An Evaluation of the Radioactive Content of Ashes Obtained from the Use of Fuels from Recycled Materials by Co-Processing in the Cement Industry
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation and Chemical Characterization
2.2. Measurement by Gamma Spectrometry
2.3. Calculation of the Natural Radionuclide Enrichment Factor of the Ashes Obtained from the Various Wastes after Use as Fuel
2.4. Assessment of the Effective Doses of the Residues Used for Workers
3. Results
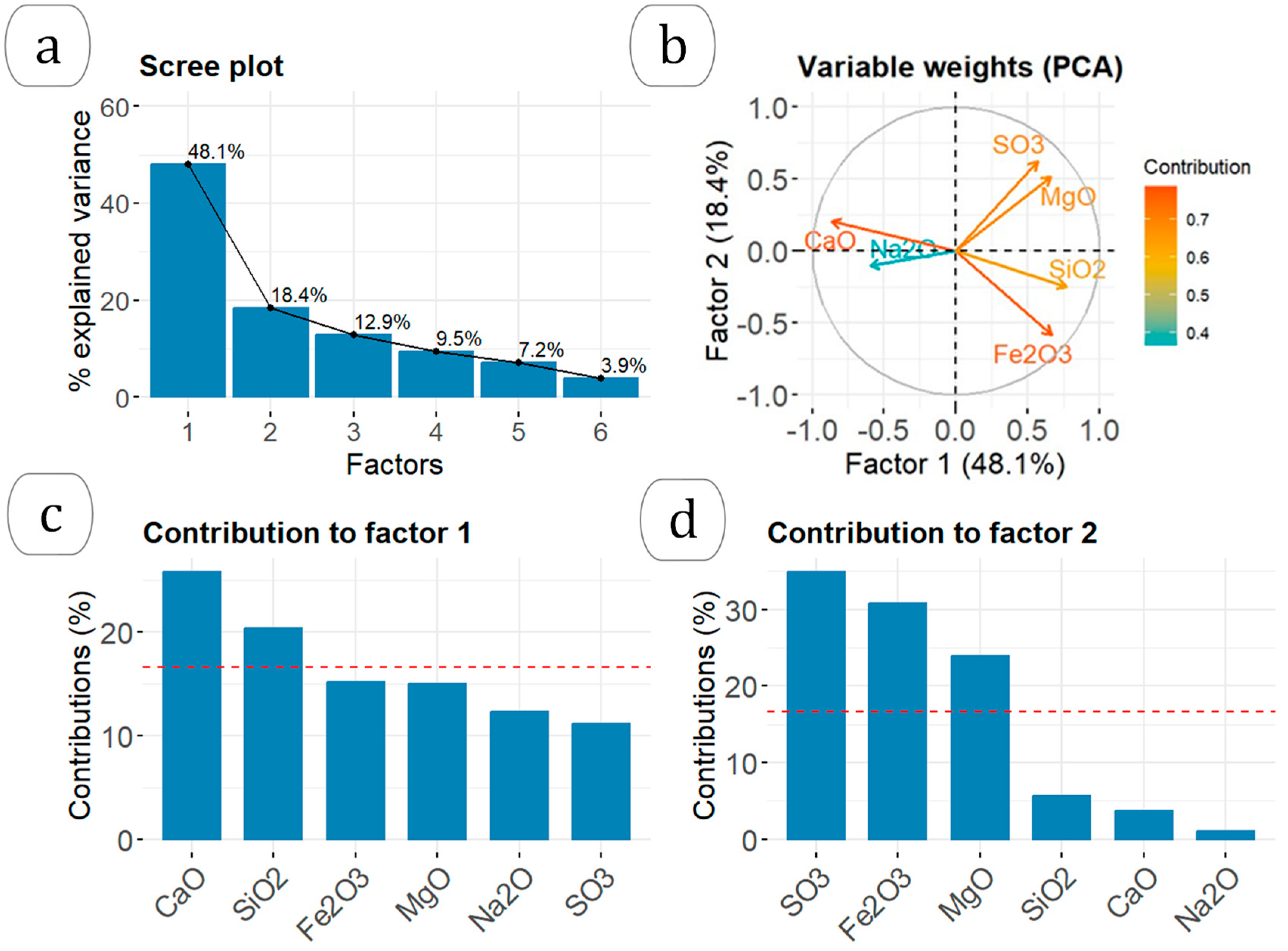
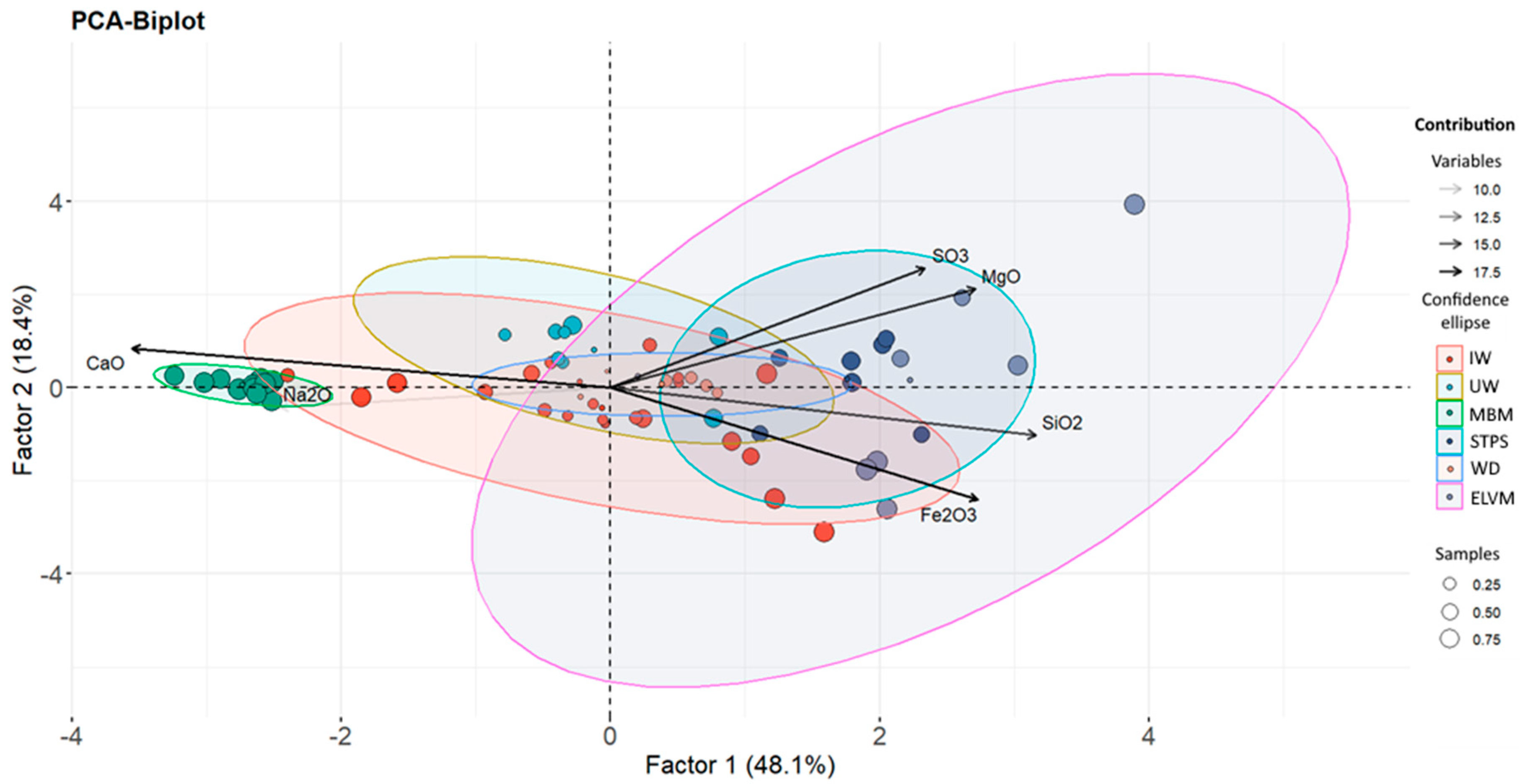
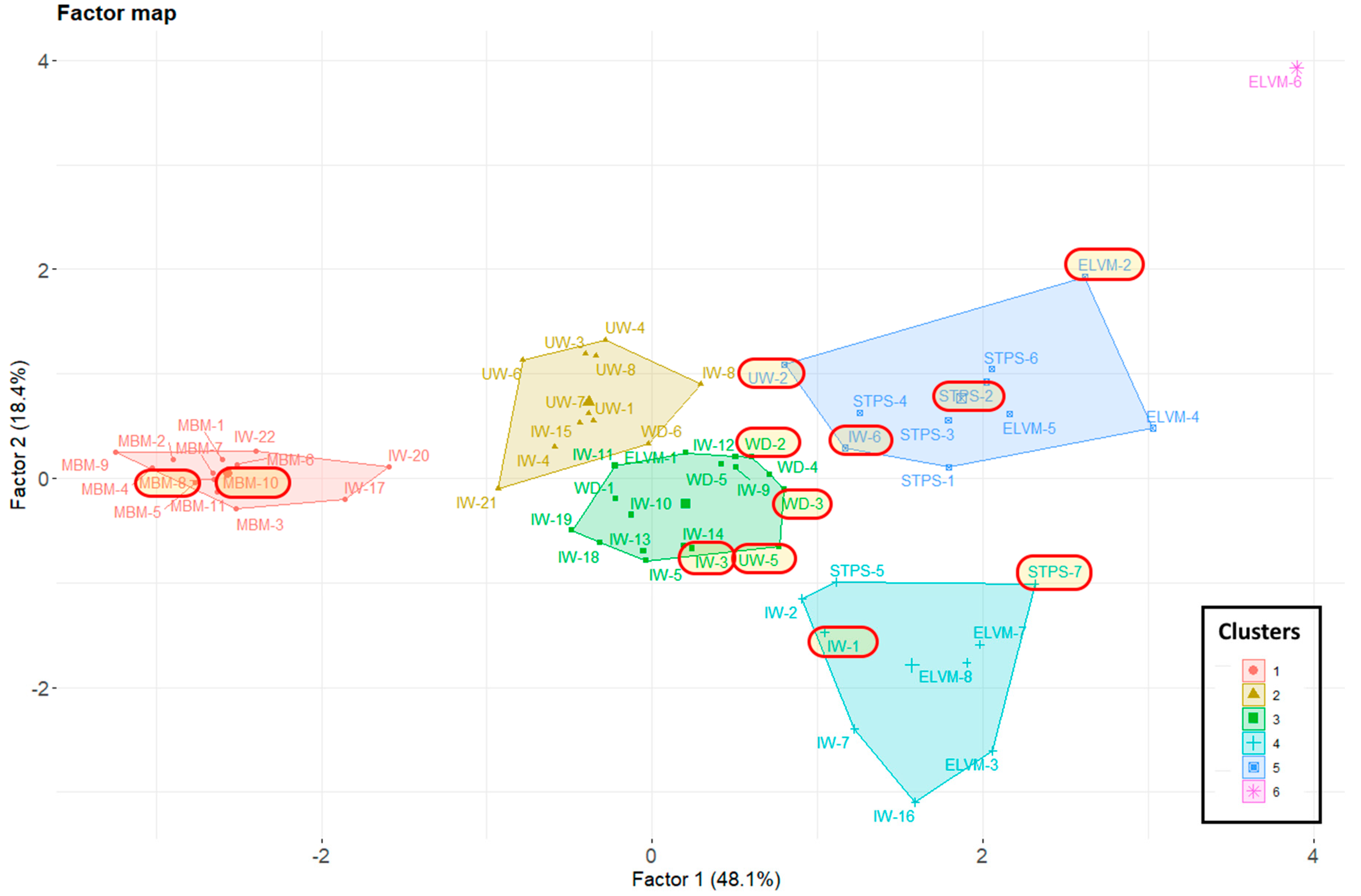
3.1. Relationship between Chemical Composition and Type of Waste
3.2. Activity Concentration of Natural Radionuclides in Different Wastes
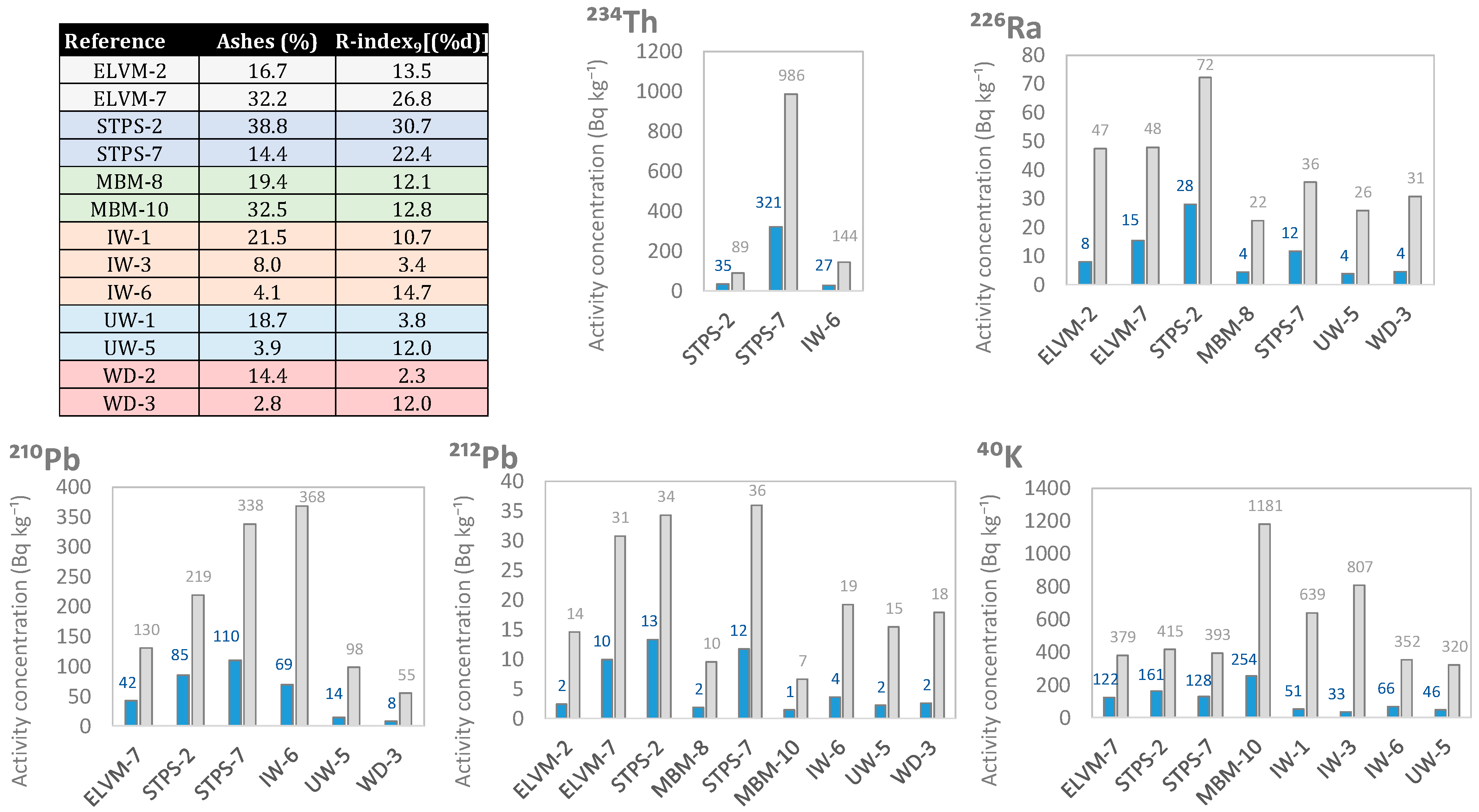
3.3. Enrichment in Natural Radionuclides of the Ashes Obtained from the Different Wastes after Use as Fuels
3.4. Effective Doses Received by Workers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viczek, S.A.; Aldrian, A.; Pomberger, R.; Sarc, R. Determination of the material-recyclable share of SRF during co-processing in the cement industry. Resour. Conserv. Recycl. 2020, 156, 104696. [Google Scholar] [CrossRef]
- Alarcón, A.; Mora, P.; Vallina, D.; Martin, M. Coprocesado: Recuperación material de la fracción mineral de los combustibles derivados de residuos en la industria cementera. Cemento Hormigón 2023, 1019, 14. [Google Scholar]
- Xu, J.; Sun, S.F.; Karstensen, K.; Yan, D.H.; Peng, Z. Co-Processing Hazardous Waste in the Chinese Cement Industry—Status 2014. Appl. Mech. Mater. 2015, 768, 679–686. [Google Scholar] [CrossRef]
- Kallio, A.; Virtanen, S.; Leikoski, N.; Iloniemi, E.; Kämäräinen, M.; Hildén, T.; Mattila, A. Radioactivity of residues from waste incineration facilities in Finland. J. Radiol. Prot. 2023, 43, 021502. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Can the incineration of Municipal Solid Waste pose occupational and environmental radiation hazards? Int. J. Occup. Environ. Saf. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gascó, C.; Morales, M.M.; Suárez-Navarro, J.A.; Zamorano, M.; Puertas, F. Olive biomass ash as an alternative activator in geopolymer formation: A study of strength, radiology and leaching behaviour. Cem. Concr. Compos. 2019, 104, 103384. [Google Scholar] [CrossRef]
- Ogundare, F.; Nwankwo, C. Radionuclide content of, and radiological hazards associated with samples from the different streams of metal recycling facilities. Radioprotection 2015, 50, 55–58. [Google Scholar] [CrossRef]
- Camacho, A.; Montaña, M.; Vallés, I.; Devesa, R.; Céspedes-Sánchez, R.; Serrano, I. Temporal evolution of radionuclides in sludge from wastewater treatment plants. J. Radioanal. Nucl. Chem. 2013, 295, 297–306. [Google Scholar] [CrossRef]
- Ozmen, S.F.; Topcuoğlu, B. Chapter Determination of Natural Radioactivity Levels of Sludges Collected From Wastewater Treatment Plants of Antalya/Türkiye; Firenze University Press: Florence, Italy, 2022. [Google Scholar]
- Yui, K.; Kuramochi, H.; Osako, M. Understanding the Behavior of Radioactive Cesium during the Incineration of Contaminated Municipal Solid Waste and Sewage Sludge by Thermodynamic Equilibrium Calculation. ACS Omega 2018, 3, 15086–15099. [Google Scholar] [CrossRef]
- Nakamura, A.; Hayabuchi, N.; Osaki, T.; Osaki, S. Output of radiopharmaceutical nuclides of known injected doses from a municipal sewage treatment system. Health Phys. 2005, 88, 163–168. [Google Scholar] [CrossRef]
- Jiménez, F.; Debán, L.; Pardo, R.; López, R.; García-Talavera, M. Levels of 131I and Six Natural Radionuclides in Sludge from the Sewage Treatment Plant of Valladolid, Spain. Water Air Soil Pollut. 2011, 217, 515–521. [Google Scholar] [CrossRef]
- Alonso, M.M.; Suarez-Navarro, J.A.; Perez-Sanz, R.; Gasco, C.; Moreno de Los Reyes, A.M.; Lanzon, M.; Blanco-Varela, M.T.; Puertas, F. Data on natural radionuclide’s activity concentration of cement-based materials. Data Brief 2020, 33, 106488. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, R.; Risica, S.; D’Alessandro, M.; Paradiso, D.; Nuccetelli, C. Natural radioactivity in building materials in the European Union: A database and an estimate of radiological significance. J. Environ. Radioact. 2012, 105, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Navarro, J.A.; Alonso, M.M.; Gascó, C.; Pachón, A.; Carmona-Quiroga, P.M.; Argiz, C.; Sanjuán, M.Á.; Puertas, F. Effect of particle size and composition of granitic sands on the radiological behaviour of mortars. Boletín Soc. Española Cerámica Vidr. 2021, 61, 561–573. [Google Scholar] [CrossRef]
- Cano, A.; Suarez-Navarro, J.A.; Puertas, F.; Fernandez-Jimenez, A.; Alonso, M.D.M. New Approach to Determine the Activity Concentration Index in Cements, Fly Ashes, and Slags on the Basis of Their Chemical Composition. Materials 2023, 16, 2677. [Google Scholar] [CrossRef]
- Coletti, C.; Brattich, E.; Cinelli, G.; Cultrone, G.; Maritan, L.; Mazzoli, C.; Mostacci, D.; Tositti, L.; Sassi, R. Radionuclide concentration and radon exhalation in new mix design of bricks produced reusing NORM by-products: The influence of mineralogy and texture. Constr. Build. Mater. 2020, 260, 119820. [Google Scholar] [CrossRef]
- UNE-EN 15443:2011; Combustibles Sólidos Recuperados. Métodos para la Preparación de la Muestra del Laboratorio. Asociación Española de Normalización (UNE): Madrid, Spain, 2011.
- UNE-EN 15410:2012; Combustibles Sólidos Recuperados. Método para la Determinación del Contenido en Elementos Principales (Al, Ca, Fe, K, Mg, Na, S, Si, Ti). Asociación Española de Normalización (UNE): Madrid, Spain, 2012.
- UNE-EN ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. Asociación Española de Normalización (UNE): Madrid, Spain, 2017.
- Hill, B.D. Sequential Kaiser-meyer-olkin Procedure as an Alternative for Determining the Number of Factors in Common-factor Analysis: A Monte Carlo Simulation; Oklahoma State University: Stillwater, OK, USA, 2011. [Google Scholar]
- Suárez-Navarro, J.A.; Moreno-Reyes, A.M.; Gascó, C.; Alonso, M.M.; Puertas, F. Gamma spectrometry and LabSOCS-calculated efficiency in the radiological characterisation of quadrangular and cubic specimens of hardened portland cement paste. Radiat. Phys. Chem. 2020, 171, 108709. [Google Scholar] [CrossRef]
- MIRION. DSA-LX Digital Signal Analyzer User’s Manual; Mirion Technologies (Canberra): Meriden, CT, USA, 2017. [Google Scholar]
- CANBERRA. Genie 2000 Spectrometry Software Customization Tools; Canberra Industries: Meriden, CT, USA, 2009. [Google Scholar]
- Currie, L.A. Limits for Qualitative Detection and Quantitative Determination. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Be, M.; Chisté, V.; Dulieu, C.; Kellett, M.; Mougeot, X.; Arinc, A.; Chechev, V.; Kuzmenko, N.; Kibédi, T.; Luca, A. Table of radionuclides (Vol. 8-A= 41 to 198); Bureau International Des Poids et Mesures (BIPM): Sèvres, France, 2016. [Google Scholar]
- Suárez-Navarro, J.A.; Gascó, C.; Alonso, M.M.; Blanco-Varela, M.T.; Lanzon, M.; Puertas, F. Use of Genie 2000 and Excel VBA to correct for γ-ray interference in the determination of NORM building material activity concentrations. Appl. Radiat. Isot. 2018, 142, 1–7. [Google Scholar] [CrossRef]
- Barba-Lobo, A.; Expósito-Suárez, V.M.; Suárez-Navarro, J.A.; Bolívar, J.P. Robustness of LabSOCS calculating Ge detector efficiency for the measurement of radionuclides. Radiat. Phys. Chem. 2023, 205, 110734. [Google Scholar] [CrossRef]
- European Commission. Radiation Protection 122. Practical Use of the Concepts of Clearance and Exemption Part II: Application of the Concepts of Exemption and Clearance to Natural Radiation Sources; Office for Official Publications of the European Communities: Luxembourg, 2001. [Google Scholar]
- Suárez-Navarro, J.A.; Lanzón, M.; Moreno-Reyes, A.M.; Gascó, C.; Alonso, M.M.; Blanco-Varela, M.T.; Puertas, F. Radiological behaviour of pigments and water repellents in cement-based mortars. Constr. Build. Mater. 2019, 225, 879–885. [Google Scholar] [CrossRef]
- Iwaoka, K.; Tabe, H.; Yonehara, H. Natural radioactivity of bedrock bath instruments and hot spring instruments in Japan. J. Radioanal. Nucl. Chem. 2013, 295, 817–821. [Google Scholar] [CrossRef]
- Janković, M.M.; Todorović, D.J. Concentrations of natural radionuclides in imported zirconium minerals. Nucl. Technol. Radiat. Prot. 2011, 26, 110–114. [Google Scholar] [CrossRef]
- Ritchie, J.C.; McHenry, J.R. Application of Radioactive Fallout Cesium-137 for Measuring Soil Erosion and Sediment Accumulation Rates and Patterns: A Review. J. Environ. Qual. 1990, 19, 215–233. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xiao, X.; Wang, X.Q.; Dong, C.Q.; Li, W.Y.; Lu, Q.; Wang, T.P. Potassium recovery from the fly ash from a grate boiler firing agro-residues: Effects of unburnt carbon and calcination pretreatment. J. Chem. Technol. Biotechnol. 2017, 92, 801–807. [Google Scholar] [CrossRef]
- Maeda, N.; Fukasawa, T.; Katakura, T.; Ito, M.; Ishigami, T.; Huang, A.-N.; Fukui, K. Existence Form of Potassium Components in Woody Biomass Combustion Ashes and Estimation Method of Its Enrichment Degree. Energy Fuels 2018, 32, 517–524. [Google Scholar] [CrossRef]
- Wolbarst, A.B.; Chiu, W.A.; Yu, C.; Aiello, K.; Bachmaier, J.T.; Bastian, R.K.; Cheng, J.-J.; Goodman, J.; Hogan, R.; Jones, A.R.; et al. Radioactive materials in biosoils: Dose modeling. Health Phys. 2006, 90, 16–30. [Google Scholar] [CrossRef]
- Cinelli, G.; De Cort, M.; Tollefsen, T. European Atlas of Natural Radiation; Publication Office of the European Union: Luxembourg, 2019. [Google Scholar]

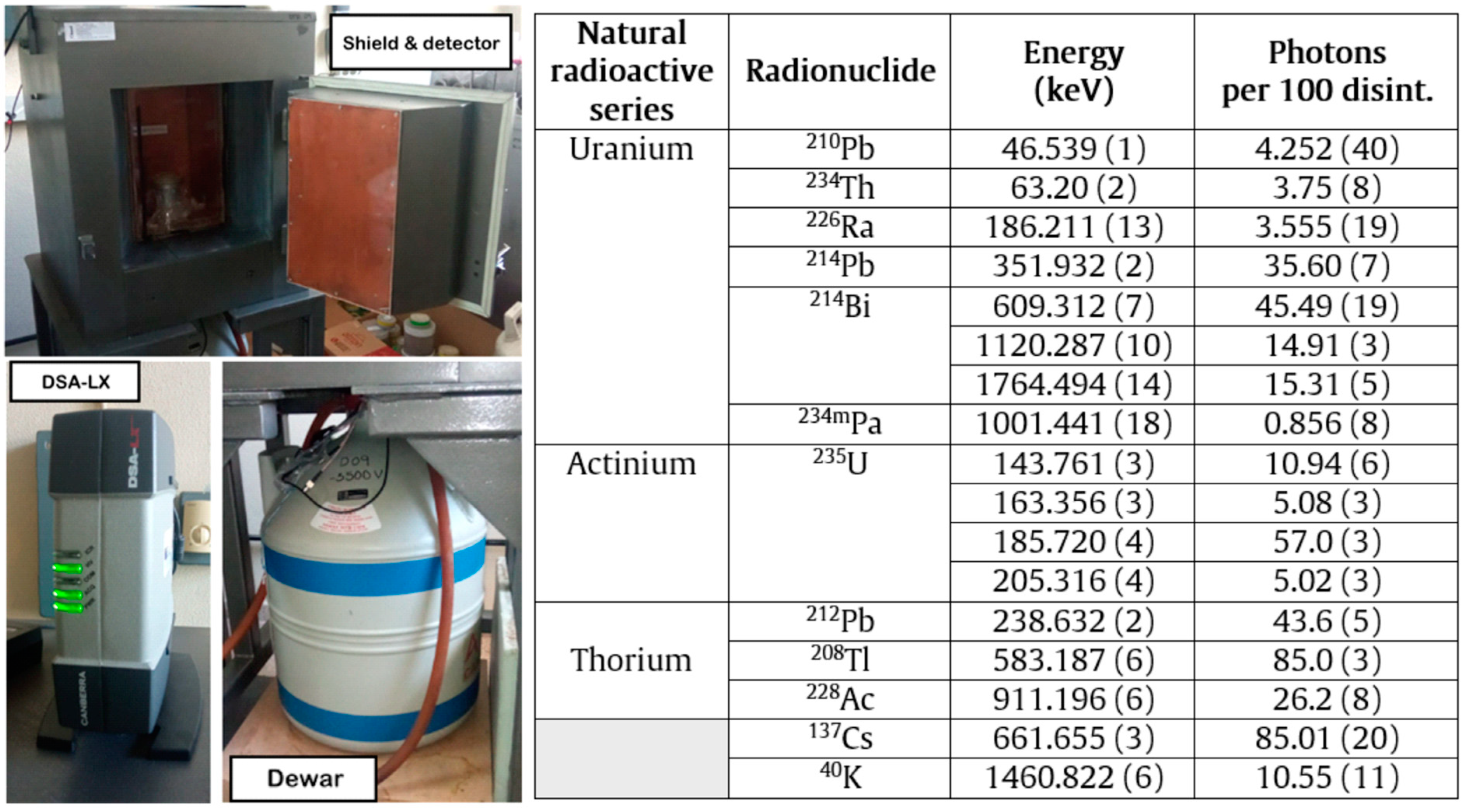

| Oxide (%) | ELVM | STPS | MBM | UW | IW | WD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Med | Min | Max | Med | Min | Max | Med | Min | Max | Med | Min | Max | Med | Min | Max | Med | |
| Al2O3 | 1.8 | 10.6 | 5.9 | 9.8 | 13.9 | 11.5 | 0.04 | 1.1 | 0.7 | 9.2 | 37.1 | 10.4 | 5.7 | 43.8 | 13.0 | 1.0 | 6.5 | 5.3 |
| CaO | 14.2 | 23.1 | 17.7 | 12.0 | 23.3 | 22.1 | 40.1 | 49.6 | 41.1 | 21.1 | 36.0 | 30.3 | 13.5 | 36.7 | 25.1 | 8.3 | 33.0 | 23.0 |
| Fe2O3 | 1.7 | 19.4 | 7.8 | 9.6 | 18.9 | 11.0 | 0.2 | 3.8 | 0.8 | 1.9 | 7.6 | 2.5 | 1.0 | 20.1 | 3.7 | 0.7 | 6.2 | 3.8 |
| K2O | 0.2 | 1.4 | 0.4 | 1.5 | 2.7 | 1.9 | 1.6 | 6.3 | 4.4 | 0.6 | 2.7 | 2.2 | 0.8 | 9.3 | 1.3 | 1.7 | 15.0 | 3.5 |
| MgO | 2.3 | 11.6 | 3.2 | 2.6 | 5.0 | 3.8 | 1.1 | 1.7 | 1.4 | 2.7 | 4.6 | 3.1 | 1.6 | 4.1 | 2.1 | 2.3 | 4.0 | 3.6 |
| Na2O | 0.3 | 3.5 | 0.8 | 0.6 | 1.8 | 1.1 | 2.8 | 5.0 | 4.3 | 1.4 | 7.4 | 3.4 | 1.7 | 10.0 | 3.0 | 0.7 | 4.7 | 3.4 |
| SO3 | 1.0 | 5.5 | 2.2 | 1.1 | 5.4 | 3.6 | 0.4 | 1.2 | 0.7 | 2.0 | 4.3 | 3.3 | 0.8 | 4.1 | 1.8 | 1.0 | 2.8 | 2.4 |
| SiO2 | 11.6 | 39.3 | 32.4 | 15.1 | 25.0 | 21.8 | 1.0 | 7.3 | 5.7 | 15.4 | 31.6 | 18.4 | 7.3 | 40.1 | 23.9 | 6.2 | 38.9 | 27.2 |
| TiO2 | 0.9 | 2.6 | 1.3 | 0.7 | 1.0 | 1.0 | 0.01 | 0.4 | 0.06 | 1.9 | 16.9 | 6.1 | 0.7 | 22.9 | 3.4 | 0.07 | 8.5 | 5.5 |
| Reference | Uranium Series | Thorium Series | 40K | 137Cs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 234Th | 226Ra | 214Pb | 214Bi | 210Pb | 228Ac | 212Pb | 208Tl | |||
| ELVM-2 | <7.1 | 7.9 ± 5.9 | <1.9 | <2.4 | <8.9 | <3.3 | 2.43 ± 0.41 | 0.91 ± 0.31 | <11.4 | <0.8 |
| ELVM-7 | <12.8 | <24.3 | 15.4 ± 4.6 | 16.8 ± 2.0 | 42 ± 12 | 11.0 ± 5.4 | 9.9 ± 2.5 | 3.8 ± 1.6 | 122 ± 24 | <1.5 |
| MBM-6 | <5.6 | <7.6 | 4.33 ± 0.72 | 4.26 ± 0.86 | <6.5 | <2.5 | 1.85 ± 0.29 | 0.73 ± 0.22 | <17.5 | <0.6 |
| MBM-10 | <11.2 | <21.6 | <3.0 | <2.7 | <11.3 | <5.0 | 1.42 ± 0.54 | <1.1 | 254 ± 26 | <1.3 |
| STPS-6 | 321 ± 38 | <16.0 | 11.6 ± 2.1 | 10.5 ± 1.1 | 110 ± 14 | 16.9 ± 1.9 | 11.7 ± 1.7 | 4.2 ± 1.2 | 128 ± 18 | <0.8 |
| STPS-2 | 34.6 ± 9.2 | 28 ± 13 | 14.7 ± 2.1 | 14.2 ± 1.9 | 85 ± 10 | 17.4 ± 2.3 | 13.3 ± 1.4 | 5.07 ± 0.82 | 161 ± 19 | <1.2 |
| IW-1 | <19.7 | <33.9 | <7.5 | <6.7 | <18.0 | <10.7 | <3.7 | <3.0 | 51 ± 13 | <2.7 |
| IW-3 | <17.9 | <27.7 | <4.8 | <4.8 | <14.7 | <6.3 | <1.7 | <2.2 | 33 ± 23 | <1.9 |
| IW-6 | 27 ± 14 | <39.0 | <5.2 | <5.8 | 69 ± 20 | <8.7 | 3.6 ± 1.1 | <2.5 | 66 ± 18 | <2.5 |
| UW-1 | <20.5 | <36.6 | <6.3 | <7.7 | <20.2 | <11.9 | <2.2 | <3.2 | <39.0 | <2.9 |
| UW-5 | <7.3 | <16.4 | 3.7 ± 1.7 | 6.0 ± 1.2 | 14.1 ± 8.8 | <2.4 | 2.22 ± 0.54 | <1.0 | 46 ± 16 | <0.7 |
| WD-2 | <14.7 | <33.5 | <4.9 | <5.1 | <14.3 | <7.0 | <2.3 | <2.2 | <26.7 | <1.3 |
| WD-3 | <5.5 | <8.7 | 4.43 ± 0.89 | 4.5 ± 1.1 | 7.9 ± 3.5 | <2.9 | 2.58 ± 0.36 | 1.12 ± 0.27 | <12.4 | <0.7 |
| Sample | Eext (mSv y−1) | Einh (mSv y−1) | Eing (mSv y−1) | EA (mSv y−1) |
|---|---|---|---|---|
| ELVM-2 | 0.028 ± 0.012 | 0.0035 ± 0.0015 | 0.00052 ± 0.00022 | 0.032 ± 0.013 |
| ELVM-7 | 0.032 ± 0.013 | 0.0071 ± 0.0029 | 0.00142 ± 0.00058 | 0.040 ± 0.016 |
| MBM-6 | 0.046 ± 0.015 | 0.0131 ± 0.0042 | 0.00208 ± 0.00066 | 0.061 ± 0.020 |
| MBM-10 | 0.0134 ± 0.0029 | 0.00220 ± 0.00047 | 0.000294 ± 0.000063 | 0.0159 ± 0.0034 |
| STPS-6 | 0.0260 ± 0.0062 | 0.063 ± 0.015 | 0.00309 ± 0.00073 | 0.092 ± 0.022 |
| STPS-2 | 0.0066 ± 0.0013 | 0.00137 ± 0.00027 | 0.000126 ± 0.000025 | 0.0081 ± 0.0016 |
| IW-1 | 0.00329 ± 0.00042 | - | - | 0.00329 ± 0.00042 |
| IW-3 | 0.0042 ± 0.0014 | - | - | 0.0042 ± 0.0014 |
| IW-6 | 0.0036 ± 0.0013 | 0.0120 ± 0.0043 | 0.00063 ± 0.00023 | 0.0162 ± 0.0058 |
| UW-1 | - | - | - | |
| UW-5 | 0.0175 ± 0.0077 | 0.0036 ± 0.0016 | 0.00074 ± 0.00033 | 0.0219 ± 0.0097 |
| WD-2 | - | - | - | - |
| WD-3 | 0.0189 ± 0.0058 | 0.0042 ± 0.0013 | 0.00088 ± 0.00027 | 0.0240 ± 0.0073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez-Navarro, J.A.; Sanjuán, M.Á.; Mora, P.; Alonso, M.d.M. An Evaluation of the Radioactive Content of Ashes Obtained from the Use of Fuels from Recycled Materials by Co-Processing in the Cement Industry. Materials 2024, 17, 2287. https://doi.org/10.3390/ma17102287
Suarez-Navarro JA, Sanjuán MÁ, Mora P, Alonso MdM. An Evaluation of the Radioactive Content of Ashes Obtained from the Use of Fuels from Recycled Materials by Co-Processing in the Cement Industry. Materials. 2024; 17(10):2287. https://doi.org/10.3390/ma17102287
Chicago/Turabian StyleSuarez-Navarro, José Antonio, Miguel Ángel Sanjuán, Pedro Mora, and María del Mar Alonso. 2024. "An Evaluation of the Radioactive Content of Ashes Obtained from the Use of Fuels from Recycled Materials by Co-Processing in the Cement Industry" Materials 17, no. 10: 2287. https://doi.org/10.3390/ma17102287
APA StyleSuarez-Navarro, J. A., Sanjuán, M. Á., Mora, P., & Alonso, M. d. M. (2024). An Evaluation of the Radioactive Content of Ashes Obtained from the Use of Fuels from Recycled Materials by Co-Processing in the Cement Industry. Materials, 17(10), 2287. https://doi.org/10.3390/ma17102287







