Aspects of Austenitization for the Bearing Steel Induction Quenching Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Spheroidization Annealing
2.3. Dilatometric Experiments for the Monitoring of Carbides Dissolution
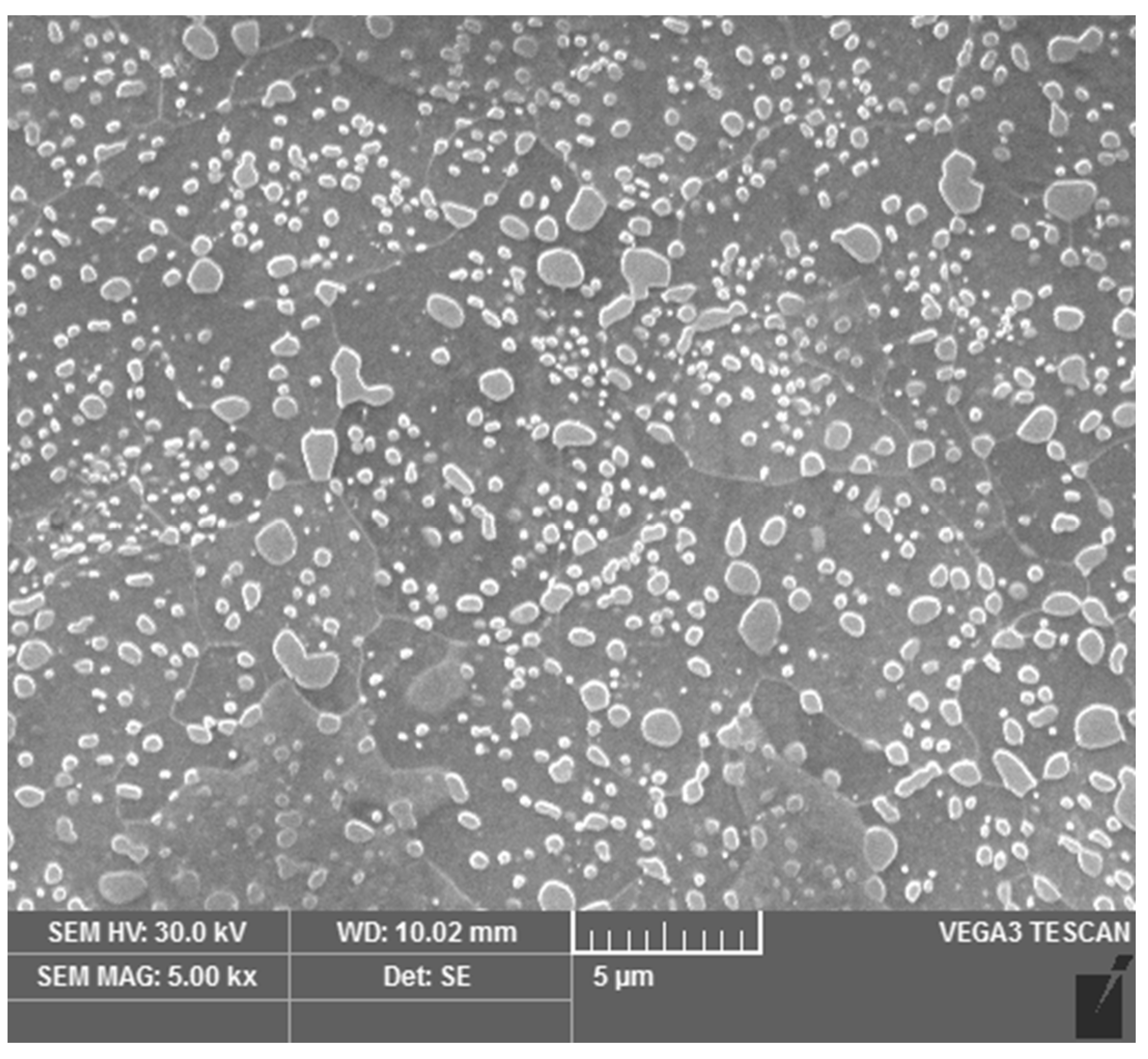
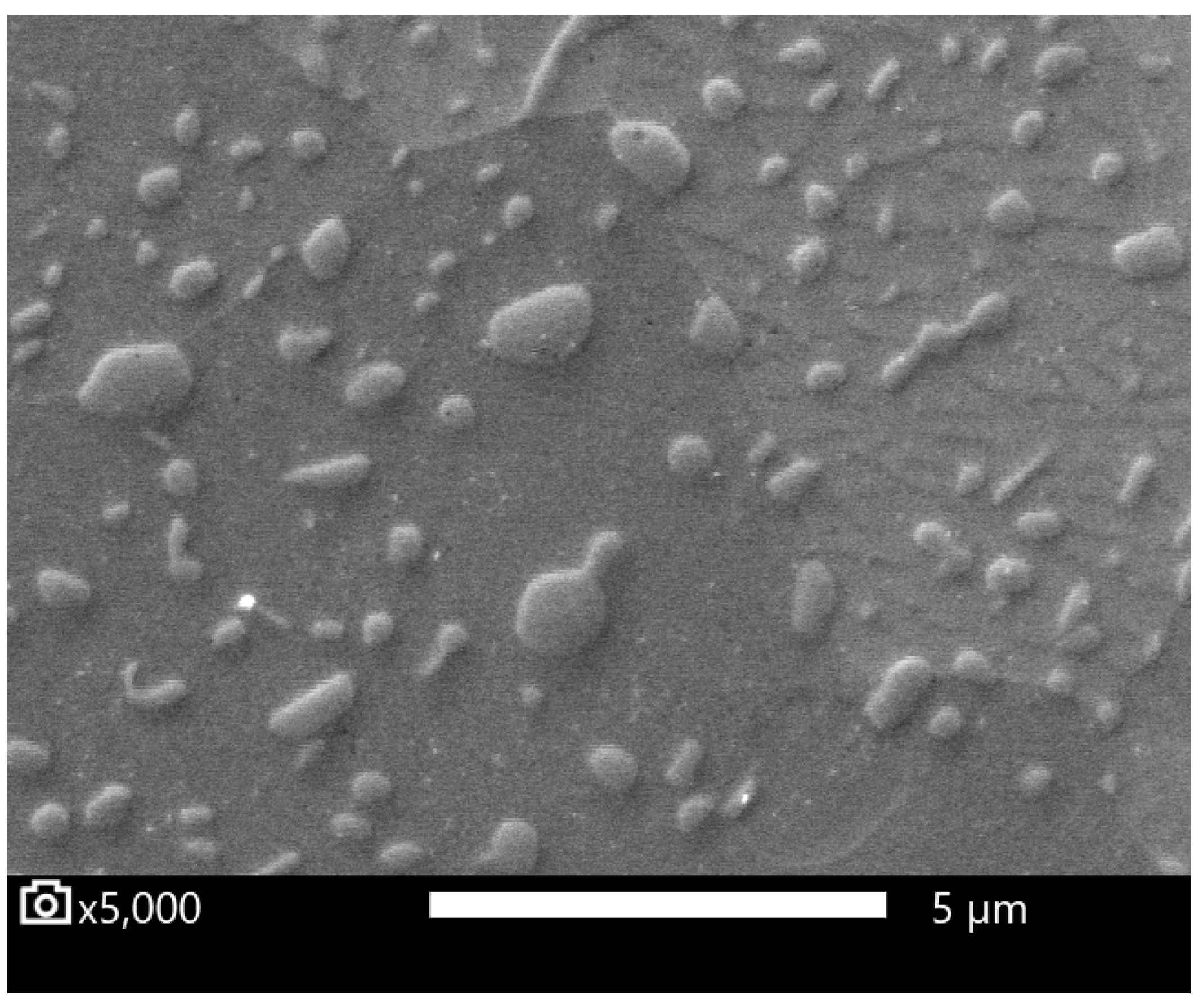
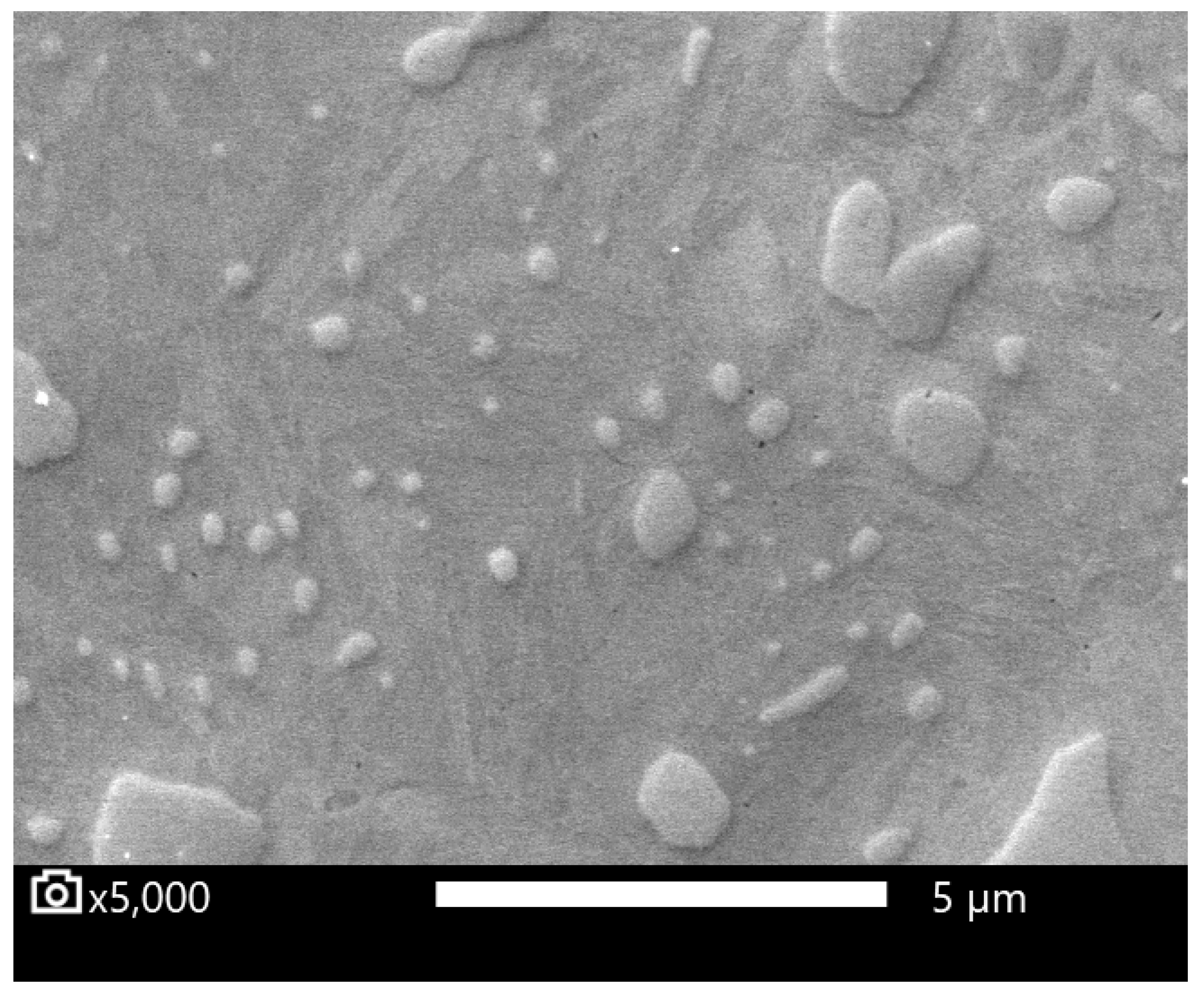
2.4. Image Analysis
3. Results and Discussion
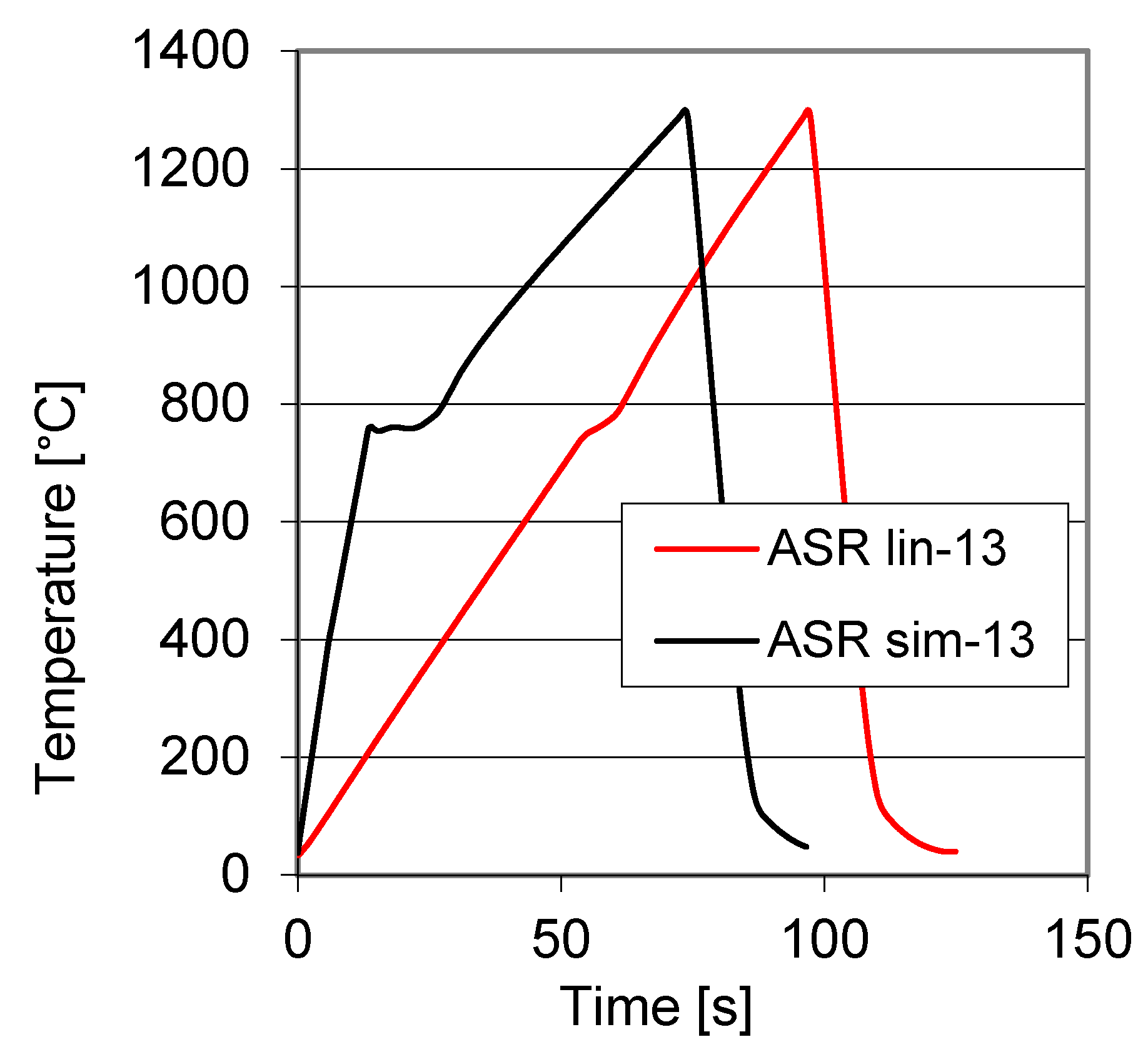
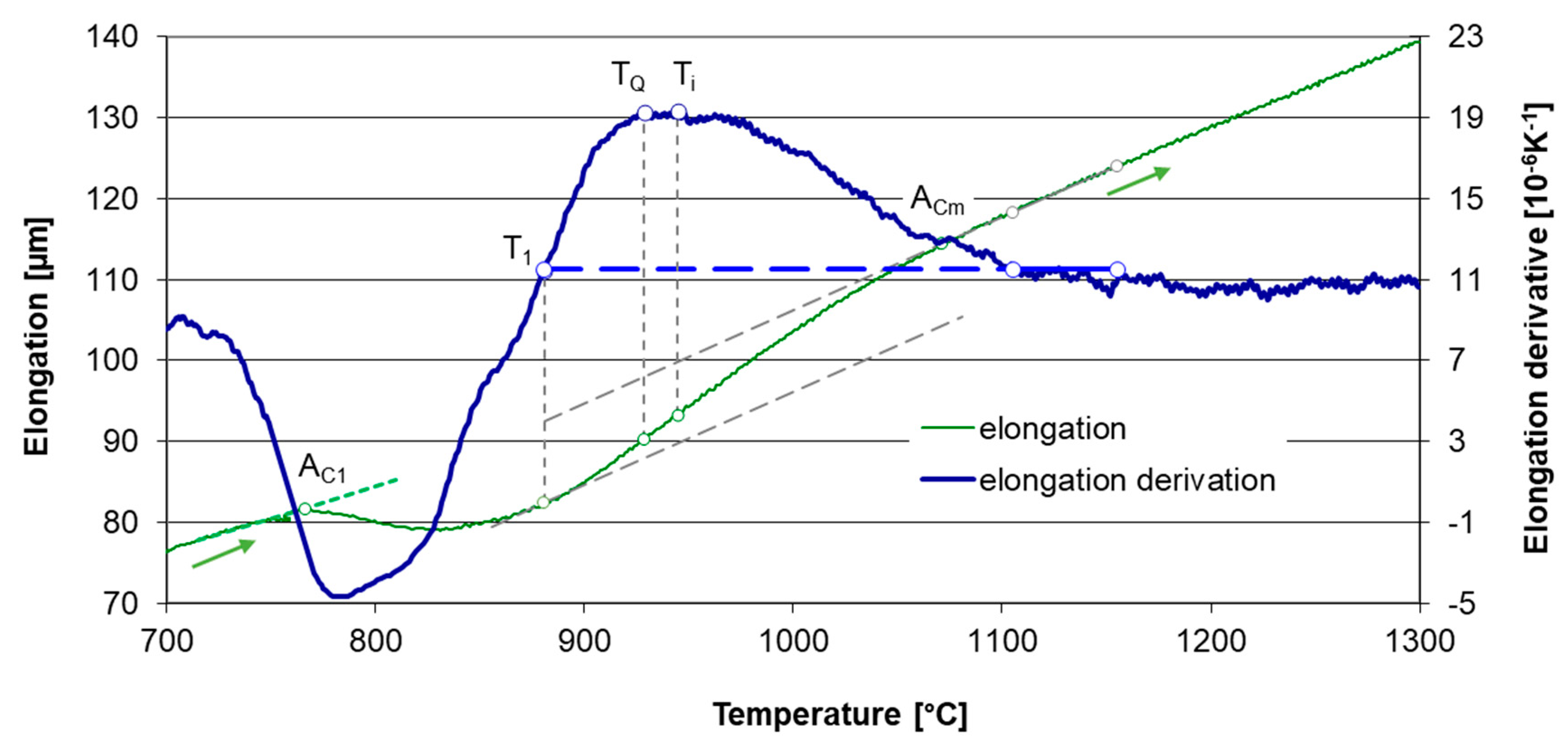
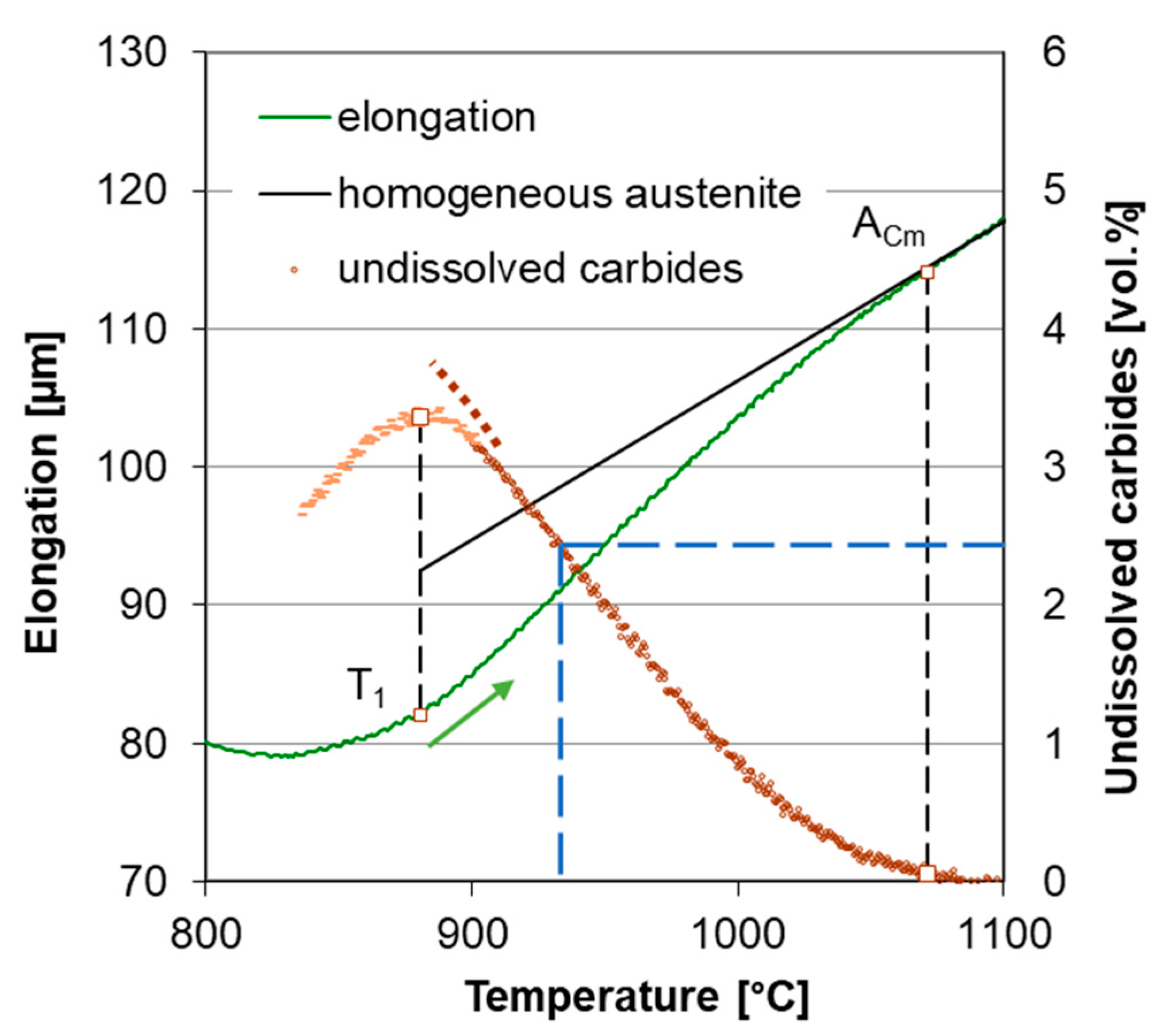
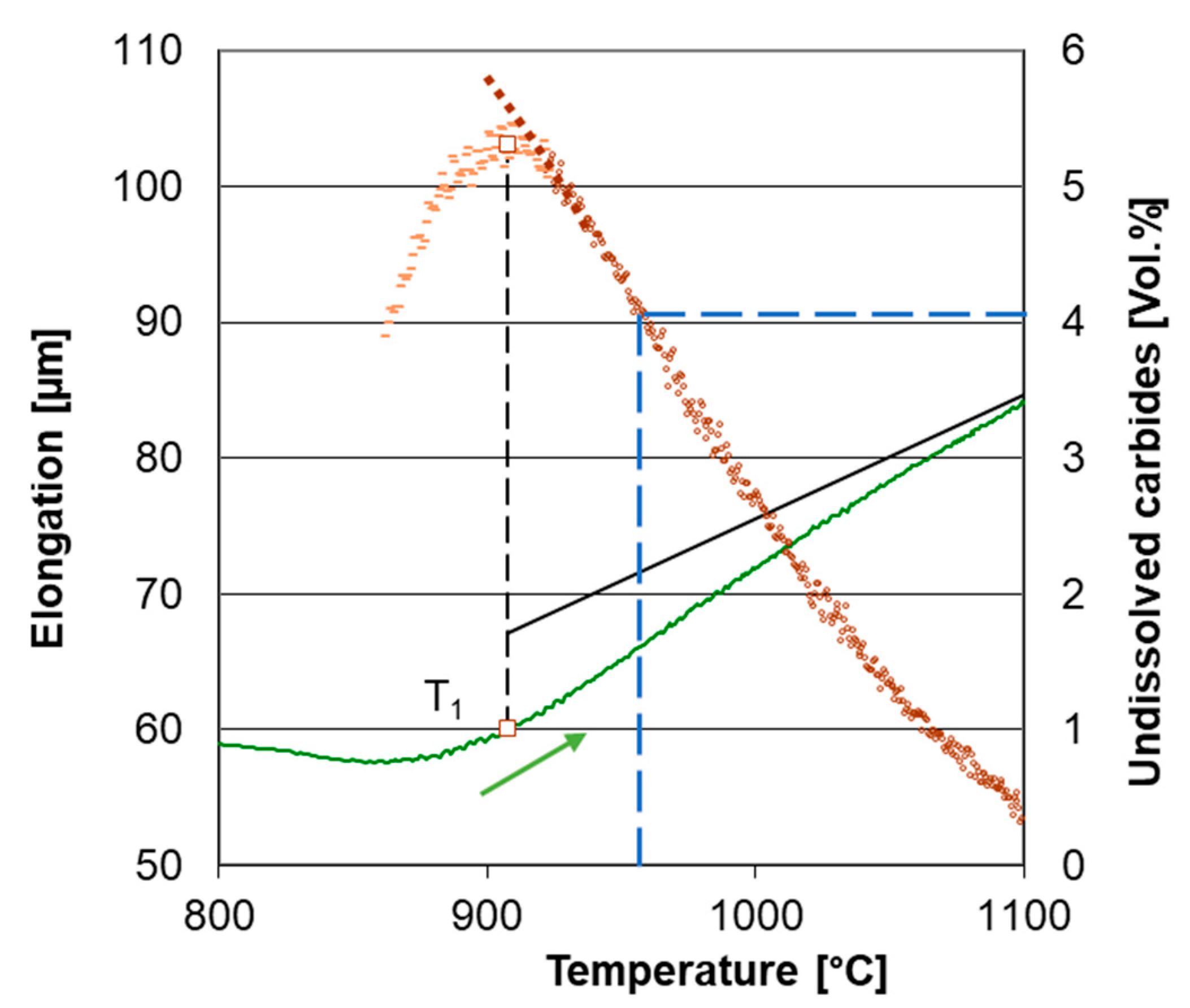
3.1. Determination of Transformation Temperatures
3.2. Carbide Dissolution Quantification
3.3. Carbide Content Quantification in Dilatometer Curve
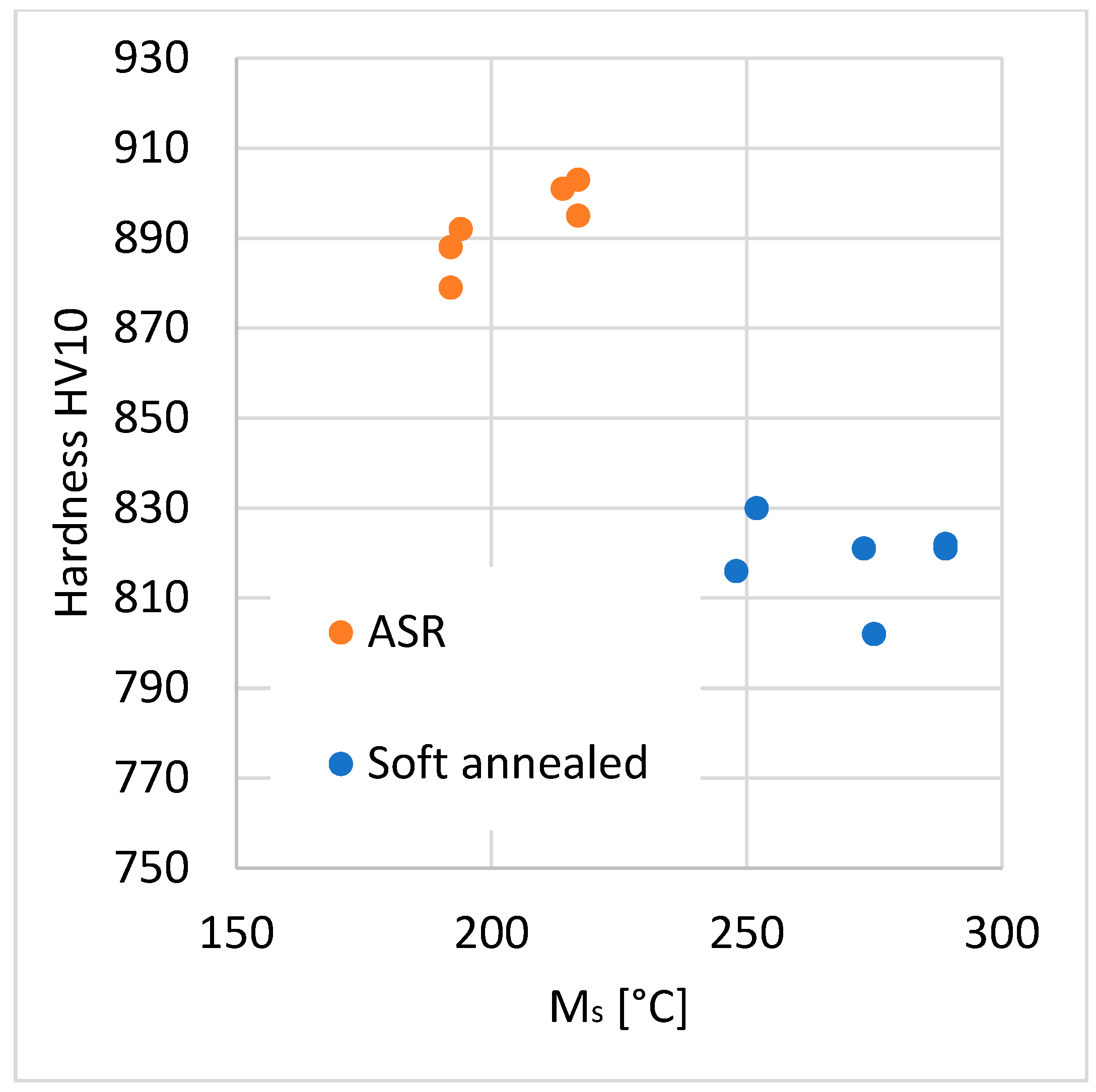
3.4. Carbide Dissolution and Final Hardness
4. Conclusions
- Dilatometer analysis can be utilized as a tool for setting parameters of induction heating. It was proved that the amount of dissolved carbides in hypereutectoid steel 100CrMnSi6-4 is represented in the dilatometric curve. The dissolution stage can be attributed to a certain point of the curve based on its general shape. Thus, it is possible to determine the quenching temperature on the actual carbon content in the austenite, regardless of the heating rate.
- The initial structure proved to have a strong impact on the carbide dissolution rate and resulting hardness. The microstructure of the austenitized steel has to be taken into account, rather than only the nominal chemical composition.
- It is possible to relate the dilatometer curve to the resulting hardness by hardness measurement and the quantification of the undissolved carbide content for specimens with interrupted heating. This can be used for the setting of an appropriate quenching temperature at the given heating rate to reach the required hardness.
- Linear heating rates normally used in dilatometer measurements can be used as a simulation of real heating regimes by industrial induction devices without any significant result distortion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Steels for Bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Lee, S.J.; Clarke, K.D. A Quantitative Investigation of Cementite Dissolution Kinetics for Continuous Heating of Hypereutectoid Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 3917–3923. [Google Scholar] [CrossRef]
- Chae, J.Y.; Jang, J.H.; Zhang, G.; Kim, K.H.; Lee, J.S.; Bhadeshia, H.K.D.H.; Suh, D.W. Dilatometric Analysis of Cementite Dissolution in Hypereutectoid Steels Containing Cr. Scr. Mater. 2011, 65, 245–248. [Google Scholar] [CrossRef]
- Eggbauer, A.; Lukas, M.; Ressel, G.; Prevedel, P.; Mendez-Martin, F.; Keckes, J.; Stark, A.; Ebner, R. In Situ Analysis of the Effect of High Heating Rates and Initial Microstructure on the Formation and Homogeneity of Austenite. J. Mater. Sci. 2019, 54, 9197–9212. [Google Scholar] [CrossRef]
- Wu, Y.X.; Wang, L.Y.; Sun, W.W.; Styles, M.J.; Studer, A.J.; Bréchet, Y.; Arlazarov, A.; Hutchinson, C.R. Austenite Formation Kinetics from Multicomponent Cementite-Ferrite Aggregates. Acta Mater. 2020, 196, 470–487. [Google Scholar] [CrossRef]
- Sidebotham, G. Heat Transfer Modeling: An Inductive Approach; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Rapoport, E.; Pleshivtseva, Y. Optimal Control of Induction Heating Processes; Mechanical Engineering; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Rudnev, V.; Loveless, D.; Cook, R.L. Handbook of Induction Heating; Manufacturing Engineering; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Haimbaugh, R.E. Practical Induction Heat Treating, 2nd ed.; ASM International: Almere, The Netherlands, 2015. [Google Scholar]
- Krauss, G. Martensite in Steel: Strength and Structure. Mater. Sci. Eng. A 1999, 273–275, 40–57. [Google Scholar] [CrossRef]
- Khajuria, A.; Kumar, R.; Bedi, R. Characterizing Creep Behaviour of Modified 9Cr1Mo Steel by Using Small Punch Impression Technique for Thermal Powerplants Effects of Heat Treatments on Grain Size and Hardness of P91 and Boron Added P91 Steels View Project FE Modelling & Analysis of Impression Creep in P91 Steels View Project. J. Mech. Mech. Eng. 2018, 4, 47–61. [Google Scholar]
- Podgornik, B.; Leskovšek, V.; Godec, M.; Senčič, B. Microstructure Refinement and Its Effect on Properties of Spring Steel. Mater. Sci. Eng. A 2014, 599, 81–86. [Google Scholar] [CrossRef]
- Dlouhy, J.; Hauserova, D.; Novy, Z. Carbide Morphology and Ferrite Grain Size after Accelerated Carbide Spheroidisation and Refinement (ASR) of C45 Steel. Mater. Tehnol. 2015, 49, 625–628. [Google Scholar] [CrossRef]
- Dlouhý, J.; Hauserová, D.; Kövér, M. Induction Quenching of Steel 100CrMnSi6-4: Coarse vs. Fine Structure in View of Transformation Kinetics. In Proceedings of the Materials Science and Technology Conference and Exhibition 2015, MS and T 2015, Columbus, OH, USA, 4–8 October 2015; Volume 2, pp. 961–968. [Google Scholar]
- Vidhyasagar, M.; Balachandran, G. Spheroidization of 100Cr6 Bearing Steel by Warm Forging. Trans. Indian Inst. Met. 2021, 74, 767–774. [Google Scholar] [CrossRef]
- Dlouhy, J.; Motycka, P. Austenitization of Lamellar Pearlite in 100CrMnSi6-4 Bearing Steel. In Proceedings of the METAL 2016—25th Anniversary International Conference on Metallurgy and Materials, Conference Proceedings, Brno, Czech Republic, 25–27 May 2016; pp. 635–640. [Google Scholar]
- Nam, W.J.; Bae, C.M. Coarsening Behavior of Cementite Particles at a Subcritical Temperature in a Medium Carbon Steel. Scr. Mater. 1999, 41, 313–318. [Google Scholar] [CrossRef]
- Luzginova, N.V.; Zhao, L.; Sietsma, J. The Cementite Spheroidization Process in High-Carbon Steels with Different Chromium Contents. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 513–521. [Google Scholar] [CrossRef]
- Tian, Y.L.; Kraft, R.W. Mechanisms of Pearlite Spheroidization. Metall. Trans. 1987, 18, 1403–1414. [Google Scholar] [CrossRef]
- Kučerová, L.; Jirková, H.; Mašek, B. Various Approaches to Accelerated Carbide Spheroidization of 54SiCr Steel. Key Eng. Mater. 2015, 647, 3–8. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chae, J.Y.; Kim, K.H.; Suh, D.W. Effects of Mn, Si and Cr Addition on the Dissolution and Coarsening of Pearlitic Cementite during Intercritical Austenitization in Fe-1mass%C Alloy. Mater. Charact. 2013, 81, 56–67. [Google Scholar] [CrossRef]
- Hauserova, D.; Dlouhy, J.; Novy, Z. Microstructure and Properties of Hardened 100CrMnSi6-4 Bearing Steel after Accelerated Carbide Spheroidization and Long-Duration Annealing. ASTM Spec. Technol. Publ. 2015, STP 1580, 389–409. [Google Scholar] [CrossRef]
- Goutam, D. Image Analysis in Quantitative Metallography. In Materials Characterization Techniques-Principles and Applications; Goswanri, N.G., Sridha, G., Chowdhunv, G., Eds.; NML: Jamshedpur, India, 1999; pp. 135–150. [Google Scholar]



| AC1 | The start temperature of austenite formation during heating |
| ACm | The temperature of the last cementite dissolution during heating |
| ASR | Accelerated carbide spheroidization and refinement |
| CEMinit | The volume of carbides in an initial microstructure |
| CEMQ | The volume of carbides in the quenched sample |
| Cγ | Carbon content in austenite |
| HRC | Rockwell hardness, C-scale |
| HV | Vickers hardness |
| Ms | Martensite start temperature during quenching |
| SA | Soft annealing |
| SEM | Scanning electron microscope |
| T1 | The point at which the elongation derivative equals to the derivative for homogeneous austenite |
| Ti | The point of maximum elongation derivative |
| TQ | The quenching temperature |
| C | Si | Mn | P | S | Cr | Ni | Al | Cu |
|---|---|---|---|---|---|---|---|---|
| 0.94 | 0.65 | 1.16 | 0.014 | 0.012 | 1.54 | 0.03 | 0.03 | 0.02 |
| Heating to 1300 °C | Heating Interrupted by Quenching at TQ | ||||||
|---|---|---|---|---|---|---|---|
| Heating Rate (°C/s) | Transformation Temperatures AC1 (°C) ACm (°C) | Quenching Temperature TQ (°C) | Martensite Start Temperature Ms (°C) | Hardness HV10 | Undissolved Carbides (vol.%) | ||
| ASR-lin-6 | 6 | 761 | 1076 | 923 | 217 | 895 | 2.5 |
| ASR-sim-6 | 6 | 748 | 1052 | 923 | 214 | 901 | 2.9 |
| ASR-lin-13 | 13 | 761 | 1110 | 943 | 194 | 892 | 2.2 |
| ASR-sim-13 | 13 | 753 | 1101 | 943 | 192 | 888 | 2.3 |
| ASR-lin-22 | 22 | 764 | 1152 | 967 | 192 | 879 | 2.3 |
| ASR-sim-22 | 22 | 768 | 1127 | 967 | 217 | 903 | 2.1 |
| SA-lin-6 | 6 | 773 | 1151 | 954 | 252 | 830 | 4.1 |
| SA-sim-6 | 6 | 762 | 1084 | 954 | 289 | 822 | 3.9 |
| SA-lin-13 | 13 | 776 | 1167 | 973 | 289 | 821 | 3.7 |
| SA-sim-13 | 13 | 775 | 1124 | 973 | 275 | 802 | 4.3 |
| SA-lin-22 | 22 | 785 | 1174 | 997 | 248 | 816 | 4.0 |
| SA-sim-22 | 22 | 783 | 1143 | 997 | 273 | 821 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachazelova, D.; Dlouhy, J.; Motycka, P.; Kotous, J. Aspects of Austenitization for the Bearing Steel Induction Quenching Design. Materials 2023, 16, 3523. https://doi.org/10.3390/ma16093523
Nachazelova D, Dlouhy J, Motycka P, Kotous J. Aspects of Austenitization for the Bearing Steel Induction Quenching Design. Materials. 2023; 16(9):3523. https://doi.org/10.3390/ma16093523
Chicago/Turabian StyleNachazelova, Daniela, Jaromir Dlouhy, Petr Motycka, and Jakub Kotous. 2023. "Aspects of Austenitization for the Bearing Steel Induction Quenching Design" Materials 16, no. 9: 3523. https://doi.org/10.3390/ma16093523
APA StyleNachazelova, D., Dlouhy, J., Motycka, P., & Kotous, J. (2023). Aspects of Austenitization for the Bearing Steel Induction Quenching Design. Materials, 16(9), 3523. https://doi.org/10.3390/ma16093523






