J.; ETIENNE, P.; Calas, S.; Duffours, L. Hybrid Organic-Inorganic photoresists, a promising class of materials for Optofluidic integration. EPJ Web Conf. 2019, 215, 16001. [Google Scholar] [CrossRef]
- Stowers, J.; Keszler, D.A. High resolution, high sensitivity inorganic resists. Microelectron. Eng. 2009, 86, 730–733. [Google Scholar] [CrossRef]
- Mamontova, E.; Favier, I.; Pla, D.; Gómez, M. Organometallic interactions between metal nanoparticles and carbon-based molecules: A surface reactivity rationale. In 40 Years of GEQO-RSEQ; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 77, pp. 43–103. [Google Scholar] [CrossRef]
- Trikeriotis, M.; Bae, W.J.; Schwartz, E.; Krysak, M.; Lafferty, N.; Xie, P.; Smith, B.; Zimmerman, P.A.; Ober, C.K.; Giannelis, E.P. Development of an inorganic photoresist for DUV, EUV, and electron beam imaging. In Proceedings of the Advances in Resist Materials and Processing Technology XXVII; Allen, R.D., Ed.; International Society for Optics and Photonics, SPIE: San Jose, CA, USA, 2010; Volume 7639, p. 76390E. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Sarma, C.; Li, L.; Giannelis, E.P.; Ober, C.K. Increasing sensitivity of oxide nanoparticle photoresists. In Proceedings Volume 9048, Extreme Ultraviolet (EUV) Lithography V; SPIE: San Jose, CA, USA, 2014; p. 90481C. [Google Scholar] [CrossRef]
- Zanchetta, E.; Giustina, G.D.; Grenci, G.; Pozzato, A.; Tormen, M.; Brusatin, G. Novel Hybrid Organic–Inorganic Spin-on Resist for Electron- or Photon-Based Nanolithography with Outstanding Resistance to Dry Etching. Adv. Mater. 2013, 25, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, I.; Zhang, Y.; Haitjema, J.; Tromp, R.M.; van der Molen, S.J.; Brouwer, A.M.; Jobst, J.; Castellanos, S. Key Role of Very Low Energy Electrons in Tin-Based Molecular Resists for Extreme Ultraviolet Nanolithography. ACS Appl. Mater. Interfaces 2020, 12, 9881–9889. [Google Scholar] [CrossRef] [PubMed]
- Sortland, M.; Hotalen, J.; Re, R.D.; Passarelli, J.; Murphy, M.C.; Kulmala, T.S.; Ekinci, Y.; Neisser, M.; Freedman, D.A.; Brainard, R.L. Platinum and palladium oxalates: Positive-tone extreme ultraviolet resists. J. Micro/Nanolithogr. Mems Moems 2015, 14, 043511. [Google Scholar] [CrossRef]
- Saifullah, M.; Asbahi, M.; Kiyani, M.; Tripathy, S.; Ong, E.; Saifullah, A.; Tan, H.R.; Dutta, T.; Ganesan, R.; Valiyaveettil, S.; et al. Direct Patterning of Zinc Sulfide on a Sub-10 Nanometer Scale via Electron Beam Lithography. Acs Nano 2017, 11, 9920–9929. [Google Scholar] [CrossRef]
- Tadano, K.I. 2.17 Selected Diastereoselective Reactions: Intramolecular Diels–Alder Reactions. In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 504–524. [Google Scholar] [CrossRef]
- Semicon, S.M. Apple Brings the World’s First 3 nm Computer Chip. 2023. Available online: https://slkor.medium.com/in-the-development-of-computer-processors-there-is-a-very-interesting-period-in-history-080e3cc4a3b3 (accessed on 11 March 2024).
- Lin, A.; Chen, M.; Tsai, T.H.; Lin, L.Y.; Lin, M.; Lee, E. Next-Generation Dry Film Photoresist for Advanced IC Substrate Applications. In Proceedings of the 2021 16th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 21–23 December 2021; pp. 164–167. [Google Scholar] [CrossRef]
- Garland, S.P.; Murphy, T.M.; Pan, T. Print-to-Pattern Dry Film Photoresist Lithography. J. Micromech. Microeng. 2014, 24, 057002. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.K.; Park, J.M.; Baek, S.I. Positive Type Dry Film Photoresist and Composition for Preparing the Same. U.S. Patent 7,749,676, 6 July 2010. [Google Scholar]
- Chen, Y.Y.; Sun, Y.; Zhu, Q.B.; Wang, B.W.; Yan, X.; Qiu, S.; Li, Q.W.; Hou, P.X.; Liu, C.; Sun, D.M.; et al. High-Throughput Fabrication of Flexible and Transparent All-Carbon Nanotube Electronics. Adv. Sci. 2018, 5, 1700965. [Google Scholar] [CrossRef]
- Kanikella, P.R. Process Development and Applications of a Dry Film Photoresist. Master’s Thesis, University of Missouri-Rolla, Rolla, MO, USA, 2007. [Google Scholar]
- Roos, M.; Winkler, A.; Nilsen, M.; Menzel, S.; Strehle, S. Towards Green 3D-Microfabrication of Bio-MEMS Devices Using ADEX Dry Film Photoresists. Int. J. Precis. Eng.-Manuf.-Green Technol. 2021, 9, 43–57. [Google Scholar] [CrossRef]
- Hinsberg, W.; Wallraff, G.M.; Larson, C.E.; Davis, B.W.; Deline, V.; Raoux, S.; Miller, D.; Houle, F.A.; Hoffnagle, J.; Sanchez, M.I.; et al. Liquid immersion lithography: Evaluation of resist issues. In Proceedings Volume 5376, Advances in Resist Technology and Processing XXI; SPIE: Bellingham, WA, USA, 2004; pp. 21–33. [Google Scholar] [CrossRef]
- Kasahara, K.; Kosma, V.; Odent, J.; Xu, H.; Yu, M.; Giannelis, E.P.; Ober, C.K. Recent progress in nanoparticle photoresists development for EUV lithography. In Proceedings Volume 9776, Extreme Ultraviolet (EUV) Lithography VII; SPIE: San Jose, CA, USA, 2016; p. 977604. [Google Scholar] [CrossRef]
- Ober, C.; Jiang, J.; Zhang, B.; Li, L.; Giannelis, E.; Chun, J.S.; Neisser, M.; Sierra-Alvares, R. New developments in ligand-stabilized metal oxide nanoparticle photoresists for EUV lithography. In Proceedings Volume 9422, Extreme Ultraviolet (EUV) Lithography VI; SPIE: San Jose, CA, USA, 2015; p. 942207. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Mojarad, N.; Gobrecht, J.; Ekinci, Y. Beyond EUV lithography: A comparative study of efficient photoresists’ performance. Sci. Rep. 2015, 5, 9235. [Google Scholar] [CrossRef]
- Levinson, H.J. Lithography in a quantum world. Jpn. J. Appl. Phys. 2023, 62, SG0802. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassaan, M.; Saleem, U.; Singh, A.; Haque, A.J.; Wang, K. Recent Advances in Positive Photoresists: Mechanisms and Fabrication. Materials 2024, 17, 2552. https://doi.org/10.3390/ma17112552
Hassaan M, Saleem U, Singh A, Haque AJ, Wang K. Recent Advances in Positive Photoresists: Mechanisms and Fabrication. Materials. 2024; 17(11):2552. https://doi.org/10.3390/ma17112552
Chicago/Turabian StyleHassaan, Muhammad, Umama Saleem, Akash Singh, Abrar Jawad Haque, and Kaiying Wang. 2024. "Recent Advances in Positive Photoresists: Mechanisms and Fabrication" Materials 17, no. 11: 2552. https://doi.org/10.3390/ma17112552
APA StyleHassaan, M., Saleem, U., Singh, A., Haque, A. J., & Wang, K. (2024). Recent Advances in Positive Photoresists: Mechanisms and Fabrication. Materials, 17(11), 2552. https://doi.org/10.3390/ma17112552