Efficient Co-Valorization of Phosphogypsum and Red Mud for Synthesis of Alkali-Activated Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AAM Synthesis
2.3. XRD Measurement and Data Analysis
2.4. SEM–EDS Measurement
3. Results and Discussion
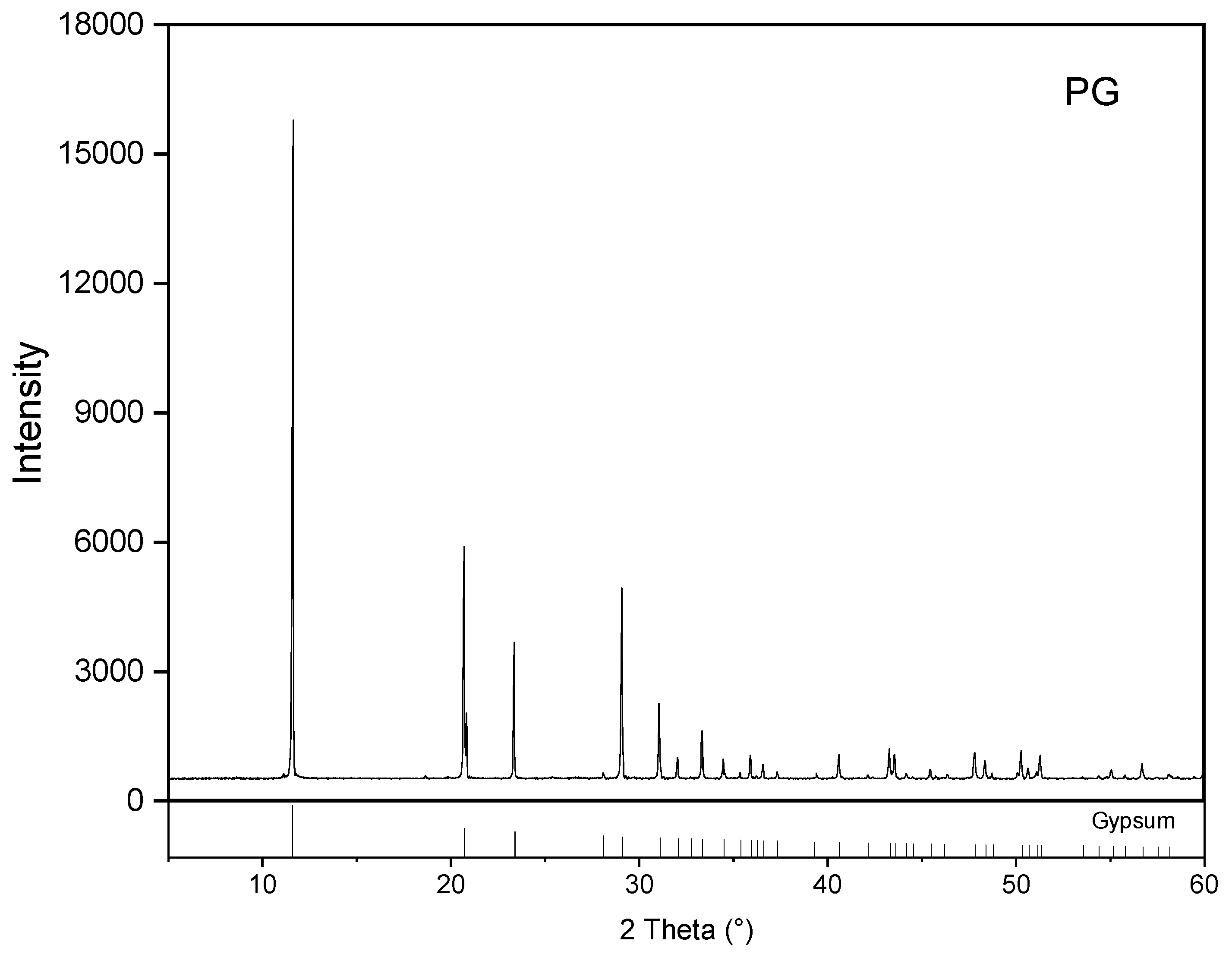
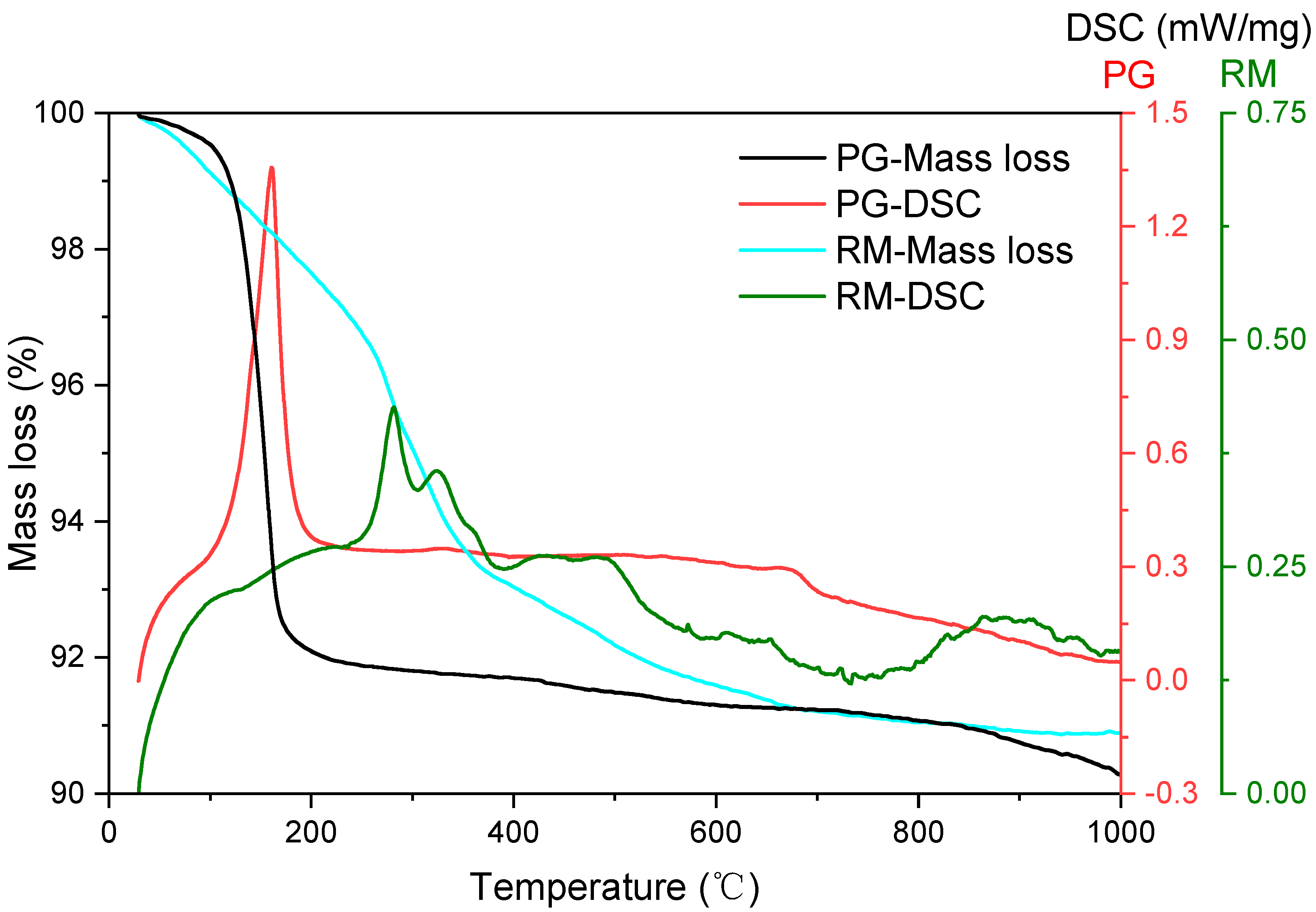
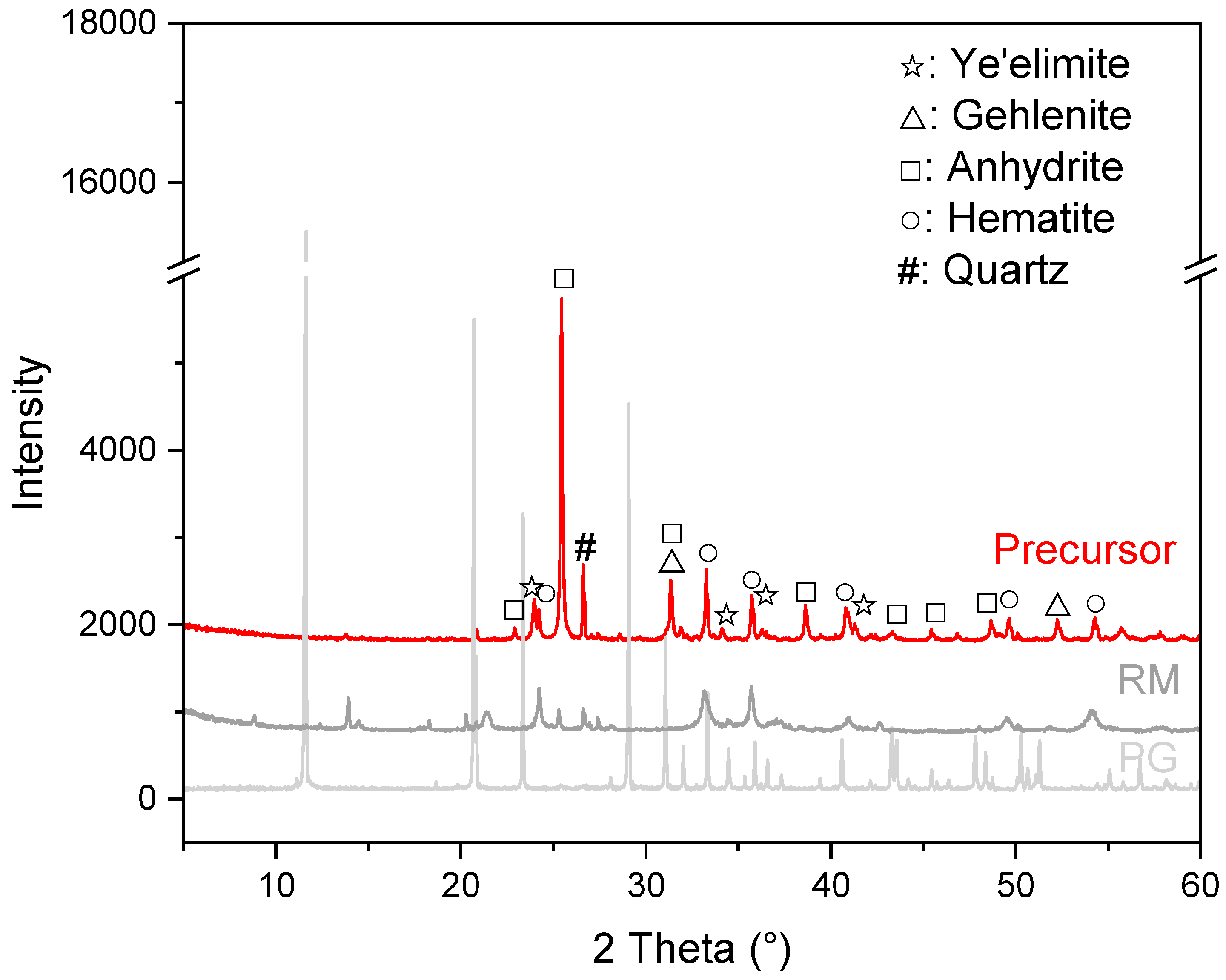
3.1. Mineral Composition of the Prepared Precursor
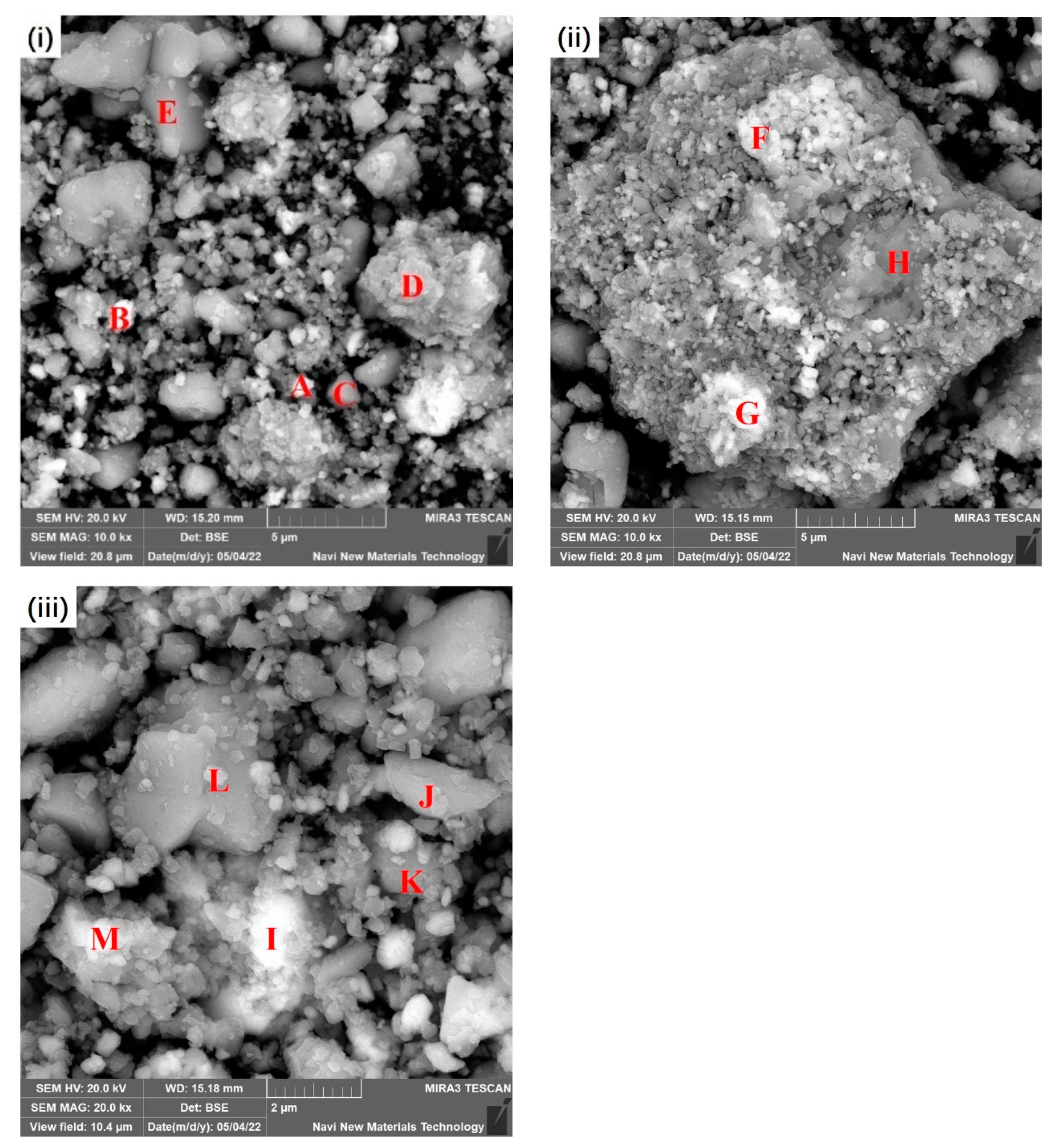
3.2. Morphology of the Prepared Precursor
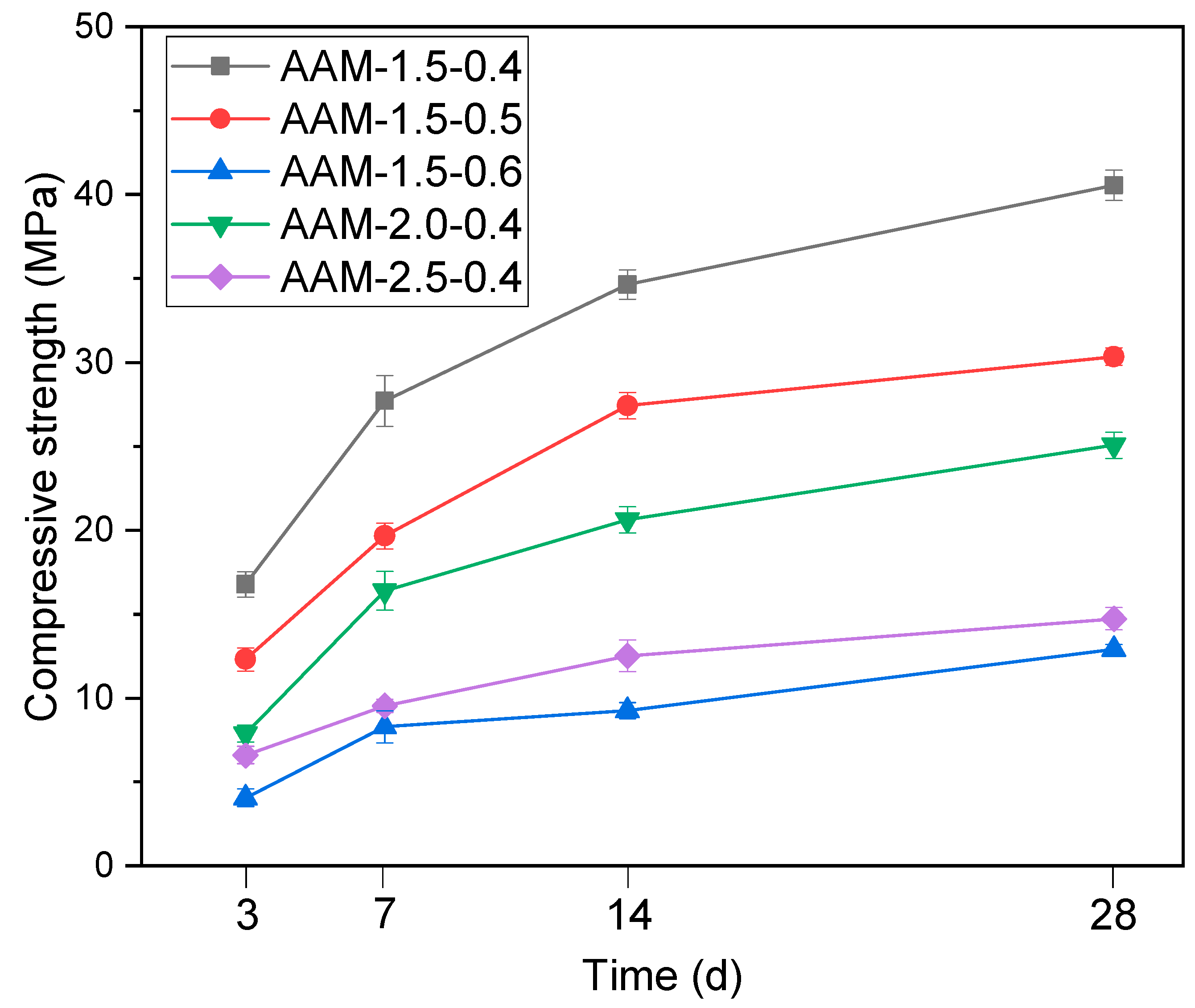
3.3. Strength of the Synthesized AAMs
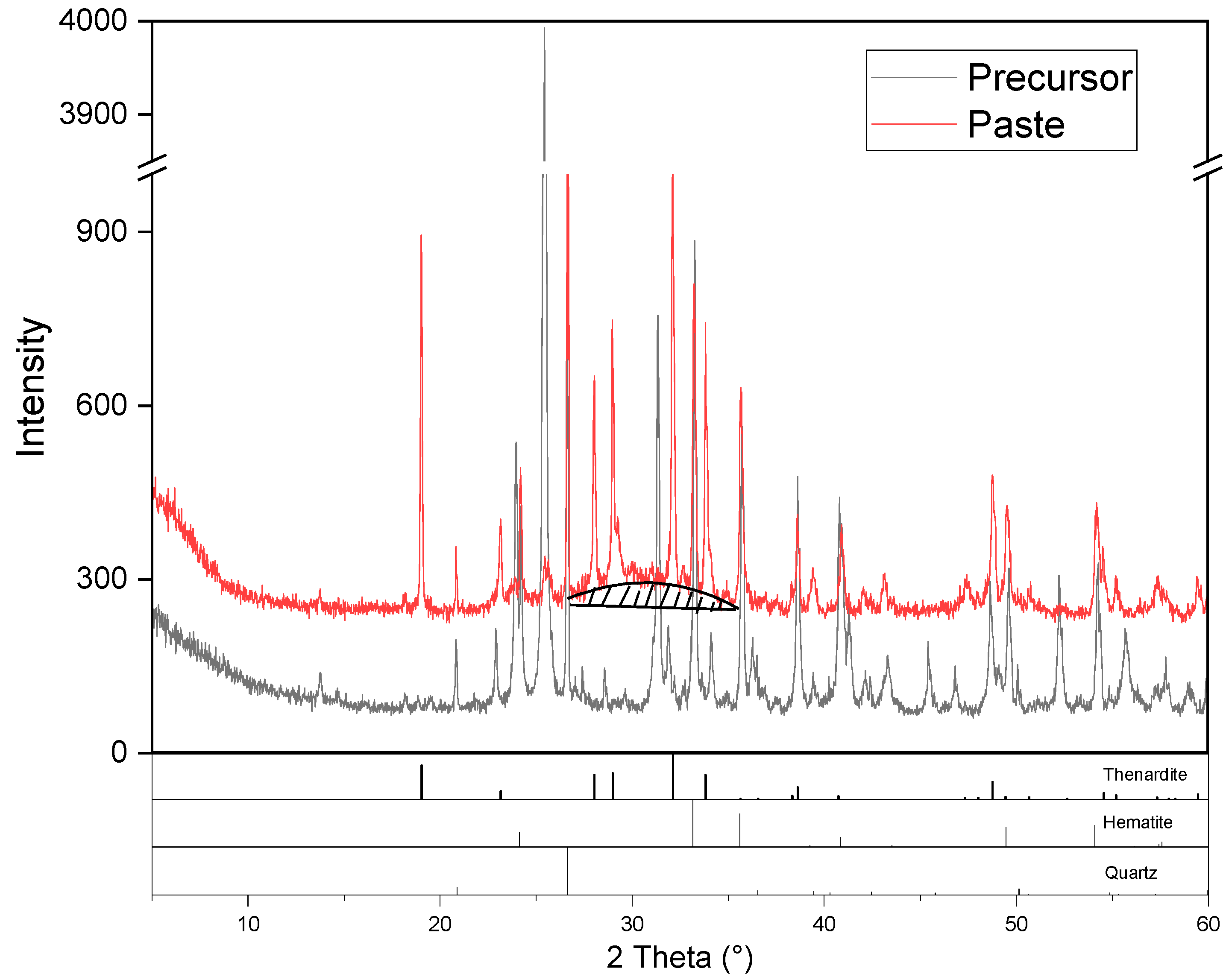
3.4. XRD of the Synthesized AAM
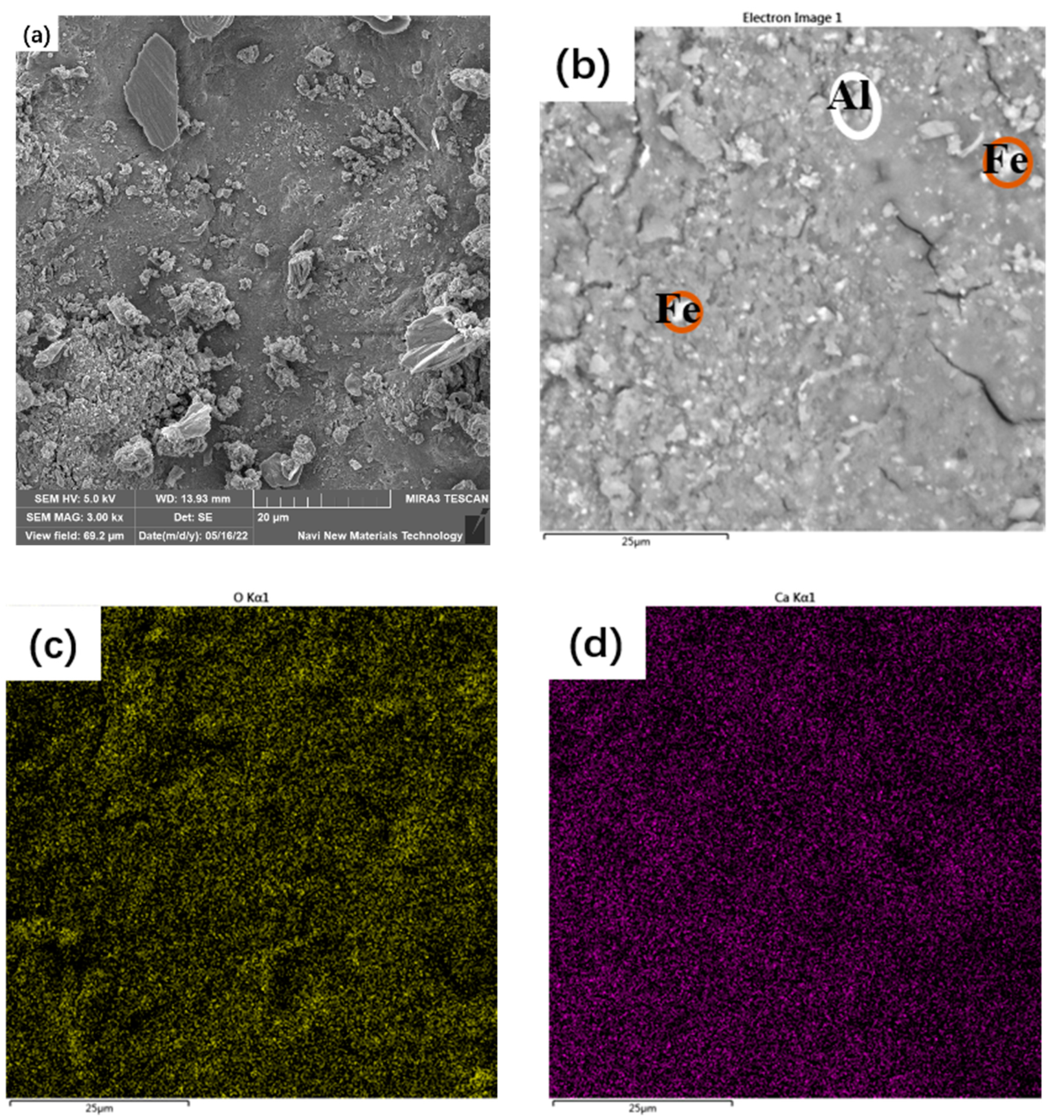
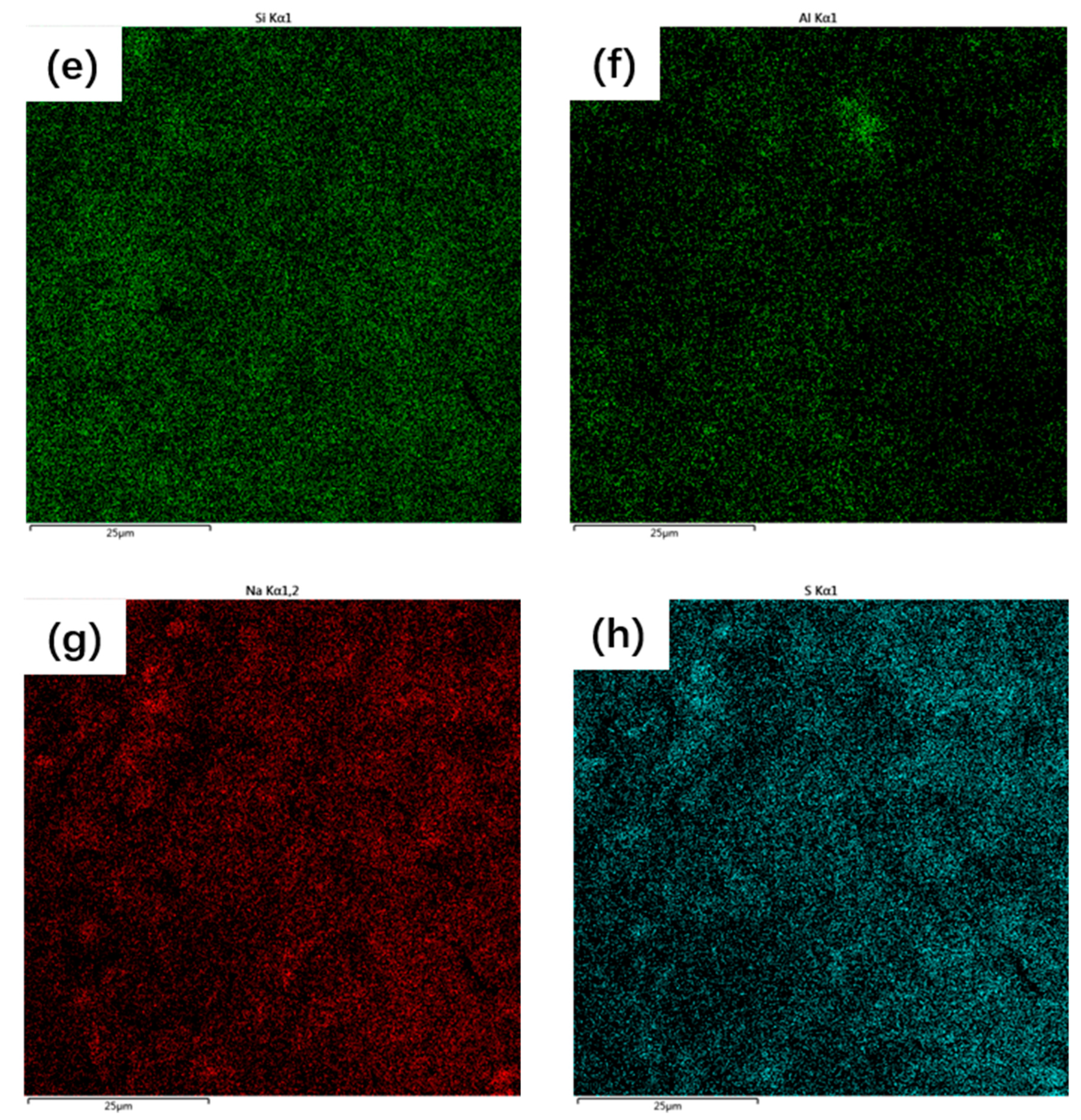
3.5. Microstructure of the Synthesized AAM
4. Summary and Conclusions
- During the calcination at 950 °C, mineral reconstruction between PG and RM took place. Gypsum, boehmite, gibbsite, sodalite and phlogopite in the source materials were converted into ye’elimite, anhydrite and gehlenite.
- The mechanical properties of the synthesized AAMs were significantly influenced by both the activator modulus and liquid–solid ratio. The 28-day compressive strength of the synthesized AAMs was up to 40.6 MPa.
- The main reaction products of the synthesized AAMs were thenardite and C-A-S-H gel. The synthesized AAMs possessed a dense microstructure in which the reaction products and unreacted particles were well interlinked by the gel matrix, contributing to the excellent mechanical performance of the synthesized AAMs.
Author Contributions
Funding
Conflicts of Interest
References
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental Impact and Management of Phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Vaičiukynienė, D.; Tamošaitis, G.; Kantautas, A.; Nizevičienė, D.; Pupeikis, D. Porous Alkali-Activated Materials Based on Municipal Solid Waste Incineration Ash with Addition of Phosphogypsum. Powder Constr. Build. Mater. 2021, 301, 123962. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kantautas, A.; Bocullo, V.; Kielė, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a Construction Material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Mashifana, T.P. Chemical Treatment of Phosphogypsum and Its Potential Application for Building and Construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Sert, Y.; Altun, İ.A. Utilization of Weathered Phosphogypsum as Set Retarder in Portland Cement. Cem. Concr. Res. 2004, 34, 677–680. [Google Scholar]
- Singh, M. Treating Waste Phosphogypsum for Cement and Plaster Manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Shen, W.; Gan, G.; Dong, R.; Chen, H.; Tan, Y.; Zhou, M. Utilization of Solidified Phosphogypsum as Portland Cement Retarder. J. Mater. Cycles Waste Manag. 2012, 14, 228–233. [Google Scholar] [CrossRef]
- Taher, M.A. Influence of Thermally Treated Phosphogypsum on the Properties of Portland Slag Cement. Resour. Conserv. Recycl. 2007, 52, 28–38. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Yan, Y. Utilization of Original Phosphogypsum as Raw Material for the Preparation of Self-Leveling Mortar. J. Clean. Prod. 2016, 127, 204–213. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Chen, F.; Shui, Z. The Chloride Permeability of Persulphated Phosphogypsum-Slag Cement Concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2016, 31, 1031–1037. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali Activated Cements and Concretes; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J. Alkali Activated Materials, State-of-the-Art Report; RILEM TC 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Liu, P.; Wang, D.; Vollpracht, A.; Oeser, M. Study of Alkali Activated Slag as Alternative Pavement Binder. Constr. Build. Mater. 2018, 186, 626–634. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Geopolymer Institute. PYRAMENT Cement Good for Heavy Traffic after 25 Years. Available online: https://www.geopolymer.org/news/pyrament-cement-good-for-heavy-traffic-after-25-years/ (accessed on 17 April 2023).
- Geopolymer Institute. World’s First Public Building with Structural Geopolymer Concrete. Available online: https://www.geopolymer.org/news/worlds-first-public-building-with-structural-geopolymer-concrete/ (accessed on 17 April 2023).
- Rashad, A.M. Potential Use of Phosphogypsum in Alkali-Activated Fly Ash Under the Effects of Elevated Temperatures and Thermal Shock Cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of Phosphogypsum on the Stability Upon Firing Treatment of Alkali-Activated Slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-Activated Binders Based on Ground Granulated Blast Furnace Slag and Phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in Red Mud Utilization-A Review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1–29. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, Hazard and Iron Recovery Technology of Red Mud-A Critical Review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Li, S.W.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Shi, Y.; Dong, T. A New Route for Separation and Recovery of Fe, Al and Ti from Red Mud. Resour. Conserv. Recycl. 2021, 168, 105314. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media—A Review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Chufarov, A.Y.; Suntsov, A.Y.; Yatsenko, S.P. High Purity Scandium Extraction from Red Mud by Novel Simple Technology. Hydrometallurgy 2021, 202, 105597. [Google Scholar] [CrossRef]
- Wei, D.; Jun-Hui, X.; Yang, P.; Si-Yue, S.; Tao, C.; Kai, Z.; Zhen, W. Extraction of Scandium and Iron from Red Mud. Miner. Process. Extr. Metall. Rev. 2022, 43, 61–68. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive Utilization Status of Red Mud in China: A Critical Review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Wei, Z.B.; Liu, Q.S.; Sun, Z.Q.; Huang, X.X.; Gan, M.; Ji, Z.Y.; Chen, X.L.; Fan, X.H. Co-Disposal of Semi-Dry Desulfurization Residue and Red Mud into High Performance Alkali Activated Material. Constr. Build. Mater. 2022, 350, 128776. [Google Scholar] [CrossRef]
- Mercury, J.M.R.; Pena, P.; De Aza, A.H.; Sheptyakov, D.; Turrillas, X. On the Decomposition of Synthetic Gibbsite Studied by Neutron Thermo Diffractometry. J. Am. Ceram. Soc. 2006, 89, 3728–3733. [Google Scholar] [CrossRef]
- Ingram-Jones, V.J.; Slade, R.C.T.; Davies, T.W.; Southern, J.C.; Salvador, S. Dehydroxylation Sequences of Gibbsite and Boehmite: Study of Differences Between Soak and Flash Calcination and of Particle-Size Effects. J. Mater. Chem. 1996, 6, 73–79. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Liu, Q.S.; Fan, X.H.; Gan, M.; Huang, X.X.; Ji, Z.Y.; Chen, X.L. A Novel Valorization Method of Multi-Source Industrial Solid Waste. CN Patent 114477804A, 13 May 2022. [Google Scholar]
- Scrivener, K.L.; Lothenbach, B.; De Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and Microstructure of Concrete with SCMs. Mater. Struct. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Sun, A.; Vollpracht, Z. Isothermal Calorimetry and In-Situ XRD Study of the Naoh Activated Fly Ash, Metakaolin and Slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One Year Geopolymerisation of One-Yearilicate Activated Fly Ash and Metakaolin Geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- El Khessaimi, Y.; El Hafiane, Y.; Smith, A. Ye’elimite Synthesis by Chemical Routes. J. Eur. Ceram. Soc. 2019, 39, 1683–1695. [Google Scholar] [CrossRef]
- El Khessaimi, Y.; El Hafiane, Y.; Smith, A. Examination of Ye’elimite Formation Mechanisms. J. Eur. Ceram. Soc. 2019, 39, 5086–5095. [Google Scholar] [CrossRef]
- Hertel, T.; Van den Bulck, A.; Onisei, S.; Sivakumar, P.P.; Pontikes, Y. Boosting the Use of Bauxite Residue (Red Mud) in Cement-Production of an Fe-rich Calciumsulfoaluminate-Ferrite Clinker and Characterisation of the Hydration. Cem. Concr. Res. 2021, 145, 106463. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious Concrete Made of Alkali Activated Slag and Geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]


| Al2O3 | SiO2 | CaO | Fe2O3 | MgO | TiO2 | Na2O | K2O | P2O5 | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| PG | 1.04 | 2.22 | 35.46 | 0.66 | 0.62 | 0.05 | 0.02 | 0.13 | 1.52 | 21.51 |
| RM | 22.1 | 15.8 | 1.7 | 37.1 | 0.1 | 5.4 | 11.6 | 0.2 | 0.03 | 0.63 |
| Areas | O | Na | Mg | Al | Si | S | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| A | 53.85 | 3.05 | 0.74 | 3.77 | 1.64 | 1.61 | 2.53 | 1.42 | 31.34 |
| B | 43.91 | 2.41 | 0.19 | 2.90 | 1.53 | 5.78 | 6.35 | 0.34 | 36.52 |
| C | 49.45 | 2.31 | 0.08 | 3.05 | 1.80 | 14.95 | 20.89 | 0.48 | 6.92 |
| D | 47.55 | 4.31 | 0.14 | 6.79 | 4.44 | 9.37 | 13.28 | 0.82 | 13.06 |
| E | 53.41 | 0.79 | 0.03 | 1.01 | 0.45 | 19.23 | 23.59 | 0.12 | 1.35 |
| F | 46.05 | 4.55 | 0.40 | 8.89 | 3.89 | 3.74 | 5.97 | 0.97 | 25.47 |
| G | 37.69 | 2.04 | 0.63 | 2.68 | 1.33 | 1.38 | 2.78 | 1.57 | 49.85 |
| H | 29.07 | 1.64 | 0.06 | 4.47 | 3.62 | 19.30 | 29.60 | 1.64 | 10.30 |
| I | 26.90 | 2.46 | 1.05 | 5.12 | 2.33 | 3.40 | 4.48 | 2.16 | 52.02 |
| J | 56.66 | 2.03 | 0.21 | 3.33 | 1.70 | 13.07 | 16.52 | 0.56 | 5.87 |
| K | 44.73 | 2.47 | 0.22 | 6.12 | 3.51 | 10.56 | 16.20 | 1.18 | 14.89 |
| L | 55.53 | 1.72 | 0.17 | 2.98 | 1.72 | 14.14 | 17.29 | 0.41 | 6.01 |
| M | 37.41 | 2.82 | 1.02 | 4.82 | 1.78 | 2.39 | 3.17 | 1.87 | 44.68 |
| Element | O | Na | Al | Si | S | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| Content | 41.19 | 16.95 | 3.07 | 11.16 | 7.37 | 11.45 | 0.71 | 7.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xue, X.; Sun, Z.; Huang, X.; Gan, M.; Ji, Z.; Chen, X.; Fan, X. Efficient Co-Valorization of Phosphogypsum and Red Mud for Synthesis of Alkali-Activated Materials. Materials 2023, 16, 3541. https://doi.org/10.3390/ma16093541
Liu Q, Xue X, Sun Z, Huang X, Gan M, Ji Z, Chen X, Fan X. Efficient Co-Valorization of Phosphogypsum and Red Mud for Synthesis of Alkali-Activated Materials. Materials. 2023; 16(9):3541. https://doi.org/10.3390/ma16093541
Chicago/Turabian StyleLiu, Qingsong, Xiangci Xue, Zengqing Sun, Xiaoxian Huang, Min Gan, Zhiyun Ji, Xuling Chen, and Xiaohui Fan. 2023. "Efficient Co-Valorization of Phosphogypsum and Red Mud for Synthesis of Alkali-Activated Materials" Materials 16, no. 9: 3541. https://doi.org/10.3390/ma16093541
APA StyleLiu, Q., Xue, X., Sun, Z., Huang, X., Gan, M., Ji, Z., Chen, X., & Fan, X. (2023). Efficient Co-Valorization of Phosphogypsum and Red Mud for Synthesis of Alkali-Activated Materials. Materials, 16(9), 3541. https://doi.org/10.3390/ma16093541





