Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization
Abstract
1. Introduction
2. PVC Characteristics
2.1. PVC’s Physical Properties
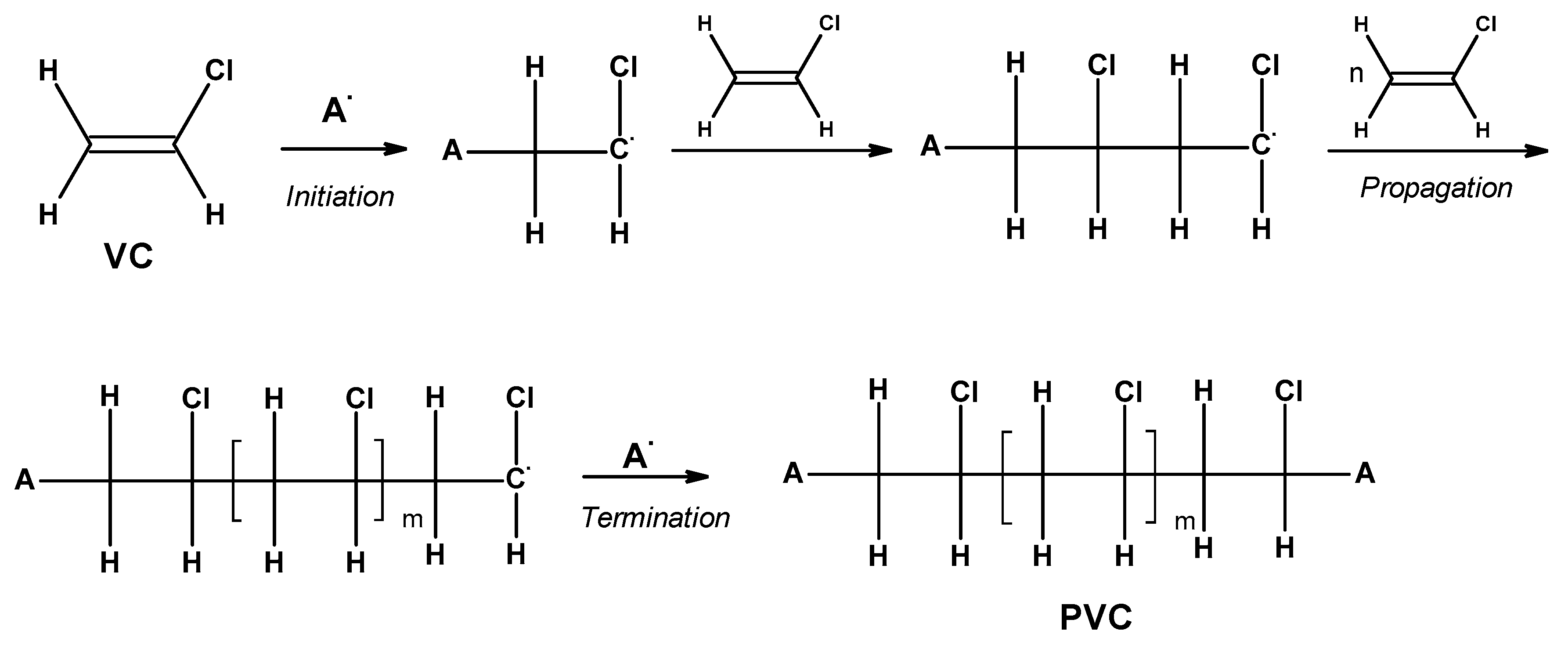
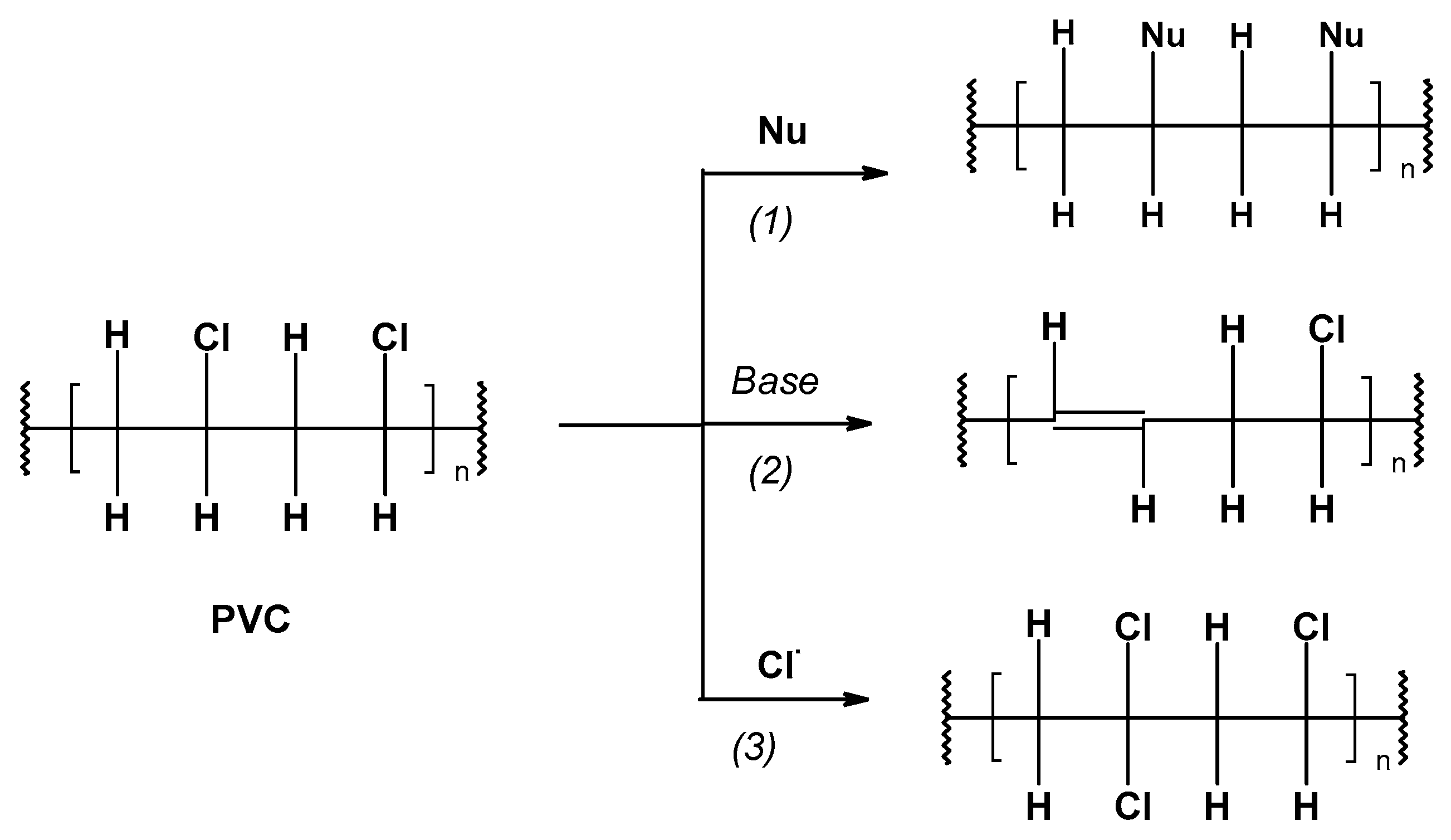
2.2. PVC’s Chemical Properties
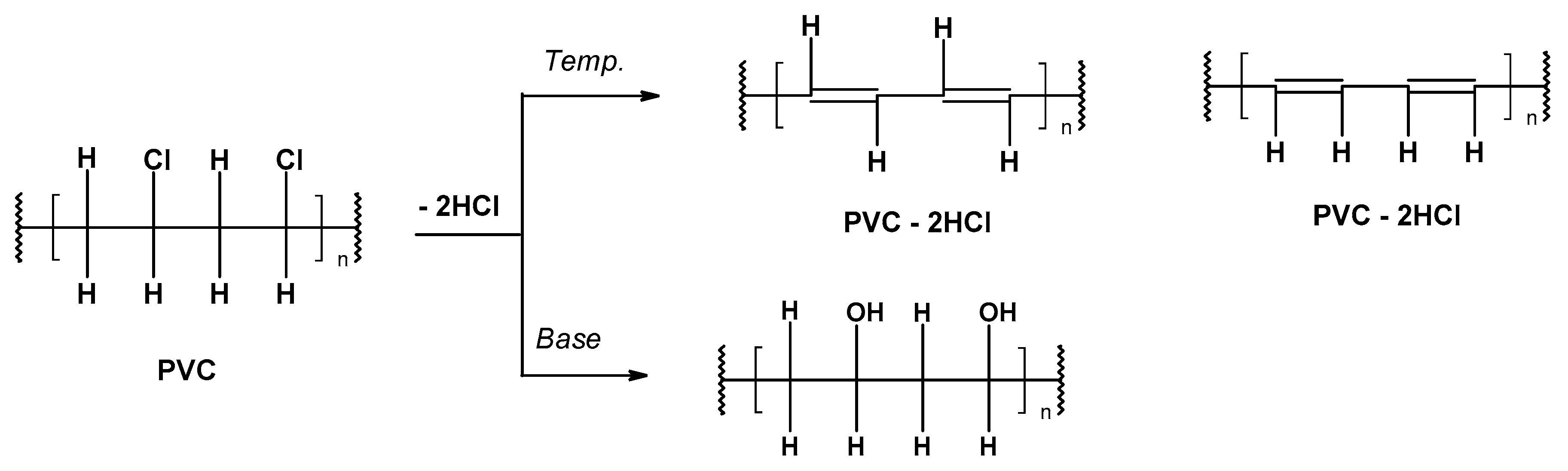
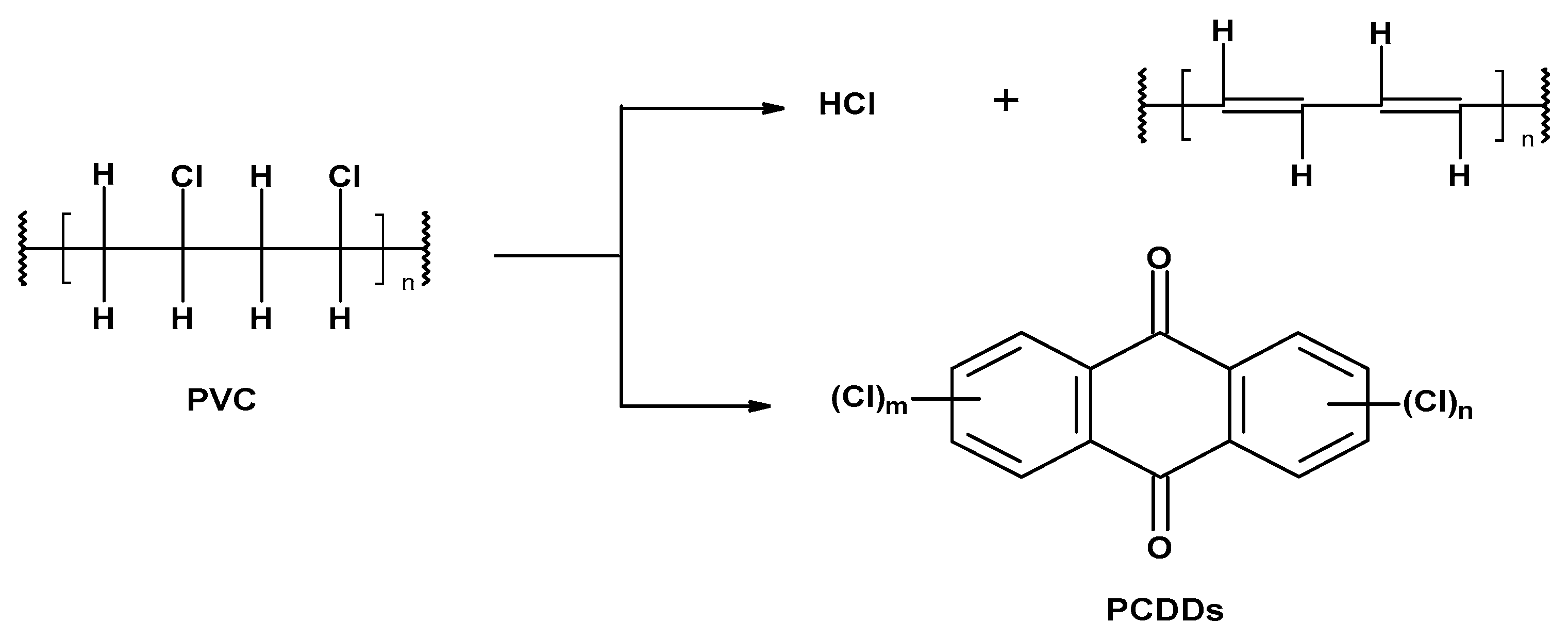
- Preliminary (occurring under the influence of temperature, called thermal) under anaerobic conditions at the molecular or ionic level,
- Secondary as a result of the increased temperature and oxygen action (thermo-oxidative degradation).
2.3. Biological Activity of Poly(vinyl chloride)
3. Threats Related to Production and Use of PVC
4. Worldwide Pollution of the Aquatic Environment by the Poly(vinyl chloride) Industry
5. Toxicity of Poly(vinyl chloride) to Surface Water Trophic Networks and Humans
- Cancer,
- Disruption of the endocrine system,
- Reproductive impairment,
- Impaired child development and birth defects,
- Neurotoxicity (damage to the brain or its function),
- Immune system suppression.
6. Contamination of Soil with PVC (and Other Plastics)
7. Disposal Methods of Poly(vinyl chloride) from the Environment
7.1. Recycling and Utilization of PVC
7.2. Biodegradation of PVC Waste
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Santillán, L. New plastic formations in the Anthropocene. Sci. Total Environ. 2021, 754, 14221–14226. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2021. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 2 October 2023).
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2023, 3, e1700782. [Google Scholar] [CrossRef]
- Bouaicha, O.; Mimmo, T.; Tiziani, R.; Praeg, N.; Polidori, C.; Lucini, L.; Vigani, G.; Terzano, R.; Sanchez-Hernandez, J.C.; Illmer, P.; et al. Microplastics make their way into the soil and rhizosphere: A review of the ecological consequences. Rhizosphere 2022, 22, 100542. [Google Scholar] [CrossRef]
- Xu, Y.; Xian, Z.-N.; Yue, W.; Yin, C.-F.; Zho, N.-Y. Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 2023, 318, 137944. [Google Scholar] [CrossRef]
- Bermúdez, J.R.; Swarzenski, P.W. A microplastic size classification scheme aligned with universal plankton survey methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef]
- Barili, S.; Bernetti, A.; Sannino, C.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pinchuk, I.; Pezzolla, D.; Turchetti, B.; Buzzini, P.; et al. Impact of PVC microplastics on soil chemical and microbiological parameters. Environ. Res. 2023, 229, 115891. [Google Scholar] [CrossRef]
- Available online: http://tts-polska.com/tts-polska2/informacje/polichlorek-winylu.html (accessed on 3 October 2023).
- Available online: https://www.globaldata.com/store/report/polyvinyl-chloride-market-analysis/ (accessed on 4 October 2023).
- Miliute-Plepiene, J.; Fråne, A.; Almasi, A.M. Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol. 2021, 4, 100246. [Google Scholar] [CrossRef]
- Uropean Commission. The Use of PVC (Poly Vinyl Chloride) in the Contex of a Non-Toxic Environment. 2022. Available online: https://op.europa.eu/en/publication-detail/-/publication/e9e7684a-906b-11ec-b4e4-01aa75ed71a1 (accessed on 5 October 2023).
- Available online: http://archive.greenpeace.org/toxics/html/contenUpvc1.html (accessed on 5 October 2023).
- Available online: http://www.greenpeace.org/usa/en/campaigns/toxics/go-pvc-free/ (accessed on 6 October 2023).
- Available online: http://www.who.inUmediacentre/factsheets/fs225/en/ (accessed on 7 October 2023).
- Available online: http://www.greenpeace.org/usa/en/campaigns/!oxics/go-pvc-free/ (accessed on 7 October 2023).
- Available online: http://www.ens-newswire.com/ens/jan2011/2011-01-21-01.htmlhttps://www.google.com/search?client=firefox-b-d&q=PVC-Free+Future%3A+A+Review+of+Restrictions+and+PVC+free+Policies+Worldwide (accessed on 9 October 2023).
- Titow, W.V. (Ed.) PVC Polymers BT—PVC Plastics: Properties, Processing, and Applications; Springer: Dordrecht, The Netherlands, 1990; pp. 53–101. [Google Scholar] [CrossRef]
- Fisher, I.; Schmitt, W.F.; Porth, H.C.; Allsopp, M.W.; Vianello, G. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Gilbert, M.; Patrick, S. Poly(Vinyl Chloride). In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 329–388. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Endo, K. Synthesis and structure of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2021–2054. [Google Scholar] [CrossRef]
- Braun, D. PVC—Origin, growth, and future. J. Vinyl Addit. Technol. 2001, 7, 168–176. [Google Scholar] [CrossRef]
- Braun, D. Poly(vinyl chloride) on the way from the 19th century to the 21st century. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 578–586. [Google Scholar] [CrossRef]
- Saeki, Y.; Emura, T. Technical progresses for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131. [Google Scholar] [CrossRef]
- Thornton, J. Environmental Impacts of Polyvinyl Chloride Building Materials. Healthy Buiding Network. 2002. Available online: https://www.google.com/search?client=firefox-b-d&q=Environmental+Impacts+of+Polyvinyl+Chloride+Building+Materials (accessed on 10 October 2023).
- Howard, M. Exploring the Global Polyvinyl Chloride (PVC) Market Size: Trends, Challenges, and Opportunities, Demand, Growth. 2030. Available online: https://Www.Zionmarketresearch.Com/Sample/Polyvinyl-Chloride-Pvc-Market (accessed on 11 October 2023).
- Moulay, S. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Skelly, P.W.; Li, L.; Braslau, R. Internal plasticization of PVC. Polym. Rev. 2022, 62, 485–528. [Google Scholar] [CrossRef]
- Lieberzeit, P.; Bekchanov, D.; Mukhamedie, M. Polyvinyl chloride modifications, properties, and applications: Review. Polym. Adv. Technol. 2022, 33, 1809–1820. [Google Scholar] [CrossRef]
- Babinsky, R. PVC additives: A global review. Plast. Addit. Compd. 2006, 8, 38–40. [Google Scholar] [CrossRef]
- Ambrogi, A.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. In Modification of Polymer Properties; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 4, pp. 87–108. [Google Scholar] [CrossRef]
- Elgharbawy, A.S. Poly Vinyl Chloride Additives and Applications—A Review. J. Risk Anal. Crisis Response 2022, 12, 143–151. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- PPI TR-19. The Plastics Pipe Institute, Inc. TR-19. Chemical Resistance of Plastic Piping Materials. 28 April 2023. Available online: https://www.plasticpipe.org/ (accessed on 11 October 2023).
- Grause, G.; Hirahashi, S.; Toyoda, H.; Kameda, T.; Yoshioka, T. Solubility parameters for determining optimal solvents for separating PVC from PVC-coated PET fibers. J. Mater. Cycles Waste Manag. 2015, 19, 612–622. [Google Scholar] [CrossRef]
- Kameda, T.; Fukuda, Y.; Grause, G.; Yoshioka, T. Chemical modification of rigid poly(vinyl chloride) by the substitution with nucleophiles. J. Appl. Polym. Sci. 2010, 116, 36–44. [Google Scholar] [CrossRef]
- Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Chemical modification of poly(vinyl chloride) by nucleophilic substitution. Polym. Degrad. Stab. 2009, 94, 107–112. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Fisch, M. Reaction mechanism of poly(vinyl chloride) degradation. Molecular orbital calculations. J. Vinyl Addit. Technol. 1995, 1, 241–249. [Google Scholar] [CrossRef]
- Ge, X.; Starnes, W.H. Chlorination of poly(vinyl chloride) model compounds in radical-complexing solvents. J. Vinyl Addit. Technol. 2016, 22, 405–409. [Google Scholar] [CrossRef]
- Zakharyan, E.M.; Petrukhina, N.N.; Dzhabarov, E.G.; Maksimov, A.L. Pathways of Chemical Recycling of Polyvinyl Chloride. Part 2. Russ. J. Appl. Chem. 2020, 93, 1445–1490. [Google Scholar] [CrossRef]
- Zakharyan, E.M.; Petrukhina, N.N.; Maksimov, A.L. Pathways of Chemical Recycling of Polyvinyl Chloride: Part 1. Russ. J. Appl. Chem. 2020, 93, 1271–1313. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef]
- Pospíšil, J.; Horák, Z.; Kruliš, Z.; Nešpůrek, S.; Kuroda, S. Degradation and aging of polymer blends I. Thermomechanical and thermal degradation. Polym. Degrad. Stab. 1999, 65, 405–414. [Google Scholar] [CrossRef]
- Carroll, W.F., Jr.; Berger, T.C.; Borrelli, F.E.; Garrity, P.J.; Jacobs, R.A.; Ledvina, J.; Lewis, J.W.; McCreedy, R.L.; Smith, T.P.; Tuhovak, D.R.; et al. Characterization of emissions of dioxins and furans from ethylene dichloride, vinyl chloride monomer and polyvinyl chloride facilities in the United States. Consolidated report. Chemosphere 2001, 43, 689–700. [Google Scholar] [CrossRef]
- Available online: https://oxoplast.com/stabilnosc-polichlorku-winylu/ (accessed on 12 October 2023).
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef]
- Chen, W.; Gong, Y.; McKie, M.; Almuhtaram, H.; Sun, J.; Barrett, H.; Yang, D.; Wu, M.; Andrews, R.C.; Peng, H. Defining the chemical additives driving in vitro toxicities of plastics. Environ. Sci. Technol. 2022, 56, 14627–14639. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.K. Toxicity of vinyl chloride and poly(vinyl chloride): A critical review. Environ. Health Perspect. 1983, 52, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Huang, K.-L.; Cheng, T.-J.; Wang, J.-D.; Hsieh, L.-L. The GST T1 and CYP2E1 genotypes are possible factors causing vinyl chloride induced abnormal liver function. Archiv. Toxicol. 1997, 71, 482–488. [Google Scholar] [CrossRef]
- Sass, J.B.; Castleman, B.; Wallinga, D. Vinyl Chloride: A Case study of data suppression and misrepresentation. Environ. Health Perspect. 2005, 113, 809–812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapp, R.W. Vinyl chloride. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 934–938. [Google Scholar] [CrossRef]
- Mahadevan, G.; Valiyaveettil, S. Comparison of genotoxicity and cytotoxicity of polyvinyl chloride and poly(methyl methacrylate) nanoparticles on normal human lung cell lines. Chem. Res. Toxicol. 2021, 34, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol. In Vitro 2021, 70, 105021. [Google Scholar] [CrossRef]
- Paul, M.B.; Fahrenson, C.; Givelet, L.; Herrmann, T.; Loeschner, K.; Böhmert, L.; Thünemann, A.F.; Braeuning, A.; Sieg, H. Beyond microplastics—Investigation on health impacts of submicron and nanoplastic particles after oral uptake in vitro. Microplastics Nanoplastics 2022, 2, 16. [Google Scholar] [CrossRef]
- Guardiola, J.J.; Beier, J.I.; Falkner, K.C.; Wheeler, B.; McClain, C.J.; Cave, M. Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. Toxicol. Appl. Pharmacol. 2016, 313, 47–56. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Oleru, U.G.; Onyekwere, C. Exposures to polyvinyl chloride, methyl ketone and other chemicals—The pulmonary and non-pulmonary effect. Int. Archiv. Occup. Environ. Health 1992, 63, 503–507. [Google Scholar] [CrossRef]
- Xu, H.; Hoet, P.H.; Nemery, B. In vitro toxicity assessment of polyvinyl chloride particles and comparison of six cellular systems. J. Toxicol. Environ. Health A 2002, 65, 1141–1159. [Google Scholar] [CrossRef]
- Zelko, I.N.; Taylor, B.S.; Das, T.P.; Watson, W.H.; Sithu, I.D.; Wahlang, B.; Malovichko, M.V.; Cave, M.C.; Srivastava, S. Effect of vinyl chloride exposure on cardiometabolic toxicity. Environ. Toxicol. 2022, 37, 245–255. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Zheng, Y.; Gao, F.; Jiang, F.; Li, J.; Sun, C. Probing the toxic interactions between polyvinyl chloride microplastics and Human Serum Albumin by multispectroscopic techniques. Sci. Total Environ. 2020, 734, 139219. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Ding, J.; Jiang, F.; Sun, C.; Jiang, F.; Sun, C. New insights into the toxic interactions of polyvinyl chloride microplastics with bovine serum albumin. Environ. Sci. Pollut. Res. 2021, 28, 5520–5531. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16S rRNA sequencing data. Sci. Total Environ. 2022, 839, 155984. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Richards, R.J.; Desai, R.; Hext, P.M.; Rose, F.A. Biological reactivity of PVC dust. Nature 1975, 5519, 664–665. [Google Scholar] [CrossRef]
- Sharman, M.; Rose, M.; Parker, I.; Mercer, A.; Castle, L.; Gilbert, J.; Startin, J. Migration from plasticized films into foods. 1. Migration of di-(2-ethylhexyl)adipate from PVC films during home-use and microwave cooking. Food Addit. Contam. 1987, 4, 385–398. [Google Scholar] [CrossRef]
- Sampson, J.; De Korte, D. Review DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 2011, 21, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Olkova, A. Toxicity of water after short-term contact with pvc materials depending on the temperature and components of the polymer composition. Ecol. Eng. Environ. Technol. 2021, 22, 119–125. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic products leach chemicals that induce in vitrotoxicity under realistic use conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L. Toxicity of the components of poly(vinylchloride) polymers additives. Prog. Clin. Biol. Res. 1983, 141, 113–136. [Google Scholar]
- Beiras, R.; Verdejo, E.; Campoy-López, P.; Vidal-Liñán, L. Aquatic toxicity of chemically defined microplastics can be explained by functional additives. J. Hazard. Mater. 2021, 406, 124338. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, X.; Guo, M.; Cao, X.; Zheng, X.; Bao, D. UV-induced microplastics (MPs) aging leads to comprehensive toxicity. Mar. Pollut. Bull. 2023, 189, 114745. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Li, W.; Li, Z. Impacts of microplastics addition on sediment environmental properties, enzymatic activities and bacterial diversity. Chemosphere 2022, 307, 135836. [Google Scholar] [CrossRef]
- Shue, M.F.; Liou, J.J.; Tasi, J.L.; Tang, H.C.; Huang, W.J.; Liao, M.H. Cytotoxicity studies on combustion gas of polyvinyl chloride (PVC) resin. Aerosol Air Qual. Res. 2009, 9, 305–308. [Google Scholar] [CrossRef]
- Sokolova, Y.; Gotlib, E.; Kozhevnikov, R.; Sokolova, A. Modification of PVC-compositions for linoleum. IOP Conf. Ser. Mater. Sci. Eng. 2018, 365, 032021. [Google Scholar] [CrossRef]
- Gotlib, E.; Sadykova, D.; Vdovina, T.; Galeeva, L.; Sokolova, A. Evaluation of bactericidal properties of PVC-compositions for linoleum production. E3S Web Conf. 2019, 97, 02001. [Google Scholar] [CrossRef]
- Tran, V.Q.C.; Le, D.V.; Yntema, D.R.; Havinga, P.J.M. A Review of Inspection Methods for Continuously Monitoring PVC Drinking Water Mains. IEEE Internet Things J. 2022, 9, 14336–14354. [Google Scholar] [CrossRef]
- Bottausci, S.; Ungureanu-Comanita, E.-D.; Gavrilescu, M.; Bonoli, A. Environmental impacts quantification of pvc production. Environ. Eng. Manag. J. 2021, 20, 1693–1702. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Murugesan, T. The chlor-alkali process: Work in progress. Clean Technol. Environ. Policy 2014, 16, 225–234. [Google Scholar] [CrossRef]
- Sustainable Solution Corporation. Life Cycle Assessment of PVC Water and Sewer Pipe and Comparative Sustainability Analysis of Pipe Materials. 2017. Available online: https://www.uni-bell.org/files/Reports/Life_Cycle_Assessment_of_PVC_Water_and_Sewer_Pipe_and_Comparative_Sustainability_Analysis_of_Pipe_Materials.pdf (accessed on 14 October 2023).
- Shi, S.Q.; Cai, L.; Weng, Y.; Wang, D.; Sun, Y. Comparative life-cycle assessment of water supply pipes made from bamboo vs. polyvinyl chloride. J. Clean. Prod. 2019, 240, 118172. [Google Scholar] [CrossRef]
- Comaniţă, E.-D.; Ghinea, C.; Roşca, M.; Simion, I.M.; MPetraru, M.; Gavrilescu, M. Environmental impacts of polyvinyl chloride (PVC) production process. In Proceedings of the 2015 E-Health and Bioengineering Conference, Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Ye, L.; Qi, C.; Hong, J.; Ma, X. Life cycle assessment of polyvinyl chloride production and its recyclability in China. J. Clean. Prod. 2017, 142, 2965–2972. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, W.; Makowski, M.; Yan, H.; Yu, Y.; Ma, T. Incorporation of life cycle emissions and carbon price uncertainty into the supply chain network management of PVC production. Ann. Oper. Res. 2021, 300, 601–620. [Google Scholar] [CrossRef]
- Marcilla, A.; García, S.; García-Quesada, J.C. Study of the migration of PVC plasticizers. J. Anal. Appl. Pyrolysis 2004, 71, 457–463. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, F.; Zhu, C.; Chen, Z.; Liu, S.; Wang, C.; Gu, C. Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors. Water Res. 2021, 204, 117597. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, P.; Wu, Y.; Zhou, Y.; Sheng, Y.; Lao, K. Microplastic acts as a vector for contaminants: The release behavior of dibutyl phthalate from polyvinyl chloride pipe fragments in water phase. Environ. Sci. Pollut. Res. 2020, 27, 42082–42091. [Google Scholar] [CrossRef]
- Skjevrak, I.; Due, A.; Gjerstad, K.O.; Herikstad, H. Volatile organic components migrating from plastic pipes (HDPE, PEX and PVC) into drinking water. Water Res. 2003, 37, 1912–1920. [Google Scholar] [CrossRef]
- Fayad, N.M.; Sheikheldin, S.Y.; Al-Malack, M.H.; El-Mubarak, A.H.; Khaja, N. Migration of vinyl chloride monomer (VCM) and additives into PVC bottled drinking water. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1997, 32, 1065–1083. [Google Scholar] [CrossRef]
- Henkel, C.; Hüffer, T.; Hofmann, T. Polyvinyl Chloride Microplastics Leach Phthalates into the Aquatic Environment over Decades. Environ. Sci. Technol. 2022, 56, 14507–14516. [Google Scholar] [CrossRef] [PubMed]
- Chong, N.S.; Abdulramoni, S.; Patterson, D.; Brown, H. Releases of Fire-Derived Contaminants from Polymer Pipes Made of Polyvinyl Chloride. Toxics 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Misidentification of PVC microplastics in marine environmental samples. TrAC Trends Anal. Chem. 2022, 153, 116649. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. 2018, 25, 11319–11332. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in seawater: Sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef] [PubMed]
- Kavya, A.N.V.L.; Sundarrajan, S.; Ramakrishna, S. Identification and characterization of micro-plastics in the marine environment: A mini review. Mar. Pollut. Bull. 2020, 160, 111704. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, H.; Chen, H.; Wang, S.; Sun, X.; Zou, Q.; Zhang, Y.; Lin, H.; Cai, S.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650 Pt 2, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, S.V.; La Spina, R.; Fumagalli, F.; Riccardi, N.; Gilliland, D.; Ponti, J. Detection of Metal-Doped Fluorescent PVC Microplastics in Freshwater Mussels. Nanomaterials 2020, 10, 2363. [Google Scholar] [CrossRef]
- Suman, K.H.; Haque, M.N.; Uddin, M.J.; Begum, M.S.; Sikder, M.H. Toxicity and biomarkers of micro-plastic in aquatic environment: A review. Biomarkers 2021, 26, 13–25. [Google Scholar] [CrossRef]
- Yin, L.; Jiang, C.; Wen, X.; Du, C.; Zhong, W.; Feng, Z.; Long, Y.; Ma, Y. Microplastic Pollution in Surface Water of Urban Lakes in Changsha, China. Int. J. Environ. Res. Public Health 2019, 16, 1650. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Wen, X.; Ren, X.; Zeng, G.; Zhang, Y.; Xiao, R. Presence of microplastics in drinking water from freshwater sources: The investigation in Changsha, China. Environ. Sci. Pollut. Res. 2021, 28, 42313–42324. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, F.; Li, J.; Wang, Z.; Sun, C.; Wang, Z.; Fu, L.; Ding, N.X.; He, C. Microplastics in the Coral Reef Systems from Xisha Islands of South China Sea. Environ. Sci. Technol. 2019, 53, 8036–8046. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zheng, K.; Zhu, Z.; Chen, G.; Peng, X. Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ. Pollut. 2019, 251, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, Q.; Chen, X.; Wang, R.; Duan, M.; Wu, C. Occurrence of microplastic in the water of different types of aquaculture ponds in an important lakeside freshwater aquaculture area of China. Chemosphere 2021, 282, 131126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yin, L.; Wen, X.; Du, C.; Wu, L.; Long, Y.; Liu, Y.; Ma, Y.; Yin, Q.; Zhou, Z.; et al. Microplastics in Sediment and Surface Water of West Dongting Lake and South Dongting Lake: Abundance, Source and Composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Chen, Y.; Wang, J. Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci. Total Environ. 2018, 633, 539–545. [Google Scholar] [CrossRef]
- Chae, D.-H.; Kim, I.-S.; Kim, S.-K.; Song, Y.K.; Shim, W.J. Abundance and Distribution Characteristics of Microplastics in Surface Seawaters of the Incheon/Kyeonggi Coastal Region. Arch. Environ. Contam. Toxicol. 2015, 69, 269–278. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Shim, W.J. Occurrence and Distribution of Microplastics in the Sea Surface Microlayer in Jinhae Bay, South Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 279–287. [Google Scholar] [CrossRef]
- Tunçer, S.; Artüz, O.B.; Demirkol, M.; Artüz, M.L. First report of occurrence, distribution, and composition of microplastics in surface waters of the Sea of Marmara, Turkey. Mar. Pollut. Bull. 2018, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Khalik, W.M.A.W.M.; Ibrahim, Y.S.; Anuar, S.T.; Govindasamy, S.; Baharuddin, N.F. Microplastics analysis in Malaysian marine waters: A field study of Kuala Nerus and Kuantan. Mar. Pollut. Bull. 2018, 135, 451–457. [Google Scholar] [CrossRef]
- Morgana, S.; Ghigliotti, L.; Estévez-Calvar, N.; Stifanese, R.; Wieckzorek, A.; Doyle, T.; Christiansen, J.S.; Faimali, M.; Garaventa, F. Microplastics in the Arctic: A case study with sub-surface water and fish samples off Northeast Greenland. Environ. Pollut. 2018, 242, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Saraux, C.; Heitz, O.; Nowaczyk, A.; Bonnet, D. Microplastics FTIR characterisation and distribution in the water column and digestive tracts of small pelagic fish in the Gulf of Lions. Mar. Pollut. Bull. 2019, 142, 510–519. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242, 1557–1565. [Google Scholar] [CrossRef]
- Zeri, C.; Adamopoulou, A.; Bojanić Varezić, D.; Fortibuoni, T.; Kovač Viršek, M.; Kržan, A.; Mandic, M.; Mazziotti, C.; Palatinus, A.; Peterlin, M.; et al. Floating plastics in Adriatic waters (Mediterranean Sea): From the macro- to the micro-scale. Mar. Pollut. Bull. 2018, 136, 341–350. [Google Scholar] [CrossRef]
- Palatinus, A.; Kovač Viršek, M.; Robič, U.; Grego, M.; Bajt, O.; Šiljić, J.; Suaria, G.; Liubartseva, S.; Coppini, G.; Peterlin, M. Marine litter in the Croatian part of the middle Adriatic Sea: Simultaneous assessment of floating and seabed macro and micro litter abundance and composition. Mar. Pollut. Bull. 2019, 139, 427–439. [Google Scholar] [CrossRef]
- de Haan, W.P.; Sanchez-Vidal, A.; Canals, M. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 523–535. [Google Scholar] [CrossRef]
- Syakti, A.D.; Bouhroum, R.; Hidayati, N.V.; Koenawan, C.J.; Boulkamh, A.; Sulistyo, I.; Lebarillier, S.; Akhlus, S.; Doumenq, P.; Wong-Wah-Chung, P. Beach macro-litter monitoring and floating microplastic in a coastal area of Indonesia. Mar. Pollut. Bull. 2017, 122, 217–225. [Google Scholar] [CrossRef]
- Su, L.; Sharp, S.M.; Pettigrove, V.J.; Craig, N.J.; Nan, B.; Du, F.; Shi, H. Superimposed microplastic pollution in a coastal metropolis. Water Res. 2020, 168, 115140. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Thompson, J.; Paris, A.; Rohindra, D.; Rico, C. Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar. Pollut. Bull. 2020, 153, 110991. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, L.D.K.; Johansson, C.; Frias, J.P.G.L.; Gardfeldt, K.; Thompson, R.C.; O’Connor, I. Deep sea sediments of the Arctic Central Basin: A potential sink for microplastics. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 145, 137–142. [Google Scholar] [CrossRef]
- Gomiero, A.; Øysæd, K.B.; Agustsson, T.; van Hoytema, N.; van Thiel, T.; Grati, F. First record of characterization, concentration and distribution of microplastics in coastal sediments of an urban fjord in south west Norway using a thermal degradation method. Chemosphere 2019, 227, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef]
- Vilakati, B.; Sivasankar, V.; Mamba, B.B.; Omine, K.; Msagati, T.A.M. Characterization of plastic micro particles in the Atlantic Ocean seashore of Cape Town, South Africa and mass spectrometry analysis of pyrolyzate products. Environ. Pollut. 2020, 265, 114859. [Google Scholar] [CrossRef]
- Lozoya, J.P.; Teixeira de Mello, F.; Carrizo, D.; Weinstein, F.; Olivera, Y.; Cedrés, F.; Pereira, M.; Fossati, M. Plastics and microplastics on recreational beaches in Punta del Este (Uruguay): Unseen critical residents? Environ. Pollut. 2016, 218, 931–941. [Google Scholar] [CrossRef]
- Bucol, L.A.; Romano, E.F.; Cabcaban, S.M.; Siplon, L.M.D.; Madrid, G.C.; Bucol, A.A.; Polidoro, B. Microplastics in marine sediments and rabbitfish (Siganus fuscescens) from selected coastal areas of Negros Oriental, Philippines. Mar. Pollut. Bull. 2020, 150, s110685. [Google Scholar] [CrossRef]
- Hara, J.; Frias, J.; Nash, R. Quantification of microplastic ingestion by the decapod crustacean Nephrops norvegicus from Irish waters. Mar. Pollut. Bull. 2020, 152, 110905. [Google Scholar] [CrossRef]
- Wootton, N.; Ferreira, M.; Gillanders, B. A comparison of microplastic in fish from Australia and Fiji. Front. Mar. Sci. 2021, 8, 690991. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, C.; Segner, H.; Mu, J.; et al. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquaculture 2019, 512, 734322. [Google Scholar] [CrossRef]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Kamari, A.; Mouneyrac, C.; Amiard, F.; Poirier, L.; Lagarde, F. Quantification and characterization of microplastics in blue mussels (Mytilus edulis): Protocol setup and preliminary data on the contamination of the French Atlantic coast. Environ. Sci. Pollut. Res. 2018, 25, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Naik, A.; Desai, A.; Nanajkar, M.; Rathore, C.; Kumar, M.; Gupta, P. Microplastics in seafood as an emerging threat to marine environment: A case study in Goa, west coast of India. Chemosphere 2021, 270, 129359. [Google Scholar] [CrossRef]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose-Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef]
- Sree, C.G.; Buddolla, V.; Lakshmi, B.A.; Kim, Y.-J. Phthalate toxicity mechanisms: An update. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109498. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Overview Report II: An Overview of Current Scientific Knowledge on the Life Cycles, Environmental Exposures, and Environmental Effects of Select Endocrine Disrupting Chemicals (EDCs) and Potential EDCs. 2017. Available online: https://Wedocs.Unep.Org/20.500.11822/25634 (accessed on 16 October 2023).
- Akovali, G. 2—Plastic materials: Polyvinyl chloride (PVC). In Toxicity of Building Materials; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Elston, UK, 2012; pp. 23–53. [Google Scholar] [CrossRef]
- Chen, L.; Qi, H.; Yu, K.; Gao, B. Increased bio-toxicity of leachates from polyvinyl chloride microplastics during the photo-aging process in the presence of dissolved organic matter. Water Sci. Technol. 2023, 88, 2465–2472. [Google Scholar] [CrossRef]
- Tekman, M.B.; Walther, B.A.; Peter, C.; Gutow, L.; Bergmann, M. Impacts of Plastic Pollution in the Oceans on Marine Species, Biodiversity and Ecosystems; WWF Germany: Berlin, Germany, 2022; pp. 1–221. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Choi, D.; Kim, C.; Kim, T.; Park, K.; Im, J.; Hong, J. Potential threat of microplastics to humans: Toxicity prediction modeling by small data analysis. Environ. Sci. Nano 2023, 10, 1096–1108. [Google Scholar] [CrossRef]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H.; et al. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and Health Risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.; Neethu, K.V.; Aneesh, B.P.; Suresh, A.; Saranya, K.S.; Bijoy Nandan, S.; Sharma, K.V. Evaluation of toxicological impacts of Polyvinyl Chloride (PVC) microplastics on fish, Etroplus suratensis (Bloch, 1790), Cochin estuary, India. Toxicol. Environ. Health Sci. 2022, 14, 131–140. [Google Scholar] [CrossRef]
- Darabi, H.; Baradaran, A.; Ebrahimpour, K. Subacute toxic effects of polyvinyl chloride microplastics (PVC-MPs) in juvenile common carp, Cyprinus carpio (Pisces: Cyprinidae). Casp. J. Environ. Sci. 2022, 20, 233–242. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Lamb, J.B.; Willis, B.L.; Fiorenza, E.A.; Couch, C.S.; Howard, R.; Rader, D.N.; True, J.D.; Kelly, L.A.; Ahmad, A.; Jompa, J.; et al. Plastic waste associated with disease on coral reefs. Science 2018, 359, 460–462. [Google Scholar] [CrossRef]
- Boyle, D.; Catarino, A.I.; Clark, N.J.; Henry, T.B. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish. Environ. Pollut. 2020, 263, 114422. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.; Yao, Y.; Artigas, F.; Huang, Q.; Zhang, W. Organotin release from polyvinyl chloride microplastics and concurrent photodegradation in water: Impacts from salinity, dissolved organic matter, and light exposure. Environ. Sci. Technol. 2019, 53, 10741–10752. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Li, W.; Lo, H.-S.; Wong, H.-M.; Zhou, M.; Wong, C.-Y.; Tam, N.F.-Y.; Cheung, S.-G. Heavy metals contamination of sedimentary microplastics in Hong Kong. Mar. Pollut. Bull. 2020, 153, 110977. [Google Scholar] [CrossRef]
- Meem, R.A.; Ahmed, A.; Maraz, K.M.; Md. Shamim Hossain Khan, R.A. A Review on the Impact of Plastic Debris on Marine Environment. Mod. Concepts Mater. Sci. 2021, 4, 1–7. [Google Scholar]
- Liu, Y.; Zhang, J.; Zhao, H.; Cai, J.; Sultan, Y.; Fang, H.; Zhang, B.; Ma, J. Effects of polyvinyl chloride microplastics on reproduction, oxidative stress and reproduction and detoxification-related genes in Daphnia magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109269. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Liang, Y.; Cao, W.; Sun, C.; Ju, P.; Zheng, L. The interactions between microplastic polyvinyl chloride and marine diatoms: Physiological, morphological, and growth effects. Ecotoxicol. Environ. Saf. 2020, 203, 111000. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Grant, M.H.; Blass, C.R.; Courtney, J.M.; Barbenel, J.C. Poly(vinyl chloride) formulations: Acute toxicity to cultured human cell lines. J. Biomater. Sci. Polym. Ed. 1996, 7, 453–459. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Rodrigues, A.C.M.; Campos, D.; Cícero, L.H.; Costa, A.P.L.; Silva, D.A.M.; Oliveira, M.; Soares, A.M.V.M.; Patrício Silva, A.L. Do microplastics affect the zoanthid Zoanthus sociatus? Sci. Total Environ. 2020, 713, 136659. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, Y.; Liu, X.; Jiang, H.; Li, W. Bladder entrance of microplastic likely induces toxic effects in carnivorous macrophyte Utricularia aurea Lour. Environ. Sci. Pollut. Res. 2020, 27, 32124–32131. [Google Scholar] [CrossRef]
- Rist, S.E.; Assidqi, K.; Zamani, N.P.; Appel, D.; Perschke, M.; Huhn, M.; Lenz, M. Suspended micro-sized PVC particles impair the performance and decrease survival in the Asian green mussel Perna viridis. Mar. Pollut. Bull. 2016, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, A.; Strafella, P.; Øysæd, K.B.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef] [PubMed]
- Renzi, M.; Grazioli, E.; Blašković, A. Effects of different microplastic types and surfactant-microplastic mixtures under fasting and feeding conditions: A case study on Daphnia magna. Bull. Environ. Contam. Toxicol. 2019, 103, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, S.C.; Odo, G.E. Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 232, 108741. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Peda, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 68, 251–259. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Hensel, F.; Gomiero, A.; Iordachescu, L.; Vianello, A.; Wittgren, H.B.; Vollertsen, J. Drinking plastics?—Quantification and qualification of microplastics in drinking water distribution systems by µFTIR and Py-GCMS. Water Res. 2021, 188, 116519. [Google Scholar] [CrossRef]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ibeto, C.N.; Enyoh, C.E.; Ofomatah, A.C.; Oguejiofor, L.A.; Okafocha, T.; Okanya, V. Microplastics pollution indices of bottled water from South Eastern Nigeria. Int. J. Environ. Anal. Chem. 2021, 103, 8176–8195. [Google Scholar] [CrossRef]
- Zhou, X.J.; Wang, J.; Li, H.Y.; Zhang, H.M.; Zhang, D.L. Microplastic pollution of bottled water in China. J. Water Process Eng. 2021, 40, 101884. [Google Scholar] [CrossRef]
- Stapleton, P.A. Microplastic and nanoplastic transfer, accumulation, and toxicity in humans. Curr. Opin. Toxicol. 2021, 28, 62–69. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Gola, D.; Kumar Tyagi, P.; Arya, A.; Chauhan, N.; Agarwal, M.; Singh, S.K.; Gola, S. The impact of microplastics on marine environment: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100552. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Wendee, N. Microplastics in Seafood: How Much Are People Eating? Environ. Health Perspect. 2023, 129, 34001. [Google Scholar] [CrossRef]
- Fischer, M.; Goßmann, I.; Scholz-Böttcher, B.M. Fleur de Sel—An interregional monitor for microplastics mass load and composition in European coastal waters? J. Anal. Appl. Pyrolysis 2019, 144, 104711. [Google Scholar] [CrossRef]
- Danopoulos, E.; Jenner, L.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of salt intended for human consumption: A systematic review and meta-analysis. SN Appl. Sci. 2020, 2, 1950. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Tan, W.; Yu, H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.; Silva, L.P.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.; Christyraj, J.R.S.S.; Karthikeyan, S.C.; Jeevanandam, M.; Ganesan, H.; Mathews, M.G.R.; Selvan Christyraj, J.D. Chapter 29—Biodegradation of microplastics and synthetic polymers in agricultural soils. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 563–573. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Lu, Y.; Qi, Y. DEHP degradation and dechlorination of polyvinyl chloride waste in subcritical water with alkali and ethanol: A comparative study. Chemosphere 2020, 249, 126138. [Google Scholar] [CrossRef] [PubMed]
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 021. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Tang, K.H.D. Effects of Microplastics on Agriculture: A Mini-review. Asian J. Environ. Ecol. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Tian, X.; Fan, H.; Wang, J.; Ippolito, J.; Li, Y.; Feng, S.; An, M.; Zhang, F.; Wang, K. Effect of polymer materials on soil structure and organic carbon under drip irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Meng, J.; Li, W.; Diao, C.; Li, Z.; Zhao, J.; Haider, G.; Zhang, H.; Xu, J.; Hu, M.; Shan, S.; et al. Microplastics drive microbial assembly, their interactions, and metagenomic functions in two soils with distinct pH and heavy metal availability. J. Hazard. Mater. 2023, 458, 131973. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, T.; Feng, Z.; Li, B.; Cai, Y.; Ouyang, D.; Gustave, W.; Ying, C.; Zhang, H. Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups. J. Hazard. Mater. 2023, 453, 131391. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Sun, Y.; Duan, H.; Ye, J.; Zhou, A.; Meng, H.; Zhu, F.; He, H.; Gu, C. Effect of PVC microplastics on soil microbial community and nitrogen availability under laboratory-controlled and field-relevant temperatures. Appl. Soil Ecol. 2023, 184, 104794. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.; Pan, J.; Zhang, Z.; Tsui, T.-H.; Luo, L.; Wang, Q. Effect of microplastics on greenhouse gas and ammonia emissions during aerobic composting. Sci. Total Environ. 2020, 737, 139856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Wang, H.; Wang, D.; Zhu, Y.; Wang, J.; He, Y.; Zheng, Q.; Zhan, X. Microplastic particles alter wheat rhizosphere soil microbial community composition and function. J. Hazard. Mater. 2022, 436, 129176. [Google Scholar] [CrossRef] [PubMed]
- Dainelli, M.; Pignattelli, S.; Bazihizina, N.; Falsini, S.; Papini, A.; Baccelli, I.; Mancuso, S.; Coppi, A.; Castellani, M.B.; Colzi, I.; et al. Can microplastics threaten plant productivity and fruit quality? Insights from Micro-Tom and Micro-PET/PVC. Sci. Total Environ. 2023, 895, 165119. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Aqeel, M.; Khalid, N.; Nazir, A.; Irshad, M.K.; Alzuaibr, F.M.; AlHaithloul, H.A.; Akhter, N.; Al-Zoubi, O.M.; Qasim, M.; et al. Microplastics in soil differentially interfere with nutritional aspects of chilli peppers. S. Afr. J. Bot. 2023, 160, 402–413. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Horvat, P.; Skalar, T.; Kržan, A. Plastic bag and facial cleanser derived microplastic do not affect feeding behaviour and energy reserves of terrestrial isopods. Sci. Total Environ. 2018, 615, 761–766. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Green Paper—Environmental Issues of PVC. 2000. Available online: https://eur-lex.europa.eu/legal-content/SL/TXT/?uri=CELEX:52000DC0469 (accessed on 16 October 2023).
- VinylPlus. Progress Report. Reporting on 2017 Activities. 2018. Available online: https://vinylplus.eu/wp-content/uploads/2021/06/VinylPlus-Progress-Report-2018-PbyP.pdf (accessed on 27 October 2018).
- Takeshita, T.; Kato, K.; Takahashi, K.K.; Sato, Y.; Nishi, S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit. Fluids 2004, 31, 185–193. [Google Scholar] [CrossRef]
- Lu, L.; Kumagai, S.; Kameda, T.; Luo, L.; Yoshioka, T. Degradation of PVC waste into a flexible polymer by chemical modification using DINP moieties. RSC Adv. 2019, 9, 28870–28875. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride)—Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. N. Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Lopes, J.A.; Gika, H.; Theodoridis, G. Polystyrene biodegradation by Tenebrio molitor larvae: Identification of generated substances using a GC-MS untargeted screening method. Polymers 2021, 13, 17. [Google Scholar] [CrossRef]
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Zhao, P.; Li, T.; Yan, W.; Yuan, L. Dechlorination of PVC wastes by hydrothermal treatment using alkaline additives. Environ. Technol. 2018, 39, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.-R.; Zhou, K.; Qi, Y. Co-treatment of PVC and used LCD panels in low-temperature subcritical water: Enhanced dechlorination and mechanism. Process Saf. Environ. Prot. 2021, 151, 10–19. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhao, Z.; Pu, Y.; Xiao, K.; Liu, R.; Cao, H.; Wang, Y.; Wang, X. Study of the photoaging process of polyvinyl chloride in different media with the electrical sensing zone method. Reg. Stud. Mar. Sci. 2023, 65, 103073. [Google Scholar] [CrossRef]
- Decker, C. Photodegradation of PVC. In Degradation and Stabilisation of PVC; Owen, E.D., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 81–136. [Google Scholar] [CrossRef]
- Sil, D.; Chakrabarti, S. Photocatalytic degradation of PVC–ZnO composite film under tropical sunlight and artificial UV radiation: A comparative study. Sol. Energy 2010, 84, 476–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Zhang, D.; Shi, Z.; Zhang, X.; Li, C.; Wang, L.; Song, J.; Lin, Q. Enhanced photodegradability of PVC plastics film by codoping nano-graphite and TiO2. Polym. Degrad. Stab. 2020, 181, 109332. [Google Scholar] [CrossRef]
- Ali, M.; Perveen, Q.; Ahmad, B.; Javed, I.; Razi-Ul-Hussnain, R.; Andleeb, S.; Atique, N.; Ghumro, P.B.; Ahmed, S.; Hameed, A. Studies on Biodegradation of Cellulose Blended Polyvinyl Chloride Films. Int. J. Agric. Biol. 2009, 11, 577–580. [Google Scholar]
- Bahl, S.; Dolma, J.; Jyot Singh, J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19. [Google Scholar]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949. [Google Scholar] [CrossRef]
- Sakhalkar, S.; Mishra, R.L. Screening and identification of soil fungi with potential of plastic degrading ability. Indian J. Appl. Res. 2013, 3, 62–64. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes 2022, 10, 1940. [Google Scholar] [CrossRef]
- Yin, F.; Zhuang, Q.; Chang, T.; Zhang, C.; Sun, H.; Sun, Q.; Wang, C.; Li, L. Study on pyrolysis characteristics and kinetics of mixed plastic waste. J. Mater. Cycles Waste Manag. 2021, 23, 1984–1994. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Baitz, M.; Kreißig, J.; Byrne, E.; Makishi, C.; Kupfer, T.; Frees, N.; Bey, N.; Hansen, M.S.; Hansen, A.; Bosch, T.; et al. Final Report: ”Life Cycle Assessment of PVC and of Principal Competing Materials”, Commissioned by the European Commission; European Commission: Luxembourg, 2004. [Google Scholar]
- Ali, M.F.; Siddiqui, M.N. Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J. Anal. Appl. Pyrolysis 2005, 74, 282–289. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Lu, J.; Ma, S.; Gao, J. Study on the Pressurized Hydrolysis Dechlorination of PVC. Energy Fuels 2002, 16, 1251–1255. [Google Scholar] [CrossRef]
- Ma, D.; Liang, L.; Hu, E.; Chen, H.; Wang, D.; He, C.; Feng, Q. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process Saf. Environ. Prot. 2021, 146, 108–117. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Amobonye, A.E.; Bhagwat, P.; Singh, S.; Pillai, S. Chapter 10—Biodegradability of Polyvinyl chloride. In Biodegradability of Conventional Plastics; Sarkar, A., Sharma, B., Shekhar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–220. [Google Scholar] [CrossRef]
- Ganesh, K.A.; Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Wei, W.; Sun, J.; Xu, Q.; Ni, B.-J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef] [PubMed]
- Klrbas, Z.; Güner, N.K.A. Biodegradation of Polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar]
- Das, G.; Bordoloi, N.K.; Rai, S.K.; Mukherjee, A.K.; Karak, N. Biodegradable and biocompatible epoxidized vegetable oil modified thermostable poly(vinyl chloride): Thermal and performance characteristics post biodegradation with Pseudomonas aeruginosa and Achromobacter sp. J. Hazard. Mater. 2012, 209–210, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J. Basic Microbiol. 2014, 54, 18–27. [Google Scholar] [CrossRef]
- Khatoon, N.; Jamal, A.; Ali, M.I. Lignin peroxidase isoenzyme: A novel approach to biodegrade the toxic synthetic polymer waste. Environ. Technol. 2019, 40, 1366–1375. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360. [Google Scholar] [CrossRef]
- Temporiti, M.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- Rad, M.M.; Moghimi, H.; Azin, E. Biodegradation of thermo-oxidative pretreated low-density polyethylene (LDPE) and polyvinyl chloride (PVC) microplastics by Achromobacter denitrificans Ebl13. Mar. Pollut. Bull. 2022, 181, 113830. [Google Scholar] [CrossRef]
- Wertz, J.T.; Béchade, B. Chapter Three—Symbiont-mediated degradation of dietary carbon sources in social herbivorous insects. In Advances in Insect Physiology; Oliver, K.M., Russell, J.A., Eds.; Mechanisms Underlying Microbial Symbiosis; Academic Press: Cambridge, MA, USA, 2020; pp. 63–109. [Google Scholar] [CrossRef]
- Bożek, M.; Hanus-Lorenz, B.; Rybak, J. The studies on waste biodegradation by Tenebrio molitor. E3S Web Conf. 2017, 17, 00011. [Google Scholar] [CrossRef]
- Xu, H.; Dinsdale, D.; Nemery, B.; Hoet, P.H.M. Role of residual additives in the cytotoxicity and cytokine release caused by polyvinyl chloride particles in pulmonary cell cultures. Toxicol. Sci. 2003, 72, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Khandare, S.D.; Chaudhary, D.R.; Jha, B. Bioremediation of polyvinyl chloride (PVC) films by marine bacteria. Mar. Pollut. Bull. 2021, 169, 112566. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Bagde, U.S. Isolation of polyvinyl chloride degrading bacterial strains from environmental samples using enrichment culture technique. Afr. J. Biotechnol. 2012, 11, 7947–7956. [Google Scholar] [CrossRef]
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal Colonization and Biodeterioration of Plasticized Polyvinyl Chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Ahmed, S.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A.; Robson, G. Biodegradation of starch blended polyvinyl chloride films by isolated Phanerochaete chrysosporium PV1. Int. J. Environ. Sci. Technol. 2014, 11, 339–348. [Google Scholar] [CrossRef]
- Saeed, S.; Iqbal, A.; Deeba, F. Biodegradation study of Polyethylene and PVC using naturally occurring plastic degrading microbes. Arch. Microbiol. 2022, 204, 497. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Rodríguez, M.L.; Zorro-Mateus, P.J.P. Biodegradation of polyvinyl chloride by Mucor sp. and Penicillium sp. isolated from soil. Rev. Investig. Desarro. Innov. 2021, 11, 387–400. [Google Scholar] [CrossRef]
- Abdel-Naby, A.S.; Al-Ghamdi, A.A. Poly(vinyl chloride) blend with biodegradable cellulose acetate in presence of N-(phenyl amino) maleimides. Int. J. Biol. Macromol. 2014, 70, 124–130. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Bajer, K. Biodegradation of plasticized poly(vinyl chloride) containing cellulose. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 903–919. [Google Scholar] [CrossRef]
- Danko, A.S.; Meizhong, L.; Bagwell, C.E.; Brigmon, R.L.; Freedman, D.L. Involvement of Linear Plasmids in Aerobic Biodegradation of Vinyl Chloride. Appl. Environ. Microbiol. 2004, 70, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; ’Izzati Ismail, N.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Shimao, M.; Tamogami, T.; Kishida, S.; Harayama, S. The gene pvaB encodes oxidized polyvinyl alcohol hydrolase of Pseudomonas sp. strain VM15C and forms an operon with the polyvinyl alcohol dehydrogenase gene pvaAThe DDBJ accession number for the sequence reported in this paper is AB008494. Microbiology 2000, 146, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2023, 13, 1001750. [Google Scholar] [CrossRef]
- Sumathi, T.; Viswanath, B.; Lakshmi, A.S.; SaiGopal, D.V.R. Production of Laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem. Res. Int. 2016, 2016, 9519527. [Google Scholar] [CrossRef]
- Wei, X.-F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of microplastics released from a biodegradable polymer during biodegradation/enzymatic hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef]

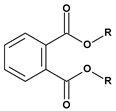 | 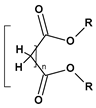 |  | 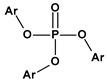 | |
| Phthalates DEHP (R = iC8) DIDP (R = iC10) DINP (R = iC9) | Adipates (n = 4) DINA (R = iC9) DIDA (R = iC10) | Sebacates (n = 8) DBS (R = C4) DOS (R = iC10) | Citrates TEC (R = Et) | Phosphates TCP (Ar = Tol) |
| St. | Steps of PVC Production Process | WI [kg] a | WsWO [kg] a |
|---|---|---|---|
| 1 | Ethylene (E) and Chlorine (Cl2) Production | ||
| 1.1. | 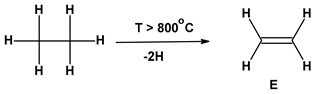 | 0 | 0 |
| 1.2 | 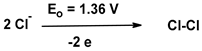 | 0 | 0 |
| 2 | VCM production process | 1.03 | 0.63 |
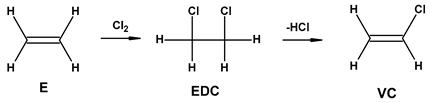 | |||
| 3 | PVC production process | 2.24 | 1.83 |
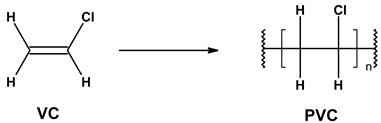 | |||
| 1-3 | Total | 2.24; 3.27 | 2.46 |
| Impact Category | Unit | Manufacturing | Use and Waste Disposal | Total Impact |
|---|---|---|---|---|
| Global warming | g CO2 eq | 272,308.0 | 223,031.1 | 495,339.1 |
| Acidification | H+ moles eq | 63,922.6 | 25,215.8 | 89,138.5 |
| Human health-cancer | g C6H6 eq | 44,720.6 | 428.5 | 45,149.1 |
| Human health-noncancer | g C7H7 eq | 56,880,581.6 | 627,435.7 | 57,508,017.3 |
| Eutrophication | g N eq | 94.2 | 47.4 | 141.7 |
| Ecotoxicity | g 2,4-D eq | 3304.5 | 223.5 | 3528.0 |
| Smog | g NOx eq | 858.7 | 214.2 | 1072.9 |
| Habitat alteration | T&E count | 1.55 × 10−13 | 4.65 × 10−13 | 6.2 × 10−13 |
| Ozone depletion | g CFC-11 eq | 0.0008 | 0.004 | 0.00 |
| Waters & Sediments | |||
|---|---|---|---|
| Water supply | Changsha (Hunan, https://pl.wikipedia.org/wiki/Hunan, accessed on 4 October 2023), China | Yin, 2019 [101], Shen, 2021 [102] | |
| NW Germany | Mintenig, 2019 [103] | ||
| Surface fresh water | Wei River, Yellow River's tributary, China | Ding, 2019 [104] | |
| Pearl River catchment, China | Fan, 2019 [105], Yan, 2019 [106] | ||
| Honghu Lake, China | Xiong, 2021 [107] | ||
| Sediments & surface fresh water | West Lakes, China | Jiang, 2018; [108] Wang, 2018 [109] | |
| Surface and sub-surface seawater | Korean coastal regions | Chae, 2015; [110] Song, 2015 [111] | |
| Marmara Sea | Tunçer, 2018 [112] | ||
| Kuantan of Malaysia | Khalik, 2018 [113] | ||
| Greenland | Morgana, 2019 [114] | ||
| Arctic Ocean | Lusher, 2015 [115] | ||
| NW Pacific | Pan, 2019 [98] | ||
| Water column | NW Mediterranean Sea | Lefebvre, 2019 [116] | |
| Bohai Sea-Yellow Sea | Dai, 2018 [117] | ||
| Floating and bottom sediment microplastics | Adriatic Sea | Zeri, 2018; [118] Palatinus, 2019 [119] | |
| W. Mediterranean Sea | de Haan, 2019 [120] | ||
| Cilacap, Java (Indonesia) | Syakti, 2017 [121] | ||
| Surface seawater and sediment | Melbourne coastal metropolis | Su, 2020 [122] | |
| Suva coastal area of Fiji | Ferreira, 2020 [123] | ||
| Bottom sediments | Arctic Ocean | Kanhai, 2019 [124] | |
| Norwegian fjords | Gomiero, 2019 [125] | ||
| Venetian islands | Vianello, 2013 [126] | ||
| Singapore coastline mangrove ecosystems | Nor, 2014 [127] | ||
| Sand seashore | Atlantic seashore, Cape Town, South Africa | Vilakati, 2020 [128] | |
| Atlantic seashore, Punta del Este, Uruguay | Lozoya, 2016 [129] | ||
| Marine Animals and Organisms | |||
| Fishes | Siganus fuscescens | Coastal sediments, Negros, Philippine | Bucol, 2020 [130] |
| Nephrops norvegicus | Coasts of Ireland. | Hara, 2020 [131] | |
| Sardines | NW Mediterranean Sea | Lefebvre, 2019 [116] | |
| Triglops nybelini , Boreogadus saida | Arctic Ocean | Lusher, 2015 [115] | |
| Various species | Australian markets | Wootton, 2021 [132] | |
| Suva coastal area, Fiji | Ferreira, 2020 [123] | ||
| Markets in Fujian, China | Fang, 2019 [133] | ||
| Shellfishes | Mytilus edulis | Mussel and oyster farming zone, Pen-Bé, France | Phuong, 2018 [134] |
| Meretrix meretrix | Markets in Fujian & Xiamen, China | Fang, 2019 [133] | |
| Various species | Sal Estuary River, Goa, India | Saha, 2021 [135] | |
| Organism | Genus/Species | PVC-MPs Conc. [Unit] | Effect of PVC-MPs on the Organism | Refs. |
|---|---|---|---|---|
| Algae | Chlamydomonas reinhardtii | 10–200 [mg/L] | Growth inhibition; reduction in chlorophyll-A level | Wang, 2020 [161]. |
| Skeletonema costatum | 1–50 [mg/L] | Inhibition of growth; inhibition of photosynthesis efficiency via decrease in chlorophyll content; adsorption, aggregation, and toxic effects on algal cells | Zhang, 2017s [163]. | |
| Corals | Zoanthus sociatus | 10 mg/L | Increase in adhesion to coral epidermis; OS induction; changes in photosynthethic efficiency | Rocha, 2020 [164]. |
| Plants | Utricularia aurea | 50 [mg/L] | Growth, length, and biomass inhibition; negative effects on physiological parameters (chlorophyll content) | Zhou, 2020 [165]. |
| Mussels | Perna viridis | 21.6–2160 [mg/L] | Decrease in clearance, respiration rates, and byssus production; decrease in median survival times with increasing pollution by PVC | Rist, 2016 [166]. |
| Mytilus galloprovincialis | - | Accumulation in the organism | Gomiero, 2019 [167]. | |
| Arthro-pods | Daphnia magna | 50 [mg/L] | Induction of mortality; increase in immobilization | Renzi, 2019 [168]. |
| Fish | Clarias gariepinus | 0.50; 1.50; 3.0 [% of diet] | Reduction of mean cell volume/cell hemoglobin values; decrease in neutrophil counts; GPx alternation (brain, gill); SOD inhibition (brain, gill); CAD reduction (brain); increase in lipid peroxidation levels (brain); AChE inhibition (brain, gill); OS induction | Iheanacho & Odo, 2020 [169]. |
| Cyprinus carpio | 10–30 [% of diet] | Growth inhibition; alternation of the antioxidant activities—inverse relationship between SOD, CAT after exposition on PVC; increase in GPx activities; reduction in MDA levels; alternation of antioxidant-related gene expression in the livers of larvae; changes in transcription; vacuolation of cytoplasm in the liver under exposure over 20% additives of PVC to diet | Xia, 2020 [170]. | |
| Dicentrarchus labrax | 100; 500 [mg/kg diet] | Increase in the phagocytic and respiratory burst activities of head kidney leucocytes; decrease in immunity and OS induction | Espinosa, 2019 [171]. | |
| Dicentrarchus labrax | 0.1 [% of diet] | Histopathological changes in the ingestine | Peda, 2016 [172]. | |
| Etroplus suratensis | 1.0–10.8 [mg/L] | Influence on SOD activity (increase at 1.03–1.8 mg/L; decrease at 3.0–10.8 mg/L); behavioral changes (fin flickering, burst swimming, and jerking movement); decrease in red and white blood cells; changes in antioxidant enzymes | Vijayaraghavan, 2022, [151]. | |
| Sparus aurata | 100; 500 [mg/kg diet] | Gene expression changes: PRDX5 (decrease); PRDX1, PRDX3 (increase); UCP1 (up-regulation). | Espinosa, 2017 [173]. |
| Method | Type | Mechanism | Refs. |
|---|---|---|---|
| chemical dechlorination | chemical neutralization | modification consisting in replacing some chlorine atoms with various nucleophilic reagents | Lu, 2019 [210] |
| hydrothermal dechlorination | physico-chemical neutralization | conducting modifications in supercritical or subcritical water which works as a solvent and reagent for reactions of organic compounds | Li, 2017 [211] |
| photodegradation | physical degradation | breaking down the chemical bonds in a polymer by ultraviolet (UV) radiation | Yousif, 2015 [212] |
| mechanical recycling | mechanical modification | recycling technique consisting in extruding and mixing the material with primary polymers | Sadat-Shojai, 2011 [213] |
| pyrolysis | physico-chemical degradation | polymer decomposition under high temperature | Yu, 2016 [21] |
| biodegradation | biological degradation | polymer decomposition by microorganisms such as bacteria and filamentous fungi, and organisms such as insects | Vivi et al. 2019 [214] Giacomucci, 2019 [215] Tsochatzis, 2021 [216] |
| biofragmentation | biological modification | the breakdown of polymers into monomers, dimers, or oligomers during a lytic process, involving decrease in the molecular weight of the polymer and oxidation of the lower-weight molecules using specific enzymes (oxidoreductases and hydrolases), as well as free radicals | Restrepo-Flórez, 2014 [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Piwowarska, D.; Festinger, N.; Chruściel, J.J. Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization. Materials 2024, 17, 173. https://doi.org/10.3390/ma17010173
Kudzin MH, Piwowarska D, Festinger N, Chruściel JJ. Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization. Materials. 2024; 17(1):173. https://doi.org/10.3390/ma17010173
Chicago/Turabian StyleKudzin, Marcin H., Dominika Piwowarska, Natalia Festinger, and Jerzy J. Chruściel. 2024. "Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization" Materials 17, no. 1: 173. https://doi.org/10.3390/ma17010173
APA StyleKudzin, M. H., Piwowarska, D., Festinger, N., & Chruściel, J. J. (2024). Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization. Materials, 17(1), 173. https://doi.org/10.3390/ma17010173







