Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
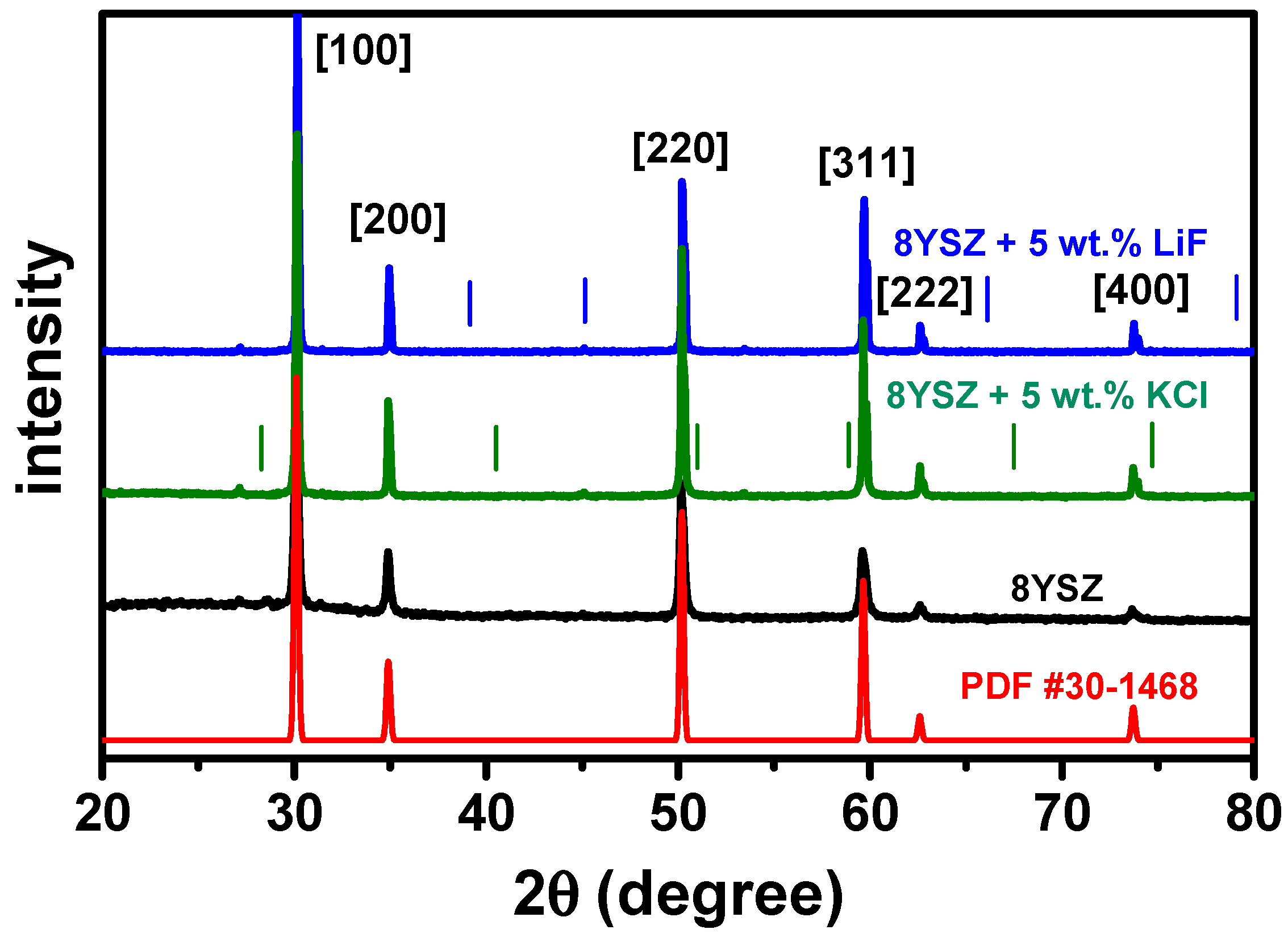
2.3. X-ray Diffraction
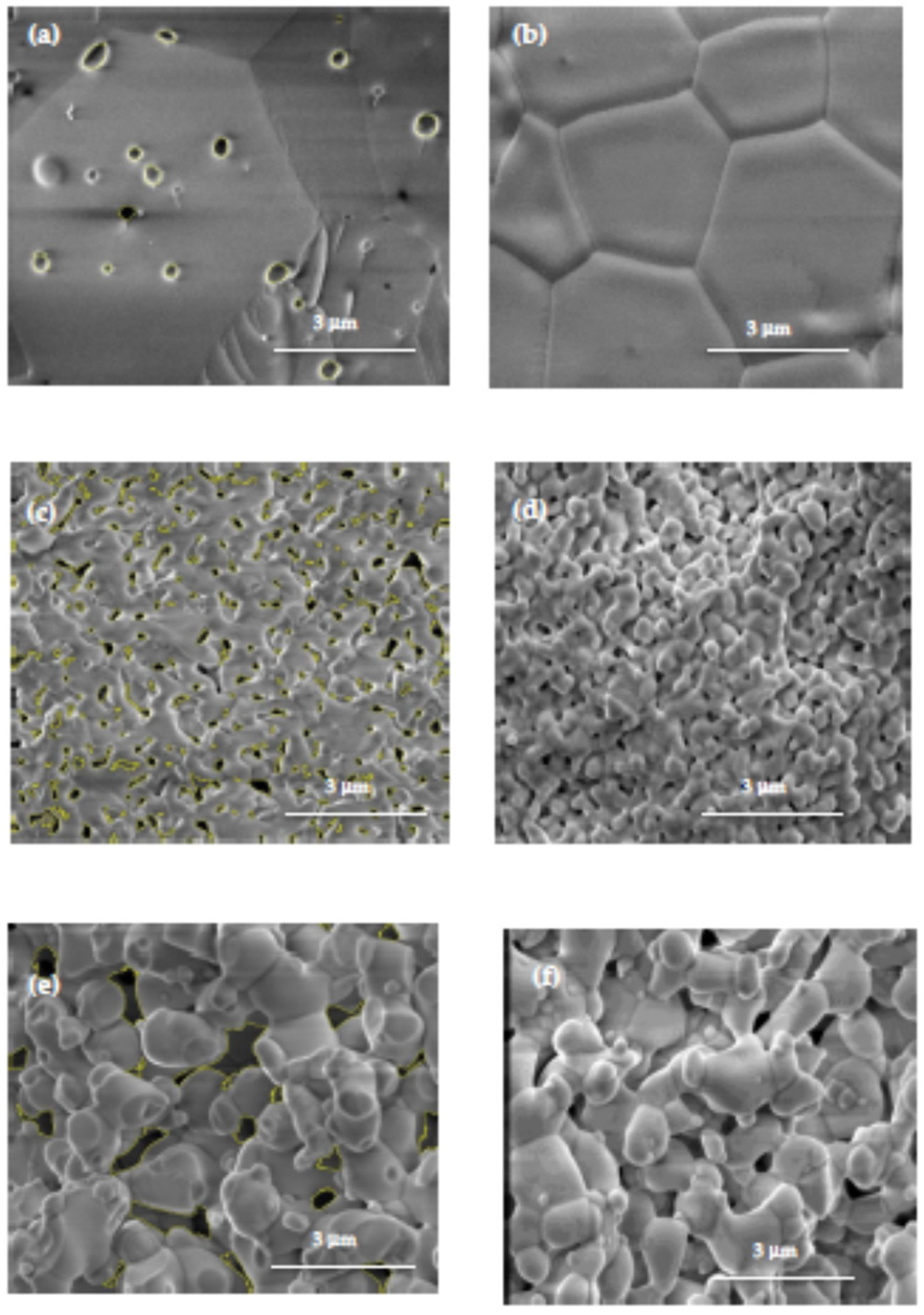
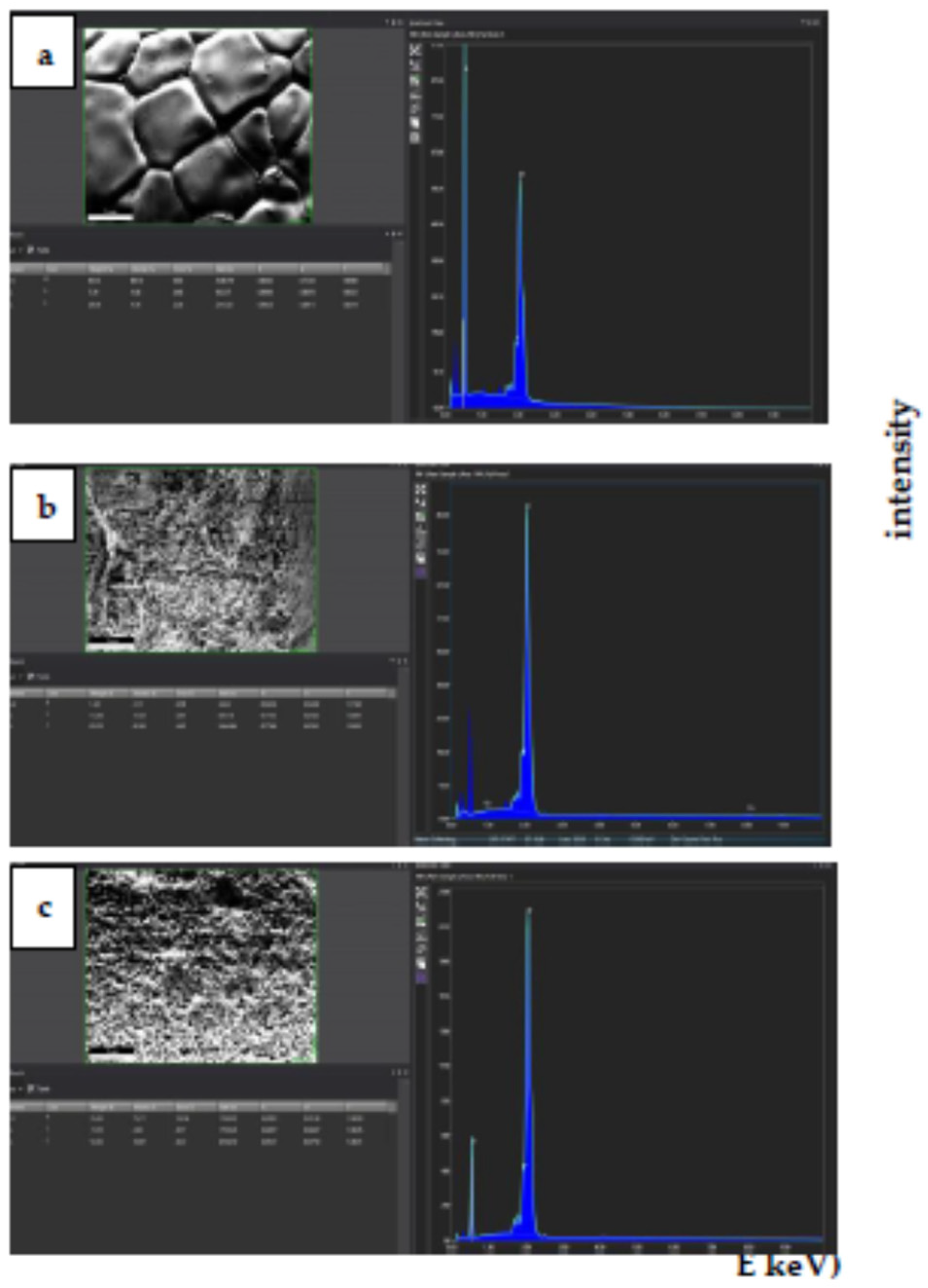
2.4. Scanning Electron Microscopy
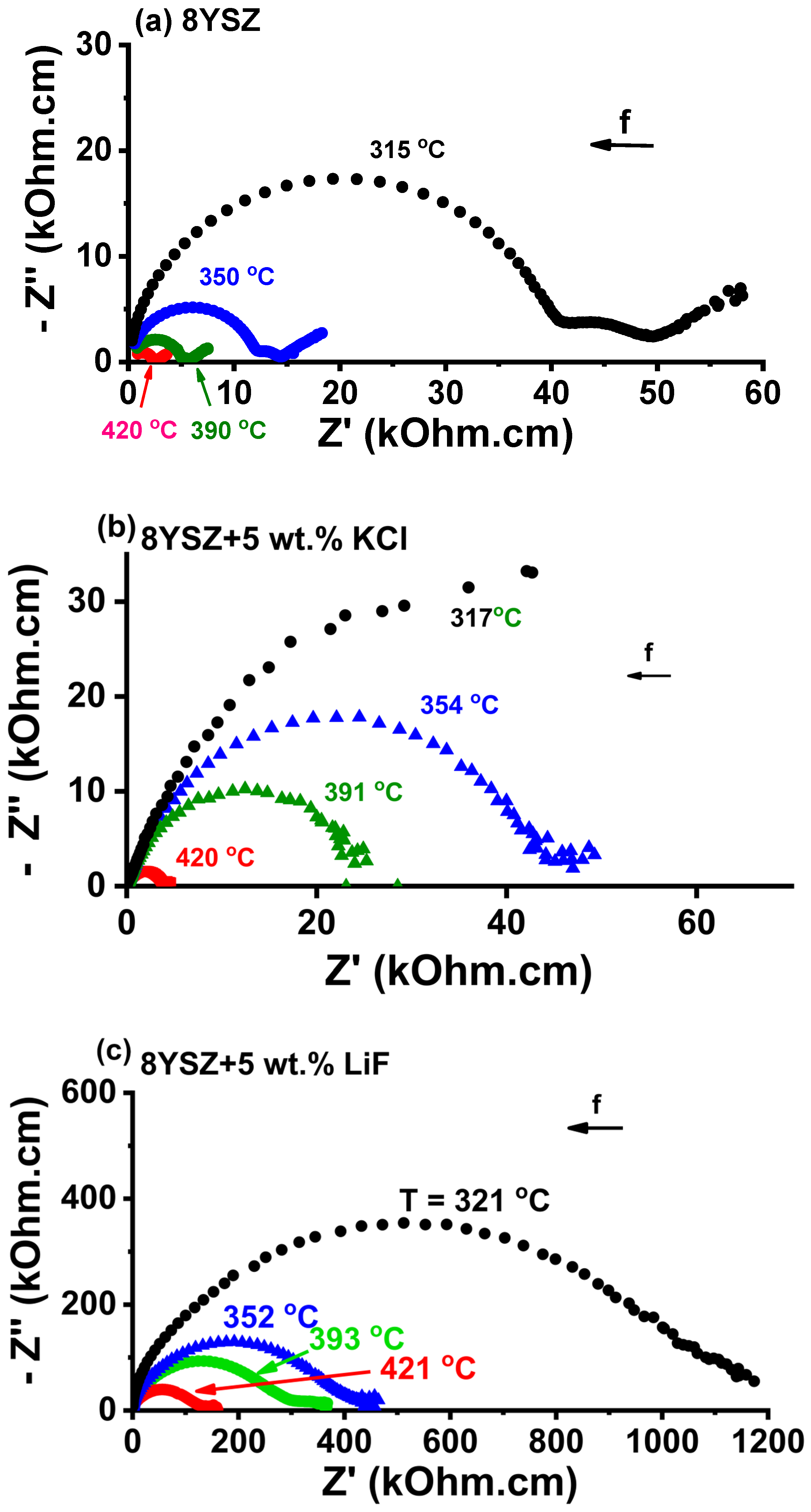
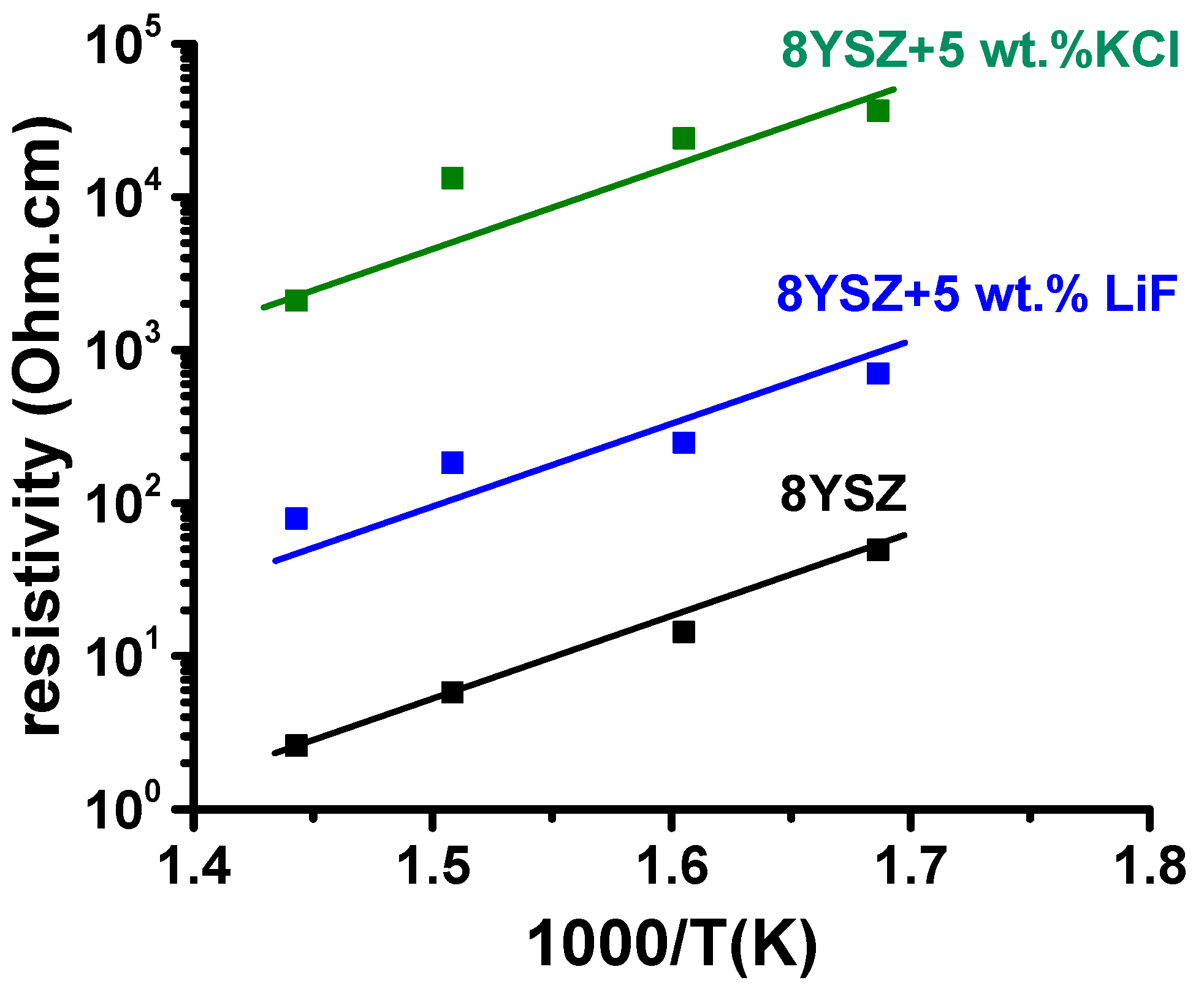
2.5. Impedance Spectroscopy
3. Results
3.1. Density and Porosity Evaluation
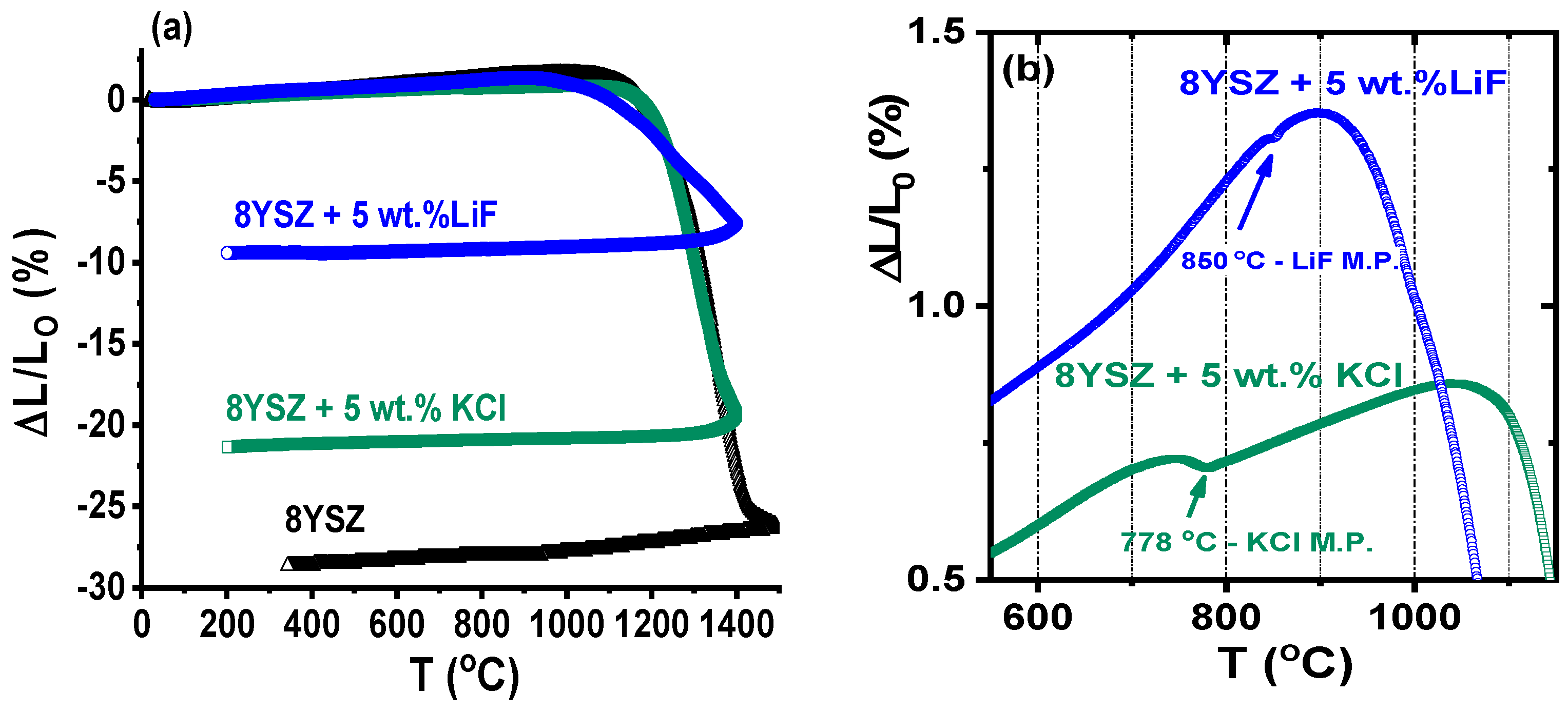
3.2. Thermal Analyses
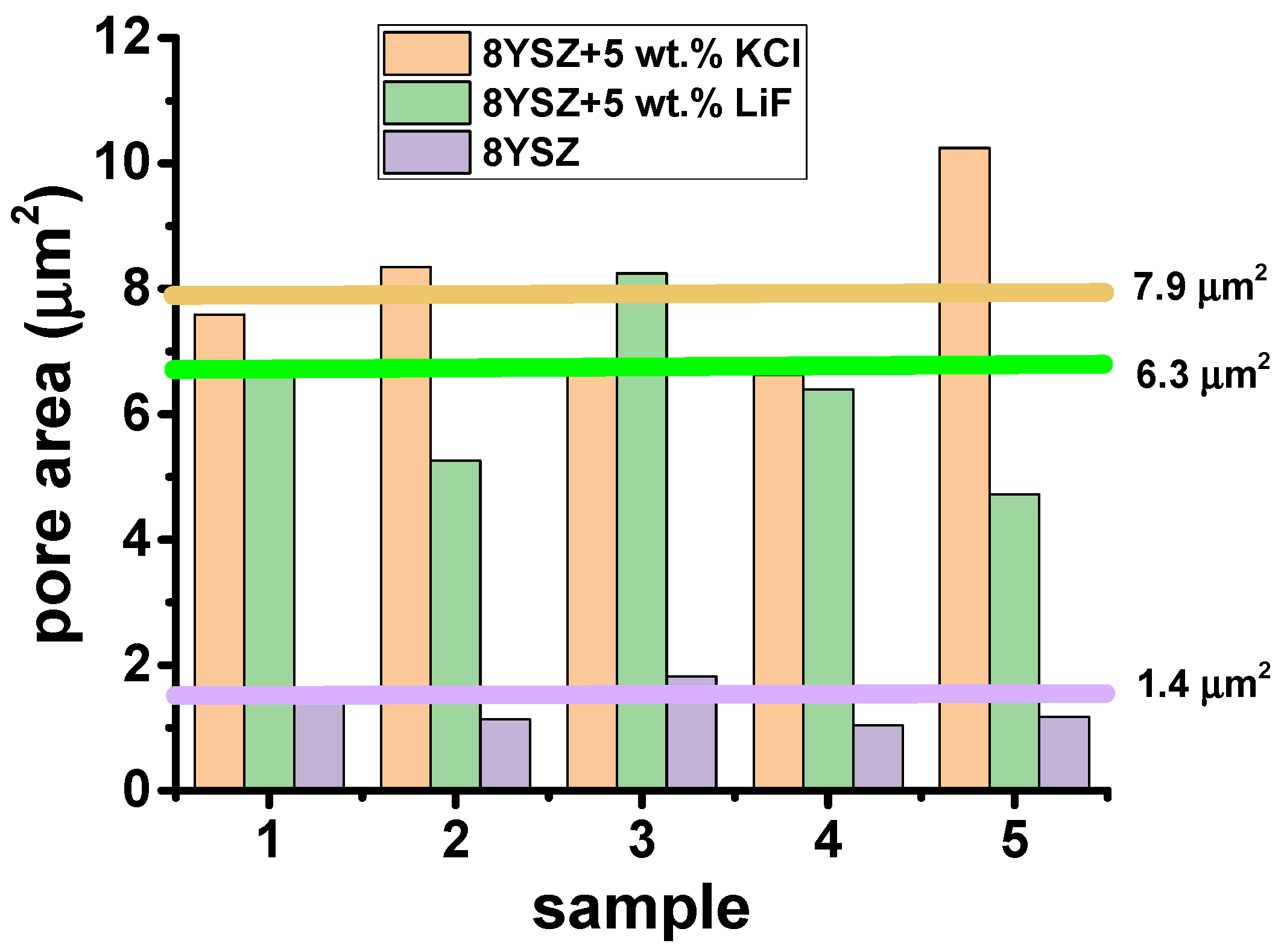
3.3. Microstructural Analyses
3.4. Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahaman, M.N. Sintering of Ceramics: Fundamentals; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rahaman, M.N. Grain growth and microstructure control. In Ceramics Processing and Sintering, 2nd ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 540–619. [Google Scholar]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Oh, U.C.; Chung, Y.S.; Kim, D.Y.; Yoon, D.N. Effect of grain growth on pore coalescence during the liquid-phase sintering of MgO-CaMgSiO4 systems. J. Am. Ceram. Soc. 1988, 71, 854–857. [Google Scholar] [CrossRef]
- Xydas, N.K.; Salam, L.A. Transient liquid phase sintering of high density Fe3Al using Fe and Fe2Al5–FeAl2 powders. Part 2-Densification mechanism analysis. Powder Metall. 2006, 49, 146–152. [Google Scholar] [CrossRef]
- German, R.M. Sintering with a Liquid Phase. In Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Woburn, MA, USA, 2014; pp. 247–303. [Google Scholar] [CrossRef]
- Van der Donk, G.S.W.; Serra, J.M.; Meulenberg, W.A. Microporous and mesoporous 8YSZ materials derived from polymeric acetylacetone-modified precursors for inorganic membrane applications. J. Noncryst. Solids 2018, 34, 3723–3731. [Google Scholar] [CrossRef]
- Van Gestel, T.; Velterop, F.; Meulenberg, W.A. Zirconia-supported hybrid organosilica microporous membranes for CO2 separation and pervaporation. Sep. Purif. Technol. 2021, 259, 118114. [Google Scholar] [CrossRef]
- Brandon, N.P.; Brett, D.J. Engineering porous materials for fuel cell applications. Phil. Trans. R. Soc. A 2006, 364, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R. Zirconia and Zirconia Ceramics; Publ. No. 113; Magnesium Elektron Ltd.: Twickenham, UK, 1986. [Google Scholar]
- Heuer, A.H.; Hobbs, L.W. (Eds.) Science and Technology of zirconia. In Advances in Ceramics; The American Ceramic Society Inc.: Columbus, OH, USA, 1981; Volume 3, ISBN 0-916094-42-1. [Google Scholar]
- Jagannathan, K.P.; Tiku, S.K.; Ray, H.S.; Ghosh, A.; Subbarao, E.C. Technological Applications of Solid Electrolytes. In Solid Electrolytes and Their Applications; Subbarao, E.C., Ed.; Springer: Boston, MA, USA, 1980; pp. 201–259. [Google Scholar]
- Prasad, N.V.K.; Prasad, K.V.; Ramesh, S.; Phanidhar, S.V.; Ratnam, K.V.; Janardhan, S.; Manjunatha, H.; Sarma, M.S.S.R.K.N.; Srinivas, K. Ceramic Sensors: A mini-review of their applications. Front. Mater. 2020, 7, 593342. [Google Scholar] [CrossRef]
- Moos, R. A brief overview on automotive exhaust gas sensors based on electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar] [CrossRef]
- Plashnitsa, V.V.; Elumalai, P.; Fujio, Y.; Miura, N. Zirconia-based electrochemical gas sensors using nano-structured sensing materials aiming at detection of automotive exhausts. Electrochim. Acta 2009, 54, 6090–6106. [Google Scholar] [CrossRef]
- Zuiykov, S.; Miura, N. Development of zirconia-based potentiometric NOx sensors for automotive and energy industries in the early 21st century: What are the prospects for sensors? Sens. Actuators B 2007, 121, 639–651. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-ion electrolytes. Ann. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Akhkozov, L.; Danilenko, I.; Podhurska, V.; Shylo, A.; Vasyliv, B.; Ostash, O.; Lyubchyk, A. Zirconia-based materials in alternative energy devices—A strategy for improving material properties by optimizing the characteristics of initial powders. Int. J. Hydrogen Energy 2022, 97, 41359–41371. [Google Scholar] [CrossRef]
- Kusnezoff, M.; Trofimenko, N.; Müller, M.; Michaelis, A. Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells. Materials 2016, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, T.; Huang, Z.; Hu, G.; Zhou, J.; Wang, S. A cathode-supported solid oxide fuel cell prepared by the phase-inversion tape casting an impregnating method. Int. J. Hydrogen Energy 2022, 47, 18810–18819. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials design for the next generation thermal barrier coatings. Ann. Rev. Mater. Sci. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Huang, J.; Chu, X.; Yang, T.; Fang, H.; Ye, D.; Wang, W.; Zhang, X.; Sun, W.; Huang, R.; Li, C.J. Achieving high anti-sintering performance of plasma-sprayed YSZ thermal barrier coatings through pore structure design. Surf. Coat. Technol. 2022, 434, 128259. [Google Scholar] [CrossRef]
- Sharma, A.; Witz, G.; LeCreux, C.; Hitchman, N. High heat flux burner-rig testing of 8YSZ thermal barrier coatings: Influence of the powder feedstock. J. Eur. Ceram. Soc. 2022, 42, 7267–7274. [Google Scholar] [CrossRef]
- Kulyk, V.; Duriagina, Z.; Vasyliv, B.; Vavrukh, V.; Kovbasiuk, T.; Lyutyy, P.; Vira, V. The Effect of Sintering Temperature on the Phase Composition, Microstructure, and Mechanical Properties of Yttria-Stabilized Zirconia. Materials 2022, 15, 2707. [Google Scholar] [CrossRef]
- Shojai, F.; Mäntylä, T. Monoclinic Zirconia Microfiltration Membranes: Preparation and Characterization. J. Porous Mater. 2001, 8, 129–142. [Google Scholar] [CrossRef]
- Diaz, J.C.C.A.; Muccillo, R. Liquid-phase flash sintering 8YSZ with alkali halide sintering aids. J. Eur. Ceram. Soc. 2020, 40, 4299–4303. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Melgar, V.M.A.; Kim, J.; Othman, M.R.; Nguyen, H.V.P.; Han, J. YSZ-carbonate dual-phase membranes for high temperature carbon dioxide separation. J. Ind. Eng. Chem. 2014, 20, 3703–3708. [Google Scholar] [CrossRef]
- Chen, D.; Dambra, C.; Dorfman, M. Process and properties of dense and porous vertically-cracked yttria stabilized zirconia thermal barrier coatings. Surf. Coat. Technol. 2020, 404, 126467. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Li, X.; Shao, J.; Zheng, J.; Bai, C.; Zhang, X.; Qiao, Y.; Colombo, P. Fabrication and application of porous materials made from coal gangue: A review. Int. J. Appl. Ceram. Technol. 2023, 1–26. [Google Scholar] [CrossRef]
- Yttria-Stabilized Zirconia Tape Cast Grade Electrolyte Powder. Available online: www.fuelcellmaterials.com (accessed on 1 January 2001).
- Available online: https://imagej.nih.gov/ij/ (accessed on 1 January 2021).
- Kleitz, M.; Kennedy, J.H. Resolution of multicomponent impedance diagrams. In Fast Ion Transport in Solids; Mundy, J.N., Shenoy, G.K., Vashishta, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1979; pp. 185–188. ISBN 0444003533. [Google Scholar]
- Muccillo, R. Impedance spectroscopy analysis of zirconia:8 mol% yttria solid electrolytes with graphite pore former. J. Mater. Res. 2009, 24, 1780–1784. [Google Scholar] [CrossRef]
- Kuma, S.; Subbarao, E.C. Complex impedance and admittance of stabilized zirconia. Solid State Ion. 1981, 5, 543–546. [Google Scholar] [CrossRef]
- Ramirez, E.B.; Huanosta, A.; Sebastian, J.P.; Huerta, L.; Ortiz, A.; Alonso, J.C. Structure, composition and electrical properties of YSZ films deposited by ultrasonic spray pyrolysis. J. Mater. Sci. 2007, 42, 901–907. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, J.C.C.A.; Muccillo, E.N.d.S.; Muccillo, R. Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives. Materials 2023, 16, 3509. https://doi.org/10.3390/ma16093509
Diaz JCCA, Muccillo ENdS, Muccillo R. Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives. Materials. 2023; 16(9):3509. https://doi.org/10.3390/ma16093509
Chicago/Turabian StyleDiaz, Julio Cesar Camilo Albornoz, Eliana Navarro dos Santos Muccillo, and Reginaldo Muccillo. 2023. "Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives" Materials 16, no. 9: 3509. https://doi.org/10.3390/ma16093509
APA StyleDiaz, J. C. C. A., Muccillo, E. N. d. S., & Muccillo, R. (2023). Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives. Materials, 16(9), 3509. https://doi.org/10.3390/ma16093509





