Influence of Disinfection Methods on Cinematographic Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Materials
2.2. Disinfection Methods
2.2.1. Butanol Vapours
2.2.2. Bacillol® AF
2.2.3. Septonex®
2.2.4. Ethylene Oxide
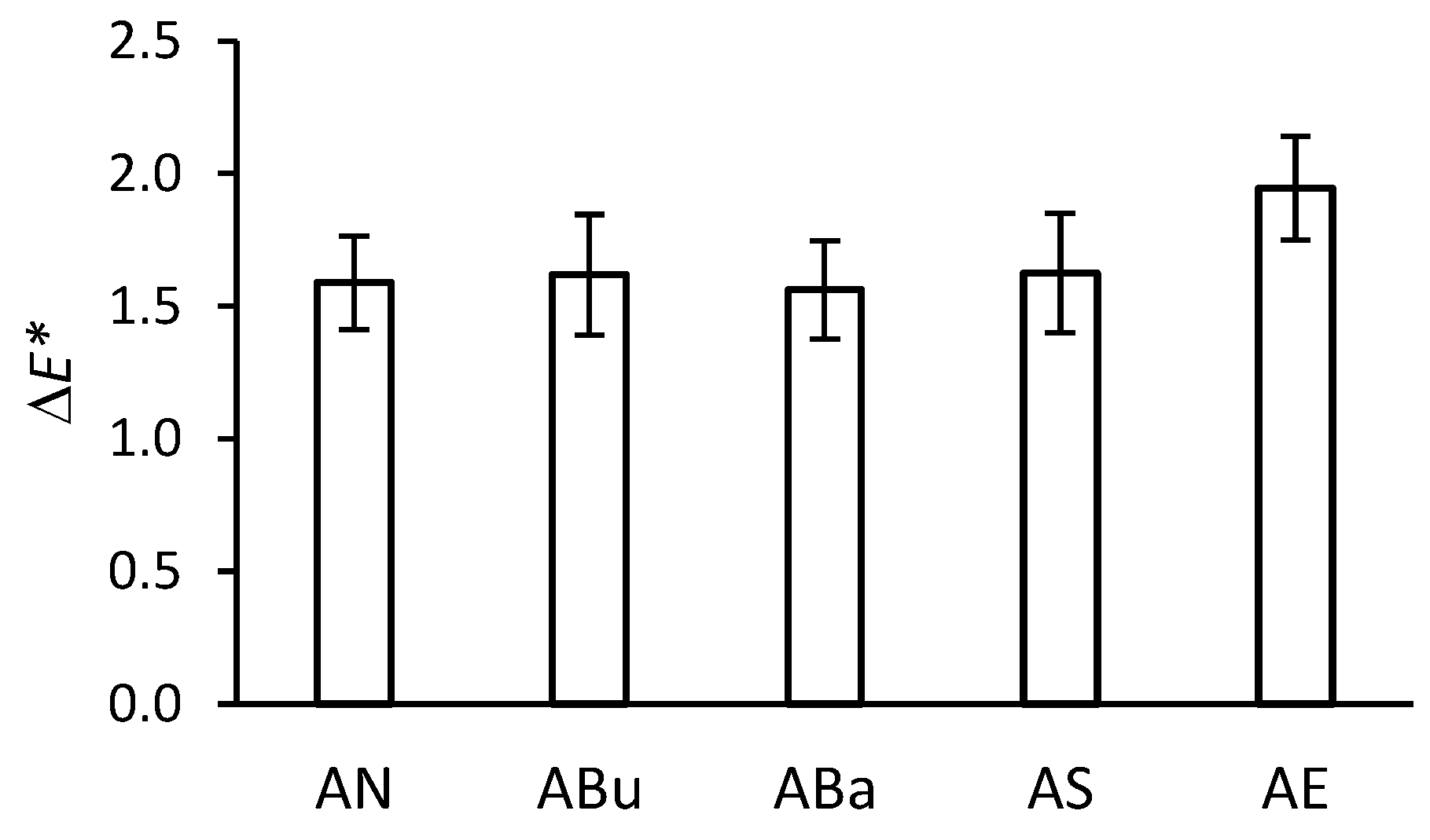
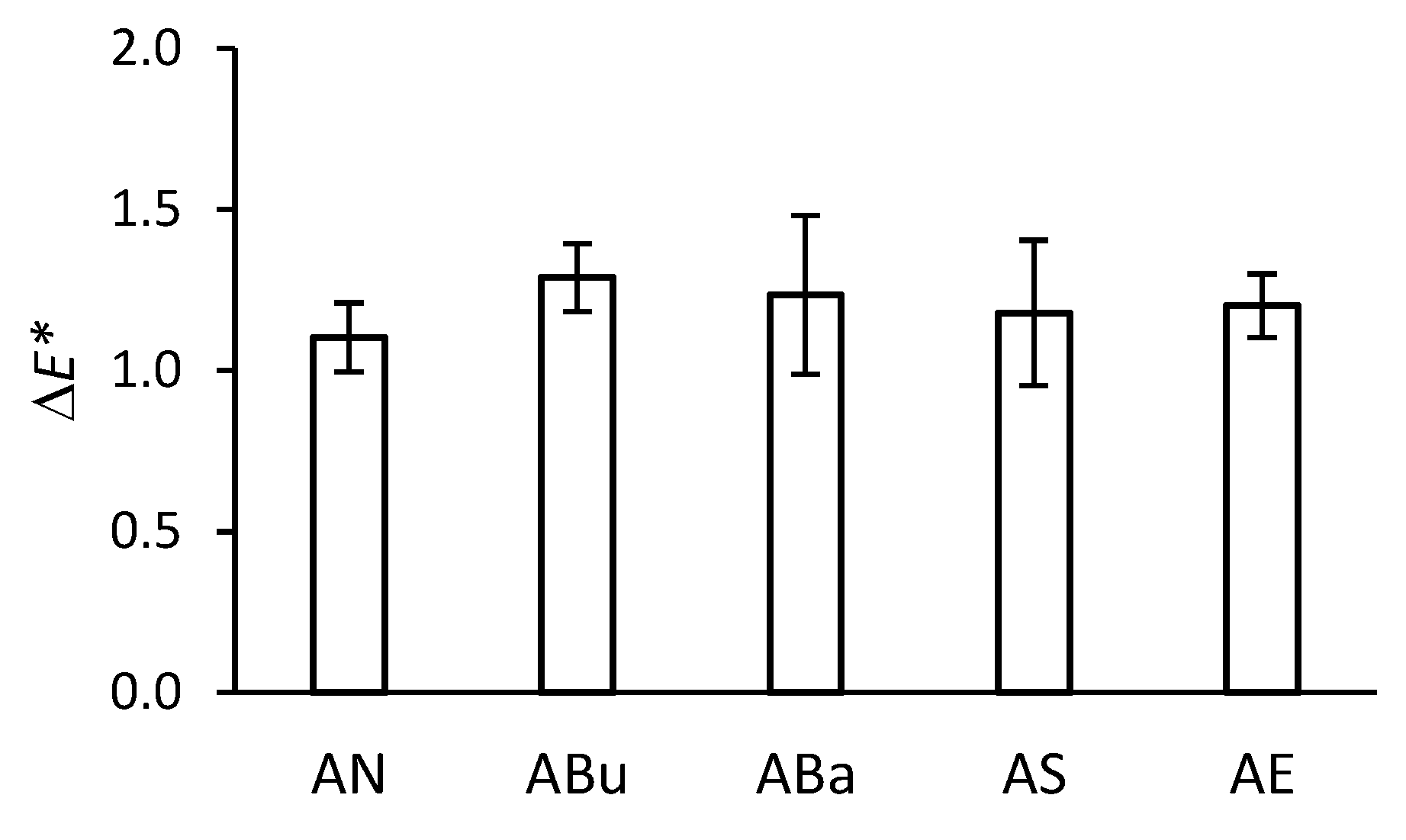
2.3. Colorimetry
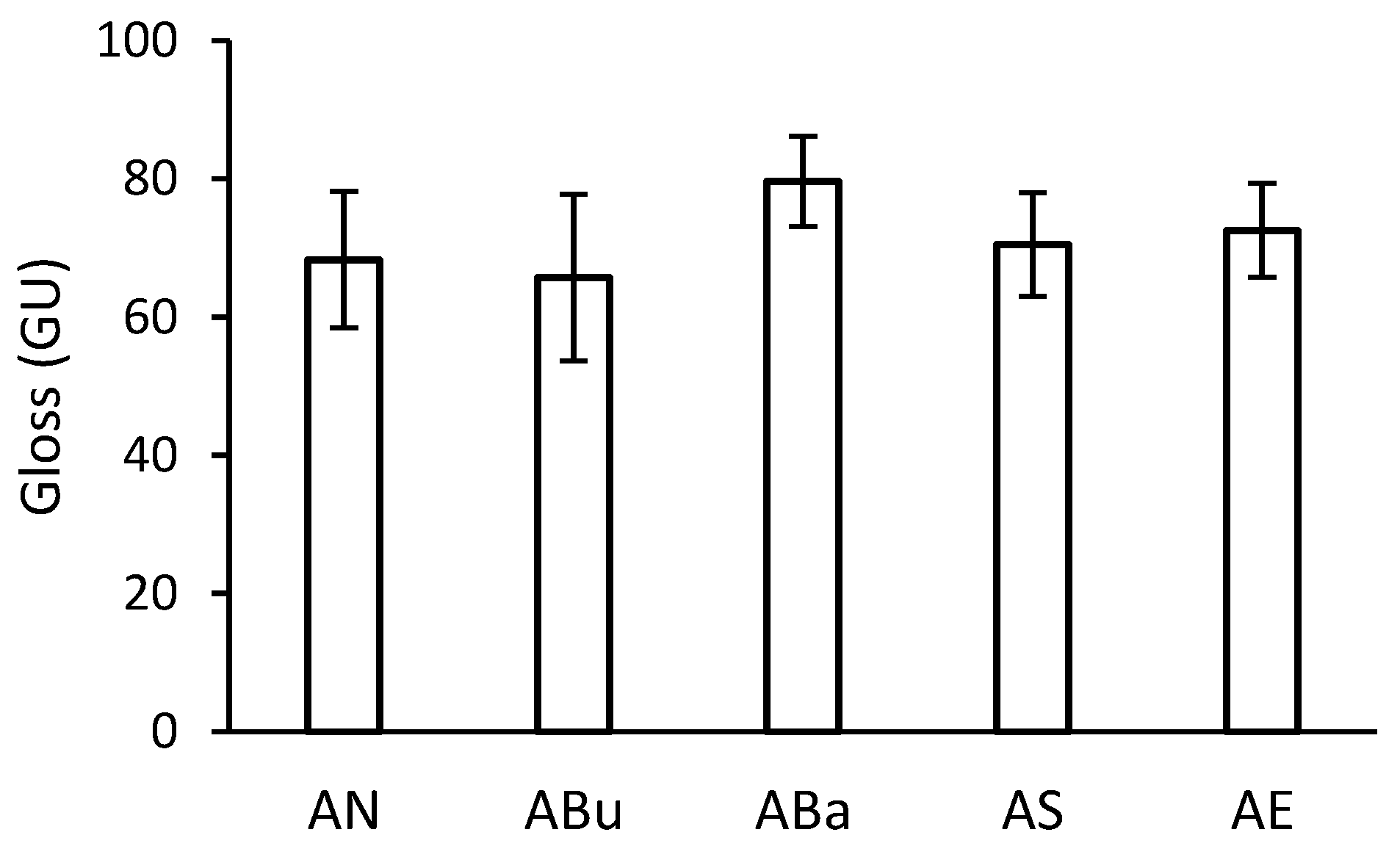
2.4. Gloss Measurement
2.5. Mechanical Properties
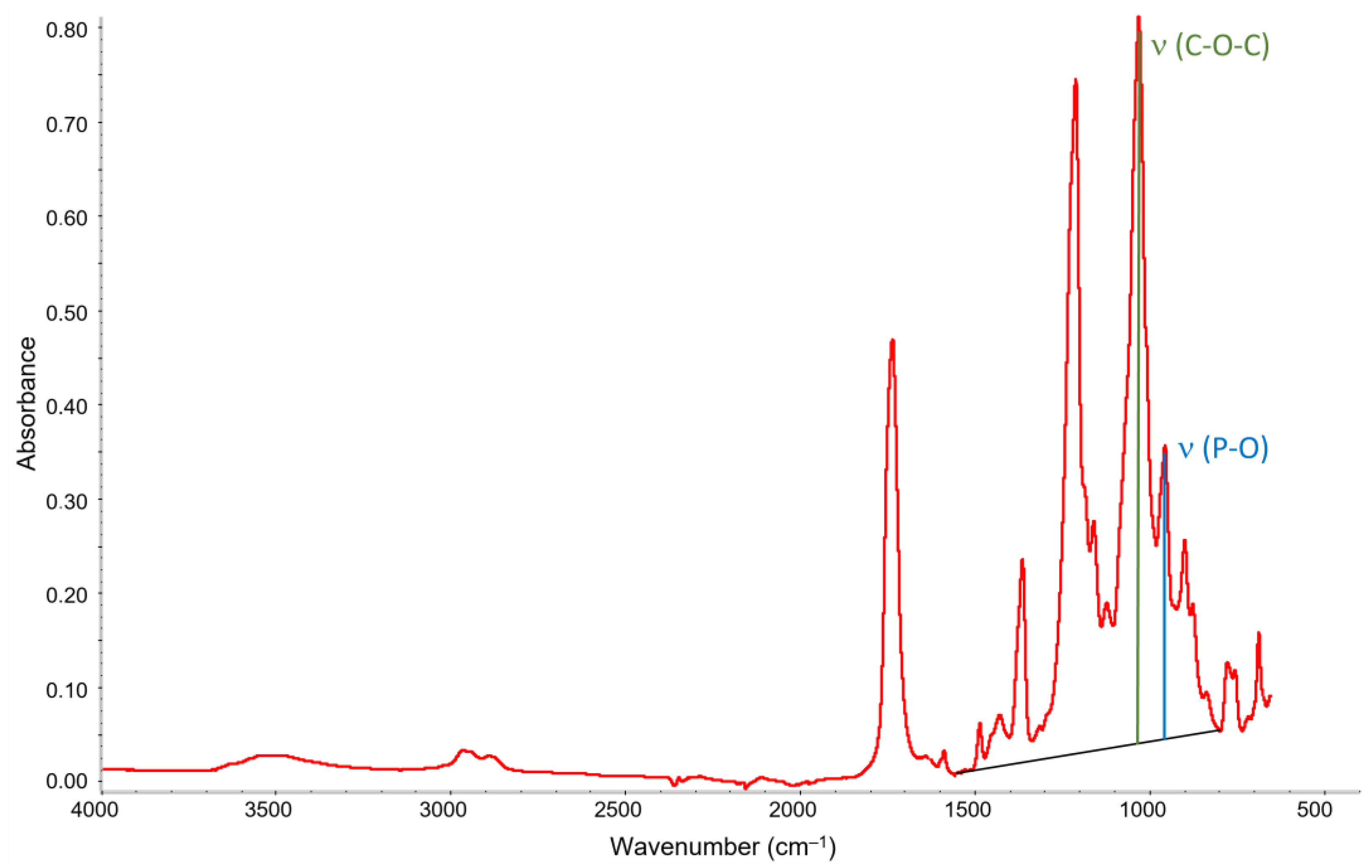
2.6. Attenuated Total Reflectance-Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
2.7. NMR Spectroscopy
2.7.1. Preparation of Samples for NMR Analyses
2.7.2. Measurement and Processing of Quantitative 1H NMR Spectra (qNMR)
2.7.3. Identification of Glucose Units
2.7.4. Estimation of Average Degree of Substitution
2.7.5. Triphenyl Phosphate Quantification
2.8. Intrinsic Viscosity
3. Results and Discussion
3.1. Colorimetry
3.2. Gloss Measurement
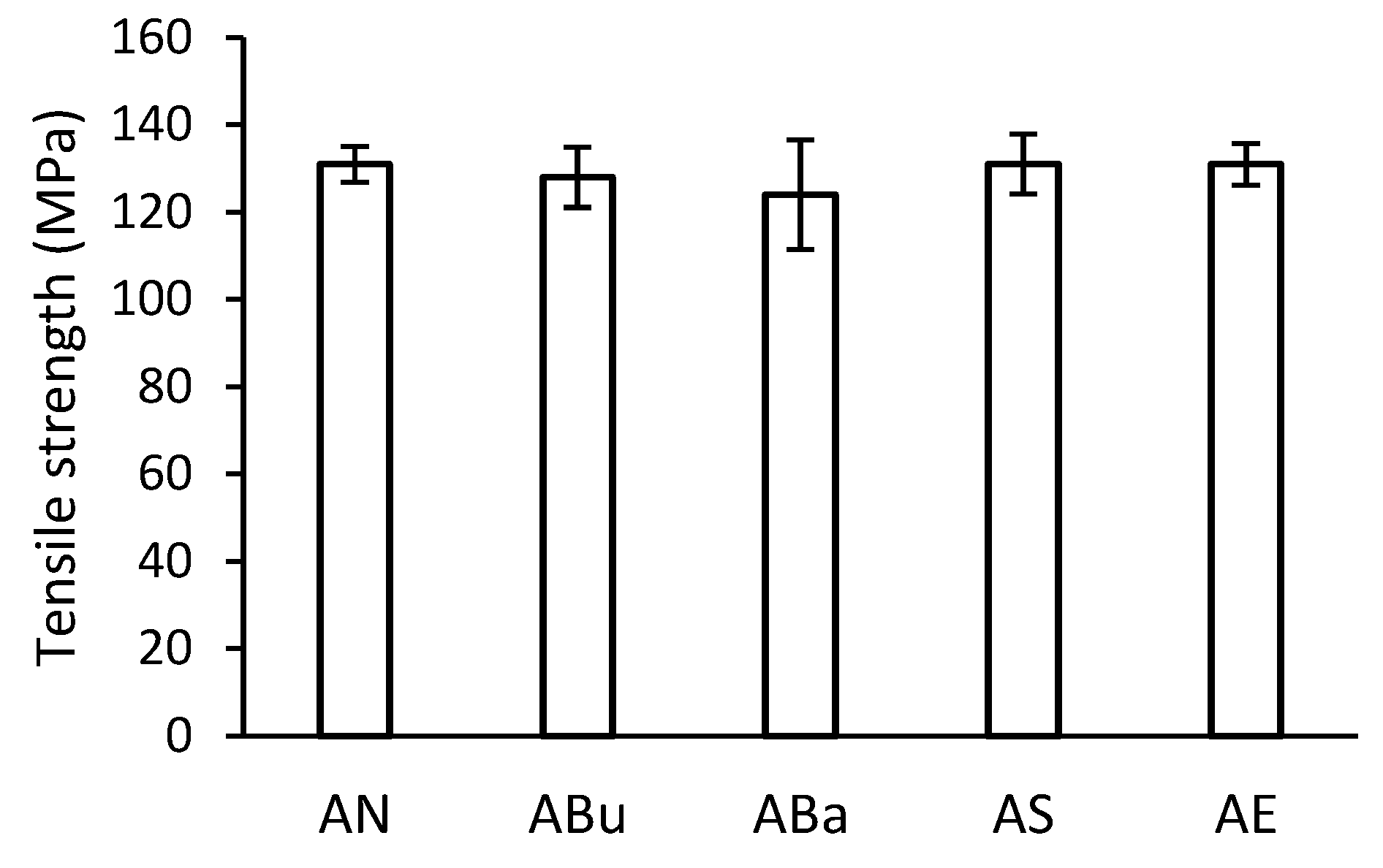
3.3. Mechanical Properties
3.4. ATR-FTIR Spectroscopy
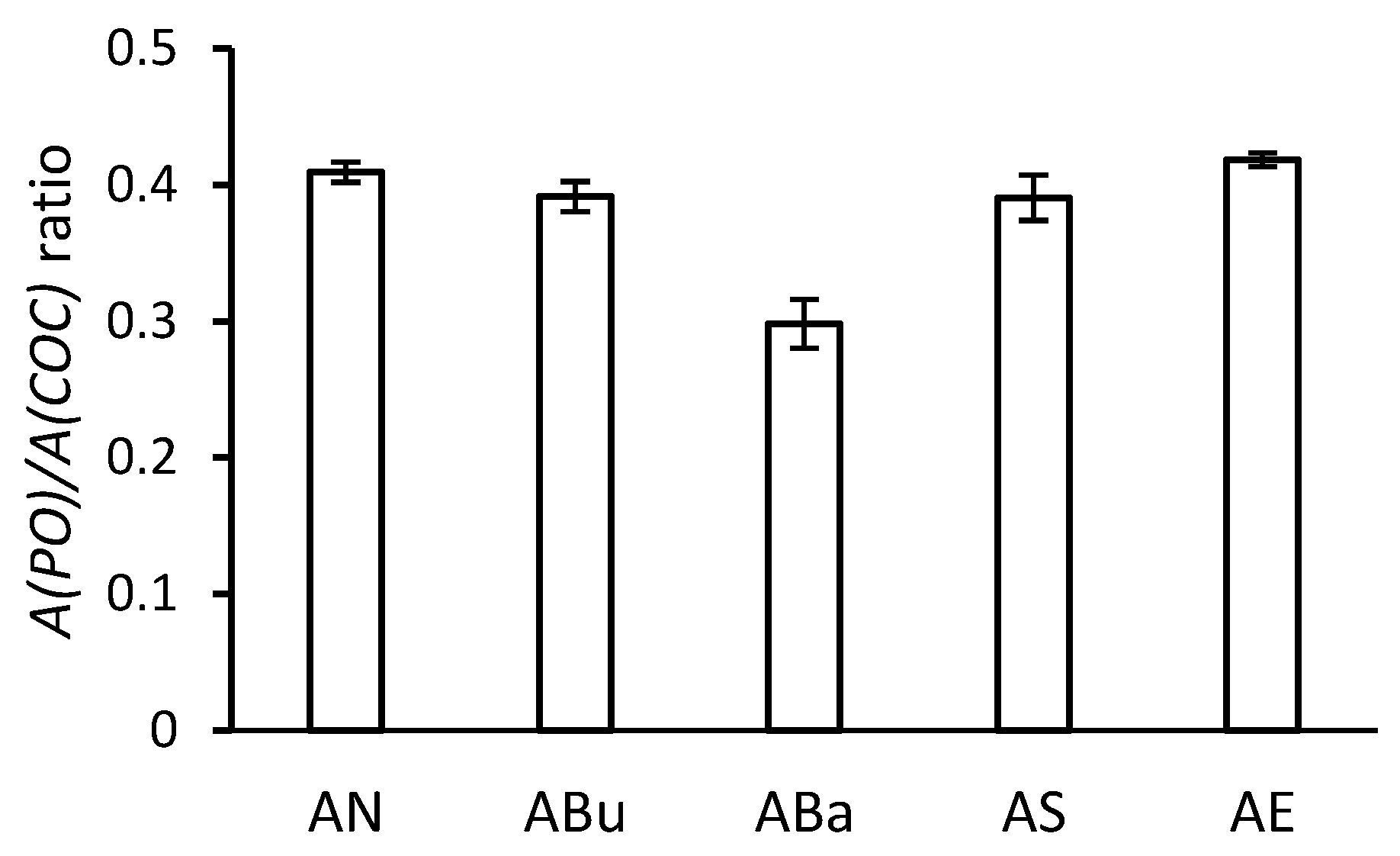
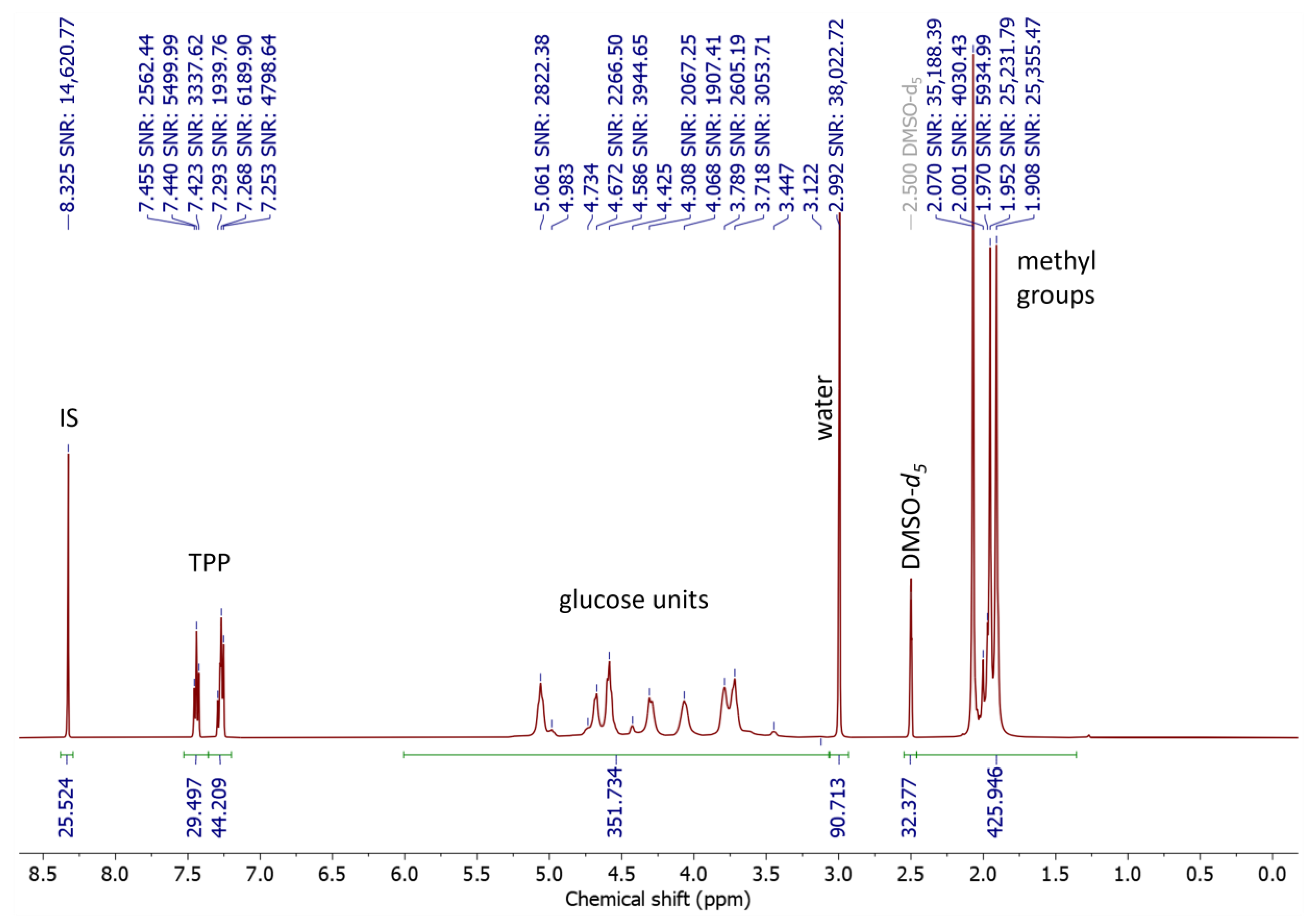
3.5. NMR Spectroscopy
3.6. Intrinsic Viscosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reilly, J.A. Celluloid Objects: Their Chemistry and Preservation. J. Am. Inst. Conserv. 1991, 30, 145–162. [Google Scholar] [CrossRef]
- Nunes, S.; Ramacciotti, F.; Neves, A.; Angelin, E.M.; Ramos, A.M.; Roldão, É.; Wallaszkovits, N.; Armijo, A.A.; Melo, M.J. A diagnostic tool for assessing the conservation condition of cellulose nitrate and acetate in heritage collections: Quantifying the degree of substitution by infrared spectroscopy. Herit. Sci. 2020, 8, 33. [Google Scholar] [CrossRef]
- Read, P.; Meyer, M.-P. Restoration of Motion Picture Film, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Abrusci, C.; Martín-González, A.; Del Amo, A.; Catalina, F.; Collado, J.; Platas, G. Isolation and identification of bacteria and fungi from cinematographic films. Int. Biodeterior. Biodegrad. 2005, 56, 58–68. [Google Scholar] [CrossRef]
- Schilling, M.; Bouchard, M.; Khanjian, H.; Learner, T.; Phenix, A.; Rivenc, R. Application of Chemical and Thermal Analysis Methods for Studying Cellulose Ester Plastics. Acc. Chem. Res. 2010, 43, 888–896. [Google Scholar] [CrossRef]
- Bingley, G.; Verran, J. Counts of fungal spores released during inspection of mouldy cinematographic film and determination of the gelatinolytic activity of predominant isolates. Int. Biodeterior. Biodegrad. 2013, 84, 381–387. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Vilmont, L.B.; Lavédrine, B. A methodology for detecting the level of fungal contamination in the French Film Archives vaults. J. Cult. Herit. 2016, 19, 454–462. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Gardner, R.M.; Komarek, R.J. Aerobic biodegradation of cellulose acetate. J. Appl. Polym. Sci. 1993, 47, 1709–1719. [Google Scholar] [CrossRef]
- Sakai, K.; Yamauchi, T.; Nakasu, F.; Ohe, T. Biodegradation of cellulose acetate by neisseria sicca. Biosci. Biotechnol. Biochem. 1996, 60, 1617–1622. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Jewitt, T.S.; Horie, C.V. Fundamental aspects of the degradation of cellulose triacetate base cinematograph film. Polym. Degrad. Stab. 1989, 25, 345–362. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Drábková, K.; Krejčí, J.; Škrdlantová, M.; Ďurovič, M.; Bacílková, B. Influence of Disinfectants on Natural Textile Fibres. Restaurator 2021, 42, 67–86. [Google Scholar] [CrossRef]
- Purkrtová, S.; Savická, D.; Kadavá, J.; Sýkorová, H.; Kováčova, N.; Kališová, D.; Nešporová, T.; Novaková, M.; Mašek Benetková, B.; Koukalová, L.; et al. Microbial Contamination of Photographic and Cinematographic Materials in Archival Funds in the Czech Republic. Microorganisms 2022, 10, 155. [Google Scholar] [CrossRef]
- Bacílková, B. Study on the Effect of Butanol Vapours and other Alcohols on Fungi. Restaurator 2006, 27, 186–199. [Google Scholar] [CrossRef]
- Zervos, S.; Alexopoulou, I. Paper conservation methods: A literature review. Cellulose 2015, 22, 2859–2897. [Google Scholar] [CrossRef]
- Stickley, F.L. The Biodegradation of Gelatin and its Problems in the Photographic Industry. J. Photogr. Sci. 1986, 34, 111–112. [Google Scholar] [CrossRef]
- Shintani, H. Ethylene Oxide Gas Sterilization of Medical Devices. Biocontrol Sci. 2017, 22, 1–16. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the fungus Aspergillus a threat to cultural heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Branyšová, T.; Teplá, B.; Demnerová, K.; Stiborová, H.; Ďurovič, M. Biodeterioration of audio-visual materials. Chem. Listy 2021, 115, 260–265. [Google Scholar]
- Tepla, B.; Demnerova, K.; Stiborova, H. History and microbial biodeterioration of audiovisual materials. J. Cult. Herit. 2020, 44, 218–228. [Google Scholar] [CrossRef]
- Vivar, I.; Borrego, S.F.; García, A.M.; Moreno, D.A. Microscopic techniques in the determination of the biodeterioration in cinematographic films. Acta Microsc. 2018, 27, 63–68. [Google Scholar]
- Vivar, I.; Borrego, S.; Ellis, G.; Moreno, D.A.; García, A.M. Fungal biodeterioration of color cinematographic films of the cultural heritage of cuba. Int. Biodeterior. Biodegrad. 2013, 84, 372–380. [Google Scholar] [CrossRef]
- Abrusci, C.; Marquina, D.; Del Amo, A.; Catalina, F. Biodegradation of cinematographic gelatin emulsion by bacteria and filamentous fungi using indirect impedance technique. Int. Biodeterior. Biodegrad. 2007, 60, 137–143. [Google Scholar] [CrossRef]
- Abrusci, C.; Marquina, D.; Santos, A.; Del Amo, A.; Corrales, T.; Catalina, F. Biodeterioration of cinematographic cellulose triacetate by Sphingomonas paucimobilis using indirect impedance and chemiluminescence techniques. Int. Biodeterior. Biodegrad. 2009, 63, 759–764. [Google Scholar] [CrossRef]
- Tisch, T. Cleaning Solutions for a Clean Environment: Developments in Motion-Picture Film-Cleaning Technology. SMPTE J. 1995, 104, 528–533. [Google Scholar] [CrossRef]
- Czerwińska, E.; Kowalik, R. Microbiodeterioration of Audiovisual Collections. Restaurator 1979, 3, 63–80. [Google Scholar] [CrossRef]
- Nagai, M.L.E.; de Souza Santos, P.; Otubo, L.; Oliveira, M.J.A.; Vasquez, P.A.S. Gamma and electron beam irradiation effects for conservation treatment of cellulose triacetate photographic and cinematographic films. Radiat. Phys. Chem. 2021, 182, 109395. [Google Scholar] [CrossRef]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization-Types, Action, and Resistance, 2nd ed.; American Society for Microbiology (ASM): Washington, DC, USA, 2017. [Google Scholar]
- Carter, E.C.; Ohno, Y.; Pointer, M.R.; Robertson, A.R.; Seve, R.; Schanda, J.D.; Witt, K. CIE Technical Report-Colorimetry, 3rd ed.; CIE Central Bureau: Vienna, Austria, 2004; Volume 15, pp. 17–18. [Google Scholar]
- Kemper, B.; Lichtblau, D.A. Extraction of plasticizers: An entire and reproducible quantification method for historical cellulose acetate material. Polym. Test. 2019, 80, 106096. [Google Scholar] [CrossRef]
- Schoenberger, T. Guideline for qNMR Analysis. Available online: http://enfsi.eu/wp-content/uploads/2017/06/qNMR-Guideline_version001.pdf (accessed on 30 August 2022).
- Da Ros, S.; Aliev, A.E.; del Gaudio, I.; King, R.; Pokorska, A.; Kearney, M.; Curran, K. Characterising plasticised cellulose acetate-based historic artefacts by NMR spectroscopy: A new approach for quantifying the degree of substitution and diethyl phthalate contents. Polym. Degrad. Stab. 2021, 183, 109420. [Google Scholar] [CrossRef]
- Riley, J.L. The Baker-Philippoff equation for cellulose acetate solutions. J. Polym. Sci. 1946, 1, 434–436. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Rodriguez, M.; Liauw, C.M.; Fontan, E. Aspects of the thermal oxidation, yellowing and stabilisation of ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2000, 71, 1–14. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A.C.; Kentridge, R.W. The perception of gloss: A review. Vis. Res. 2015, 109, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Kono, H.; Hashimoto, H.; Shimizu, Y. NMR characterization of cellulose acetate: Chemical shift assignments, substituent effects, and chemical shift additivity. Carbohydr. Polym. 2015, 118, 91–100. [Google Scholar] [CrossRef]
- Hikichi, K.; Kakuta, Y.; Katoh, T. 1H NMR Study on Substituent Distribution of Cellulose Diacetate. Polym. J. 1995, 27, 659–663. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation markers and plasticizer loss of cellulose acetate films during ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Deng, W.; Liu, M.; Zhang, Q.; Wang, Y. Direct transformation of cellulose into methyl and ethyl glucosides in methanol and ethanol media catalyzed by heteropolyacids. Catal. Today 2011, 164, 461–466. [Google Scholar] [CrossRef]
- Sequeira, S.; Cabrita, E.J.; Macedo, M.F. Antifungals on paper conservation: An overview. Int. Biodeterior. Biodegrad. 2012, 74, 67–86. [Google Scholar] [CrossRef]


| Disinfection Method | Without Disinfection | Butanol Vapours | Bacillol® AF | Septonex® | Ethylene Oxide |
|---|---|---|---|---|---|
| Label | AN | ABu | ABa | AS | AE |
| AN | ABu | ABa | AS | AE | |
|---|---|---|---|---|---|
| DS | 2.85 | 2.84 | 2.85 | 2.85 | 2.84 |
| TPP content (%) | 10.50 ± 0.03 | 10.41 ± 0.03 | 9.49 ± 0.02 | 10.52 ± 0.01 | 10.54 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knotek, V.; Ďurovič, M.; Dolenský, B.; Hrdlička, Z. Influence of Disinfection Methods on Cinematographic Film. Materials 2023, 16, 3493. https://doi.org/10.3390/ma16093493
Knotek V, Ďurovič M, Dolenský B, Hrdlička Z. Influence of Disinfection Methods on Cinematographic Film. Materials. 2023; 16(9):3493. https://doi.org/10.3390/ma16093493
Chicago/Turabian StyleKnotek, Vítězslav, Michal Ďurovič, Bohumil Dolenský, and Zdeněk Hrdlička. 2023. "Influence of Disinfection Methods on Cinematographic Film" Materials 16, no. 9: 3493. https://doi.org/10.3390/ma16093493
APA StyleKnotek, V., Ďurovič, M., Dolenský, B., & Hrdlička, Z. (2023). Influence of Disinfection Methods on Cinematographic Film. Materials, 16(9), 3493. https://doi.org/10.3390/ma16093493







