Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Methods
3. Results and Discussion
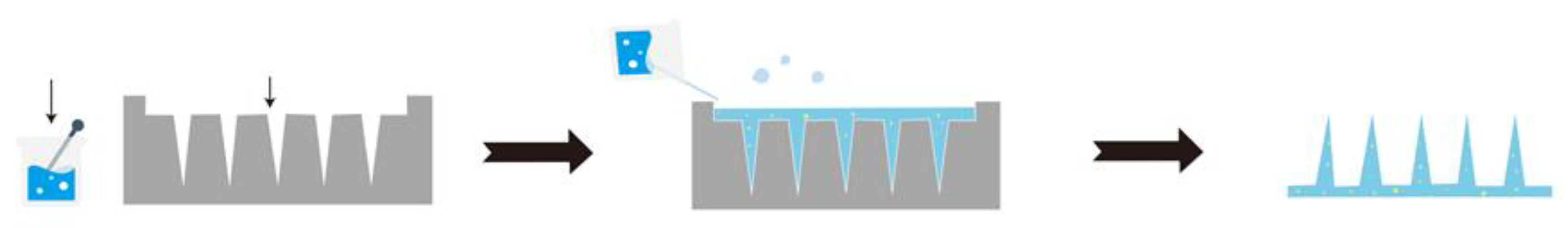
3.1. Preparation of MNs
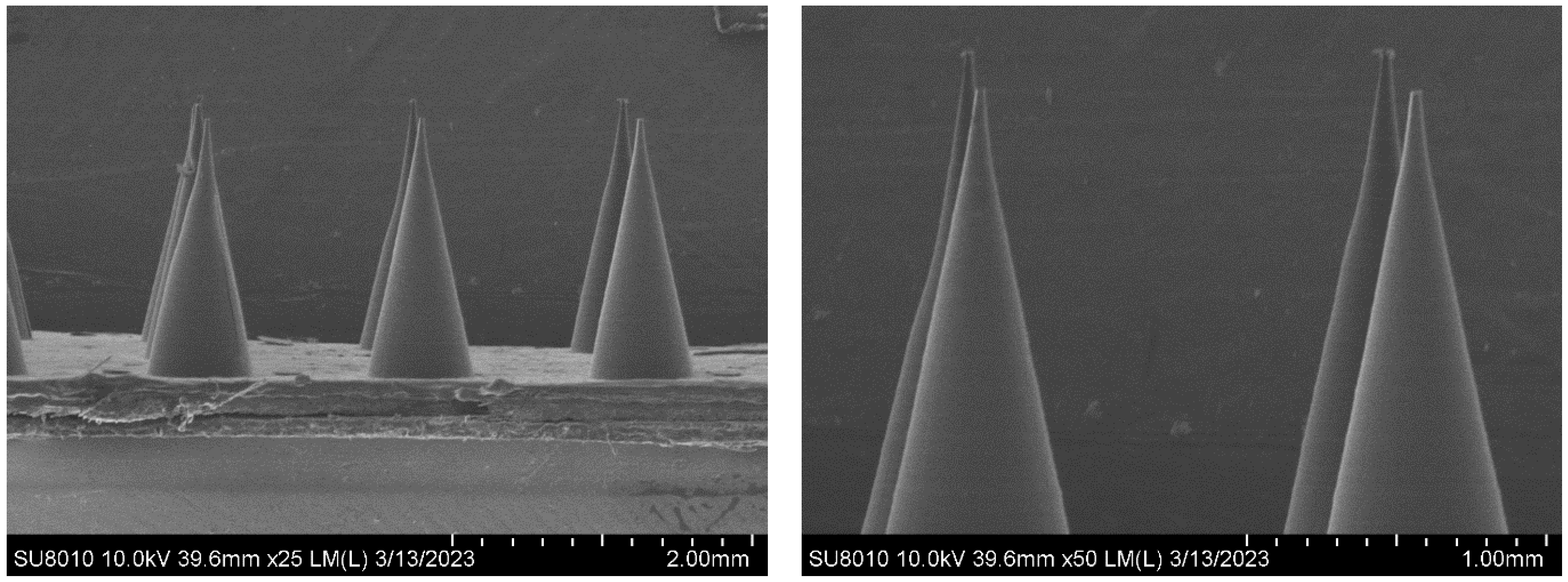
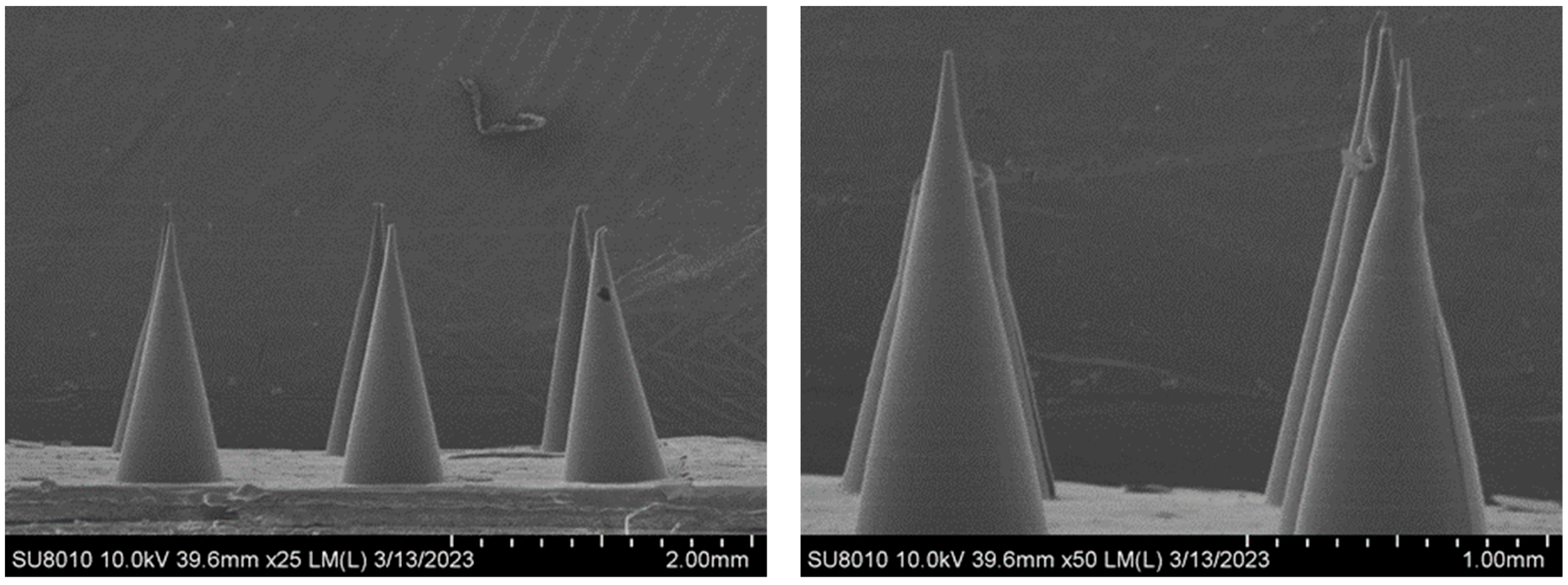
3.2. Morphology of the MNs
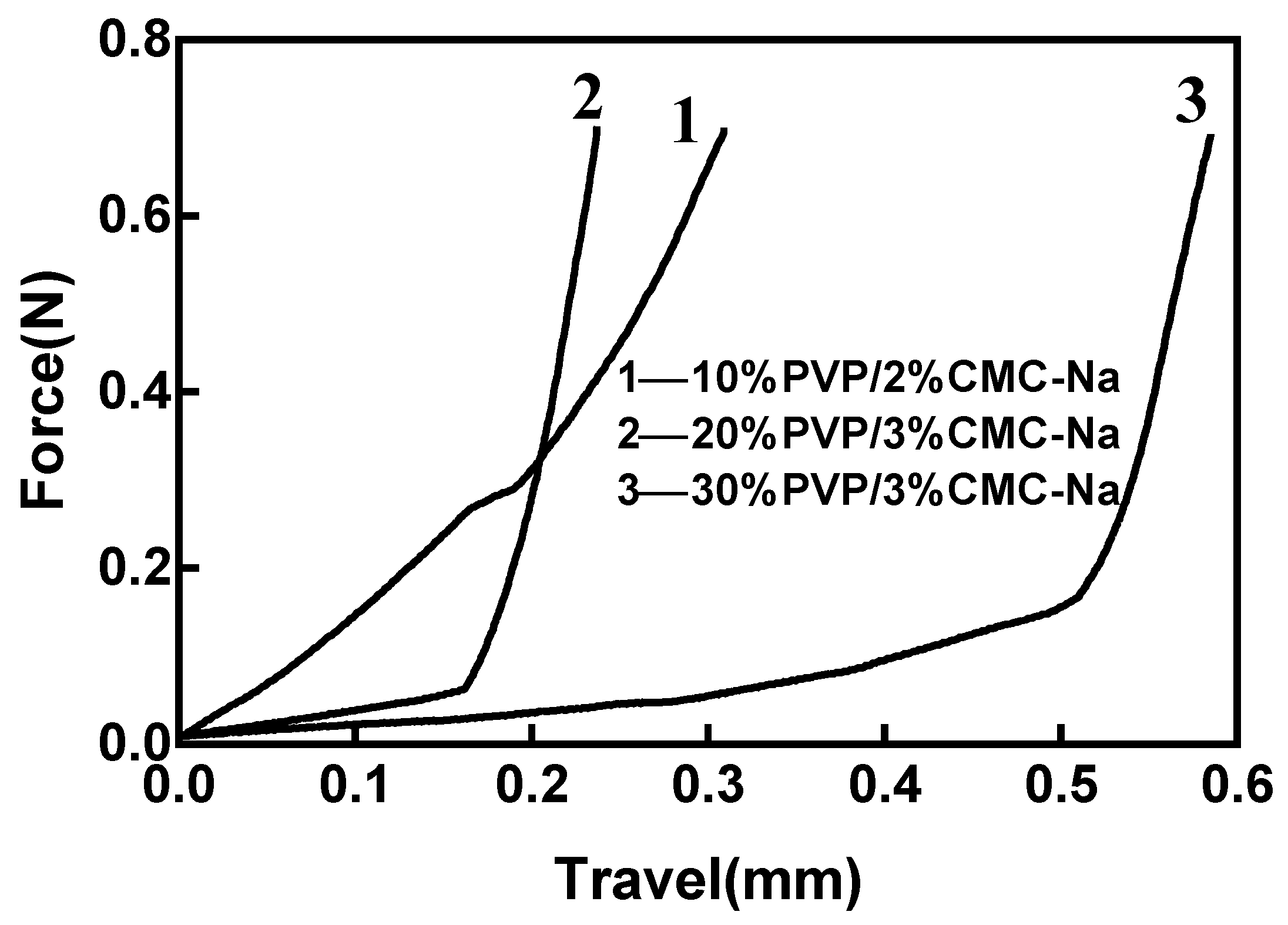
3.3. Mechanical Properties of MNs
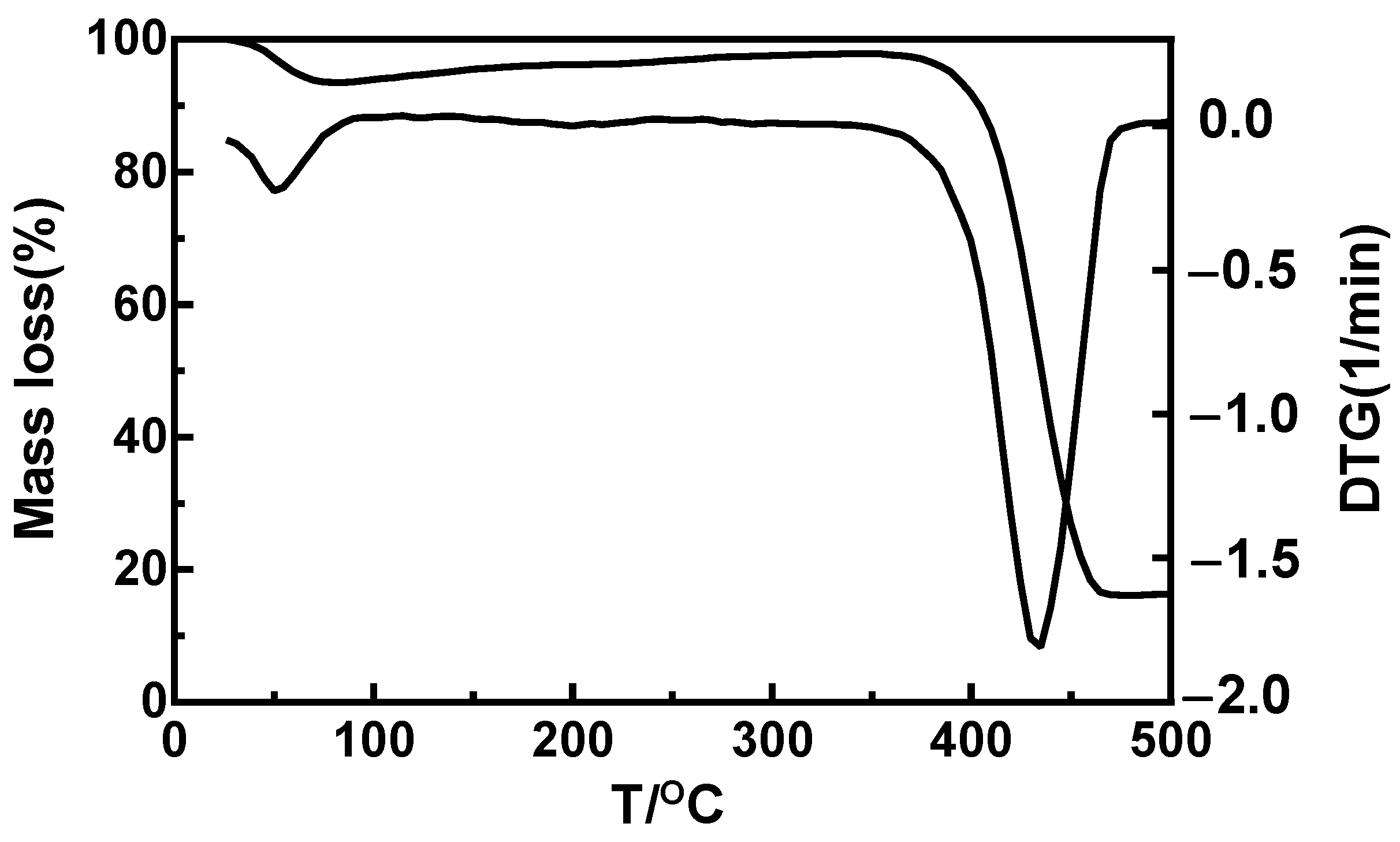
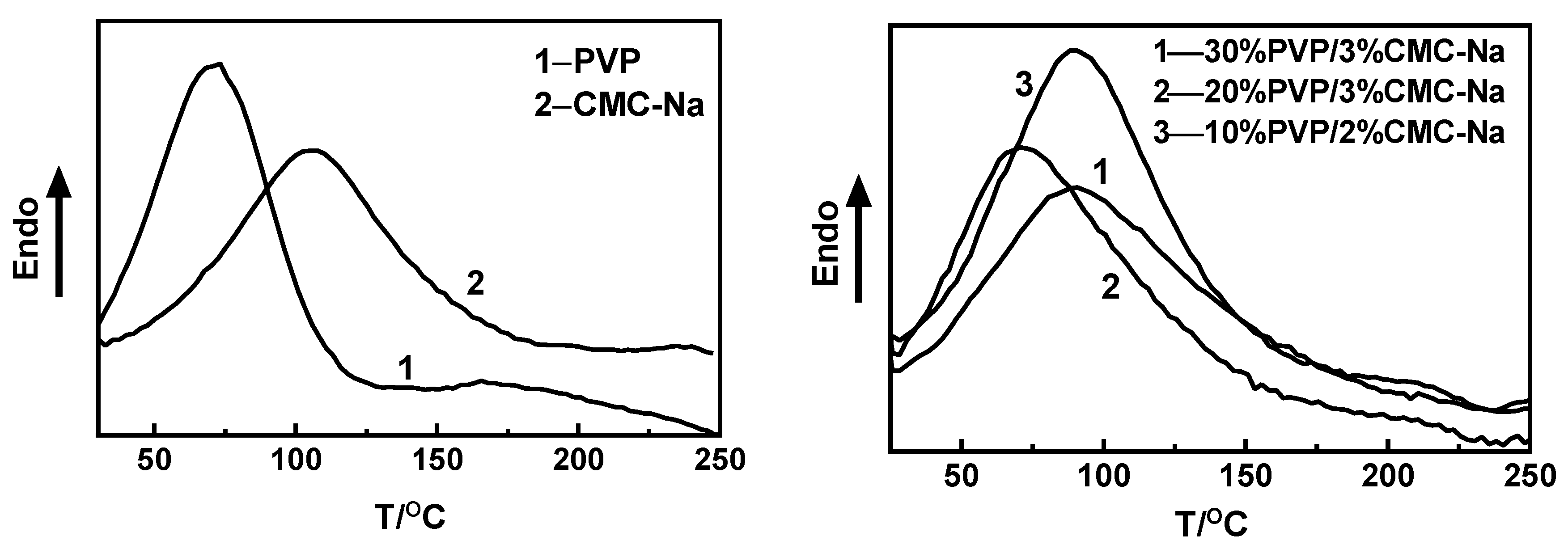
3.4. Thermodynamic Properties of MNs
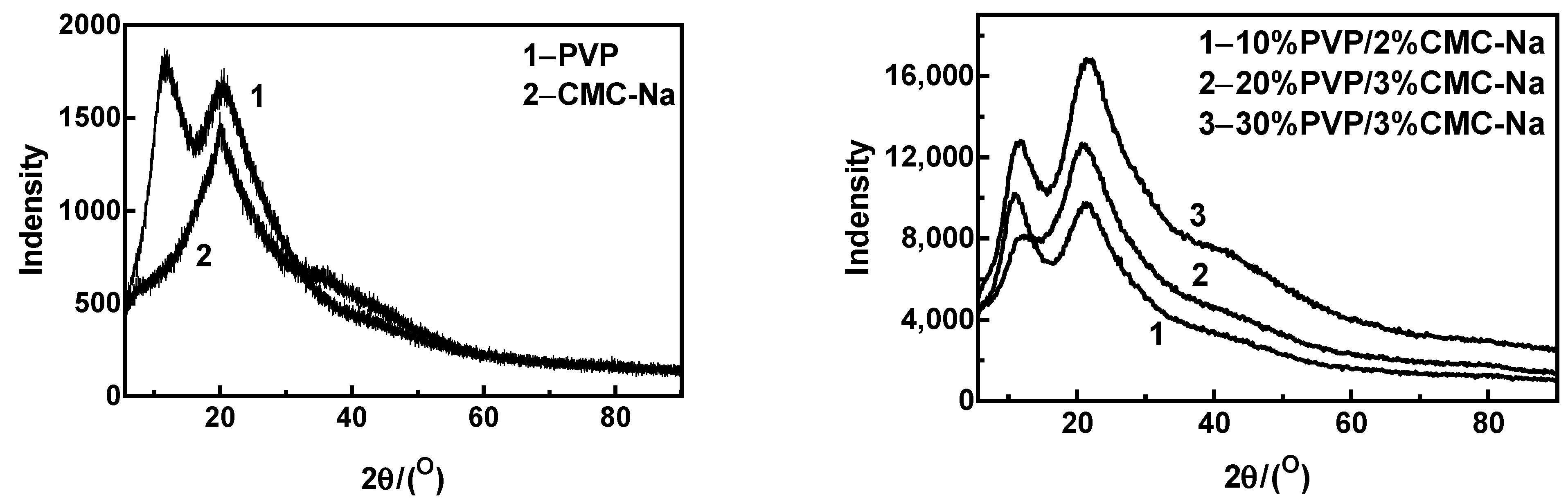
3.5. X-ray Diffractometry of MNs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Priya, S.; Singhvi, G. Microneedles-based drug delivery strategies: A breakthrough approach for the management of pain. Biomed. Pharmacother. 2022, 155, 113717. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.J.; Permana, A.D.; Volpe-Zanutto, F.; Amir, M.N.; Vora, L.K.; Tekko, I.A.; Akhavein, N.; Weber, A.D.; Larrañeta, E.; Donnelly, R.F. Ring inserts as a useful strategy to prepare tip-loaded microneedles for long-acting drug delivery with application in HIV pre-exposure prophylaxis. Mater. Des. 2022, 224, 111416. [Google Scholar] [CrossRef]
- Ahmed Saeed AL-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A microneedle patch for measles and rubella vaccination: A game changer for achieving elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Vos, P.J.; Saelens, X.; van der Maaden, K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses. Eur. J. Pharm. Biopharm. 2019, 136, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Priester, M.I.; Romeijn, S.; Nejadnik, M.R.; Mönkäre, J.; O’Mahony, C.; Jiskoot, W.; Kersten, G.; Bouwstra, J.A. Hyaluronan-based dissolving microneedles with high antigen content for intradermal vaccination: Formulation, physicochemical characterization and immunogenicity assessment. Eur. J. Pharm. Biopharm. 2019, 134, 49–59. [Google Scholar] [CrossRef]
- Yu, J.; Xia, Y.; Zhang, H.; Pu, X.; Gong, T.; Zhang, Z.; Deng, L. A semi-interpenetrating network-based microneedle for rapid local anesthesia. J. Drug Deliv. Sci. Technol. 2022, 78, 103984. [Google Scholar] [CrossRef]
- Zhan, H.; Ma, F.; Huang, Y.; Zhang, J.; Jiang, X.; Qian, Y. Application of composite dissolving microneedles with high drug loading ratio for rapid local anesthesia. Eur. J. Pharm. Sci. 2018, 121, 330–337. [Google Scholar] [CrossRef]
- Smith, F.; Sabri, A.H.; Heppel, M.; Fonseca, I.; Chowdhury, F.; Cheung, K.; Willmor, S.; Rawson, F.; Marlow, M. The clinical and translational prospects of microneedle devices, with a focus on insulin therapy for diabetes mellitus as a case study. Int. J. Pharm. 2022, 628, 122234. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose controlin diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Wang, L.; Shen, D.; Ni, Z.; Ren, S.; Lu, Y.; Chen, X.; Yang, J.; Hong, Y. Research progress on cosmetic microneedle systems: Preparation, property and application. Eur. Polym. J. 2022, 163, 110942. [Google Scholar] [CrossRef]
- Rai, V.K.; Saha, I.; Alam, M.; Nishchaya, K.; Ghosh, G.; Rath, G. Microneedle arrays for cutaneous and transcutaneous drug delivery, disease diagnosis, and cosmetic aid: Microneedle patches of cutaneous and transcutaneous uses. J. Drug Deliv. Sci. Technol. 2023, 79, 104058. [Google Scholar] [CrossRef]
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving microneedles: Applications and growing therapeutic potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Azim, H.; Tekko, I.A.; Ali, A.; Ramadan, A.; Nafee, N.; Khalafallah, N.; Rahman, T.; Mcdaid, W.; Aly, R.G.; Vora, L.K.; et al. Hollow microneedle assisted intradermal delivery of hypericin lipid nanocapsules with light enabled photodynamic therapy against skin cancer. J. Control. Release 2022, 348, 849–869. [Google Scholar] [CrossRef]
- Chandbadshah SB, V.J.; Mannayee, G. Structural analysis and simulation of solid microneedle array for vaccine delivery applications. Mater. Today Proc. 2022, 65, 3774–3779. [Google Scholar] [CrossRef]
- Evens, T.; Malek, O.; Castagne, S.; Seveno, D.; Van Bael, A. A novel method for producing solid polymer microneedles using laser ablated moulds in an injection moulding process. Manuf. Lett. 2020, 24, 29–32. [Google Scholar] [CrossRef]
- Shu, W.; Heimark, H.; Bertollo, N.; Tobin, D.J.; O’Cearbhaill, E.D.; Annaidh, A.N. Insights into the mechanics of solid conical microneedle array insertion into skin using the finite element method. Acta Biomater. 2021, 135, 403–413. [Google Scholar] [CrossRef]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef]
- Mönkäre, J.; Pontier, M.; van Kampen, E.E.M.; Du, G.; Leone, M.; Romeijn, S.; Nejadnik, M.R.; O’Mahony, C.; Slütter, B.; Jiskoot, W.; et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. 2018, 129, 111–121. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, H.; Jiang, J.; Xu, X.; Zeng, Z.; Ren, L.; Liu, Q.; Chen, M.; Zhang, T.; Ding, X.; et al. pH-activatable oxidative stress amplifying dissolving microneedles for combined chemo-photodynamic therapy of melanoma. Asian J. Pharm. Sci. 2022, 17, 679–696. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wang, C.; Zhu, L.; Wang, F.; Jiao, W.; Zhuang, X.; Xie, F.; Du, L.; Jin, Y. Cinnarizine dissolving microneedles against microwave-induced brain injury. Biomed. Pharmacother. 2022, 155, 113779. [Google Scholar] [CrossRef]
- Huang, C.; Gou, K.; Yue, X.; Zhao, S.; Zeng, R.; Qu, Y.; Zhang, C. A novel hyaluronic acid-based dissolving microneedle patch loaded with ginsenoside Rg3 liposome for effectively alleviate psoriasis. Mater. Des. 2022, 224, 111363. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Y.; Kang, Y.; Dai, G.; Liu, Z.; Xu, K.; Zhong, W. The effects of molecular weight of hyaluronic acid on transdermal delivery efficiencies of dissolving microneedles. Eur. J. Pharm. Sci. 2022, 168, 106075. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Prinz, G.; Powell, J.; Greenlee, L.; Almodovar, J. Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Mater. Sci. Eng. C 2021, 118, 111544. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Ronnander, P.; Simon, L.; Koch, A. Experimental and mathematical study of the transdermal delivery of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur. J. Pharm. Biopharm. 2020, 146, 32–40. [Google Scholar] [CrossRef]
- Wu, L.; Shrestha, P.; Iapichino, M.; Cai, Y.; Kim, B.; Stoeber, B. Characterization method for calculating diffusion coefficient of drug from polylactic acid (PLA) microneedles into the skin. J. Drug Deliv. Sci. Technol. 2021, 61, 102192. [Google Scholar] [CrossRef]
- Jeong, J.O.; Lim, Y.M.; Lee, J.Y.; Park, J.S. Polyvinylpyrrolidone based graphene oxide hydrogels by radiation crosslinking for conductive microneedle patches. Eur. Polym. J. 2023, 184, 111726. [Google Scholar] [CrossRef]
- Hosseini, M.; Amiri, M.; Ghanbari, M.; Mahdi, M.A.; Abdulsahib, W.K.; Salavati-Niasari, M. Drug delivery based on chitosan, β-cyclodextrin and sodium carboxymethyl cellulose as well as nanocarriers for advanced leukemia treatment. Biomed. Pharmacother. 2022, 153, 113369. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen TT, D.; Van Vo, G. Advances of microneedles in hormone delivery. Biomed. Pharmacother. 2022, 145, 112393. [Google Scholar] [CrossRef]
- Bauleth-Ramos, T.; El-Sayed, N.; Fontana, F.; Lobita, M.; Shahbazi, M.A.; Santos, H.A. Recent approaches for enhancing the performance of dissolving microneedles in drug delivery applications. Mater. Today 2023, 63, 239–287. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.T.; Wang, C.A.; Shen, Y.; Zhang, Z.H.; Cheng, J.; Wu, S.Z.; Ye, Y.H.; Shen, R.Q. Positive effects of PVP in MIC: Preparation and characterization of Al-Core heterojunction fibers. Def. Technol. 2023, 19, 52–62. [Google Scholar] [CrossRef]
- Alsulami, Q.A.; Rajeh, A. Synthesis of the SWCNTs/TiO2 nanostructure and its effect study on the thermal, optical, and conductivity properties of the CMC/PEO blend. Results Phys. 2021, 28, 104675. [Google Scholar] [CrossRef]


| Microneedle Material (Mass Percentage) | Centrifugal Condition | Drying Temperature | Drying Time | Molding Microneedle Content | Microneedle Formability |
|---|---|---|---|---|---|
| 10%PVP 2%CMC-Na | 2000 r/min (3 min) +2500 r/min (2 min) | 45 °C | 120 min | 85% | √ |
| 15%PVP 3%CMC-Na | 2000 r/min (3 min) +2500 r/min (2 min) | 45 °C | 150 min | 70% | × |
| 15%PVP 2%CMC-Na | 2000 r/min (3 min) +2500 r/min (3 min) | 45 °C | 100 min | 20% | × |
| 20%PVP 3%CMC-Na | 2500 r/min (5 min) +2000 r/min (3 min) | 45 °C | 180 min | 100% | √ |
| 25%PVP 3%CMC-Na | 2500 r/min (5 min) +2000 r/min (5 min) | 45 °C | 200 min | 100% | × |
| 30%PVP 3%CMC-Na | 2500 r/min (5 min) +2000 r/min (5 min) | 45 °C | 240 min | 100% | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, N.; Liu, Z.; Lu, M.; Yang, Y.; Liao, F.; Feng, Y.; Liu, G.; Qiu, S. Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles. Materials 2023, 16, 3417. https://doi.org/10.3390/ma16093417
Qiang N, Liu Z, Lu M, Yang Y, Liao F, Feng Y, Liu G, Qiu S. Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles. Materials. 2023; 16(9):3417. https://doi.org/10.3390/ma16093417
Chicago/Turabian StyleQiang, Na, Zhu Liu, Ming Lu, Yong Yang, Fangli Liao, Ying Feng, Guocong Liu, and Si Qiu. 2023. "Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles" Materials 16, no. 9: 3417. https://doi.org/10.3390/ma16093417
APA StyleQiang, N., Liu, Z., Lu, M., Yang, Y., Liao, F., Feng, Y., Liu, G., & Qiu, S. (2023). Preparation and Properties of Polyvinylpyrrolidone/Sodium Carboxymethyl Cellulose Soluble Microneedles. Materials, 16(9), 3417. https://doi.org/10.3390/ma16093417






