Improvements in Wear and Corrosion Resistance of Ti-W-Alloyed Gray Cast Iron by Tailoring Its Microstructural Properties
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
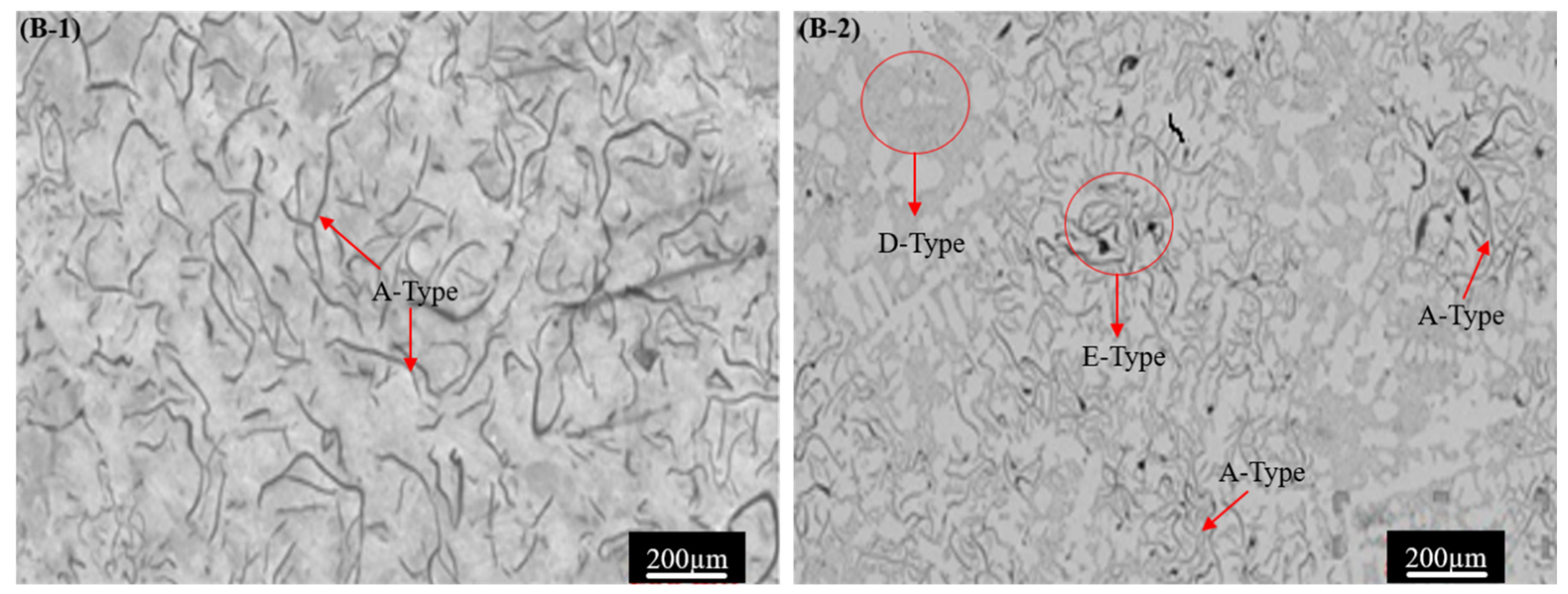
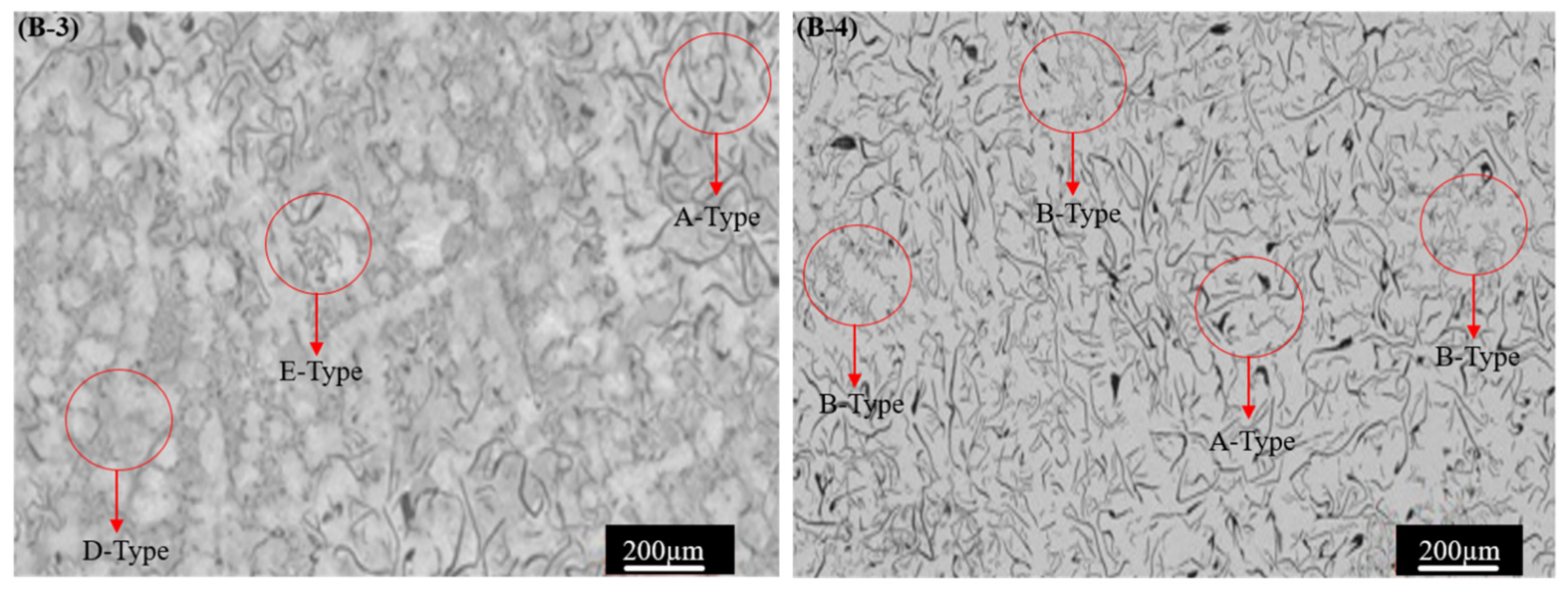
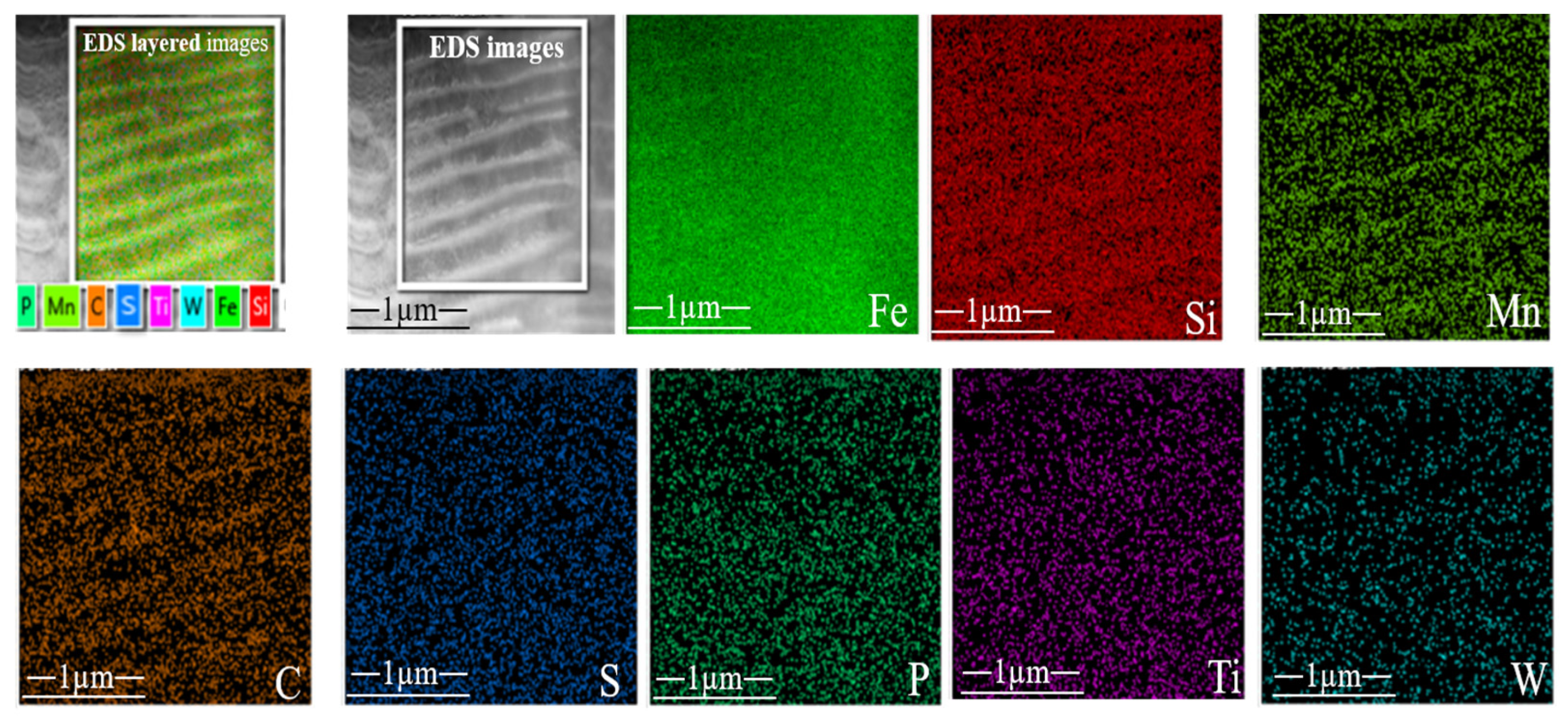
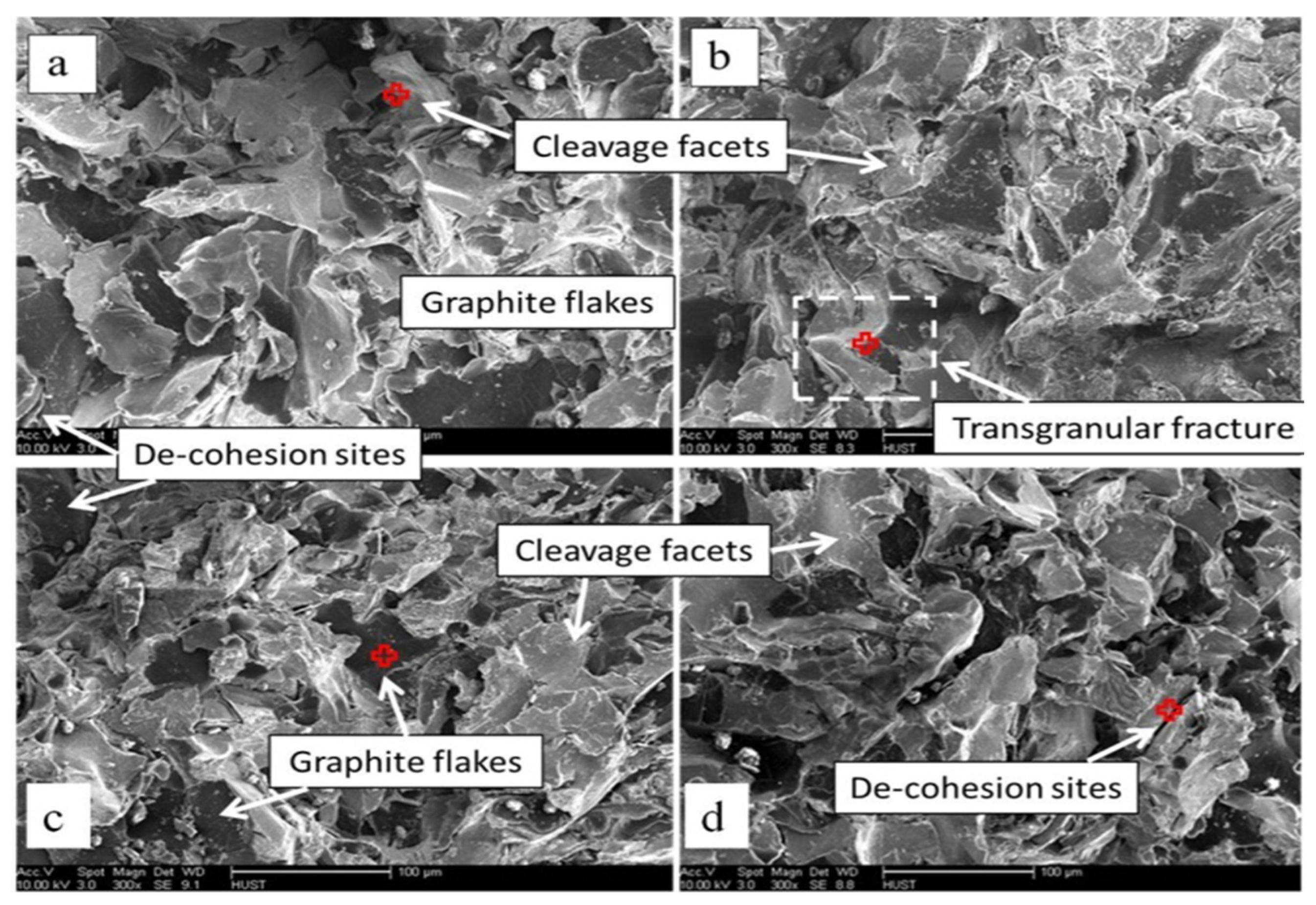
3.1. Microstructure
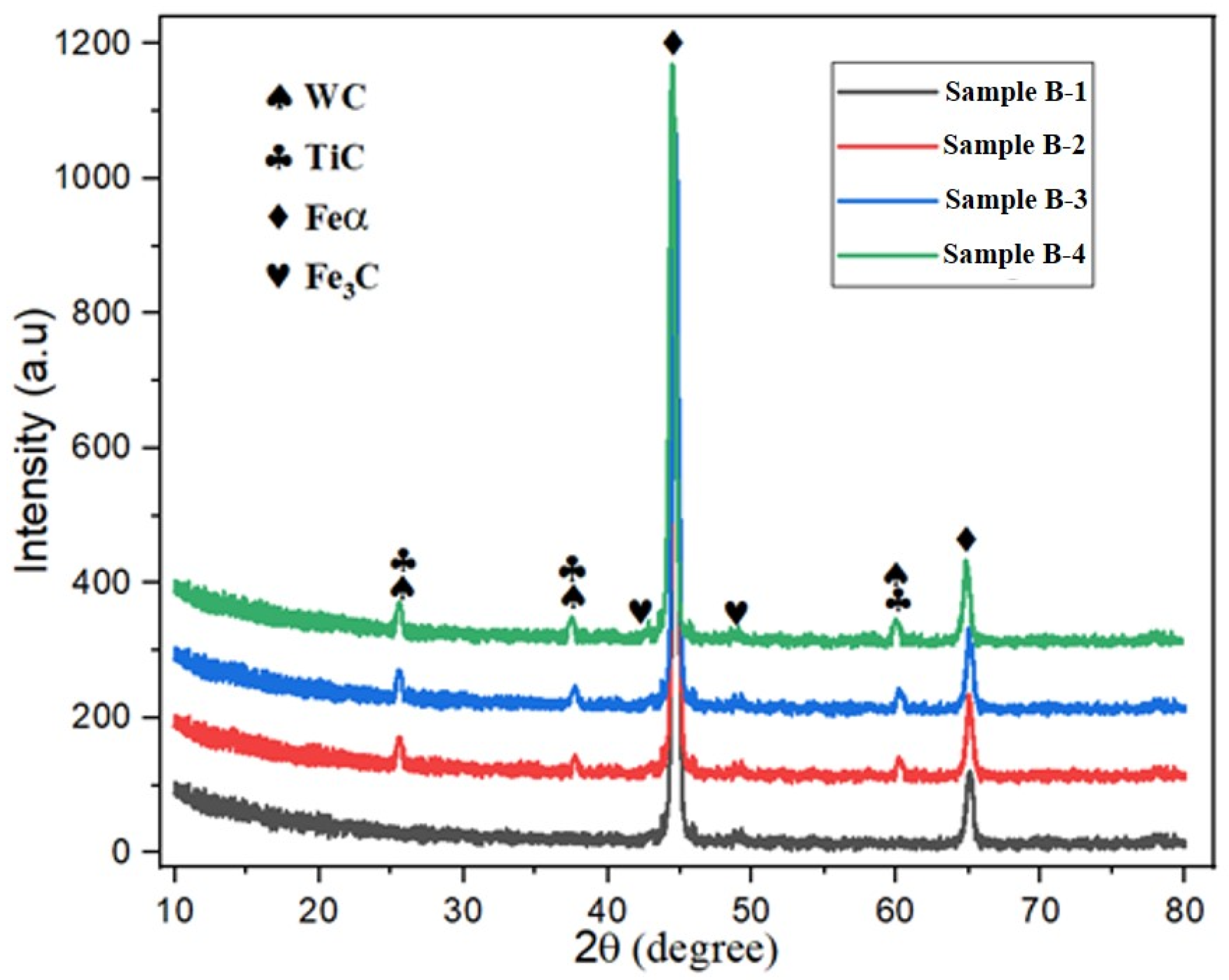
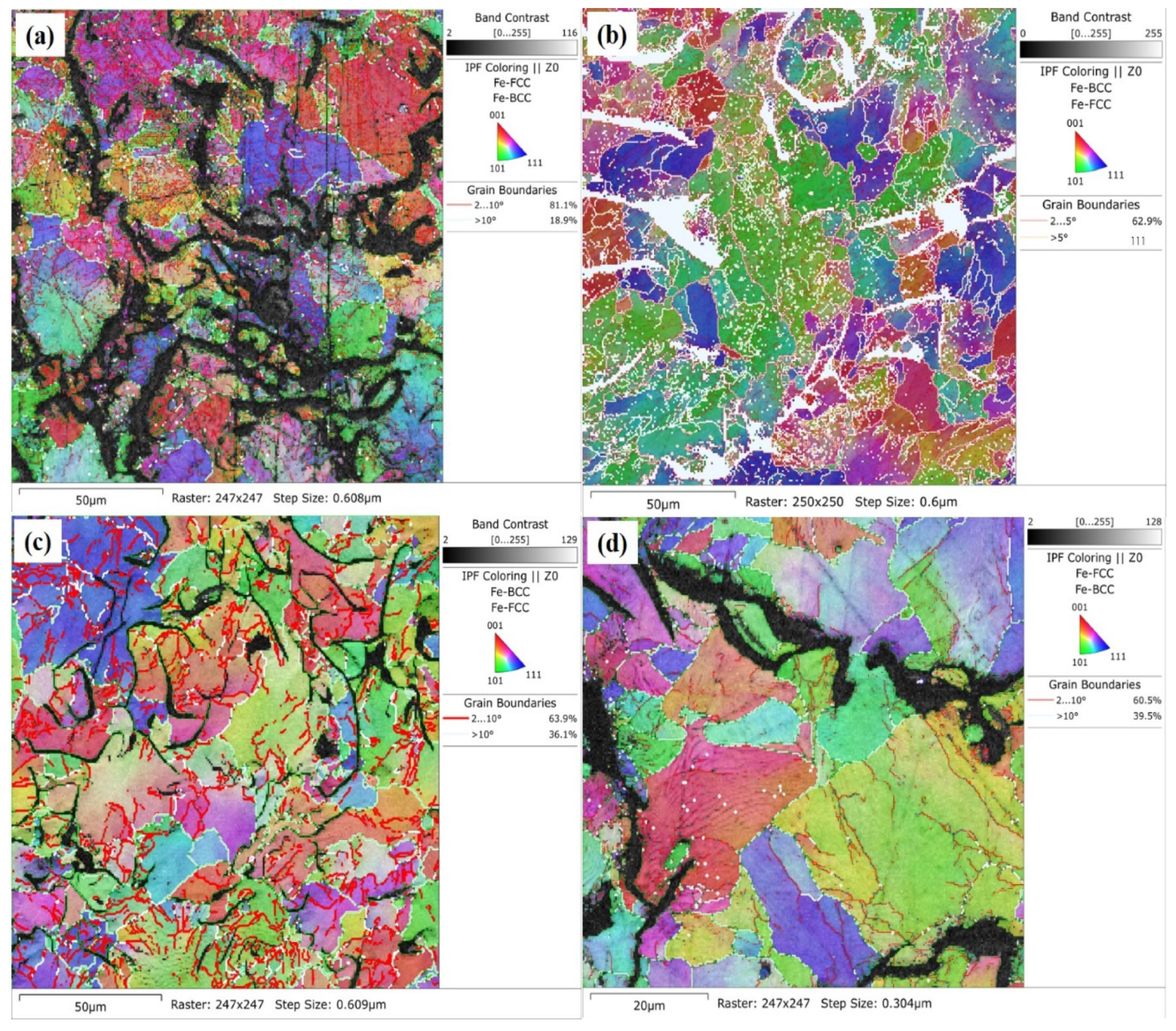
3.2. Analysis of Alterations in Alloyed GCI Crystallographic Texture
3.3. Mechanical Properties
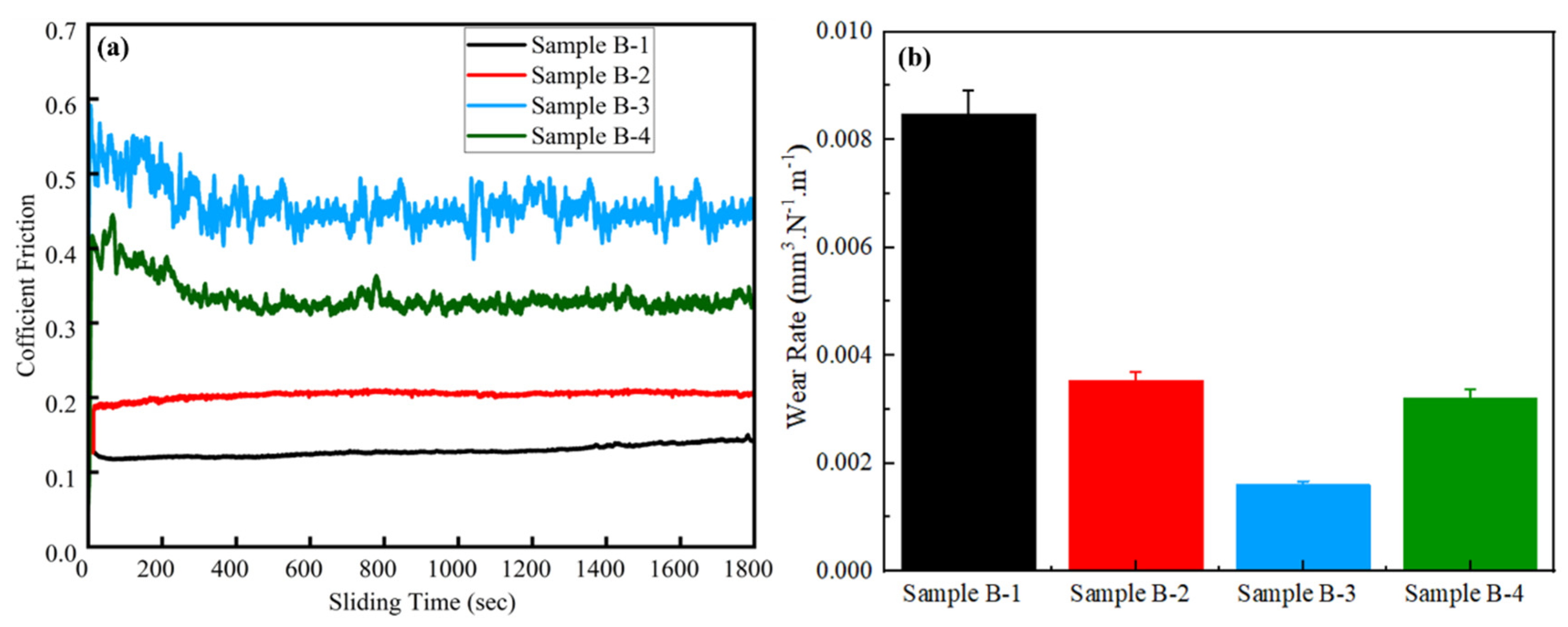
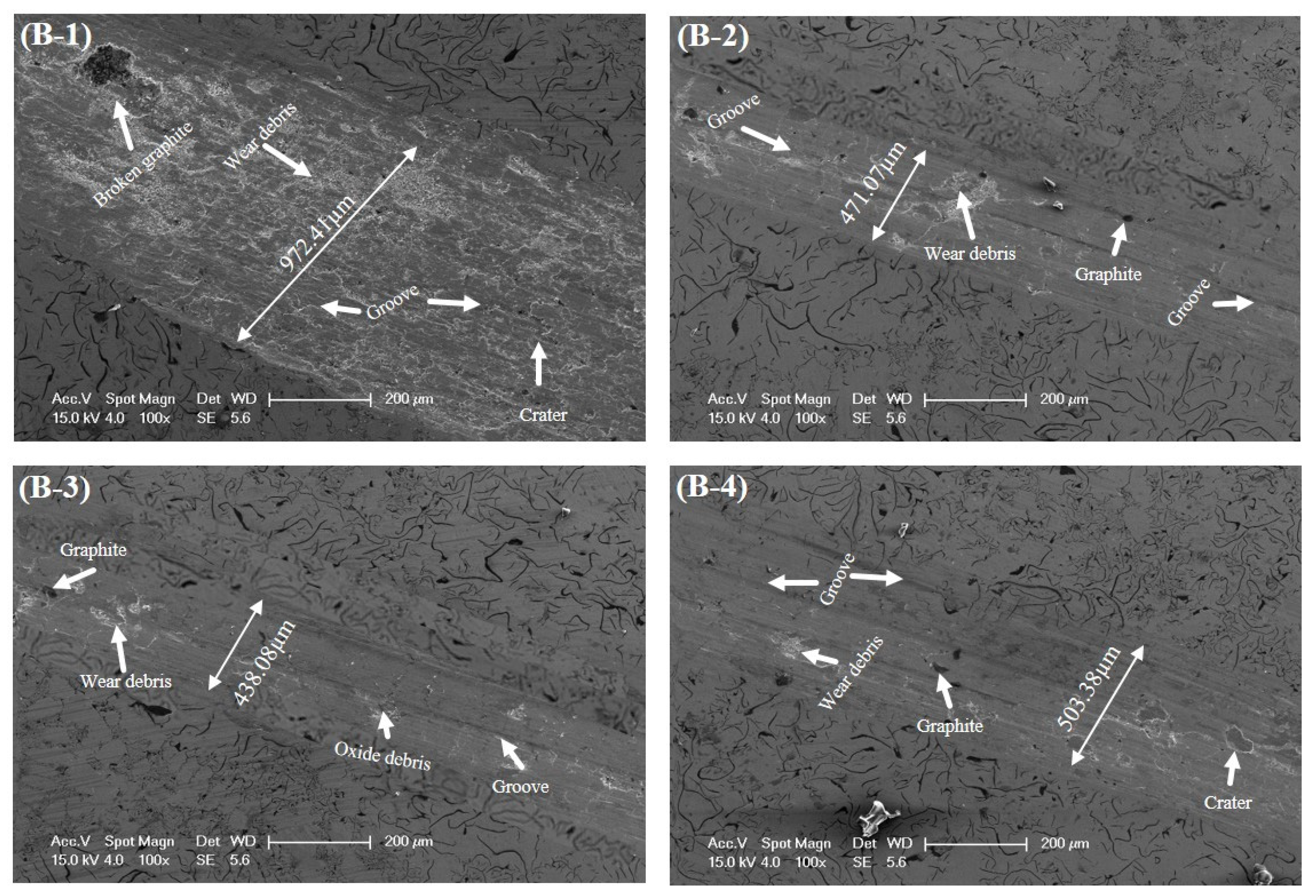
3.4. Tribological Properties
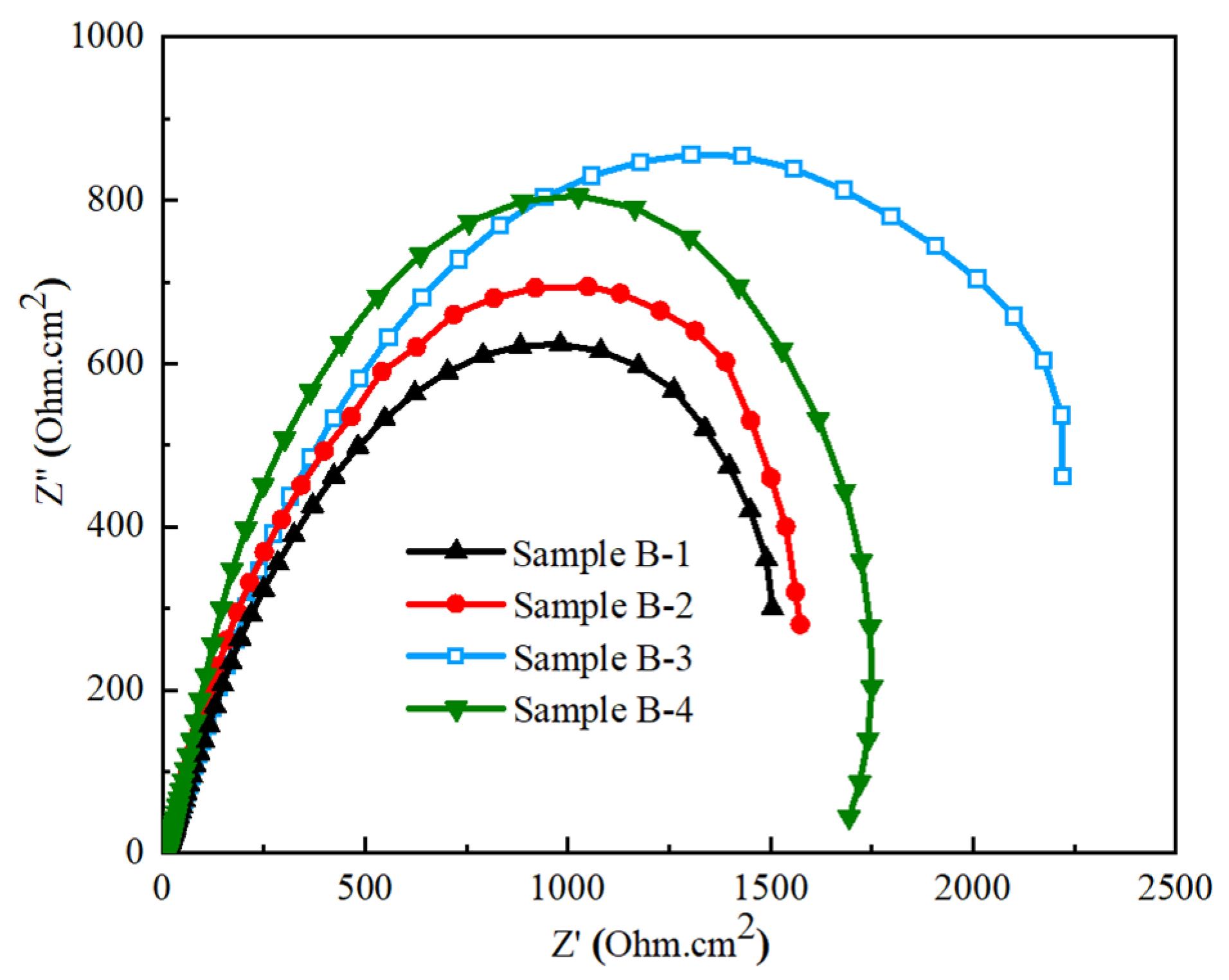
3.5. Corrosion Analysis
3.5.1. Potentiodynamic Polarization Analysis
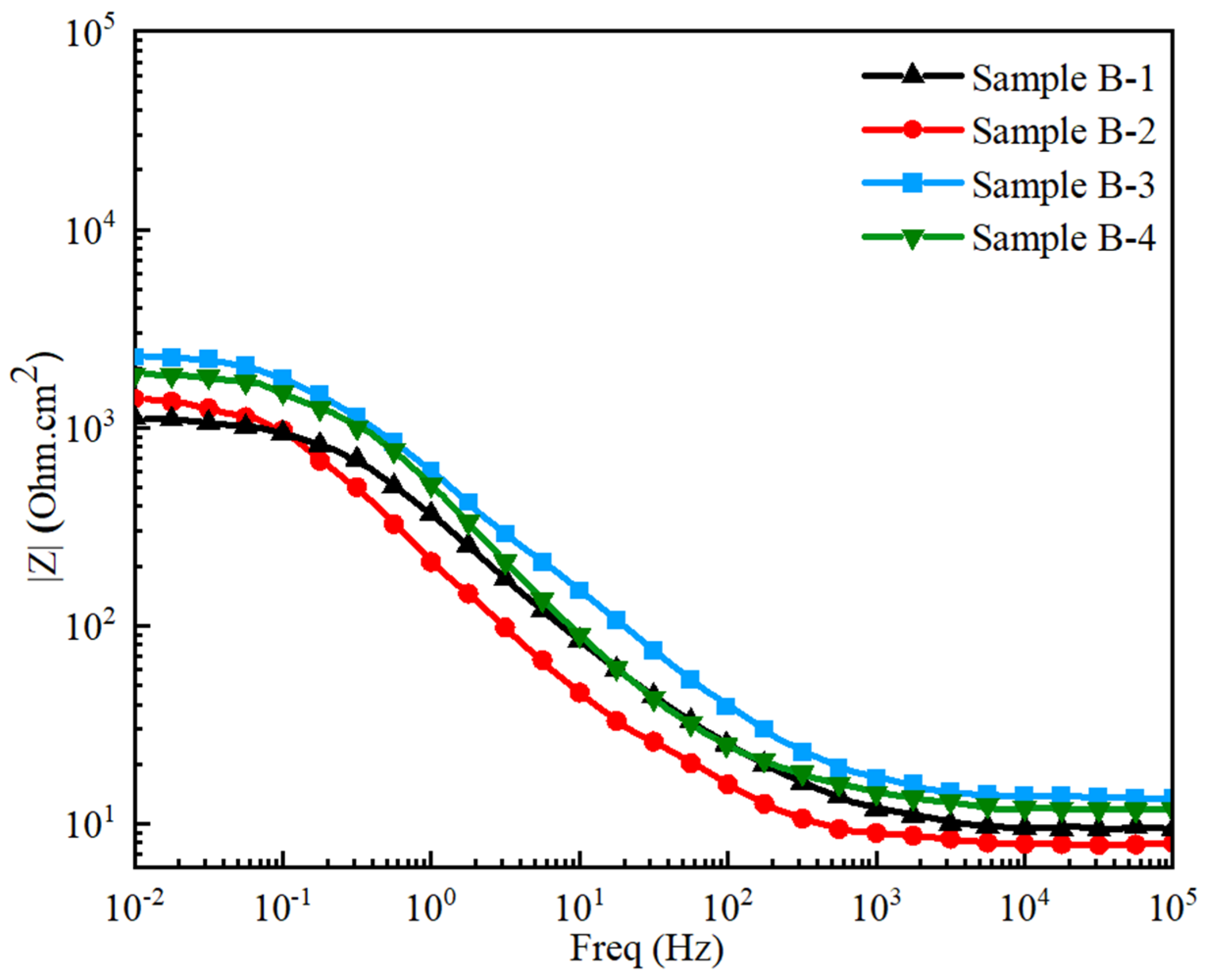
3.5.2. Electrochemical Impedance Spectroscopy
4. Conclusions
- (1)
- The addition of Ti and W has transformed the A-type graphite flaky microstructure into B + E + D-type, which resulted in an improvement in the pearlite volume percentage and the refinement of grain size. The improvement in the mechanical properties can be attributed to the enhancement of the volume fractions of pearlite from 67% to 87%, reducing the average grain size from 378 µm to 349 µm and maximum graphite length from 356 µm to 297 µm.
- (2)
- The hardness of the alloyed GCI sample was improved from 213 BHN to 272 BHN, which demonstrated a reduced wear rate from 8.49 × 10−3 mm3/N·m to 1.59 × 10−3 mm3/N·m and surface roughness from 297.625 nm to 192.553 nm, which indicated a significant improvement in the wear resistance of alloyed GCI.
- (3)
- The lower corrosion resistance, ~1545 Ohm.cm2, of the pristine GCI sample with a coarse grain size and high ferrite volume fraction enhanced the formation of graphitization in the metal matrix. The alloying of tungsten and titanium enhanced the corrosion resistance to ~2290 Ohm·cm2, owning to the refinement of the microstructure, improvement in the pearlites volume fraction, and scarcity of ferrites in GCI.
- (4)
- The crystallographic study showed the misoriented microstructure with grain and sub-grain boundaries distributed at high and low angles. A successfully tailored microstructure of GCI by alloying Ti-W was observed due to the nucleation of grain and sub-grain boundaries, which improved the mechanical, wear, and corrosion properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olejnik, E.; Sobczak, J.J.; Szala, M.; Kurtyka, P.; Tokarski, T.; Janas, A. Dry sliding, slurry abrasion and cavitation erosion of composite layers reinforced by TiC fabricated in situ in cast steel and gray cast iron. J. Mater. Process. Technol. 2022, 308, 117688. [Google Scholar]
- Pang, Z.; Zhou, H.; Xie, G.; Cong, D.; Meng, C.; Ren, L. Effect of bionic coupling units׳ forms on wear resistance of gray cast iron under dry linear reciprocating sliding condition. Opt. Laser Technol. 2015, 70, 89–93. [Google Scholar] [CrossRef]
- Salawu, E.Y.; Ajayi, O.O.; Inegbenebor, A.O.; Akinlabi, S.; Popoola AP, I.; Akinlabi, E.; Uyo, U.O. Electrochemical study and gravimetric behaviour of gray cast iron in varying concentrations of blends as alternative material for gears in ethanol environment. J. Mater. Res. Technol. 2020, 9, 7529–7539. [Google Scholar] [CrossRef]
- Brklay, A. Investigating the Properties of Low Carbon Gray Cast Iron. Eurasian J. Chem. Med. Pet. Res. 2023, 2, 217–224. [Google Scholar]
- Li, Q.; Zhang, Y.; Zhang, Y.; Liu, H.; Ren, H.; Zhong, Y.; Huang, W. Influence of Sn and Nb additions on the microstructure and wear characteristics of a gray cast iron. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Sugishita, J.; Fujiyoshi, S. The effect of cast iron graphites on friction and wear performance I: Graphite film formation on grey cast iron surfaces. Wear 1981, 66, 209–221. [Google Scholar] [CrossRef]
- Ren, J.; Ren, F.; Li, F.; Cui, L.; Xiong, Y.; Volinsky, A.A. Effects of Microstructure, Mechanical and Physical Properties on Machinability of Graphite Cast Irons. Metals 2020, 10, 285. [Google Scholar] [CrossRef]
- Cárcel-Carrasco, F.-J.; Pascual-Guillamón, M.; Salas-Vicente, F.; Donderis-Quiles, V. Influence of Heat Treatment in the Microstructure of a Joint of Nodular Graphite Cast Iron when Using the Tungsten Inert Gas Welding Process with Perlitic Grey Cast Iron Rods as Filler Material. Metals 2019, 9, 48. [Google Scholar] [CrossRef]
- Tonolini, P.; Montesano, L.; Pola, A.; Bontempi, G.; Gelfi, M. Wear Behavior of Nb Alloyed Gray Cast Iron for Automotive Brake Disc Application. Metals 2023, 13, 365. [Google Scholar] [CrossRef]
- Hamatschek, C.; Augsburg, K.; Schobel, D.; Gramstat, S.; Stich, A.; Gulden, F.; Hesse, D. Comparative Study on the Friction Behaviour and the Particle Formation Process between a Laser Cladded Brake Disc and a Conventional Grey Cast Iron Disc. Metals 2023, 13, 300. [Google Scholar] [CrossRef]
- González-Pociño, A.; Asensio-Lozano, J.; Álvarez-Antolín, F.; García-Diez, A. Evaluation of hardness, sliding wear and strength of a hypoeutectic white iron with 25% cr after heat treatments. Metals 2021, 11, 947. [Google Scholar] [CrossRef]
- Fontana, M.G. Cast-iron resistance Corrosion. Ind. Eng. Chem. 1951, 43, 71A–74A. [Google Scholar] [CrossRef]
- Shepelev, Y.S.; Rudenko, L. Mechanism of gray cast iron corrosion. Sov. Mater. Sci. Fiz. Khim. Mekh. Mater./Acad. Sci. Ukr. SSR 1978, 14, 86–87. [Google Scholar] [CrossRef]
- Wang, B.; Han, X.; Barber, G.C.; Pan, Y. Wear behavior of austempered and quenched and tempered gray cast irons under similar hardness. Metals 2019, 9, 1329. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Vadiraj, A.; Balachandran, G.; Kamaraj, M. Effect of misch metal inoculation on microstructure, mechanical and wear properties of hypoeutectic gray cast irons. Mater. Des. 2009, 30, 4488–4492. [Google Scholar] [CrossRef]
- Razaq, A.; Yin, Y.; Zhou, J.; Shen, X.; Ji, X.; Ullah, I. Influence of alloying elements Sn and Ti on the microstructure and mechanical properties of gray cast iron. Procedia Manuf. 2019, 37, 353–359. [Google Scholar] [CrossRef]
- Niu, L.B.; Xu, Y.H.; Peng, J.H.; Wu, H. Effects of heat treatment on mechanical properties and microstructure of tungsten fiber reinforced grey cast iron matrix composites. China Foundry 2009, 6, 333–338. [Google Scholar]
- Anijdan, S.M.; Bahrami, A.; Varahram, N.; Davami, P. Effects of tungsten on erosion–corrosion behavior of high chromium white cast iron. Mater. Sci. Eng. A 2007, 454, 623–628. [Google Scholar] [CrossRef]
- Katiyar, P.K. A comprehensive review on synergy effect between corrosion and wear of cemented tungsten carbide tool bits: A mechanistic approach. Int. J. Refract. Met. Hard Mater. 2020, 92, 105315. [Google Scholar] [CrossRef]
- Günen, A.; Soylu, B.; Karakaş, Ö. Titanium carbide coating to improve surface characteristic, wear and corrosion resistance of spheroidal graphite cast irons. Surf. Coat. Technol. 2022, 437, 128280. [Google Scholar] [CrossRef]
- Güney, B. Corrosion and wear behaviour of HVOF spraying WC-12% Ni coating on gray cast-iron. Indian J. Eng. Mater. Sci. (IJEMS) 2021, 28, 73–81. [Google Scholar]
- Mohammadi, J.; Eslami, A.; Ashrafizadeh, F.; Fadaeifard, F.; Hadizadeh, B.; Nazari, B. An Investigation on Room and High Temperature Wear Performance of Fe-45% WC Hardfaced Coating Applied on Ductile Cast Iron. J. Mater. Eng. Perform. 2024, 33, 1358–1368. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Y.; Liu, Y.; Barber, G.C.; Qiu, F.; Hu, M. Wear behavior of composite strengthened gray cast iron by austempering and laser hardening treatment. J. Mater. Res. Technol. 2020, 9, 2037–2043. [Google Scholar] [CrossRef]
- Sai, Q.; Hao, J.; Wang, S.; Wang, Z. Improving the Properties of Gray Cast Iron by Laser Surface Modification. Materials 2023, 16, 5533. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, W.; Zhang, H. Corrosion and wear resistance characteristics of NiCr coating by laser alloying with powder feeding on grey iron liner. Wear 2006, 260, 1349–1355. [Google Scholar] [CrossRef]
- Feldshtein, E.; Devojno, O.; Wojciechowski, S.; Kardapolava, M.; Kasiakova, I. On the microstructure, micro-hardness and wear behavior of gray cast iron surface layer after laser strengthening. Materials 2022, 15, 1075. [Google Scholar] [CrossRef]
- Catalán, N.; Ramos-Moore, E.; Boccardo, A.; Celentano, D. Surface laser treatment of cast irons: A review. Metals 2022, 12, 562. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Ogundare, O.D.; Oluwafemi, O.M.; Ebisike, K.; Nageri, A.K.; Akinwamide, S.O.; Olubambi, P.A. A Review on Heat Treatment of Cast Iron: Phase Evolution and Mechanical Characterization. Materials 2022, 15, 7109. [Google Scholar] [CrossRef]
- Oyetunji, A.; Talabi, H.K. Effects of heat treatment process on mechanical and microstructural properties of gray cast iron. Acta Tech. Corviniensis-Bull. Eng. 2016, 9, 117. [Google Scholar]
- Woodward, R.G.; Toumpis, A.; Galloway, A. The influence of tempering and annealing on the microstructure and sliding wear response of G350 grey cast iron. Wear 2022, 496, 204283. [Google Scholar] [CrossRef]
- Olawale, J.; Ibitoye, S.; Oluwasegun, K. Processing techniques and productions of ductile iron: A review. Int. J. Sci. Eng. Res. 2016, 7, 397–423. [Google Scholar]
- Srivastava, A.K.; Das, K. Microstructure and abrasive wear study of (Ti, W) C-reinforced high-manganese austenitic steel matrix composite. Mater. Lett. 2008, 62, 3947–3950. [Google Scholar] [CrossRef]
- Gregorutti, R.W.; Grau, J.E. Mechanical properties of compacted graphite cast iron with different microstructures. Int. J. Cast Met. Res. 2014, 27, 275–281. [Google Scholar] [CrossRef]
- Aranke, O.; Algenaid, W.; Awe, S.; Joshi, S. Coatings for automotive gray cast iron brake discs: A review. Coatings 2019, 9, 552. [Google Scholar] [CrossRef]
- Priyan, M.S.; Hariharan, P. Wear and corrosion resistance of Fe based coatings by HVOF sprayed on gray cast-iron for automotive application. Tribol. Ind. 2014, 36, 394. [Google Scholar]
- ASTM E8/E8M-24; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- Brown, J.R. Chapter 10—Melting and Treatment of Steel for Casting. In Foseco Ferrous Foundryman’s Handbook, 11th ed.; Brown, J.R., Ed.; Butterworth-Heinemann: Oxford, UK, 2000; pp. 121–129. [Google Scholar]
- Razaq, A.; Zhou, J.-X.; Hussain, T.; Tu, Z.-X.; Yin, Y.-J.; Ji, X.-Y.; Xiao, G.; Shen, X. Effect of alloying elements W, Ti, Sn on microstructure and mechanical properties of gray iron 220. China Foundry 2019, 16, 393–398. [Google Scholar] [CrossRef]
- Kardak, A.; Sinclair, G. Stress concentration factors for ASTM E8/E8M-16a standard round specimens for tension testing. J. Test. Eval. 2020, 48, 711–719. [Google Scholar] [CrossRef]
- Gabauer, W. The Estimation of Uncertainties in Hardness Measurements; Standard Measurements and Testing: Middlesex, UK, 2000. [Google Scholar]
- Górny, M.; Kawalec, M. Effects of titanium addition on microstructure and mechanical properties of thin-walled compacted graphite iron castings. J. Mater. Eng. Perform. 2013, 22, 1519–1524. [Google Scholar] [CrossRef]
- Fraś, E.; Lopez, H.F.; Kawalec, M.; Gorny, M. Role of Alloying Additions in the Solidification Kinetics and Resultant Chilling Tendency and Chill of Cast Iron. Metals 2015, 5, 256–288. [Google Scholar] [CrossRef]
- Seikh, A.H.; Sarkar, A.; Singh, J.K.; Mohammed SM, K.; Alharthi, N.; Ghosh, M. Corrosion characteristics of copper-added austempered gray cast iron (AGCI). Materials 2019, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Tamta, K.; Karunakar, D.B. Grain refinement of cast alloys: A review. Int. J. Mech. Eng. Robot. Res. 2014, 1, 199–212. [Google Scholar]
- Zhao, J.; Lee, T.; Lee, J.H.; Jiang, Z.; Lee, C.S. Effects of tungsten addition on the microstructure and mechanical properties of microalloyed forging steels. Metall. Mater. Trans. A 2013, 44, 3511–3523. [Google Scholar] [CrossRef]
- Qiu, K.; Xiao, B. Effect of Mechanical Vibration on Microstructure and Mechanical Properties of Gray Cast Iron in Lost Foam Casting. Math. Probl. Eng. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Wang, C.; Yan, R.; Li, F.; Wang, J.; Wang, J. Study on friction and wear behavior of gray cast iron with different carbon content at different temperatures. Mater. Res. Express 2024, 11, 046505. [Google Scholar] [CrossRef]
- Tada, N.; Uemori, T.; Sakamoto, J. Prediction of the fracture location by tensile tests of gray cast iron based on the dimensional changes of graphite flakes. J. Press. Vessel Technol. 2021, 143, 021502. [Google Scholar] [CrossRef]
- Lin, M.-B.; Wang, C.-J.; Volinsky, A.A. High temperature oxidation behavior of flake and spheroidal graphite cast irons. Oxid. Met. 2011, 76, 161–168. [Google Scholar] [CrossRef]
- Saidi, A. Reaction synthesis of Fe–(W, Ti) C composites. J. Mater. Process. Technol. 1999, 89, 141–144. [Google Scholar] [CrossRef]
- Wiengmoon, A. Carbides in high chromium cast irons. Naresuan Univ. Eng. J. 2011, 6, 64–71. [Google Scholar]
- Chen, X.; Li, Y.; Zhang, H. Microstructure and mechanical properties of high boron white cast iron with about 4 wt% chromium. J. Mater. Sci. 2011, 46, 957–963. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Effect of heat treatment on microstructure and mechanical properties of high boron white cast iron. Mater. Sci. Eng. A 2010, 528, 770–775. [Google Scholar] [CrossRef]
- Abdel-Aziz, K.; El-Shennawy, M.; Omar, A.A. Microstructural characteristics and mechanical properties of heat treated High-Cr white cast iron alloys. Int. J. Appl. Eng. Res. 2017, 12, 4675–4686. [Google Scholar]
- Kopyciński, D.; Siekaniec, D.; Szczęsny, A.; Guzik, E.; Nowak, A. The effect of Fe-Ti inoculation on solidification, structure and mechanical properties of high chromium cast iron. Arch. Metall. Mater. 2017, 26, 2183–2187. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Liu, Y.; Zhou, S.; Dang, X. Effect of Ti-V-Nb-Mo addition on microstructure of high chromium cast iron. China Foundry 2012, 9, 148–153. [Google Scholar]
- Sun, P.; Qiu, C. Influence of addition of TiAl particles on microstructural and mechanical property development in a selectively laser melted stainless steel. Mater. Sci. Eng. A 2021, 826, 141925. [Google Scholar] [CrossRef]
- Siekaniec, D.; Kopyciński, D.; Guzik, E.; Szczęsny, A. Effect of inoculation treatment on number of primary austenite grains in hypoeutectic chromium cast iron: EBSD imaging and mathematical structure prediction. Materials 2022, 15, 6318. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.J. The effect of tin on the mechanical and physical properties of gray cast iron. Master’s Thesis, Lehigh University, Bethlehem, PA, USA, 1975. [Google Scholar]
- Bockus, S. A study of the microstructure and mechanical properties of continuously cast iron products. Metalurgija 2006, 45, 287–290. [Google Scholar]
- Lacaze, J.; Sertucha, J.; Larrañaga, P.; Suarez, R. Combined effects of copper and tin at intermediate level of manganese on the structure and properties of as-cast nodular graphite cast iron. Arch. Metall. Mater. 2017, 62, 825–831. [Google Scholar] [CrossRef]
- Hoare, W.E. Modifying the Structure of Cast-iron by Additions of Tin. Tin Uses 1959, 47, 4–8. [Google Scholar]
- Gonzaga, R. Influence of ferrite and pearlite content on mechanical properties of ductile cast irons. Mater. Sci. Eng. A 2013, 567, 1–8. [Google Scholar] [CrossRef]
- Xiao, B.T.; Fan, Z.T.; Jiang, W.M.; Xiang, J.H.; Yan, X.F. Effect of vibration frequency on primary phase and properties of grey cast iron fabricated by lost foam casting. China Foundry 2020, 17, 1–7. [Google Scholar] [CrossRef]
- Razaq, A.; Peng, X.; Hafeez, M.A.; Ali, W.; Shehabeldeen, T.A.; Yin, Y.J.; Zhou, J.X. Failure analysis of oil refinery heater treater’s fractured fire tube. Eng. Fail. Anal. 2023, 154, 107672. [Google Scholar] [CrossRef]
- Wu, S.C.; Wene, H.C.; Tseng, K.H.; Yau, W.H.; Wu, M.J.; Chou, C.P.; Hsu, W.K. Sliding wear resistance of tempered D6ac steel. Vacuum 2013, 87, 89–94. [Google Scholar] [CrossRef]
- Feldshtein, E.; Devojno, O.; Patalas-Maliszewska, J.; Kardapolava, M.; Kasiakova, I. On the Efficiency of Laser Alloying of Grey Cast Iron with Tungsten and Silicon Carbides. Materials 2023, 16, 6230. [Google Scholar] [CrossRef]
- Minhas, B.; Hanif, Z.; Nadeem, M.H.; Batool, S.A.; Ahmad, K.; Aizaz, A.; Manzur, J.; Rehman, M.A.U. The electrochemical and in-vitro study on electrophoretic deposition of chitosan/gelatin/hydroxyapatite coating on 316L stainless steel. Carbohydr. Polym. Technol. Appl. 2023, 5, 100322. [Google Scholar] [CrossRef]
- Rouein, Z.; Jafari, H.; Pishbin, F.; Mohandes, F.; Simchi, A. In vitro bioactivity and biocompatibility of magnesium implants coated with poly (methyl methacrylate)-bioactive glass composite. Mater. Today Commun. 2022, 33, 104872. [Google Scholar] [CrossRef]
- Sobhani, A.; Salimi, E. Low temperature preparation of diopside nanoparticles: In-vitro bioactivity and drug loading evaluation. Scientific Reports. 2023, 13, 16330. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, H.; Lelito, J.; Mróz, M.; Radoń, M. Influence of Heat Treatment Parameters of Austempered Ductile Iron on the Microstructure, Corrosion and Tribological Properties. Materials 2023, 16, 4107. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.A.; Ahmad, K.; Irfan, M.; Ur Rehman, M.A. Zn–Mn-doped mesoporous bioactive glass nanoparticle-loaded zein coatings for bioactive and antibacterial orthopedic implants. J. Funct. Biomater. 2022, 13, 97. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Khoie, S.M.; Aparicio, M.; López-Elvira, E.; Méndez, J.; Gago, R. Structural properties and corrosion resistance of tantalum nitride coatings produced by reactive DC magnetron sputtering. RSC Adv. 2016, 6, 89061–89072. [Google Scholar] [CrossRef]
- Minhas, B.; Dino, S.; Qian, H.; Zuo, Y. An approach for high stability TiO2 in strong acid environments at high temperature. Surf. Coat. Technol. 2020, 395, 125932. [Google Scholar] [CrossRef]
- García, E.; Flores-Martínez, M.; Rivera, L.P.; Camps-Carvajal, E.E.; Muhl, S. The Effects of the Addition of Zr on the Mechanical and Tribological Properties of TaN Coating. J. Mater. Eng. Perform. 2023, 1–11. [Google Scholar] [CrossRef]
- Palumbo, G.; Górny, M.; Bitka, A. Influence of the Cooling Rate on Mechanical and Corrosion Properties of Ferritic Si–Mo Ductile Cast Iron. Metall. Mater. Trans. A 2023, 54, 2362–2373. [Google Scholar] [CrossRef]
- Ahmad, K.; Manzur, J.; Tahir, M.; Hussain, R.; Khan, M.; Wadood, A.; Avcu, E.; Rehman, M.A. Electrophoretic deposition, microstructure and selected properties of zein/cloves coatings on 316L stainless steel. Prog. Org. Coat. 2023, 176, 107407. [Google Scholar] [CrossRef]
- Gong, L.; Fu, H.; Zhi, X. Corrosion wear of hypereutectic high chromium cast iron: A review. Metals 2023, 13, 308. [Google Scholar] [CrossRef]



| Sample | C | Si | Mn | P | S | Ti | W |
|---|---|---|---|---|---|---|---|
| B-1 | 3.321 | 2.206 | 0.567 | 0.025 | 0.081 | 0 | 0 |
| B-2 | 3.435 | 2.109 | 0.542 | 0.031 | 0.082 | 0.612 | 0.516 |
| B-3 | 3.395 | 2.157 | 0.576 | 0.029 | 0.092 | 0.762 | 0.785 |
| B-4 | 3.294 | 2.125 | 0.593 | 0.024 | 0.089 | 0.985 | 1.351 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Applied load (N) | 20 | Revolution (rpm) | 50 |
| Sliding time (s) | 1800 | Wear track radius (mm) | 3 |
| Atmosphere | Dry | Temperature °C | 30 °C ± 5 °C |
| Sample # | Structure Type | Graphite Content, % | Average Graphite Length, µm | Max Graphite Length, µm | Average Grain Size, µm | Pearlite and Ferrite, % |
|---|---|---|---|---|---|---|
| B-1 | Type A | 10.2 ± 2.3 | 31.9 ± 2.5 | 356 ± 31 | 378 ± 18 | 67/33 |
| B-2 | Type A + D + E | 12.9 ± 2.1 | 33.7 ± 2.4 | 319 ± 19 | 363 ± 21 | 79/21 |
| B-3 | Type A + D + E | 12.2 ± 1.7 | 35.2 ± 3.7 | 297 ± 16 | 349 ± 19 | 87/13 |
| B-4 | Type A + B | 13.3 ± 4.2 | 36.3 ± 3.2 | 324 ± 09 | 358 ± 31 | 74/26 |
| Sample # | Tensile Strength (MPa) | Yield Strength (MPa) | Brinell Hardness (BHN) |
|---|---|---|---|
| B-1 | 346 ± 12 | 260 ± 3 | 213 ± 7 |
| B-2 | 362 ± 9 | 286± 11 | 241 ± 4 |
| B-3 | 375 ± 7 | 310± 2 | 272 ± 8 |
| B-4 | 351 ± 10 | 271± 6 | 243 ± 3 |
| Sample | COF | Wear Rate (mm3/N·m) | Wear Width (µm) | Surface Roughness (nm) |
|---|---|---|---|---|
| B-1 | 0.12 | 8.49 × 10−3 | 972.41 | 297.625 |
| B-2 | 0.19 | 5.52 × 10−3 | 471.07 | 260.727 |
| B-3 | 0.51 | 1.59 × 10−3 | 438.08 | 192.55 |
| B-4 | 0.40 | 3.21 × 10−3 | 503.38 | 238.794 |
| Sample ID | i (A/cm2) | Ecorr (V) | RP (Ohm·cm2) |
|---|---|---|---|
| B-1 | −5.6924 | −0.5832 | 1545 |
| B-2 | −5.7008 | −0.5767 | 1654 |
| B-3 | −5.9561 | −0.4813 | 2290 |
| B-4 | −5.8168 | −0.5427 | 1856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razaq, A.; Yu, P.; Khan, A.R.; Ji, X.-Y.; Yin, Y.-J.; Zhou, J.-X.; Shehabeldeen, T.A. Improvements in Wear and Corrosion Resistance of Ti-W-Alloyed Gray Cast Iron by Tailoring Its Microstructural Properties. Materials 2024, 17, 2468. https://doi.org/10.3390/ma17102468
Razaq A, Yu P, Khan AR, Ji X-Y, Yin Y-J, Zhou J-X, Shehabeldeen TA. Improvements in Wear and Corrosion Resistance of Ti-W-Alloyed Gray Cast Iron by Tailoring Its Microstructural Properties. Materials. 2024; 17(10):2468. https://doi.org/10.3390/ma17102468
Chicago/Turabian StyleRazaq, Abdul, Peng Yu, Adnan Raza Khan, Xiao-Yuan Ji, Ya-Jun Yin, Jian-Xin Zhou, and Taher A. Shehabeldeen. 2024. "Improvements in Wear and Corrosion Resistance of Ti-W-Alloyed Gray Cast Iron by Tailoring Its Microstructural Properties" Materials 17, no. 10: 2468. https://doi.org/10.3390/ma17102468
APA StyleRazaq, A., Yu, P., Khan, A. R., Ji, X.-Y., Yin, Y.-J., Zhou, J.-X., & Shehabeldeen, T. A. (2024). Improvements in Wear and Corrosion Resistance of Ti-W-Alloyed Gray Cast Iron by Tailoring Its Microstructural Properties. Materials, 17(10), 2468. https://doi.org/10.3390/ma17102468






