Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyurethane Glycerolysate
2.3. Preparation of Rubber Mixtures and Rubber Vulcanizates
2.4. Testing Methods
2.5. Accelerated Thermal Aging of Natural Rubber Vulcanizates
3. Results and Discussion
3.1. Vulcanization Kinetics
3.2. Equilibrium Swelling and Cross-Linking Density
3.3. Thermo-Mechanical Properties
3.4. Thermal Stability
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molero, C.; de Lucas, A.; Rodríguez, J.F. Recovery of polyols from flexible polyurethane foam by “split-phase” glycolysis: Study on the influence of reaction parameters. Polym. Degrad. Stab. 2008, 93, 353–361. [Google Scholar] [CrossRef]
- Datta, J. Effect of glycols used as glycolysis agents on chemical structure and thermal stability of the produced glycolysates. J. Therm. Anal. Calorim. 2012, 109, 517–520. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; De Lucas, A.; Rodríguez, J.F. Glycolysis of flexible polyurethane wastes containing polymeric polyols. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Datta, J.; Haponiuk, J.T. Influence of Glycols on the Glycolysis Process and the Structure and Properties of Polyurethane Elastomers. J. Elastomers Plast. 2011, 43, 529–541. [Google Scholar] [CrossRef]
- Simón, D.; García, M.T.; De Lucas, A.; Borreguero, A.M.; Rodríguez, J.F. Glycolysis of flexible polyurethane wastes using stannous octoate as the catalyst: Study on the influence of reaction parameters. Polym. Degrad. Stab. 2013, 98, 144–149. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer Through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef]
- Jutrzenka Trzebiatowska, P.; Beneš, H.; Datta, J. Evaluation of the glycerolysis process and valorisation of recovered polyol in polyurethane synthesis. React. Funct. Polym. 2019, 139, 25–33. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Glycolysis of high resilience flexible polyurethane foams containing polyurethane dispersion polyol. Polym. Degrad. Stab. 2016, 133, 119–130. [Google Scholar] [CrossRef]
- del Amo, J.; Borreguero, A.M.; Ramos, M.J.; Rodríguez, J.F. Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers 2021, 13, 1418. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane wastes using different carboxylic acids via acidolysis to produce wood adhesives. J. Polym. Sci. 2021, 59, 697–705. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane scraps via acidolysis. Chem. Eng. J. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of different types of polyurethane foam wastes via acidolysis to produce polyurethane coatings. Sustain. Mater. Technol. 2021, 29, e00330. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Norakankorn, C.; Pimpan, V. Chemical Recycling of Rigid Polyurethane Foam Scrap via Base Catalyzed Aminolysis. J. Met. Mater. Miner. 2002, 12, 19–22. [Google Scholar]
- Grdadolnik, M.; Zdovc, B.; Drinčić, A.; Onder, O.C.; Utroša, P.; Ramos, S.G.; Ramos, E.D.; Pahovnik, D.; Žagar, E. Chemical Recycling of Flexible Polyurethane Foams by Aminolysis to Recover High-Quality Polyols. ACS Sustain. Chem. Eng. 2023, 11, 10864–10873. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Kim, Y.; Kim, S. Flame retardant properties of polyurethane produced by the addition of phosphorous containing polyurethane oligomers (II). J. Ind. Eng. Chem. 2009, 15, 888–893. [Google Scholar] [CrossRef]
- Troev, K.; Atanasov, V.I.; Tsevi, R.; Grancharov, G.; Tsekova, A. Chemical degradation of polyurethanes. Degradation of microporous polyurethane elastomer by dimethyl phosphonate. Polym. Degrad. Stab. 2000, 67, 159–165. [Google Scholar] [CrossRef]
- Troev, K.; Tsekova, A.; Tsevi, R. Chemical degradation of polyurethanes2. Degradation of flexible polyether foam by dimethyl phosphonate. Polym. Degrad. Stab. 2000, 67, 397–405. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R. Chemical degradation of polyurethanes 3. Degradation of microporous polyurethane elastomer by diethyl phosphonate and tris(1-methyl-2-chloroethyl) phosphate. Polym. Degrad. Stab. 2000, 70, 43–48. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Beneš, H.; Rösner, J.; Holler, P.; Synková, H.; Kotek, J.; Horák, Z. Glycolysis of flexible polyurethane foam in recycling of car seats. Polym. Adv. Technol. 2007, 18, 149–156. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Li, J.-K. Glycolysis of rigid polyurethane from waste refrigerators. Polym. Degrad. Stab. 2002, 75, 413–421. [Google Scholar] [CrossRef]
- Shin, S.-R.; Kim, H.-N.; Liang, J.-Y.; Lee, S.-H.; Lee, D.-S. Sustainable rigid polyurethane foams based on recycled polyols from chemical recycling of waste polyurethane foams. J. Appl. Polym. Sci. 2019, 136, 47916. [Google Scholar] [CrossRef]
- Zhu, P.; Cao, Z.B.; Chen, Y.; Zhang, X.J.; Qian, G.R.; Chu, Y.L.; Zhou, M. Glycolysis recycling of rigid waste polyurethane foam from refrigerators. Environ. Technol. 2014, 35, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Datta, J. Synthesis and Investigation of Glycolysates and Obtained Polyurethane Elastomers. J. Elastomers Plast. 2010, 42, 117–127. [Google Scholar] [CrossRef]
- Włoch, M.; Ostaszewska, U.; Datta, J. The Effect of Polyurethane Glycolysate on the Structure and Properties of Natural Rubber/Carbon Black Composites. J. Polym. Environ. 2019, 27, 1367–1378. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, X.; Li, H.; Lai, X.; Xie, H. Synthesis and antioxidative properties in natural rubber of novel macromolecular hindered phenol antioxidants containing thioether and urethane groups. Polym. Degrad. Stab. 2015, 111, 232–238. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Flexible polyurethane foams synthesized employing recovered polyols from glycolysis: Physical and structural properties. J. Appl. Polym. Sci. 2017, 134, 45087. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Xie, W.-Y.; He, M.-F.; Zhu, D.; Liu, S.; Zhang, L.; Liao, S. The role of non-rubber components acting as endogenous antioxidants on thermal-oxidative aging behavior of natural rubber. Polym. Test. 2022, 111, 107614. [Google Scholar] [CrossRef]
- Alexander, M.; Thachil, E.T. The Effectiveness of Cardanol as Plasticiser, Activator, and Antioxidant for Natural Rubber Processing. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 107–124. [Google Scholar] [CrossRef]
- Gregorová, A.; Košíková, B.; Moravčík, R. Stabilization effect of lignin in natural rubber. Polym. Degrad. Stab. 2006, 91, 229–233. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Yan, Z.; Lu, T.; Liu, R.; Han, X.; Cai, C.; Zhao, S.; Wang, H. Preparation of lignin--based filling antioxidant and its application in styrene--butadiene rubber. J. Appl. Polym. Sci. 2021, 138, 51281. [Google Scholar] [CrossRef]
- Sukatta, U.; Rugthaworn, P.; Seangyen, W.; Tantaterdtam, R.; Smitthipong, W.; Chollakup, R. Prospects for rambutan peel extract as natural antioxidant on the aging properties of vulcanized natural rubber. SPE Polym. 2021, 2, 199–209. [Google Scholar] [CrossRef]
- Komethi, M.; Othman, N.; Ismail, H.; Sasidharan, S. Comparative study on natural antioxidant as an aging retardant for natural rubber vulcanizates. J. Appl. Polym. Sci. 2012, 124, 1490–1500. [Google Scholar] [CrossRef]
- Alexander, M.; Kurian, P.; Thachil, E.T. Effectiveness of Cardanol as Plasticizer for Silica-Filled Natural Rubber. Prog. Rubber Plast. Recycl. Technol. 2007, 23, 43–55. [Google Scholar] [CrossRef]
- Fard-Zolfaghari, G.; Abbasian, A.; Razzaghi-Kashani, M. Insights into the compatibility of vegetable-based plasticizers on the performance of filled rubber vulcanizates. Polym. Eng. Sci. 2021, 61, 1379–1391. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Zhang, L.; Zhao, Y.; Vyzhimov, R.; Tan, T.; Fong, H. Investigation of Palm Oil as Green Plasticizer on the Processing and Mechanical Properties of Ethylene Propylene Diene Monomer Rubber. Ind. Eng. Chem. Res. 2016, 55, 2784–2789. [Google Scholar] [CrossRef]
- Fernandez, S.S.; Kunchandy, S.; Ghosh, S. Linseed Oil Plasticizer Based Natural Rubber/Expandable Graphite Vulcanizates: Synthesis and Characterizations. J. Polym. Environ. 2015, 23, 526–533. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Ionescu, M.; Milić, J.; Halladay, J.R. Soybean oil plasticizers as replacement of petroleum oil in rubber. Rubber Chem. Technol. 2013, 86, 233–249. [Google Scholar] [CrossRef]
- Xu, H.; Fan, T.; Ye, N.; Wu, W.; Huang, D.; Wang, D.; Wang, Z.; Zhang, L. Plasticization effect of bio-based plasticizers from soybean oil for tire tread rubber. Polymers 2020, 12, 623. [Google Scholar] [CrossRef]
- Khalaf, A.I.; Ward, A.A.; Abd El-Kader, A.E.; El-Sabbagh, S.H. Effect of selected vegetable oils on the properties of acrylonitrile-butadiene rubber vulcanizates. Polimery/Polymers 2015, 60, 43–56. [Google Scholar] [CrossRef]
- Colom, X.; Marín-Genescà, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Phiri, M.M.; Phiri, M.J.; Formela, K.; Wang, S.; Hlangothi, S.P. Grafting and reactive extrusion technologies for compatibilization of ground tyre rubber composites: Compounding, properties, and applications. J. Clean. Prod. 2022, 369, 133084. [Google Scholar] [CrossRef]
- Ren, T.; Song, P.; Yang, W.; Formela, K.; Wang, S. Reinforcing and plasticizing effects of reclaimed rubber on the vulcanization and properties of natural rubber. J. Appl. Polym. Sci. 2023, 140, 53580. [Google Scholar] [CrossRef]
- Formela, K.; Wąsowicz, D.; Formela, M.; Hejna, A.; Haponiuk, J. Curing characteristics, mechanical and thermal properties of reclaimed ground tire rubber cured with various vulcanizing systems. Iran. Polym. J. 2015, 24, 289–297. [Google Scholar] [CrossRef]
- Dębek, C. Oil from tyre pyrolysis as a plasticizer in rubber compounds. Polimery 2019, 64, 530–537. [Google Scholar] [CrossRef]
- Cataldo, F. Evaluation of Pyrolytic Oil from Scrap Tires as Plasticizer of Rubber Compounds. Prog. Rubber Plast. Recycl. Technol. 2006, 22, 243–252. [Google Scholar] [CrossRef]
- Paul, S.; Rahaman, M.; Ghosh, S.K.; Das, P.; Katheria, A.; Ghosh, T.; Das, N.C. Effects of tire—Derived pyrolytic carbon black and pyrolytic heavy oil on the curing and mechanical properties of styrene—Butadiene rubber composites. Polym. Eng. Sci. 2023, 63, 2942–2957. [Google Scholar] [CrossRef]
- ISO 3417; Rubber. Measurement of Vulcanization Characteristics with the Oscillating Disc Curemeter. International Organization for Standardization: Geneva, Switzerland, 2008.
- Datta, J.; Kosiorek, P.; Włoch, M. Effect of high loading of titanium dioxide particles on the morphology, mechanical and thermo-mechanical properties of the natural rubber-based composites. Iran. Polym. J. 2016, 25, 1021–1035. [Google Scholar] [CrossRef]
- ISO 6721-1; Plastics. Determination of Dynamic Mechanical Properties. Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 37; Rubber, Vulcanized or Thermoplastic. Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 868; Plastics and Ebonite. Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 4662; Rubber, Vulcanized or Thermoplastic. Determination of Rebound Resilience. International Organization for Standardization: Geneva, Switzerland, 2017.
- Alam, M.N.; Kumar, V.; Potiyaraj, P.; Lee, D.-J.; Choi, J. Mutual dispersion of graphite–silica binary fillers and its effects on curing, mechanical, and aging properties of natural rubber composites. Polym. Bull. 2022, 79, 2707–2724. [Google Scholar] [CrossRef]
- Nellen, A.H.; Sellers, H.M. Correlation between Geer Oven and Natural Aging of Selected Tire Compounds. Ind. Eng. Chem. 1929, 21, 1019–1020. [Google Scholar] [CrossRef]
- Ngolemasango, E.F.; Bennett, M.; Clarke, J. Kinetics of the effect of ageing on tensile properties of a natural rubber compound. J. Appl. Polym. Sci. 2006, 102, 3732–3740. [Google Scholar] [CrossRef]
- Ahagon, A.; Kida, M.; Kaidou, H. Aging of Tire Parts during Service. I. Types of Aging in Heavy-Duty Tires. Rubber Chem. Technol. 1990, 63, 683–697. [Google Scholar] [CrossRef]
- Pimolsiriphol, V.; Saeoui, P.; Sirisinha, C. Relationship Among Thermal Ageing Degradation, Dynamic Properties, Cure Systems, and Antioxidants in Natural Rubber Vulcanisates. Polym. Plast. Technol. Eng. 2007, 46, 113–121. [Google Scholar] [CrossRef]
- Rocco, J.A.F.F.; Lima, J.E.S.; Lourenço, V.L.; Batista, N.L.; Botelho, E.C.; Iha, K. Dynamic mechanical properties for polyurethane elastomers applied in elastomeric mortar. J. Appl. Polym. Sci. 2012, 126, 1461–1467. [Google Scholar] [CrossRef]




| Sample Code | NRM-REF | NRM-G2 | NRM-G4 | NRM-G6 |
|---|---|---|---|---|
| natural rubber | 100 | 100 | 100 | 100 |
| stearic acid | 3 | 3 | 3 | 3 |
| zinc oxide | 5 | 5 | 5 | 5 |
| glycerolysate | - | 2 | 4 | 6 |
| accelerator T | 0.5 | 0.5 | 0.5 | 0.5 |
| sulfur | 2 | 2 | 2 | 2 |
| Sample Code | T | ML | MH | ΔM | ts2 | t90 | CRI | R300 |
|---|---|---|---|---|---|---|---|---|
| [°C] | [dNm] | [dNm] | [dNm] | [min] | [min] | [min−1] | [%] | |
| NRM-REF | 150 | 1.85 | 21.55 | 19.7 | 3.92 | 5.66 | 57.5 | 2.3 |
| NRM-GR2 | 150 | 1.91 | 21.92 | 20.01 | 2.85 | 4.91 | 48.5 | 2.0 |
| NRM-GR4 | 150 | 1.60 | 21.80 | 20.20 | 2.48 | 4.48 | 50.0 | 2.0 |
| NRM-GR6 | 150 | 1.79 | 21.30 | 19.51 | 2.24 | 4.46 | 45.0 | 2.4 |
| Material Code | Aging Time | SR | Vr | ·10−1 | Mc |
|---|---|---|---|---|---|
| [Days] | [%] | [-] | [mol/dm3] | [g/mol] | |
| NR-REF | 0 | 351.3 ± 3.4 | 0.21167 ± 0.00158 | 0.17514 ± 0.00301 | 5711 ± 99 |
| 7 | 349.4 ± 2.4 | 0.21286 ± 0.00112 | 0.17741 ± 0.00216 | 5637 ± 69 | |
| 14 | 368.3 ± 3.8 | 0.20382 ± 0.00171 | 0.16061 ± 0.00306 | 6228 ± 120 | |
| NR-G2 | 0 | 342.5 ± 0.1 | 0.21269 ± 0.00006 | 0.17708 ± 0.00011 | 5647 ± 4 |
| 7 | 326.7 ± 1.9 | 0.22083 ± 0.00095 | 0.19317 ± 0.00193 | 5177 ± 52 | |
| 14 | 350.3 ± 1.4 | 0.20890 ± 0.00066 | 0.16990 ± 0.00123 | 5886 ± 43 | |
| NR-G4 | 0 | 347.7 ± 2.4 | 0.20668 ± 0.00114 | 0.16580 ± 0.00209 | 6032 ± 76 |
| 7 | 325.5 ± 1.5 | 0.21794 ± 0.00072 | 0.18735 ± 0.00144 | 5338 ± 41 | |
| 14 | 334.1 ± 1.4 | 0.21368 ± 0.00070 | 0.17898 ± 0.00136 | 5587 ± 42 | |
| NR-G6 | 0 | 356.5 ± 2.2 | 0.19920 ± 0.00098 | 0.15244 ± 0.00170 | 6560 ± 74 |
| 7 | 336.4 ± 0.5 | 0.20897 ± 0.00024 | 0.17004 ± 0.00045 | 5881 ± 16 | |
| 14 | 336.0 ± 0.6 | 0.20940 ± 0.00030 | 0.17084 ± 0.00056 | 5853 ± 19 |
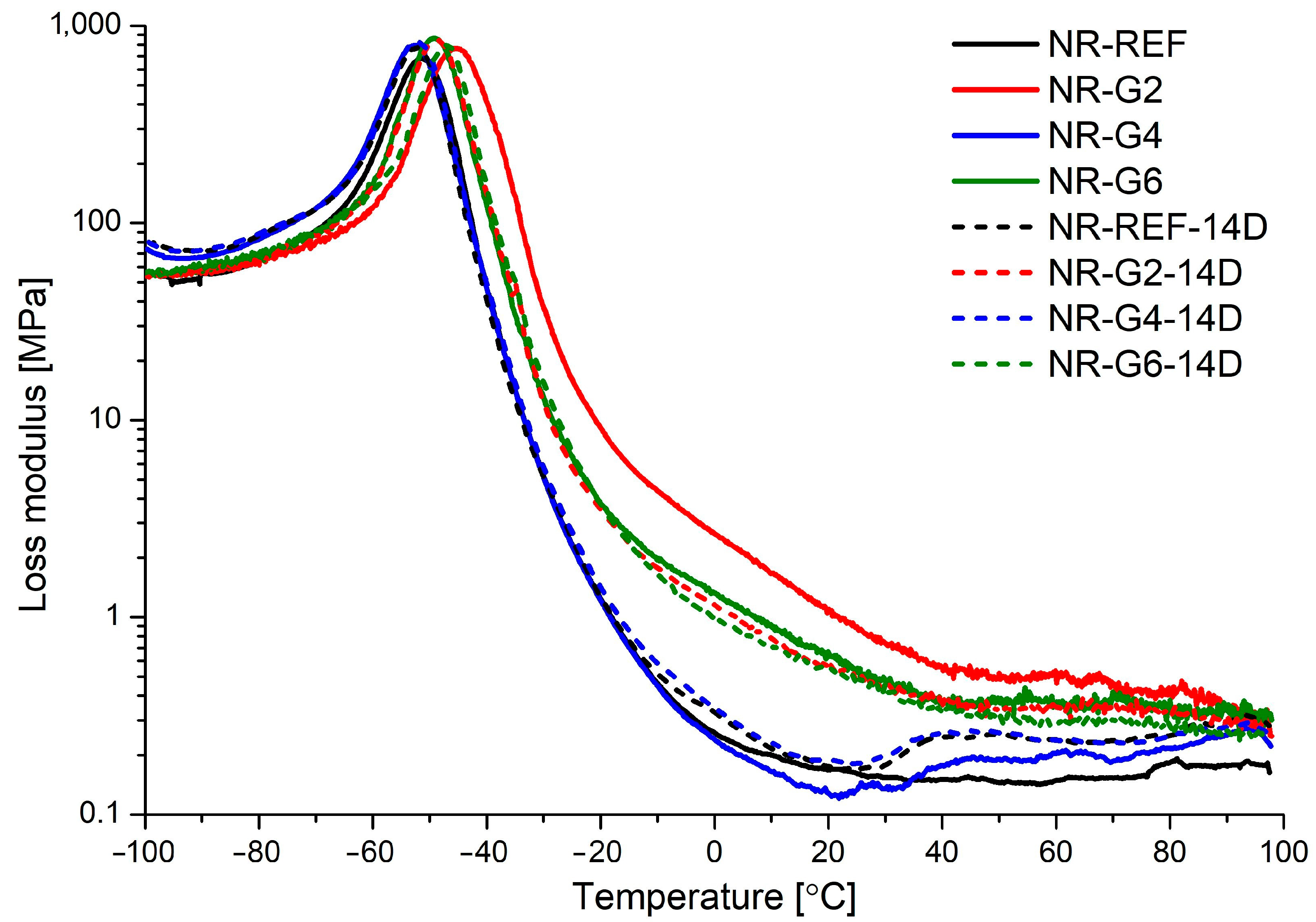
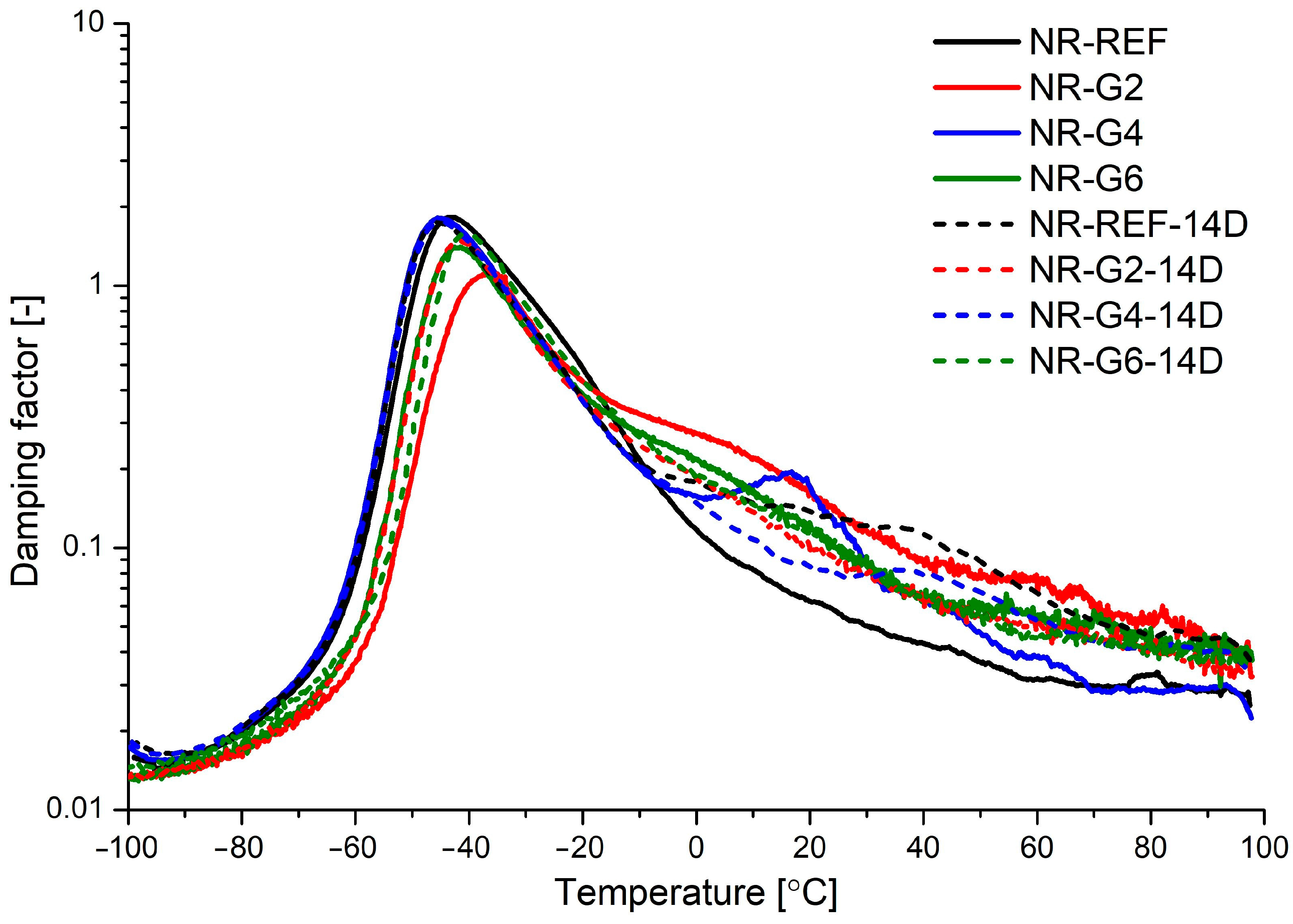
| Material | Aging Time | E′max | E′ @ 21 °C | E″max | TE″max | Tg | tanδ @ Tg |
|---|---|---|---|---|---|---|---|
| [Days] | [MPa] | [MPa] | [MPa] | [°C] | [°C] | [-] | |
| NR-REF | 0 | 3500 | 2.73 | 683 | −51.5 | −42.8 | 1.820 |
| 14 | 4074 | 6.61 | 768 | −45.7 | −35.8 | 1.122 | |
| NR-G2 | 0 | 4278 | 0.81 | 797 | −52.8 | −45.5 | 1.809 |
| 14 | 4188 | 5.42 | 863 | −49.1 | −42.5 | 1.406 | |
| NR-G4 | 0 | 4426 | 1.27 | 780 | −52.3 | −45.6 | 1.743 |
| 14 | 4060 | 5.55 | 850 | −49.0 | −41.5 | 1.486 | |
| NR-G6 | 0 | 4425 | 2.22 | 832 | −52.4 | −44.7 | 1.751 |
| 14 | 3847 | 4.76 | 797 | −47.2 | −40.3 | 1.585 |
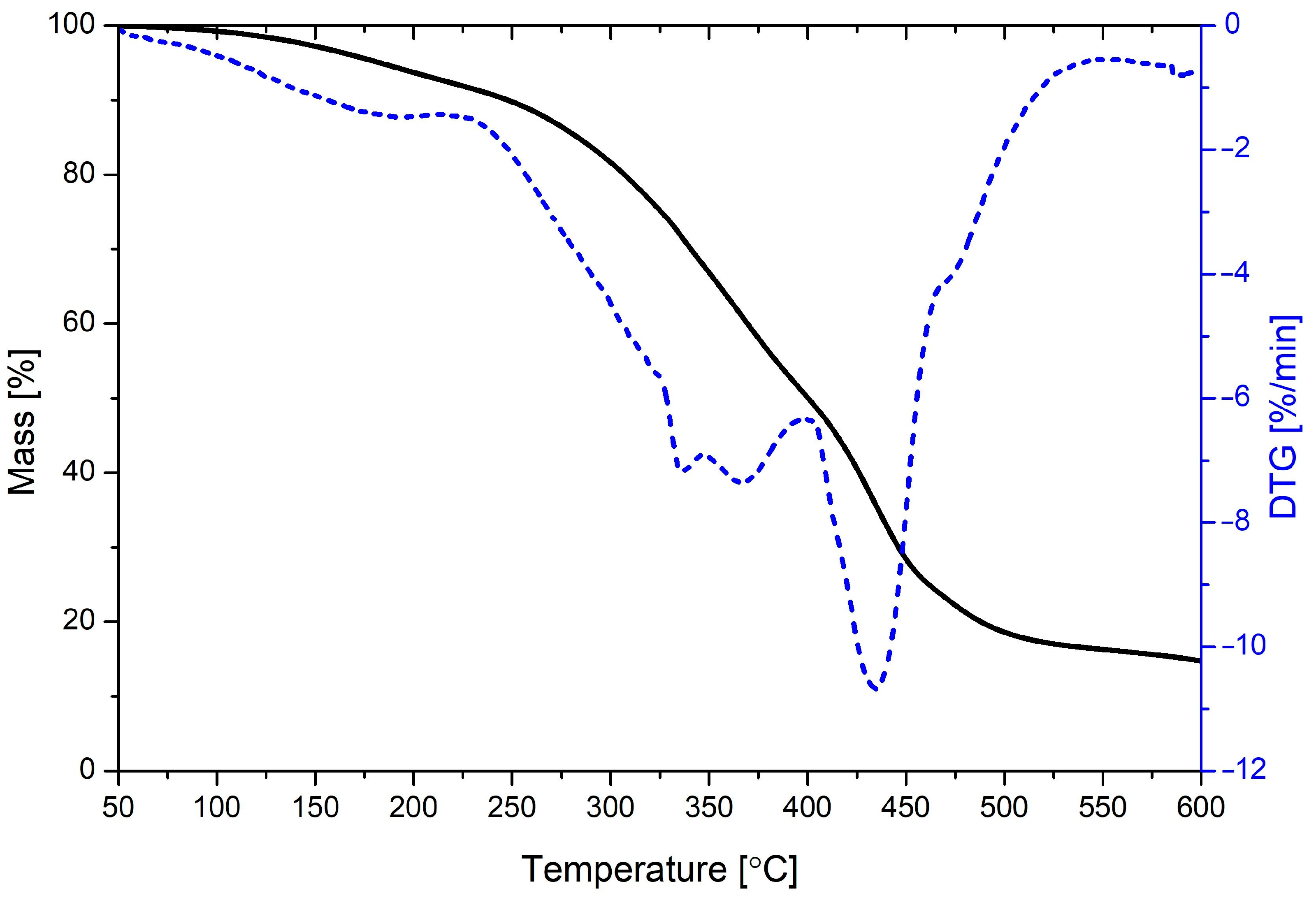
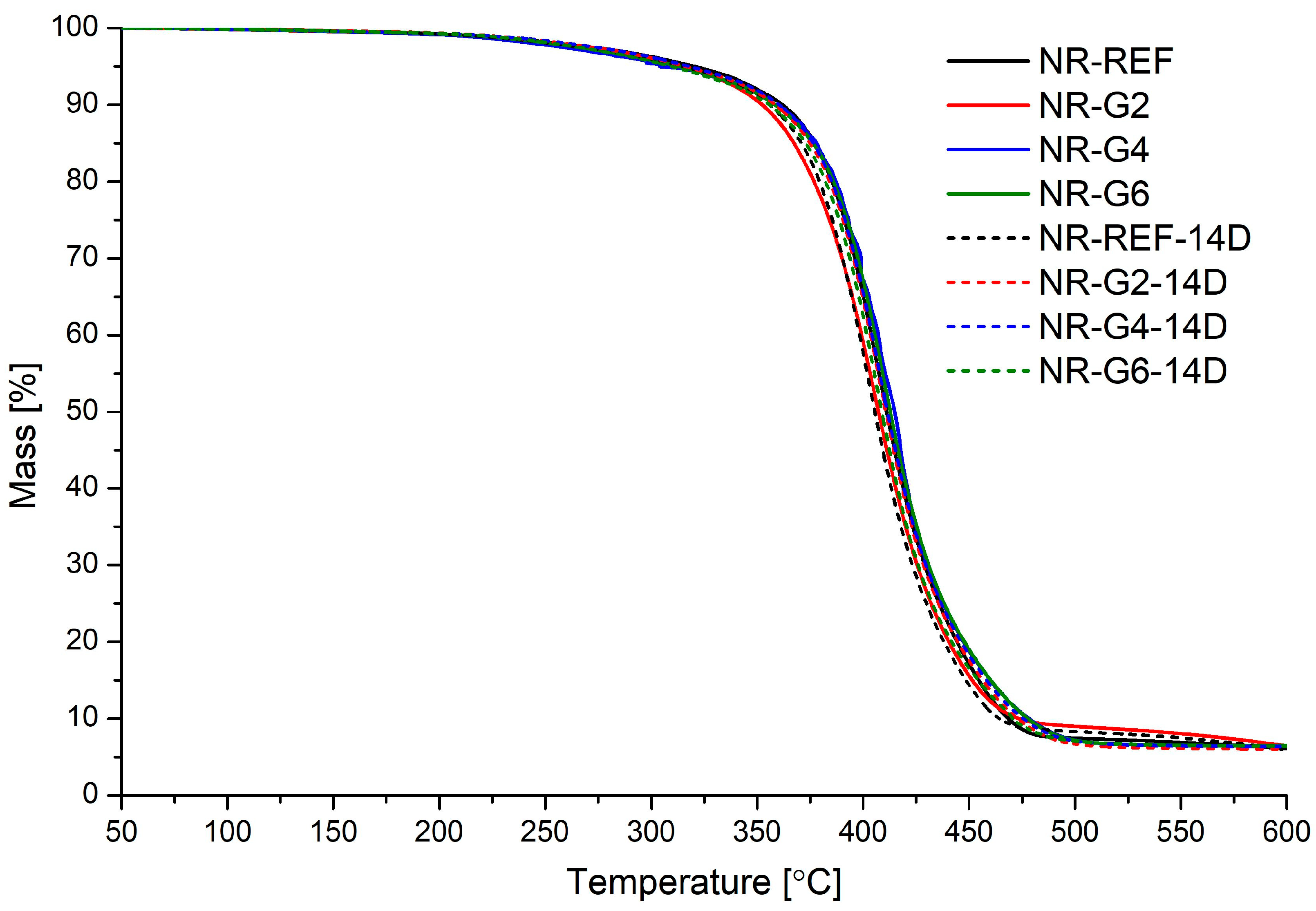
| Material | Aging Time | Td5 | Td10 | Td max | Char Yield at 600 °C |
|---|---|---|---|---|---|
| [Days] | [°C] | [°C] | [°C] | [%] | |
| GLYCEROLYSATE | - | 182.6 | 247.6 | 335.6/434.8 | 14.8 |
| NR-REF | 0 | 320.6 | 362.0 | 407.8 | 6.1 |
| 14 | 315.1 | 355.7 | 402.6 | 6.0 | |
| NR-G2 | 0 | 313.7 | 352.4 | 406.4 | 6.4 |
| 14 | 316.2 | 358.6 | 408.3 | 6.1 | |
| NR-G4 | 0 | 304.0 | 358.8 | 413.6 | 6.4 |
| 14 | 318.1 | 360.5 | 408.4 | 6.4 | |
| NR-G6 | 0 | 313.4 | 358.4 | 410.7 | 6.5 |
| 14 | 309.6 | 355.6 | 406.7 | 6.4 |
| Material | Aging Time | TSb | εb | M100 | M200 | M300 | M400 | M500 | M600 | εt |
|---|---|---|---|---|---|---|---|---|---|---|
| [Days] | [MPa] | [%] | [MPa] | [MPa] | [MPa] | [MPa] | [MPa] | [MPa] | [%] | |
| NR-REF | 0 | 21.0 ± 2.6 | 647 ± 37 | 0.86 | 1.44 | 2.31 | 3.92 | 7.87 | 16.13 | 8.6 ± 1.2 |
| 7 | 17.5 ± 0.9 | 648 ± 38 | 0.84 | 1.43 | 2.28 | 3.80 | 7.25 | 14.46 | 7.9 ± 1.1 | |
| 14 | 1.8 ± 0.4 | 268 ± 56 | 0.76 | 1.32 | 2.14 | - | - | - | 0.6 ± 0.5 | |
| NR-G2 | 0 | 22.5 ± 0.8 | 651 ± 8 | 0.97 | 1.55 | 2.30 | 3.56 | 6.95 | 15.10 | 14.3 ± 1.5 |
| 7 | 19.5 ± 1.7 | 543 ± 22 | 1.02 | 1.83 | 3.17 | 6.16 | 14.21 | - | 12.4 ± 2.2 | |
| 14 | 12.1 ± 1.5 | 482 ± 18 | 0.90 | 1.72 | 3.18 | 6.57 | - | - | 5.4 ± 1.9 | |
| NR-G4 | 0 | 21.9 ± 0.3 | 609 ± 19 | 0.88 | 1.50 | 2.39 | 4.29 | 10.36 | 21.63 | 15.3 ± 3.7 |
| 7 | 20.2 ± 0.2 | 571 ± 20 | 0.96 | 1.78 | 3.15 | 6.24 | 14.45 | - | 10.9 ± 1.4 | |
| 14 | 11.2 ± 0.7 | 495 ± 5 | 0.84 | 1.55 | 2.72 | 5.17 | - | - | 3.3 ± 0.2 | |
| NR-G6 | 0 | 23.1± 0.9 | 638 ± 9 | 0.81 | 1.40 | 2.25 | 4.05 | 9.75 | 20.57 | 14.3 ± 0.8 |
| 7 | 18.8 ± 1.7 | 607 ± 7 | 0.89 | 1.50 | 2.41 | 4.05 | 8.12 | 16.72 | 10.6 ± 1.3 | |
| 14 | 16.8 ± 0.5 | 556 ± 18 | 0.86 | 1.61 | 2.90 | 5.70 | 12.71 | - | 5.5 ± 1.4 |
| Material | Aging Time | Retention of TSb | Retention of εb |
|---|---|---|---|
| [Days] | [%] | [%] | |
| NR-REF | 7 | 83.3 | 100 |
| 14 | 8.6 | 41.4 | |
| NR-G2 | 7 | 86.7 | 83.4 |
| 14 | 53.8 | 74.0 | |
| NR-G4 | 7 | 92.2 | 93.8 |
| 14 | 51.1 | 81.3 | |
| NR-G6 | 7 | 81.4 | 95.1 |
| 14 | 72.7 | 87.1 |
| Material | Aging Time | Hardness | Rebound Resilience |
|---|---|---|---|
| [Days] | H [°Sh A] | RR [%] | |
| NR-REF | 0 | 44.8 ± 0.1 | 77.0 ± 0.2 |
| 7 | 42.1 ± 0.3 | 73.4 ± 0.6 | |
| 14 | 41.7 ± 0.2 | 73.1 ± 0.4 | |
| NR-G2 | 0 | 45.7 ± 0.5 | 79.4 ± 0.9 |
| 7 | 43.5 ± 0.2 | 75.9 ± 0.5 | |
| 14 | 44.8 ± 0.1 | 74.8 ± 0.5 | |
| NR-G4 | 0 | 45.2 ± 0.2 | 78.6 ± 0.7 |
| 7 | 43.4 ± 0.2 | 78.2 ± 0.4 | |
| 14 | 44.4 ± 0.3 | 75.5 ± 0.7 | |
| NR-G6 | 0 | 44.8 ± 0.4 | 78.7 ± 0.6 |
| 7 | 43.5 ± 0.2 | 77.3 ± 0.8 | |
| 14 | 44.1 ± 0.2 | 75.9 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włoch, M.; Toruńczak, M.; Datta, J. Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates. Materials 2024, 17, 62. https://doi.org/10.3390/ma17010062
Włoch M, Toruńczak M, Datta J. Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates. Materials. 2024; 17(1):62. https://doi.org/10.3390/ma17010062
Chicago/Turabian StyleWłoch, Marcin, Maksymilian Toruńczak, and Janusz Datta. 2024. "Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates" Materials 17, no. 1: 62. https://doi.org/10.3390/ma17010062
APA StyleWłoch, M., Toruńczak, M., & Datta, J. (2024). Polyurethane Glycerolysate as a Modifier of the Properties of Natural Rubber Mixtures and Vulcanizates. Materials, 17(1), 62. https://doi.org/10.3390/ma17010062







