Highly Reinforced Acrylic Resins for Hard Tissue Engineering and Their Suitability to Be Additively Manufactured through Nozzle-Based Photo-Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Compression Test
2.1.1. Specimen Preparation
2.1.2. Compression Test Set-Up
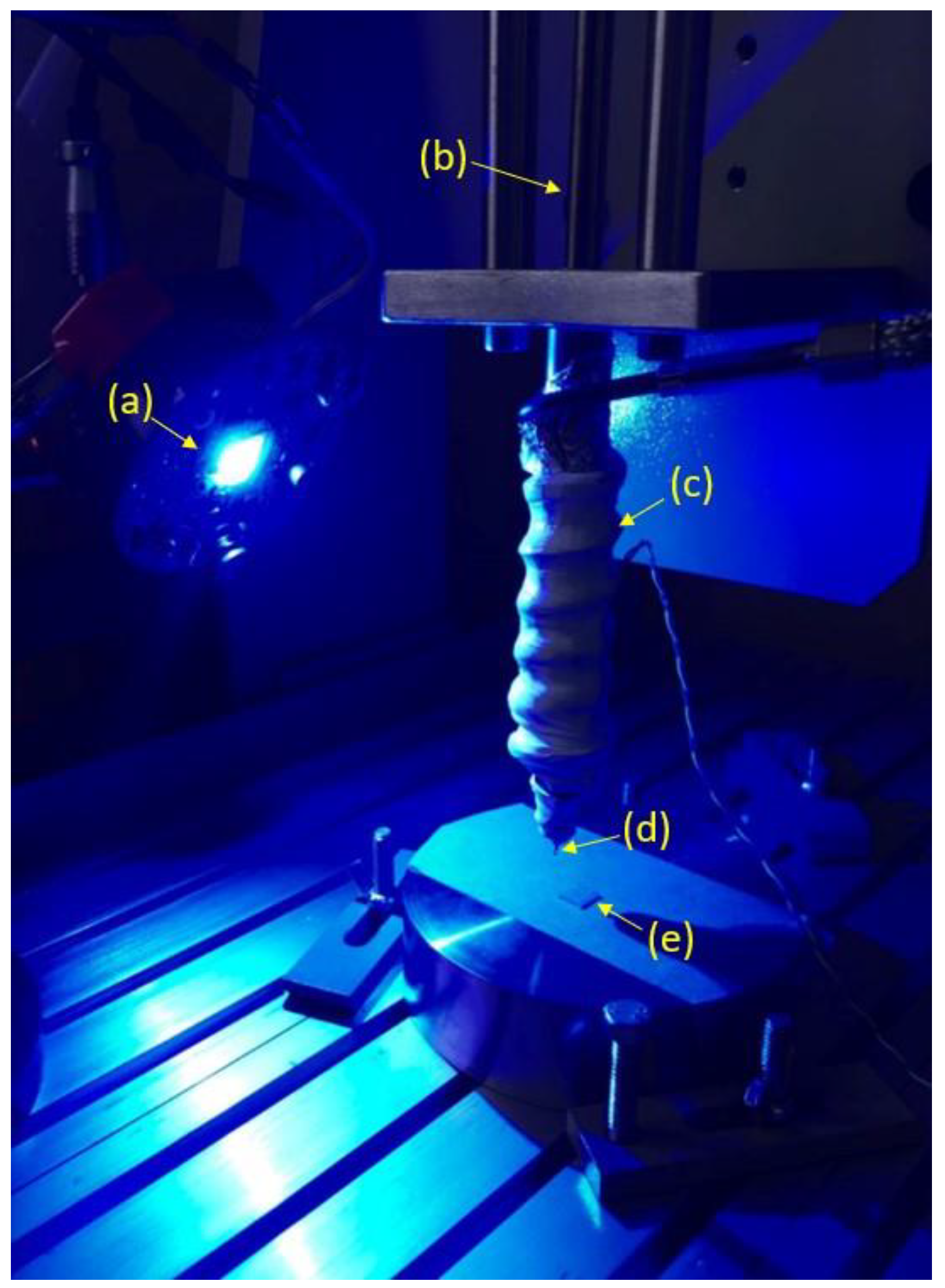
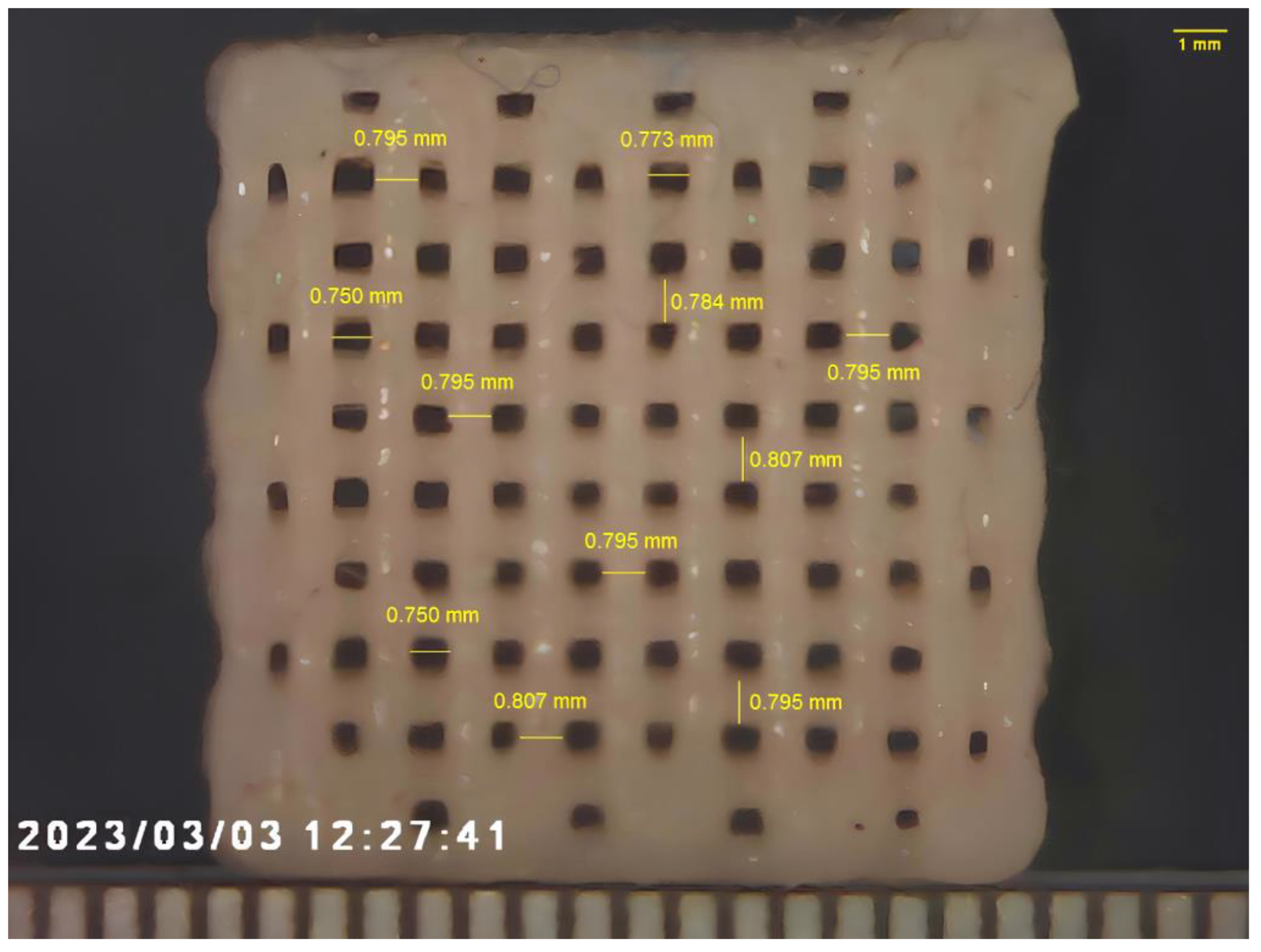
2.2. Additive Manufacturing of Composites
3. Results and Discussions
4. Conclusions
- It is possible to tailor mechanical properties of particulate composites by altering the total resin matrix percentage and the filler content.
- The stiffness and the strength of the composite material increase as the filler fraction increases, but a reduction of ductility (i.e., break strain) is observed as the filler fraction increases.
- Zirconium oxide nanoparticles allow one to achieve mechanical properties higher than those observed for the basic formulations.
- It is possible to process highly reinforced photopolymerizable composite materials using additive manufacturing technologies consisting of 3D fiber deposition through extrusion in conjunction with photo-polymerization. Further analyses will consist of manufacturing more components by varying process parameters, and by studying the results in terms of accuracy and structural behavior.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Santis, R.; Papallo, I.; Onofrio, I.; Peluso, V.; Gallicchio, V.; Rega, A.; D’Antò, V.; Improta, G.; Catauro, M.; Gloria, A.; et al. Analyzing the Role of Magnetic Features in Additive Manufactured Scaffolds for Enhanced Bone Tissue Regeneration. Macromol. Symp. 2021, 396, 2000314. [Google Scholar] [CrossRef]
- Tunesi, M.; Raimondi, I.; Russo, T.; Colombo, L.; Micotti, E.; Brandi, E.; Cappelletti, P.; Cigada, A.; Negro, A.; Ambrosio, L.; et al. Hydrogel-based delivery of Tat-fused protein Hsp70 protects dopaminergic cells in vitro and in a mouse model of Parkinson’s disease. NPG Asia Mater. 2019, 11, 28. [Google Scholar] [CrossRef]
- Arifin, N.; Sudin, I.; Ngadiman, N.H.A.; Ishak, M.S.A. A comprehensive review of biopolymer fabrication in additive manufacturing processing for 3D-tissue-engineering scaffolds. Polymers 2022, 14, 2119. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gloria, A.; Raucci, M.G.; Ambrosio, L. Hydrogel-Based Platforms for the Regeneration of Osteochondral Tissue and Intervertebral Disc. Polymers 2012, 4, 1590–1612. [Google Scholar] [CrossRef]
- Maietta, S.; Russo, T.; De Santis, R.; Ronca, D.; Riccardi, F.; Catauro, M.; Martorelli, M.; Gloria, A. Further Theoretical In-sight into the Mechanical Properties of Polycaprolactone Loaded with Organic–Inorganic Hybrid Fillers. Materials 2018, 11, 312. [Google Scholar] [CrossRef]
- Reitmaier, S.; Shirazi-Adl, A.; Bashkuev, M.; Wilke, H.J.; Gloria, A.; Schmidt, H. In vitro and in silico investigations of disc nucleus replacement. J. R. Soc. Interface 2012, 9, 1869–1879. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Gloria, A.; Mayol, L.; Dickinson, S.; Miot, S.; Martin, I.; Ambrosio, L. Natural/synthetic porous scaffold designs and properties for fibro-cartilaginous tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 437–451. [Google Scholar] [CrossRef]
- Currey, J.D.; Brear, K. Hardness, Young’s modulus and yield stress in mammalian mineralized tissues. J. Mater. Sci. Mater. Med. 1990, 1, 14–20. [Google Scholar] [CrossRef]
- De Santis, R.; Ambrosio, L.; Mollica, F.; Netti, P.; Nicolais, L. Mechanical properties of human mineralized connective tissues. In Modeling of Biological Materials; Birkhäuser Boston: Boston, MA, USA, 2007; pp. 211–261. [Google Scholar]
- Freilich, M.A.; Karmaker, A.C.; Burstone, C.J.; Goldberg, A.J. Development and clinical applications of a light-polymerized fiber-reinforced composite. J. Prosthet. Dent. 1998, 80, 311–318. [Google Scholar] [CrossRef]
- De Santis, R.; Guarino, V.; Ambrosio, L. Composite biomaterials for bone repair. In Bone Repair Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 273–299. [Google Scholar]
- Kabir, S.F.; Mathur, K.; Seyam, A.F.M. A critical review on 3D printed continuous fiber-reinforced composites: History, mechanism, materials and properties. Compos. Struct. 2020, 232, 111476. [Google Scholar] [CrossRef]
- Watt, J.P.; Davies, G.F.; O’Connell, R.J. The elastic properties of composite materials. Rev. Geophys. 1976, 14, 541–563. [Google Scholar] [CrossRef]
- Butcher, R.J.; Rousseau, C.E.; Tippur, H.V. A functionally graded particulate composite: Preparation, measurements and failure analysis. Acta Mater. 1998, 47, 259–268. [Google Scholar] [CrossRef]
- Jiang, F.; Drummer, D. Analysis of UV curing strategy on reaction heat control and part accuracy for additive manufacturing. Polymers 2022, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Khan, S.B.; Chen, S.; Aiyiti, W.; Zhou, J.; Lu, B. Promising New Horizons in Medicine: Medical Advancements with Nanocomposite Manufacturing via 3D Printing. Polymers 2023, 15, 4122. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Lee, J.H.; Lin-Yip, M.J.; Chiang, S.; Levaslot, A.; Giffney, T.; Aw, K.C. A new photopolymer extrusion 5-axis 3D printer. Addit. Manuf. 2018, 23, 355–361. [Google Scholar] [CrossRef]
- Generalova, A.N.; Demina, P.A.; Akasov, R.A.; Khaydukova, E.V. Photopolymerization in 3D printing of tissue-engineered constructs for regenerative medicine. Russ. Chem. Rev. 2023, 92, RCR5068. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef]
- Das, A.; Awasthi, P.; Jain, V.; Banerjee, S.S. 3D printing of maxillofacial prosthesis materials: Challenges and opportunities. Bioprinting 2023, 32, e00282. [Google Scholar] [CrossRef]
- Kontou, E.; Christopoulos, A.; Koralli, P.; Mouzakis, D.E. The Effect of Silica Particle Size on the Mechanical Enhancement of Polymer Nanocomposites. Nanomaterials 2023, 13, 1095. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E. Modeling of the elastic stiffness of biobased polymer nanocomposites. J. Reinf. Plast. Compos. 2014, 33, 942–952. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Göstemeyer, G.; Blunck, U.; Paris, S.; Hsu, L.Y.; Tu, Y.K. Directly placed restorative materials: Review and network meta-analysis. J. Dent. Res. 2016, 95, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, V.; Lodato, V.; De Santis, R.; Rengo, S. Fracture Strength and failure modes of endodontically treated premolars restored with compact and hollow composite posts subjected to cyclic fatigue. Materials 2022, 15, 1141. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, D.C.; Neme, A.M.; Pink, F.E.; Hughes, P.J. The effect of three finishing systems on four esthetic restorative materials. Oper. Dent. 1998, 23, 36–42. [Google Scholar] [PubMed]
- Bowen, R.L. Crystalline dimethacrylate monomers. J. Dent. Res. 1970, 49, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Skrtic, D. N-Acetyl cysteine modulates the inflammatory and oxidative stress responses of rescued growth-arrested dental pulp microtissues exposed to TEGDMA in ECM. Int. J. Mol. Sci. 2020, 21, 7318. [Google Scholar] [CrossRef]
- Krifka, S.; Petzel, C.; Bolay, C.; Hiller, K.A.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials 2011, 32, 1787–1795. [Google Scholar] [CrossRef]
- Bagis, Y.H.; Rueggeberg, F.A. The effect of post-cure heating on residual, unreacted monomer in a commercial resin composite. Dent. Mater. 2000, 16, 244–247. [Google Scholar] [CrossRef]
- Hansen, E.K.; Asmussen, E. Marginal adaptation of posterior resins: Effect of dentin-bonding agent and hygroscopic expansion. Dent. Mater. 1989, 5, 122–126. [Google Scholar] [CrossRef]
- Buonocore, M.G.; Matsui, A.; Gwinnett, A.J. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch. Oral Biol. 1968, 13, 61–67, IN17–IN18, 69–70, IN19–IN20. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Mitchem, J.C.; Condon, J.R.; Todd, R. Wear and marginal breakdown of composites with various degrees of cure. J. Dent. Res. 1997, 76, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.A. Applications of nanotechnology. Pract. Proced. Aesthet. Dent. 2004, 16, 220–222. [Google Scholar] [PubMed]
- Nicolais, L. Mechanics of composites: Particulate and fiber polymeric laminate properties. Polym. Eng. Sci. 1975, 15, 137–149. [Google Scholar] [CrossRef]
- Gloria, A.; Ronca, D.; Russo, T.; D’Amora, U.; Chierchia, M.; De Santis, R.; Nicolais, L.; Ambrosio, L. Technical features and criteria in designing fiber-reinforced composite materials: From the aerospace and aeronautical field to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Elkhouly, H.I.; Ali, E.M.; El-Sheikh, M.N.; Hassan, A.E.M. An investigated organic and inorganic reinforcement as an effective economical filler of poly (methyl methacrylate) nanocomposites. Sci. Rep. 2022, 12, 16416. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M. Fundamental mechanical behaviors of dental restorative materials resin composites due to compressive and flexural loading and internal damage evaluation. Int. J. Damage Mech. 2017, 26, 826–839. [Google Scholar] [CrossRef]
- Lourenço, A.L.; De Jager, N.; Prochnow, C.; Dutra, D.A.M.; Kleverlaan, C.J. Young’s modulus and Poisson ratio of composite materials: Influence of wet and dry storage. Dent. Mater. J. 2020, 39, 657–663. [Google Scholar] [CrossRef]
- Száva, D.T.; Száva, I.; Vlase, S.; Száva, A. Experimental Investigations of the Dental Filling Materials: Establishing Elastic Moduli and Poisson’s Ratios. Materials 2023, 16, 3456. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Najim, W.A. A comparison of fracture strength of posterior composite and ceramic inlay materials. J. Bagh. College Dent. 2009, 21, 18–22. [Google Scholar]
- Moezzyzadeh, M. Evaluation of the compressive strength of hybrid and nanocomposites. J. Dent. Sch. 2012, 30, 24–29. [Google Scholar]
- Gill, R.; Millar, B.J.; Deb, S. Properties of a Bulk-Fill Flowable Composite Resin with High Depth of Cure. Open J. Stomatol. 2017, 7, 377–387. [Google Scholar] [CrossRef][Green Version]
- Lemon, D.J.; Chen, W.; Smith, T.; Ford, A.A.; Moffett, S.X.; Hoyle, J.T.; Hamlin, N.J.; Hwang, Y.Y. The effect of simulated field storage conditions on dental restorative materials for military field use. Mil. Med. 2020, 185, e831–e838. [Google Scholar] [CrossRef] [PubMed]
- von Gehren, M.O.; Rüttermann, S.; Romanos, G.E.; Herrmann, E.; Gerhardt-Szép, S. A 23-Year Observational Follow-Up Clinical Evaluation of Direct Posterior Composite Restorations. Dent. J. 2023, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Taher, R.M.; Moharam, L.M.; Amin, A.E.; Zaazou, M.H.; El-Askary, F.S.; Ibrahim, M.N. The effect of radiation exposure and storage time on the degree of conversion and flexural strength of different resin composites. Bull. Natl. Res. Cent. 2021, 45, 1–11. [Google Scholar] [CrossRef]
- Gradinaru, I.; Ignat, L.; Doroftei, F.; Ignat, M.E.; Antohe, M.E. Researches Regarding the Improvement of Different Dental Contemporary Composite Materials Structure. Environment 2018, 69, 1774–1778. [Google Scholar]
- Li, J.; Li, H.; Fok, A.S.; Watts, D.C. Numerical evaluation of bulk material properties of dental composites using two-phase finite element models. Dent. Mater. 2012, 28, 996–1003. [Google Scholar] [CrossRef]
- Albanés-Ojeda, E.A.; Calderón-Olvera, R.M.; García-Hipólito, M.; Chavarría-Bolaños, D.; Vega-Baudrit, R.; Álvarez-Perez, M.A.; Alvarez-Fregoso, O. Physical and chemical characterization of PLA nanofibres and PLA/ZrO2mesoporous composites synthesized by air-jet spinning. Indian J. Fibre Text. Res. 2020, 45, 57. [Google Scholar]
- Masouras, K.; Silikas, N.; Watts, D.C. Correlation of filler content and elastic properties of resin-composites. Dent. Mater. 2008, 24, 932–939. [Google Scholar] [CrossRef]
- Khosravani, M.R. Mechanical behavior of restorative dental composites under various loading conditions. J. Mech. Behav. Biomed. Mater. 2019, 93, 151–157. [Google Scholar] [CrossRef]
- Encalada-Alayola, J.J.; Veranes-Pantoja, Y.; Uribe-Calderón, J.A.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Effect of type and concentration of nanoclay on the mechanical and physicochemical properties of Bis-GMA/TTEGDMA dental resins. Polymers 2020, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.S.; Durmus, A.; Kurt, B.Z.; Koymen, S.S.; Donmez, N. Depth of cure, mechanical properties and morphology of dual-cure bulk-Fill composites. Odovtos-Int. J. Dent. Sci. 2023, 25, 72–87. [Google Scholar]
- Main, K.; Khan, M.A.; Nuutinen, J.P.; Young, A.M.; Liaqat, S.; Muhammad, N. Evaluation of modified dental composites as an alternative to Poly (methyl methacrylate) bone cement. Polym. Bull. 2023, 80, 13143–13158. [Google Scholar] [CrossRef]
- Khan, M.A.; Delgado, A.H.; Young, A.M. Modifying dental composites to formulate novel methacrylate-based bone cements with improved polymerisation kinetics, and mechanical properties. Dent. Mater. 2023, 39, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Hassanzadeh Tabrizi, S.A.; Ismail, A.F.; Berto, F. Poly (methyl methacrylate) bone cement, its rise, growth, downfall and future. Polym. Int. 2021, 70, 1182–1201. [Google Scholar] [CrossRef]
- Turner, B.N.; Gold, S.A. A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Salmi, M.; Paloheimo, K.S.; Tuomi, J.; Wolff, J.; Mäkitie, A. Accuracy of medical models made by additive manufacturing (rapid manufacturing). J. Cranio-Maxillofac. Surg. 2013, 41, 603–609. [Google Scholar] [CrossRef]



| Material | Resin 1 (%) * | Resin 2 (%) ** | Premix (%) *** | SiO2 (%) | TiO2 (%) | ZrO2 Nanoparticles (%) | |
|---|---|---|---|---|---|---|---|
| Group 1 | UX_1 | 18 | 13 | - | 5 | 64 | - |
| UX_2 | 20 | 12 | - | 5 | 63 | - | |
| UX_3 | 20 | 13 | - | 5 | 62 | - | |
| Group 2 | UX_P1 | 17 | 12 | 6 | 4 | 60 | 1 |
| UX_P5 | 16 | 11 | 6 | 4 | 58 | 5 | |
| UX_P10 | 15 | 10 | 6 | 4 | 55 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallicchio, V.; Spinelli, V.; Russo, T.; Marino, C.; Spagnuolo, G.; Rengo, C.; De Santis, R. Highly Reinforced Acrylic Resins for Hard Tissue Engineering and Their Suitability to Be Additively Manufactured through Nozzle-Based Photo-Printing. Materials 2024, 17, 37. https://doi.org/10.3390/ma17010037
Gallicchio V, Spinelli V, Russo T, Marino C, Spagnuolo G, Rengo C, De Santis R. Highly Reinforced Acrylic Resins for Hard Tissue Engineering and Their Suitability to Be Additively Manufactured through Nozzle-Based Photo-Printing. Materials. 2024; 17(1):37. https://doi.org/10.3390/ma17010037
Chicago/Turabian StyleGallicchio, Vito, Vincenzo Spinelli, Teresa Russo, Ciro Marino, Gianrico Spagnuolo, Carlo Rengo, and Roberto De Santis. 2024. "Highly Reinforced Acrylic Resins for Hard Tissue Engineering and Their Suitability to Be Additively Manufactured through Nozzle-Based Photo-Printing" Materials 17, no. 1: 37. https://doi.org/10.3390/ma17010037
APA StyleGallicchio, V., Spinelli, V., Russo, T., Marino, C., Spagnuolo, G., Rengo, C., & De Santis, R. (2024). Highly Reinforced Acrylic Resins for Hard Tissue Engineering and Their Suitability to Be Additively Manufactured through Nozzle-Based Photo-Printing. Materials, 17(1), 37. https://doi.org/10.3390/ma17010037











