Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion
Abstract
1. Introduction
2. Materials and Methods
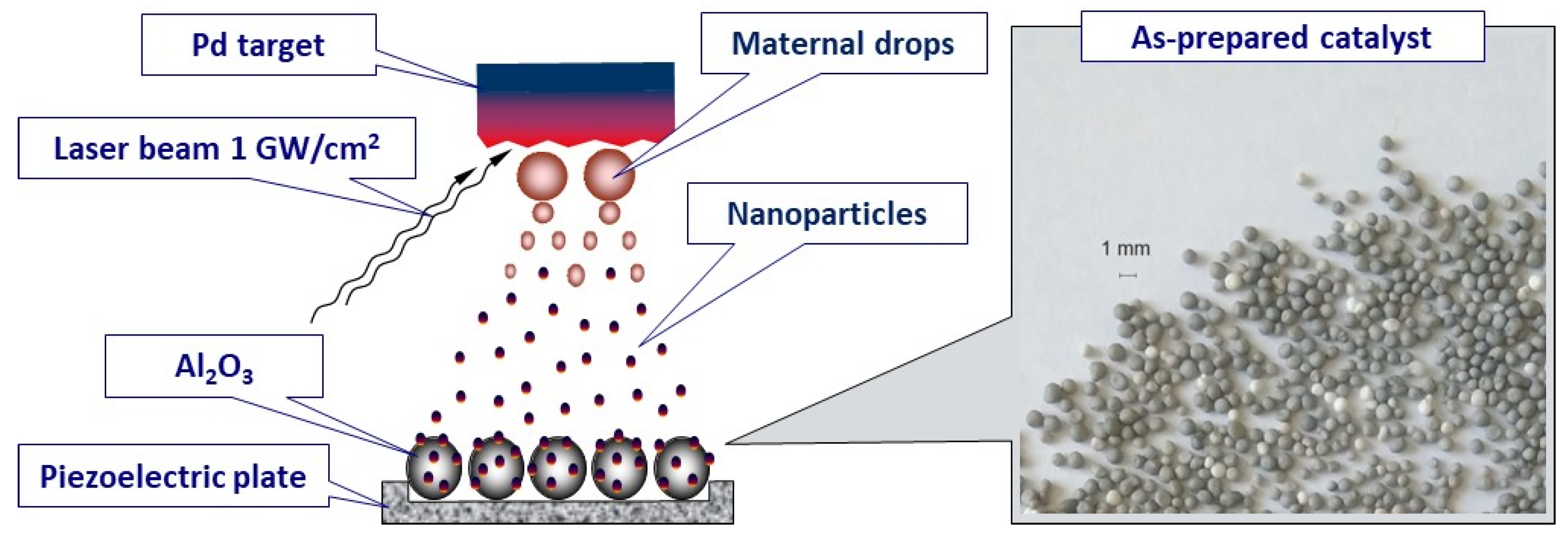
2.1. Preparation of the Catalysts
2.2. Testing the Catalytic Activity
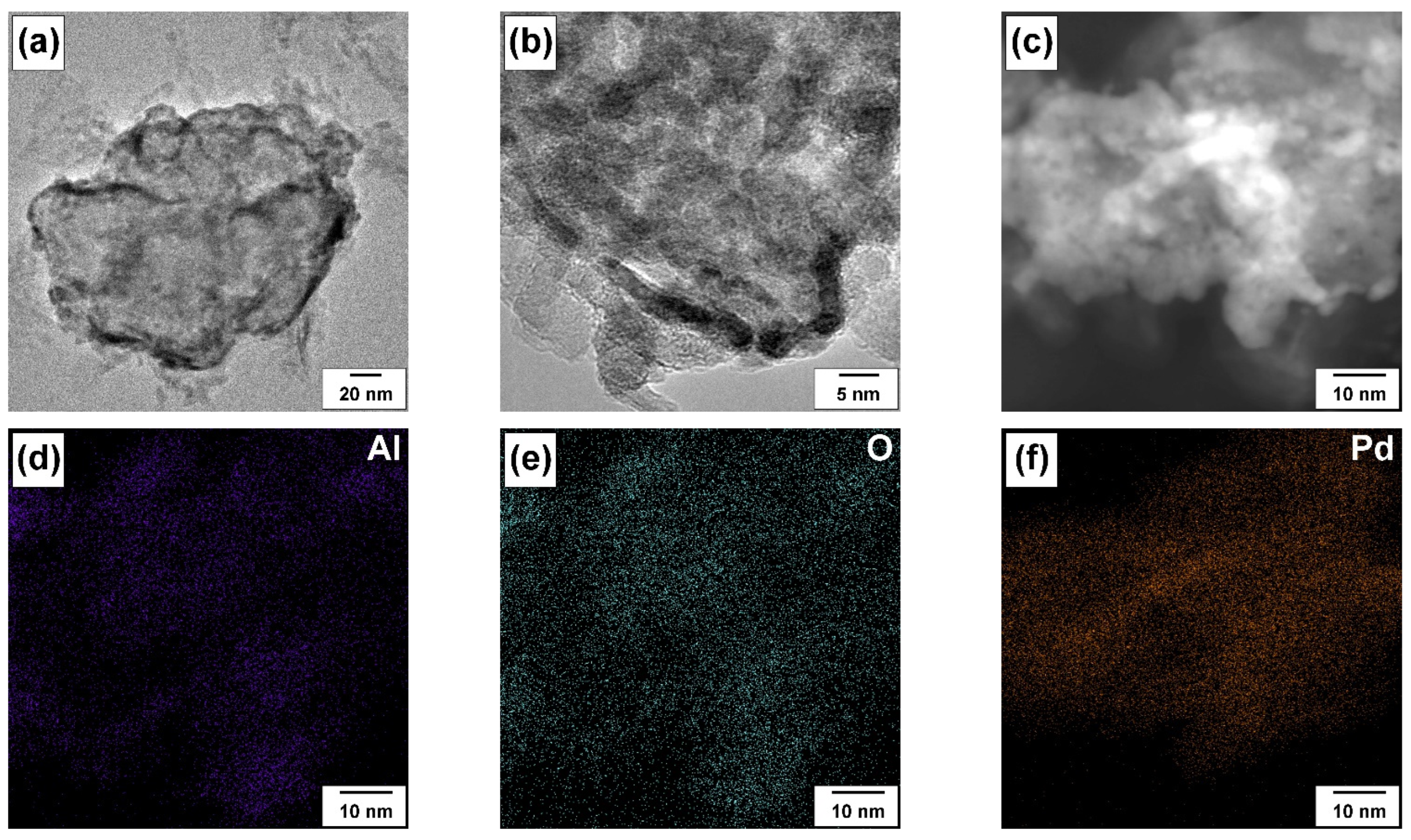
2.3. Characterization of the Materials
3. Results and Discussion
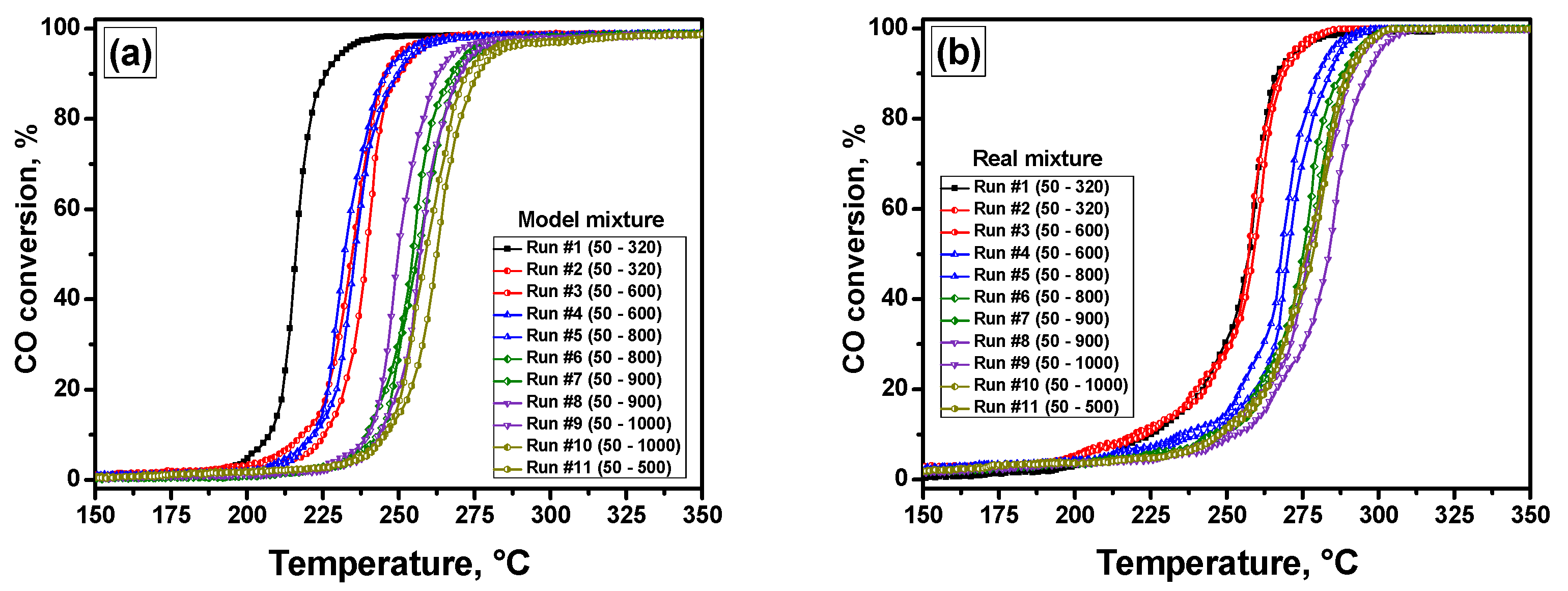
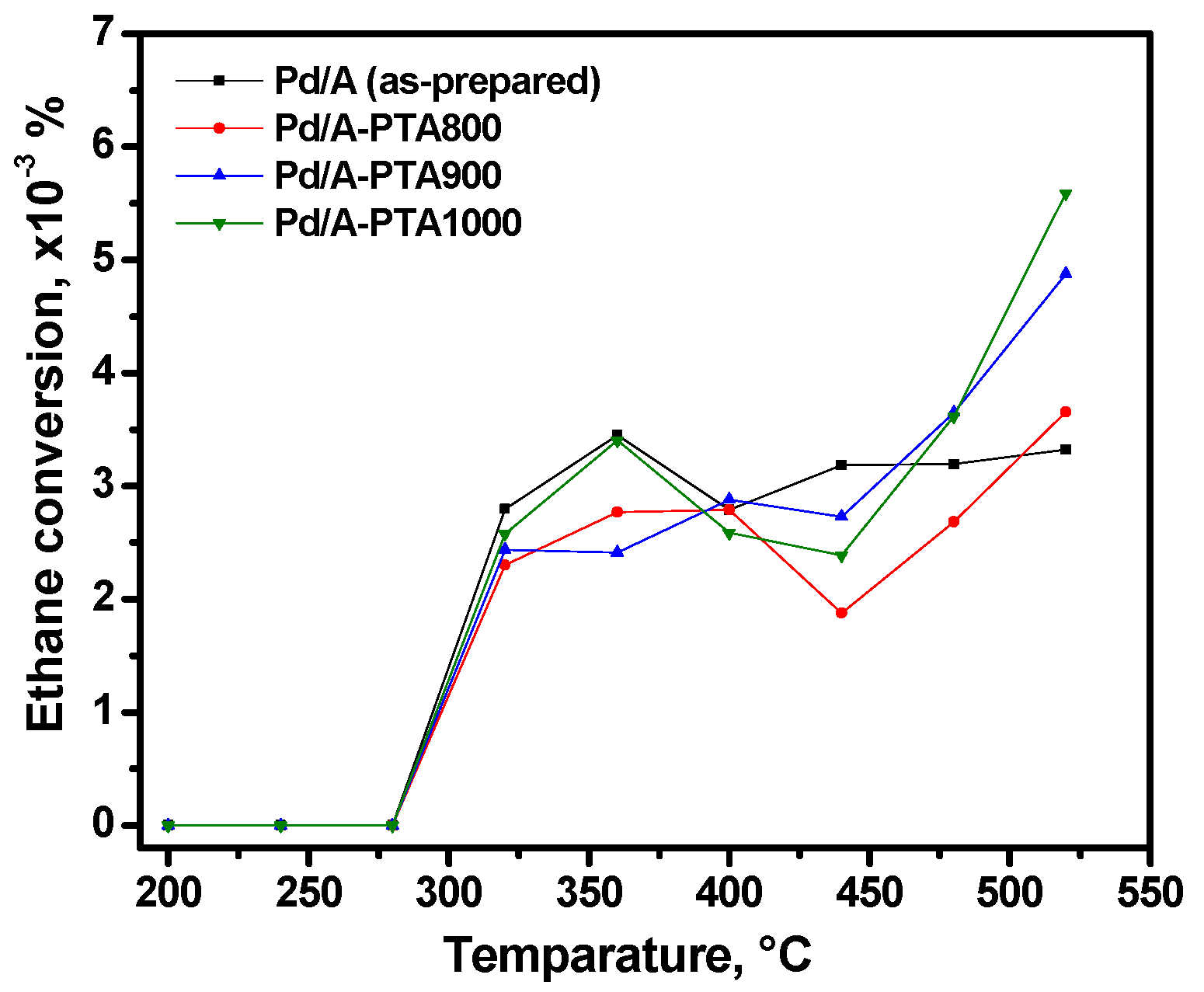
3.1. Catalytic Activity and Thermal Stability of the Samples
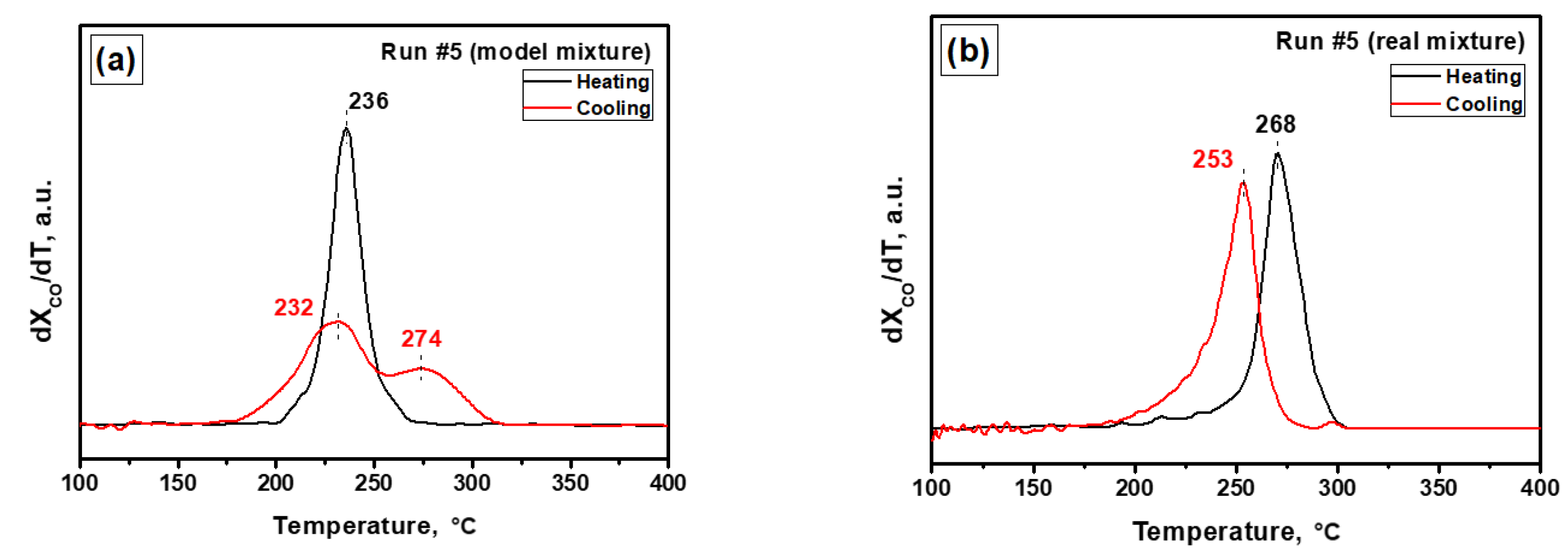
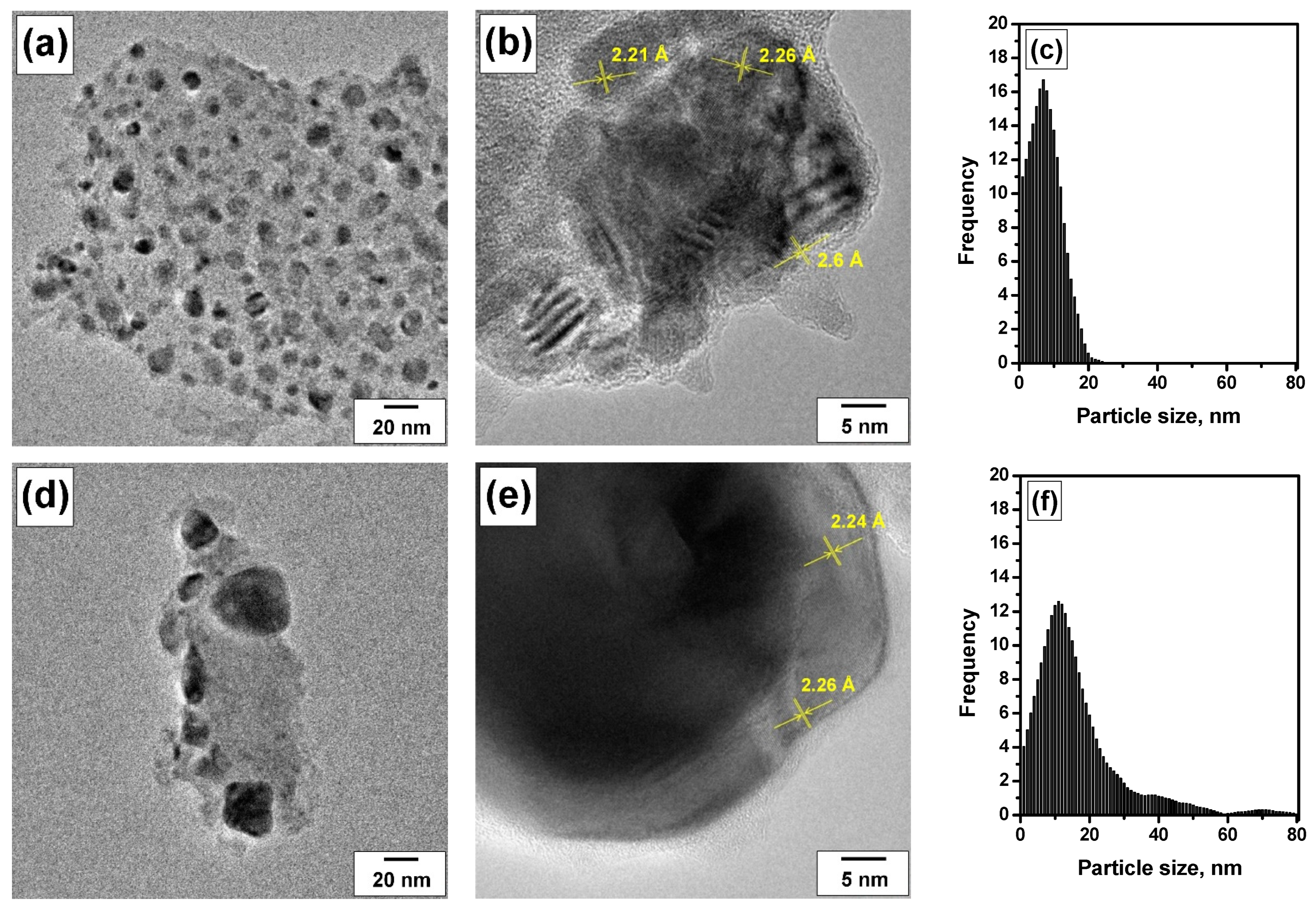
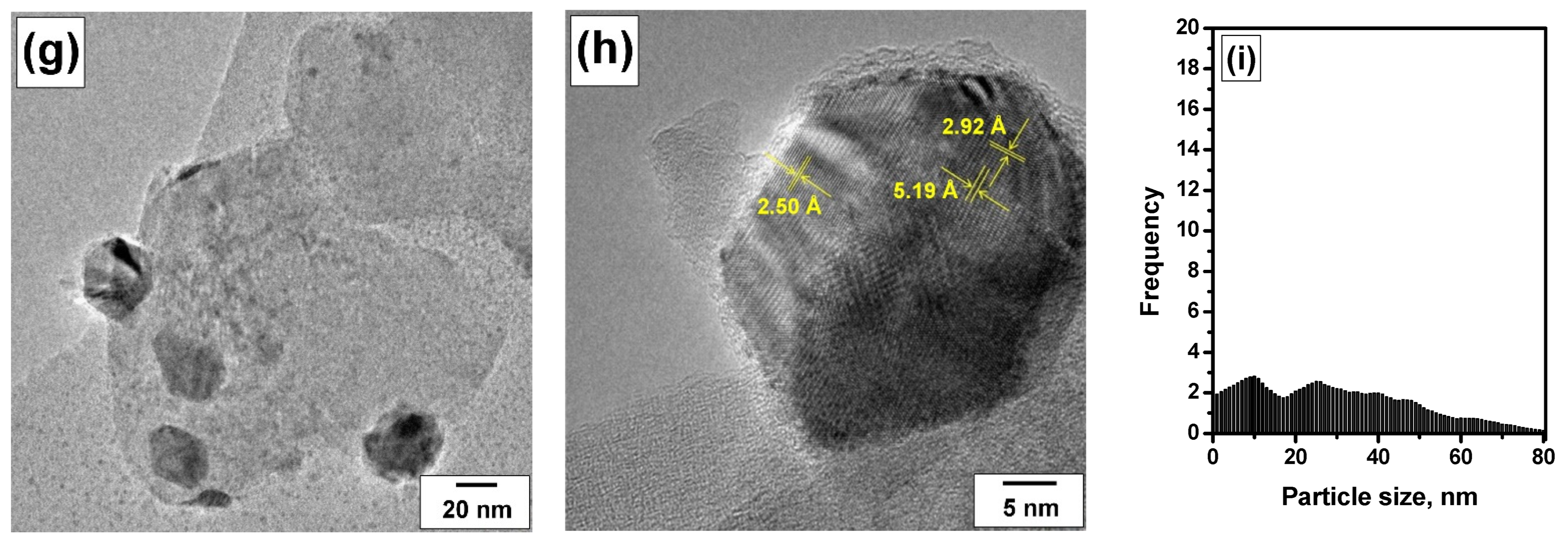
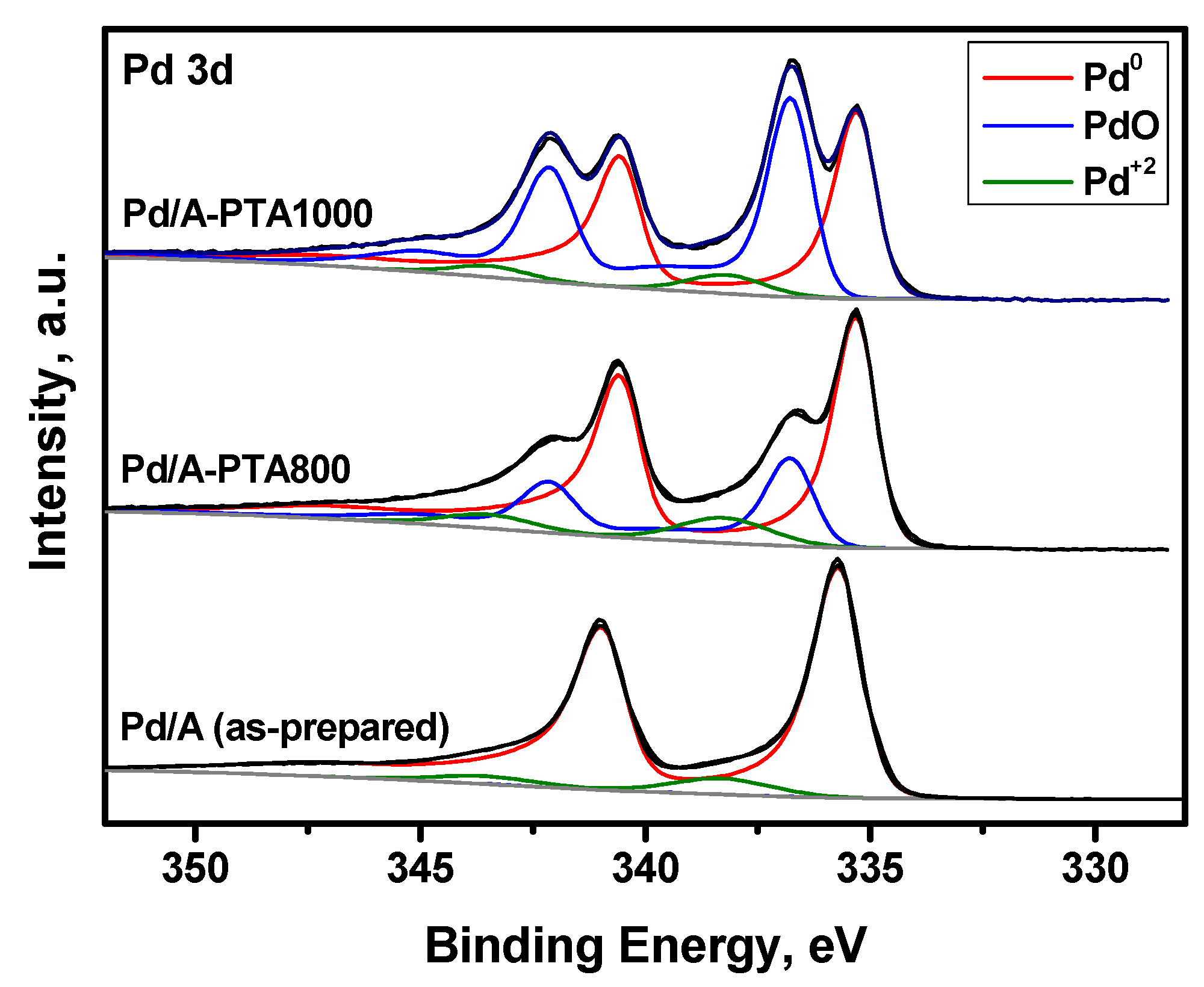
3.2. Evolution of the Catalyst during the Prompt Thermal Aging Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V. Controlling automotive exhaust emissions: Successes and underlying science. Philos. Trans. R. Soc. A 2005, 363, 1013–1033. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 1107–1112. [Google Scholar] [CrossRef]
- Hoek, G.; Brunekreef, B.; Goldbohm, S.; Fischer, P.; van den Brandt, P.A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet 2002, 360, 1203–1209. [Google Scholar] [CrossRef]
- Brunekreef, B.; Janssen, N.A.; de Hartog, J.; Harssema, H.; Knape, M.; van Vliet, P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology 1997, 8, 298–303. [Google Scholar] [CrossRef]
- Brauer, M.; Hoek, G.; Van Vliet, P.; Meliefste, K.; Fischer, P.H.; Wijga, A.; Koopman, L.P.; Neijens, H.J.; Gerritsen, J.; Kerkhof, M.; et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am. J. Resp. Crit. Care Med. 2002, 166, 1092–1098. [Google Scholar] [CrossRef]
- De Sarkar, A.; Khanra, B. CO oxidation and NO reduction over supported Pt-Rh and Pd-Rh nanocatalysts: A comparative study. J. Mol. Catal. A Chem. 2005, 229, 25–29. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A review on the Pd-based three-way catalyst. Catal. Rev. 2015, 57, 79–144. [Google Scholar] [CrossRef]
- Hangas, J.; Chen, A.E. Comparative Analytical Study of Two Pt–Rh Three-way Catalysts. Catal. Lett. 2006, 108, 103–111. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 2006, 36, 433–457. [Google Scholar] [CrossRef]
- Ozawa, M.; Takahashi-Morita, M.; Kobayashi, K.; Haneda, M. Core-shell type ceria zirconia support for platinum and rhodium three way catalysts. Catal. Today 2017, 281, 482–489. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2020, 234, 936–949. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V. Effect of alumina phase transformation on stability of low-loaded Pd-Rh catalysts for CO oxidation. Top. Catal. 2017, 60, 152–161. [Google Scholar] [CrossRef]
- Beck, D.D.; Carr, C.J. Effects of High-Temperature Aging on the Dispersion of Rh/Al2O3. J. Catal. 1993, 144, 296–310. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.W.; Chun, W.; McCabe, R.W. High-Temperature-Aging-Induced Encapsulation of Metal Particles by Support Materials: Comparative Results for Pt, Pd, and Rh on Cerium–Zirconium Mixed Oxides. J. Catal. 1999, 182, 228–233. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; Botas, J.A.; Ferret, R.; Pilar González-Marcos, M.; Marc, J.-L.; Gutiérrez-Ortiz, M.A. Thermal aging of Pd/Pt/Rh automotive catalysts under a cycled oxidizing–reducing environment. Catal. Today 2000, 59, 395–402. [Google Scholar] [CrossRef]
- Winkler, A.; Ferri, D.; Hauert, R. Influence of aging effects on the conversion efficiency of automotive exhaust gas catalysts. Catal. Today 2010, 155, 140–146. [Google Scholar] [CrossRef]
- Kang, S.B.; Han, S.J.; Nam, S.B.; Nam, I.-S.; Cho, B.K.; Kim, C.H.; Oh, S.H. Effect of Aging Atmosphere on Thermal Sintering of Modern Commercial TWCs. Top. Catal. 2013, 56, 298–305. [Google Scholar] [CrossRef]
- Behafarid, F.; Roldan Cuenya, B. Towards the Understanding of Sintering Phenomena at the Nanoscale: Geometric and Environmental Effects. Top. Catal. 2013, 56, 1542–1559. [Google Scholar] [CrossRef]
- Ozawa, M.; Kimura, M. Effect of cerium addition on the thermal stability of gamma alumina support. J. Mater. Sci. Lett. 1990, 9, 291–293. [Google Scholar] [CrossRef]
- Zhou, Z.; Harold, M.P.; Luss, D. Comparison of Pt-BaO/Al2O3 and Pt-CeO2/Al2O3 for NOx storage and reduction: Impact of cycling frequency. Appl. Catal. B Environ. 2019, 255, 117742. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Kenzhin, R.M.; Tashlanov, M.Y.; Stoyanovskii, V.O.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V.; Kalinkin, A.V.; Smirnov, M.Y.; Bukhtiyarov, V.I. Synthesis and study of bimetallic Pd-Rh system supported on zirconia-doped alumina as a component of three-way catalysts. Emiss. Control Sci. Technol. 2019, 5, 363–377. [Google Scholar] [CrossRef]
- Gandhi, H.; Williamson, W.; Logothetis, E.; Tabock, J.; Peters, C.; Hurley, M.; Shelef, M. Affinity of lead for noble metals on different supports. Surf. Interface Anal. 1984, 6, 149–161. [Google Scholar] [CrossRef]
- Gandhi, H.; Shelef, M. Effects of sulphur on noble metal automotive catalysts. Appl. Catal. 1991, 77, 175–186. [Google Scholar] [CrossRef]
- Shen, M.; Lin, F.; Wei, G.; Wang, J.; Zhu, S. Improved sulfur-resistant ability on CO oxidation of Pd/Ce0.75Zr0.25O2 over Pd/CeO2-TiO2 and Pd/CeO2. J. Rare Earths 2015, 33, 56–61. [Google Scholar] [CrossRef]
- Zheng, T.; Lu, B.; Harle, G.; Yang, D.; Wang, C.; Zhao, Y. A comparative study of Rh-only, Pd-only and Pd/Rh catalysts. Appl. Catal. A Gen. 2020, 602, 117649. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, M.; Pi, Z.; Gong, M.; Chen, Y. Preparation of Ce-Zr-La-Al2O3 and Supported Single Palladium Three-Way Catalyst. Chin. J. Catal. 2008, 29, 108–112. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Zhao, B.; Li, G.; Zhou, R. Effect of synthesis method on the properties of ceria–zirconia modified alumina and the catalytic performance of its supported Pd-only three-way catalyst. J. Mol. Catal. A Chem. 2011, 344, 132–137. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Lin, S.; Zhou, R. Effects of the addition of small quantities of ceria on the catalytic behavior of Pd-only close-coupled catalysts during automobile exhaust elimination. Chin. J. Catal. 2014, 35, 1267–1280. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Zhao, B.; Shen, M.; Zhou, R. The effect of La doping on the structure of Ce0.2Zr0.8O2 and the catalytic performance of its supported Pd-only three-way catalyst. Appl. Catal. B Environ. 2010, 101, 150–159. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Zhao, B.; Zhou, R. Synthesis of La modified ceria–zirconia solid solution by advanced supercritical ethanol drying technology and its application in Pd-only three-way catalyst. Appl. Catal. B Environ. 2010, 100, 516–528. [Google Scholar] [CrossRef]
- Li, W.-J.; Wey, M.-Y. Design of a thermally resistant core@shell/halloysite catalyst with optimized structure and surface properties for a Pd-only three-way catalyst. Appl. Catal. A Gen. 2020, 602, 117732. [Google Scholar] [CrossRef]
- Chen, K.; Wan, J.; Lin, J.; Zhou, R. Comparative study of three-way catalytic performance over Pd/CeO2-ZrO2-Al2O3 and Pd/La-Al2O3 catalysts: New insights into microstructure and thermal stability. Mol. Catal. 2022, 526, 112361. [Google Scholar] [CrossRef]
- Ferri, D.; Elsener, M.; Kröcher, O. Methane oxidation over a honeycomb Pd-only three-way catalyst under static and periodic operation. Appl. Catal. B Environ. 2018, 220, 67–77. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064. [Google Scholar] [CrossRef]
- Zheng, Q.; Farrauto, R.; Deeba, M. Part II: Oxidative thermal aging of Pd/Al2O3 and Pd/CexOy-ZrO2 in automotive three way catalysts: The effects of fuel shutoff and attempted fuel rich regeneration. Catalysts 2015, 5, 1797–1814. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.; Seo, C.Y.; Schwank, J.W.; McCabe, R.W. Aging, re-dispersion, and catalytic oxidation characteristics of model Pd/Al2O3 automotive three-way catalysts. Appl. Catal. B Environ. 2015, 163, 499–509. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Martínez-Arias, A.; Fernández-García, M. Metal–promoter interface in Pd/(Ce, Zr)Ox/Al2O3 catalysts: Effect of thermal aging. J. Catal. 2004, 221, 148–161. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Dahlberg, K.A.; Seo, C.Y.; Fisher, G.B.; Peczonczyk, S.L.; Rhodes, K.; Jagner, M.J.; Haack, L.P. Pd model catalysts: Effect of aging environment and lean redispersion. Appl. Catal. B Environ. 2016, 183, 343–360. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Chen, X.; Peczonczyk, S.L.; Drews, A.R. Pd model catalysts: Effect of aging duration on lean redispersion. Appl. Catal. B Environ. 2016, 185, 189–202. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Hangas, J.; Peczonczyk, S.L.; Paxton, W.A. Pd model catalysts: Effect of air pulse length during redox aging on Pd redispersion. Appl. Catal. B Environ. 2018, 223, 76–90. [Google Scholar] [CrossRef]
- Lieske, H.; Voelter, J. Palladium redispersion by spreading of palladium(II) oxide in oxygen treated palladium/alumina. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lampert, J.K.; Hobson, M.C.; Waterman, E.M. Thermal decomposition and reformation of PdO catalysts; support effects. Appl. Catal. B Environ. 1995, 6, 263–270. [Google Scholar] [CrossRef]
- Chen, X.; Schwank, J.W.; Fisher, G.B.; Cheng, Y.; Jagner, M.; McCabe, R.W.; Katz, M.B.; Graham, G.W.; Pan, X. Nature of the two-step temperature-programmed decomposition of PdO supported on alumina. Appl. Catal. A Gen. 2014, 475, 420–426. [Google Scholar] [CrossRef]
- Cao, Y.; Ran, R.; Wu, X.; Si, Z.; Kang, F.; Weng, D. Progress on metal-support interactions in Pd-based catalysts for automobile emission control. J. Environ. Sci. 2023, 125, 401–426. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Uzio, D.; Berhault, G. Factors governing the catalytic reactivity of metallic nanoparticles. Catal. Rev. 2010, 52, 106–131. [Google Scholar] [CrossRef]
- Lokteva, E.S.; Golubina, E.V. Metal-support interactions in the design of heterogeneous catalysts for redox processes. Pure Appl. Chem. 2019, 91, 609–631. [Google Scholar] [CrossRef]
- Duan, H.; You, R.; Xu, S.; Li, Z.; Qian, K.; Cao, T.; Huang, W.; Bao, X. Pentacoordinated Al3+-Stabilized Active Pd Structures on Al2O3-Coated Palladium Catalysts for Methane Combustion. Angew. Chem. Int. Ed. 2019, 58, 12043–12048. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, O.A.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I. Metal–support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl. Catal. B Environ. 2010, 97, 57–71. [Google Scholar] [CrossRef]
- Haneda, M.; Todo, M.; Nakamura, Y.; Hattori, M. Effect of Pd dispersion on the catalytic activity of Pd/Al2O3 for C3H6 and CO oxidation. Catal. Today 2017, 281, 447–453. [Google Scholar] [CrossRef]
- Murata, K.; Mahara, Y.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. The Metal–Support Interaction Concerning the Particle Size Effect of Pd/Al2O3 on Methane Combustion. Angew. Chem. Int. Ed. 2017, 56, 15993–15997. [Google Scholar] [CrossRef]
- Murata, K.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Methane Combustion over Pd/Al2O3 Catalysts in the Presence of Water: Effects of Pd Particle Size and Alumina Crystalline Phase. ACS Catal. 2020, 10, 8149–8156. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Stoyanovskii, V.O.; Kenzhin, R.M.; Slavinskaya, E.M.; Plyusnin, P.E.; Shubin, Y.V. Purification of gasoline exhaust gases using bimetallic Pd–Rh/δ-Al2O3 catalysts. Reac. Kinet. Mech. Catal. 2019, 127, 137–148. [Google Scholar] [CrossRef]
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107. [Google Scholar] [CrossRef]
- Hoost, T.E.; Otto, K.; Laframboise, K.A. FTIR Spectroscopy of Nitric Oxide Adsorption on Pd/Al2O3: Evidence of Metal-Support Interaction. J. Catal. 1995, 155, 303–311. [Google Scholar] [CrossRef]
- Cao, Y.; Ran, R.; Wu, X.; Zhao, B.; Wan, J.; Weng, D. Comparative study of ageing condition effects on Pd/Ce0.5Zr0.5O2 and Pd/Al2O3 catalysts: Catalytic activity, palladium nanoparticle structure and Pd-support interaction. Appl. Catal. A Gen. 2013, 457, 52–61. [Google Scholar] [CrossRef]
- Yang, M.; Shen, M.; Wang, J.; Wen, J.; Zhao, M.; Wang, J.; Wang, W. Pd-Supported Interaction-Defined Selective Redox Activities in Pd−Ce0.7Zr0.3O2−Al2O3 Model Three-Way Catalysts. J. Phys. Chem. C 2009, 113, 12778–12789. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Guo, Y.; Zhang, L.; Li, T.; Zang, K.; Liu, F.; Li, L.; Luo, J.; Qiao, B.; et al. Oxidative strong metal–support interactions (OMSI) of supported platinum-group metal catalysts. Chem. Sci. 2018, 9, 6679–6684. [Google Scholar] [CrossRef]
- Murata, K.; Eleeda, E.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Identification of active sites in CO oxidation over a Pd/Al2O3 catalyst. Phys. Chem. Chem. Phys. 2019, 21, 18128–18137. [Google Scholar] [CrossRef] [PubMed]
- Casapu, M.; Fischer, A.; Gänzler, A.M.; Popescu, R.; Crone, M.; Gerthsen, D.; Türk, M.; Grunwaldt, J.-D. Origin of the Normal and Inverse Hysteresis Behavior during CO Oxidation over Pt/Al2O3. ACS Catal. 2017, 7, 343–355. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Svetlichnyi, V.A.; Kulinich, S.A. Green laser ablation-based synthesis of functional nanomaterials for generation, storage, and detection of hydrogen. Curr. Opin. Green Sustain. Chem. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Muller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J. Coll. Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Rostovshchikova, T.; Lokteva, E.; Shilina, M.; Golubina, E.; Maslakov, K.; Krotova, I.; Bryzhin, A.; Tarkhanova, I.; Udalova, O.; Kozhevin, V. Laser Electrodispersion of Metals for the Synthesis of Nanostructured Catalysts: Achievements and Prospects. Russ. J. Phys. Chem. A 2021, 95, 451–474. [Google Scholar] [CrossRef]
- Lokteva, E.S.; Rostovshchikova, T.N.; Kachevskii, S.A.; Golubina, E.V.; Smirnov, V.V.; Stakheev, A.Y.; Telegina, N.S.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A. High catalytic activity and stability of palladium nanoparticles prepared by the laser electrodispersion method in chlorobenzene hydrodechlorination. Kinet. Catal. 2008, 49, 748–755. [Google Scholar] [CrossRef]
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.A.; Shilina, M.I.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Slavinskaya, E.M. Role of surface coverage of alumina with Pt nanoparticles deposited by laser electrodispersion in catalytic CO oxidation. Appl. Surf. Sci. 2021, 536, 147656. [Google Scholar] [CrossRef]
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.A.; Egorova, T.B.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Yermakov, A.Y. Chlorobenzene hydrodechlorination on bimetallic catalysts prepared by laser electrodispersion of NiPd alloy. Pure Appl. Chem. 2018, 90, 1685–1701. [Google Scholar] [CrossRef]
- Matieva, Z.M.; Nikolaev, S.A.; Snatenkova, Y.M.; Gurevich, S.A.; Yavsin, D.A.; Ezzhelenko, D.I.; Kolesnichenko, N.V. Conversion of dimethyl ether to liquid hydrocarbons over the nano-Pd-ZnHZSM-5 catalyst obtained by laser electrodispersion of the metal. J. Chem. Technol. Biotechnol. 2022, 97, 1792–1802. [Google Scholar] [CrossRef]
- Shilina, M.; Krotova, I.; Nikolaev, S.; Gurevich, S.; Yavsin, D.; Udalova, O.; Rostovshchikova, T. Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture. Hydrogen 2023, 4, 154–173. [Google Scholar] [CrossRef]
- Rostovshchikova, T.; Nikolaev, S.; Krotova, I.; Maslakov, K.; Udalova, O.; Gurevich, S.; Yavsin, D.; Shilina, M. ZSM-5 and BEA zeolites modified with Pd nanoparticles by laser electrodispersion. The structure and catalytic activity in CO and CH4 oxidation. Russ. Chem. Bull. 2022, 71, 1179–1193. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Shilina, M.I.; Gurevich, S.A.; Yavsin, D.A.; Veselov, G.B.; Vedyagin, A.A. New Approaches to the Synthesis of Ultralow-Palladium Automotive Emission Control Catalysts. Doklady Phys. Chem. 2022, 506, 123–130. [Google Scholar] [CrossRef]
- Bryzhin, A.A.; Golubina, E.V.; Maslakov, K.I.; Lokteva, E.S.; Tarkhanova, I.G.; Gurevich, S.A.; Yavsin, D.A.; Rostovshchikova, T.N. Bimetallic Nanostructured Catalysts Prepared by Laser Electrodispersion: Structure and Activity in Redox Reactions. ChemCatChem 2020, 12, 4396–4405. [Google Scholar] [CrossRef]
- Aznarez, A.; Gil, A.; Korili, S. Performance of palladium and platinum supported on alumina pillared clays in the catalytic combustion of propene. RSC Adv. 2015, 5, 82296–82309. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Knözinger, H. Nature and Estimation of Functional Groups on Solid Surfaces. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 39–207. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Amorphous Semiconductors. In Amorphous and Liquid Semiconductors; Tauc, J., Ed.; Springer: Boston, MA, USA, 1974; pp. 159–220. [Google Scholar]
- Sinfelt, J.; Yates, D.J.C. Catalytic hydrogenolysis of ethane over the noble metals of Group VIII. J. Catal. 1967, 8, 82–90. [Google Scholar] [CrossRef]
- Sinfelt, J. Kinetics of ethane hydrogenolysis. J. Catal. 1972, 27, 468–471. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stadnichenko, A.I.; Salanov, A.N.; Chumakov, G.A.; Boronin, A.I. Inverse temperature hysteresis and self-sustained oscillations in CO oxidation over Pd at elevated pressures of reaction mixture: Experiment and mathematical modeling. Chem. Eng. Sci. 2020, 212, 115312. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Myint, M.T.Z.; Göthelid, M.A.; Dutta, J. CO Oxidation Efficiency and Hysteresis Behavior over Mesoporous Pd/SiO2 Catalyst. Catalysts 2021, 11, 131. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, J.; Fan, Y.; Zhan, Y.; Chen, C.; Li, D.; Jiang, L. Mg–Al hydrotalcite-supported Pd catalyst for low-temperature CO oxidation: Effect of Pdn+ species and surface hydroxyl groups. Dalton Trans. 2018, 47, 14938–14944. [Google Scholar] [CrossRef] [PubMed]
- Strømsheim, M.D.; Knudsen, J.; Farstad, M.H.; Sørvik, L.; Guo, X.; Venvik, H.J.; Borg, A. Near Ambient Pressure XPS Investigation of CO Oxidation Over Pd3Au(100). Top. Catal. 2017, 60, 1439–1448. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stonkus, O.A.; Gulyaev, R.V.; Stadnichenko, A.I.; Chumakov, G.A.; Boronin, A.I.; Demidenko, G.V. Self-sustained oscillations in CO oxidation reaction on PdO/Al2O3 catalyst. Chem. Eng. Sci. 2012, 83, 149–158. [Google Scholar] [CrossRef]
- Etheridge, J.E.; Watling, T.C. Is reactor light-off data sufficiently discriminating between kinetic parameters to be used for developing kinetic models of automotive exhaust aftertreatment catalysts? The effect of hysteresis induced by strong self inhibition. Chem. Eng. J. 2015, 264, 376–388. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Chumakov, G.A.; Boronin, A.I. Self-sustained oscillations within the temperature hysteresis in CO oxidation over Pd: Mathematical model of a cascade of continuous stirred-tank reactors. Int. J. Chem. Kinet. 2019, 51, 918–930. [Google Scholar] [CrossRef]
- Dubbe, H.; Eigenberger, G.; Nieken, U. Hysteresis Phenomena on Pt- and Pd-Diesel Oxidation Catalysts: Experimental Observations. Top. Catal. 2016, 59, 1054–1058. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O. The attractiveness of the ternary Rh-Pd-Pt alloys for CO oxidation process. Processes 2020, 8, 928. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O.; Volodin, A.M. Prospect of using nanoalloys of partly miscible rhodium and palladium in three-way catalysis. Top. Catal. 2019, 62, 305–314. [Google Scholar] [CrossRef]
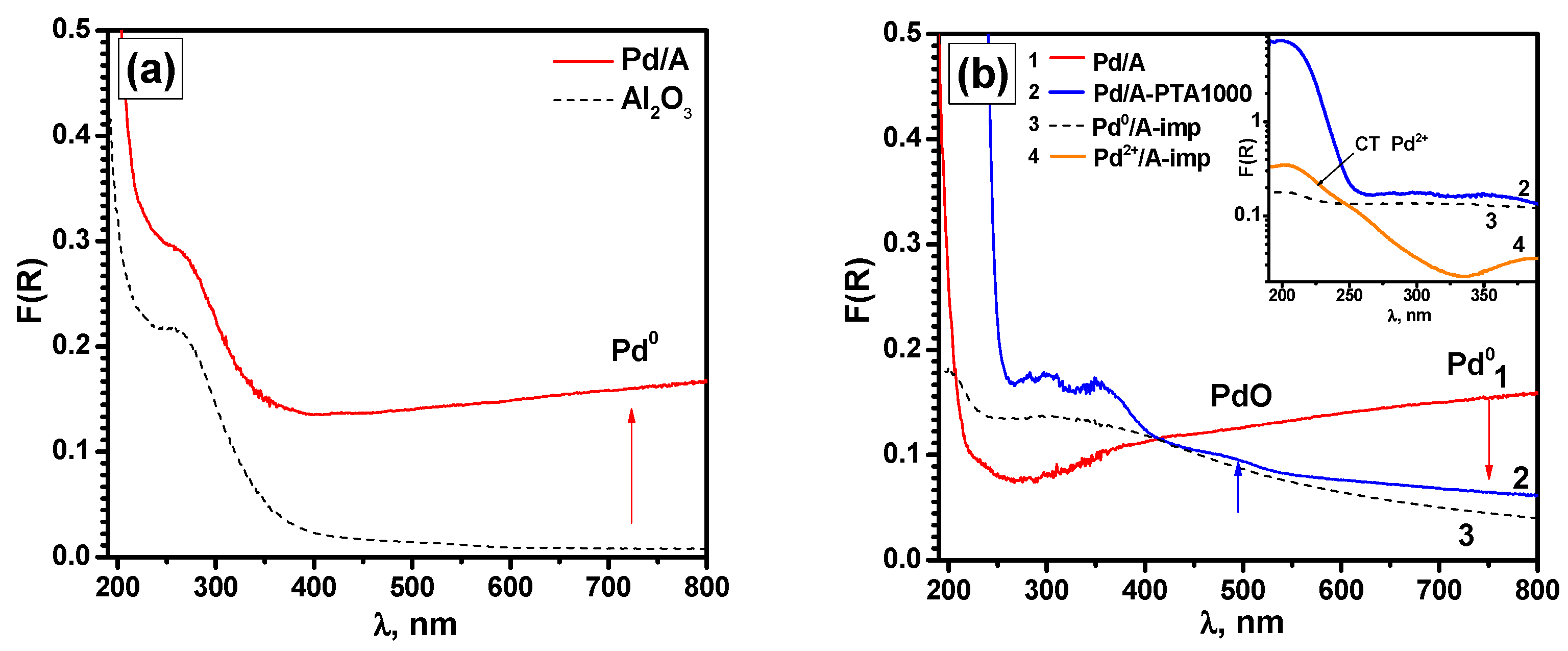

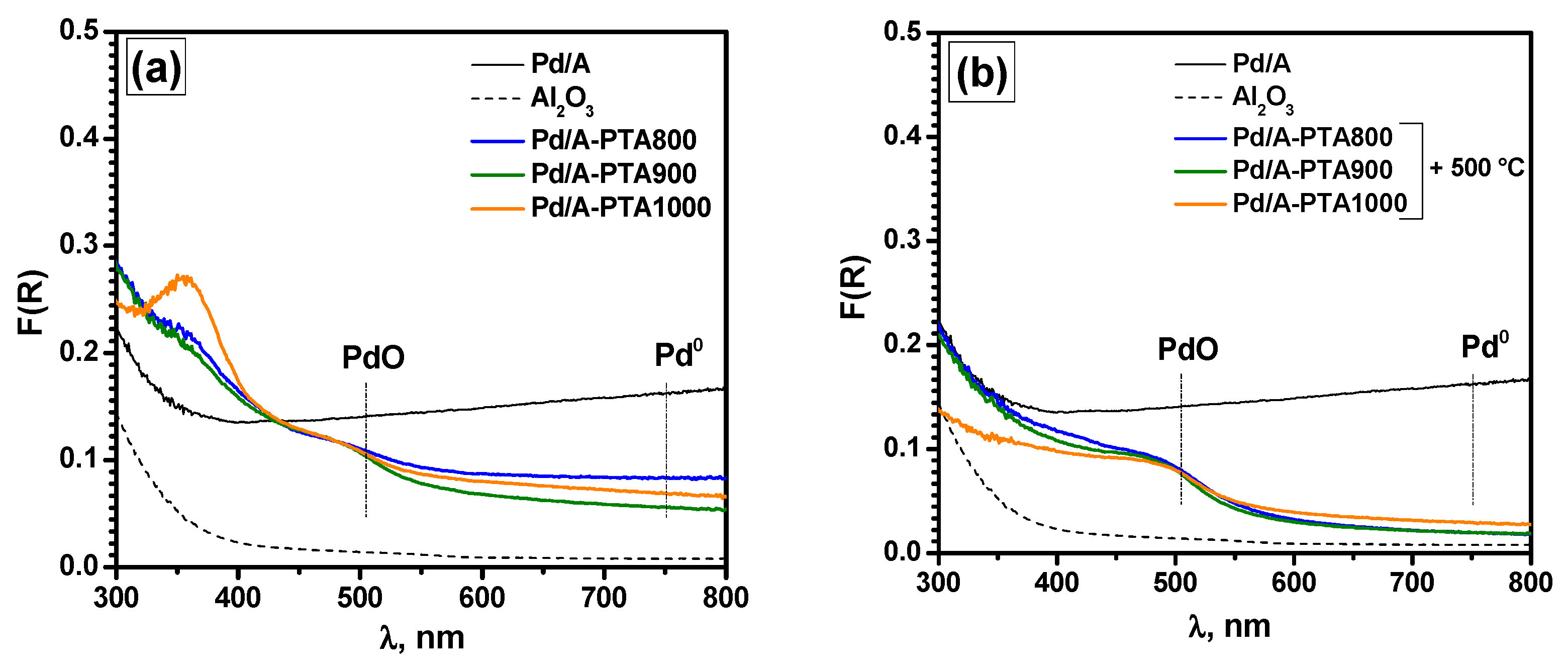
| Run Number | Reaction Conditions | |||
|---|---|---|---|---|
| Model Mixture | Real Mixture | |||
| Heating | Cooling | Heating | Cooling | |
| 1 | 216 | 212 | 259 | 240 |
| 2 | 235 | 220 | 258 | 240 |
| 3 | 240 | 218, 247 | 261 | 248 |
| 4 | 231 | 219, 257 | 268 | 252 |
| 5 | 236 | 232, 274 | 268 | 253 |
| 6 | 255 | 232, 275 | 278 | 258 |
| 7 | 255 | 227, 251 | 280 | 257 |
| 8 | 249 | 228, 257 | 276 | 260 |
| 9 | 257 | 225, 245 | 286 | 258 |
| 10 | 258 | 225, 247 | 283 | 259 |
| 11 | 263 | 282 | 282 | 258 |
| Sample | Run Number | Ref. | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 11 | ||
| 0.028 wt.% Pd/Al2O3 | 257 | 259 | 270 | 277 | 283 | 278 | This work |
| 0.21 wt.% Pd/Al2O3 | 160 | 238 | 258 | 268 | 269 | 278 | [74] |
| 0.21 wt.% Pd/Al2O3 + La2O3 * | 164 | 200 | 231 | 232 | - | - | [90] |
| 0.3 wt.% Pd/Al2O3 * | 229 | 265 | 278 | 278 | - | - | [91] |
| 0.24 wt.% Pd/δ-Al2O3 * | - | 260 | 269 | 274 | - | - | [56] |
| 0.12 wt.% Pd/Al2O3 * | 243 | 261 | 272 | 274 | - | - | [15] |
| Sample | SSA, m2/g | Vtotal, cm3/g | Dav, nm | Ref. |
|---|---|---|---|---|
| Al2O3 | 167 | 0.53 | 11 | [74] |
| Pd/A (as-prepared) | 191 | 0.55 | 11 | [74] |
| Pd/A-PTA800 | 187 | 0.56 | 12 | This work |
| Pd/A-PTA900 | 173 | 0.55 | 12 | This work |
| Pd/A-PTA1000 | 145 | 0.49 | 13 | This work |
| Sample | [Pd]/[Al] | BE (Pd3d5/2), eV | ν (Pd), % | ||||
|---|---|---|---|---|---|---|---|
| Pd0 | PdO | Pd2+ | Pd0 | PdO | Pd2+ | ||
| Pd/A (as-prepared) | 4.65 | 335.7 | 336.9 | 338.3 | 92 | 0 | 8 |
| Pd/A-PTA800 | 0.45 | 335.3 | 336.8 | 338.2 | 66 | 25 | 9 |
| Pd/A-PTA1000 | 0.11 | 335.3 | 336.8 | 338.2 | 47 | 48 | 5 |
| Sample | F(R) at 750 nm | Eg, eV | |
|---|---|---|---|
| PTA | PTA + 500 °C | ||
| Pd/A-PTA800 | 0.103 | 0.020 | 2.25 |
| Pd/A-PTA900 | 0.056 | 0.020 | 2.28 |
| Pd/A-PTA1000 | 0.069 | 0.029 | 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostovshchikova, T.N.; Shilina, M.I.; Gurevich, S.A.; Yavsin, D.A.; Veselov, G.B.; Stoyanovskii, V.O.; Vedyagin, A.A. Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion. Materials 2023, 16, 3501. https://doi.org/10.3390/ma16093501
Rostovshchikova TN, Shilina MI, Gurevich SA, Yavsin DA, Veselov GB, Stoyanovskii VO, Vedyagin AA. Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion. Materials. 2023; 16(9):3501. https://doi.org/10.3390/ma16093501
Chicago/Turabian StyleRostovshchikova, Tatiana N., Marina I. Shilina, Sergey A. Gurevich, Denis A. Yavsin, Grigory B. Veselov, Vladimir O. Stoyanovskii, and Aleksey A. Vedyagin. 2023. "Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion" Materials 16, no. 9: 3501. https://doi.org/10.3390/ma16093501
APA StyleRostovshchikova, T. N., Shilina, M. I., Gurevich, S. A., Yavsin, D. A., Veselov, G. B., Stoyanovskii, V. O., & Vedyagin, A. A. (2023). Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion. Materials, 16(9), 3501. https://doi.org/10.3390/ma16093501







