Comparing the Properties of Bio-Polyols Based on White Mustard (Sinapis alba) Oil Containing Boron and Sulfur Atoms Obtained by Various Methods and Checking Their Influence on the Flammability of Rigid Polyurethane/Polyisocyanurate Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of New Bio-Polyols
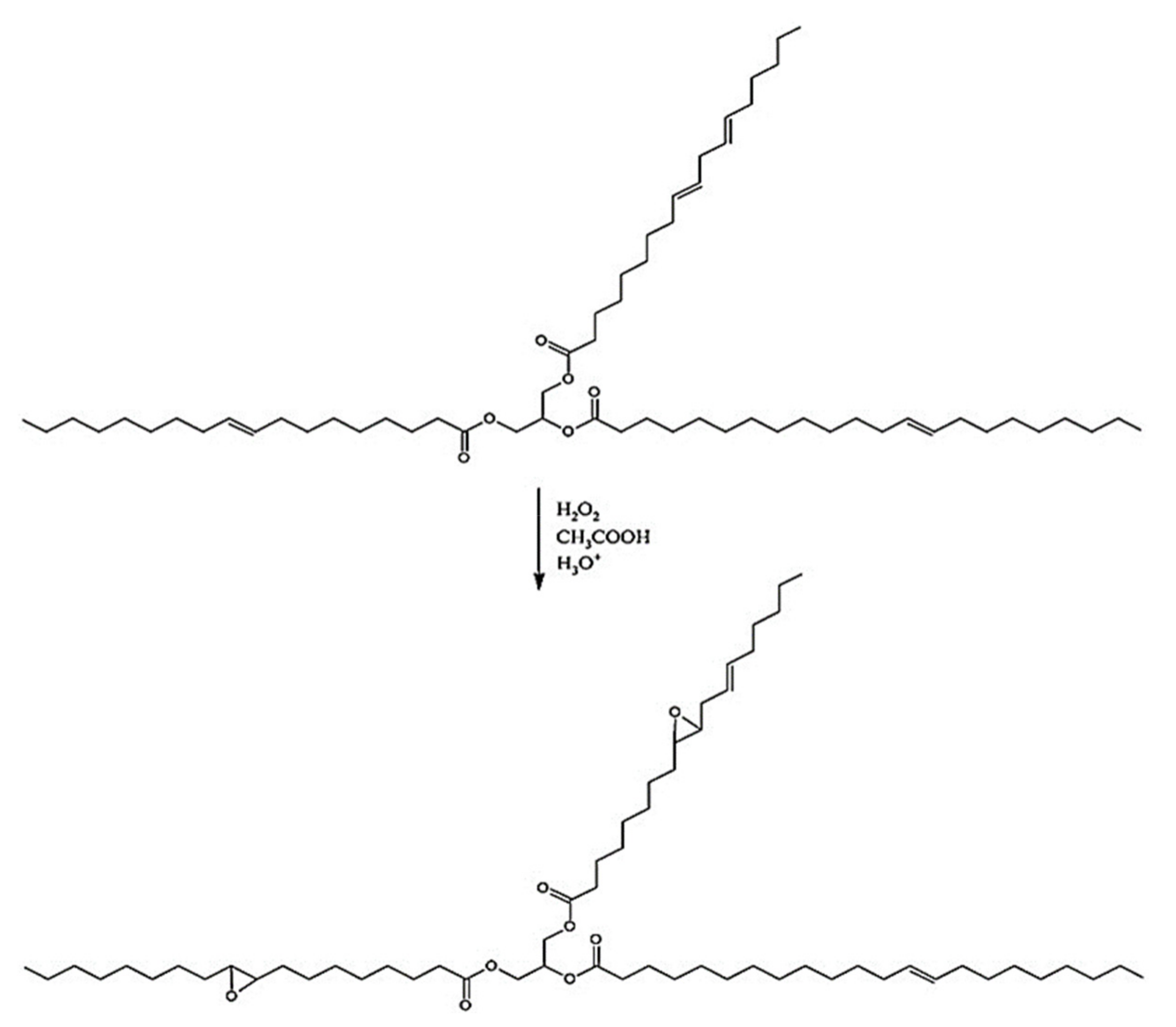
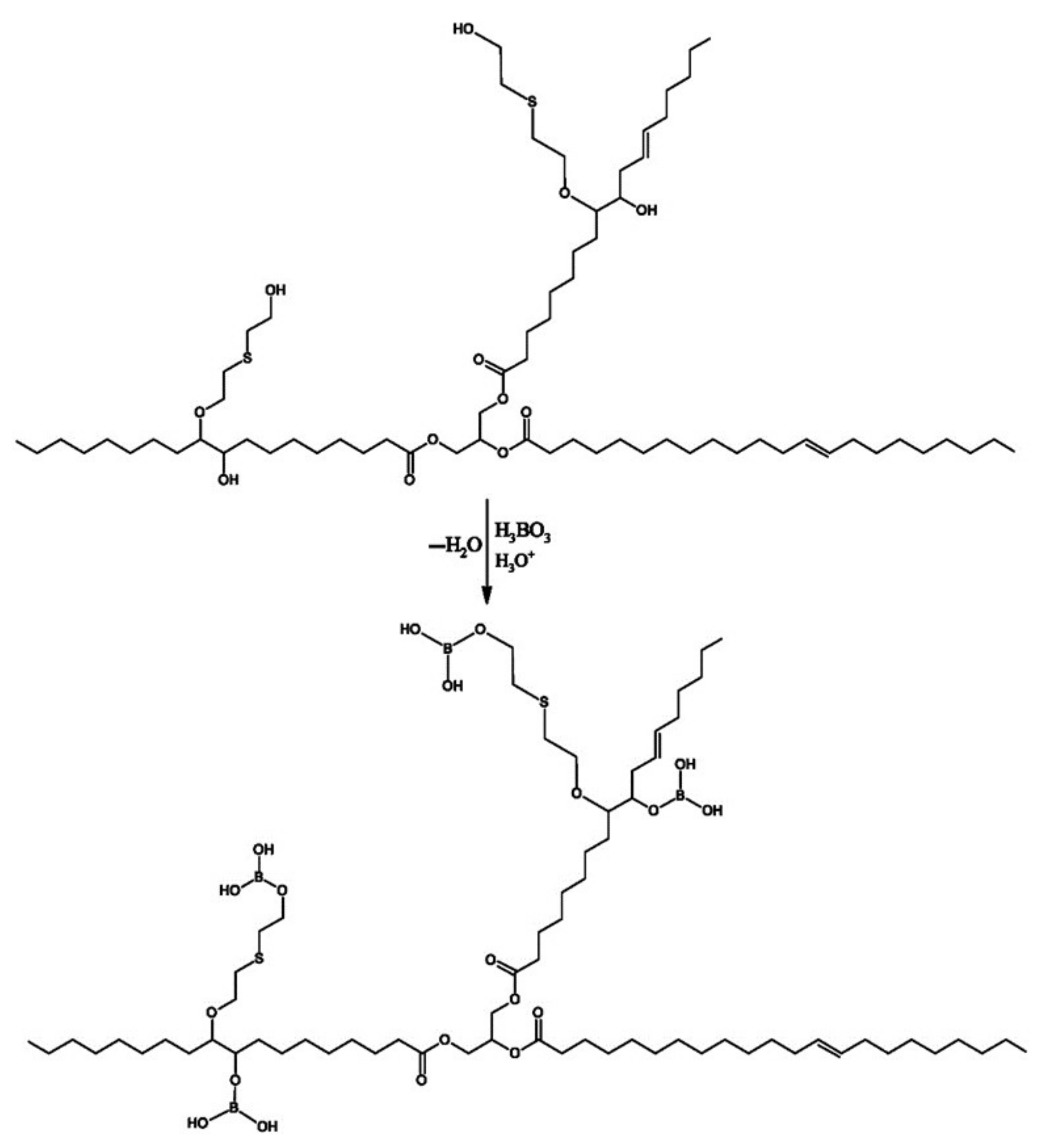
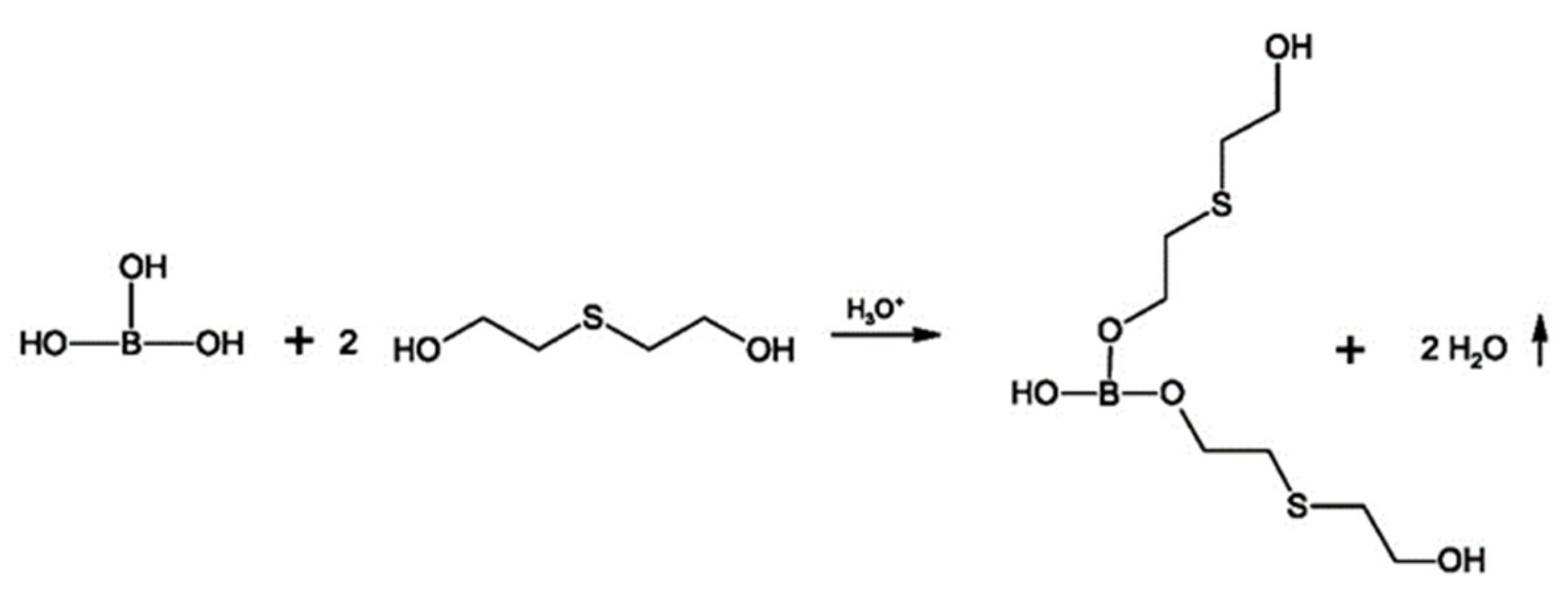
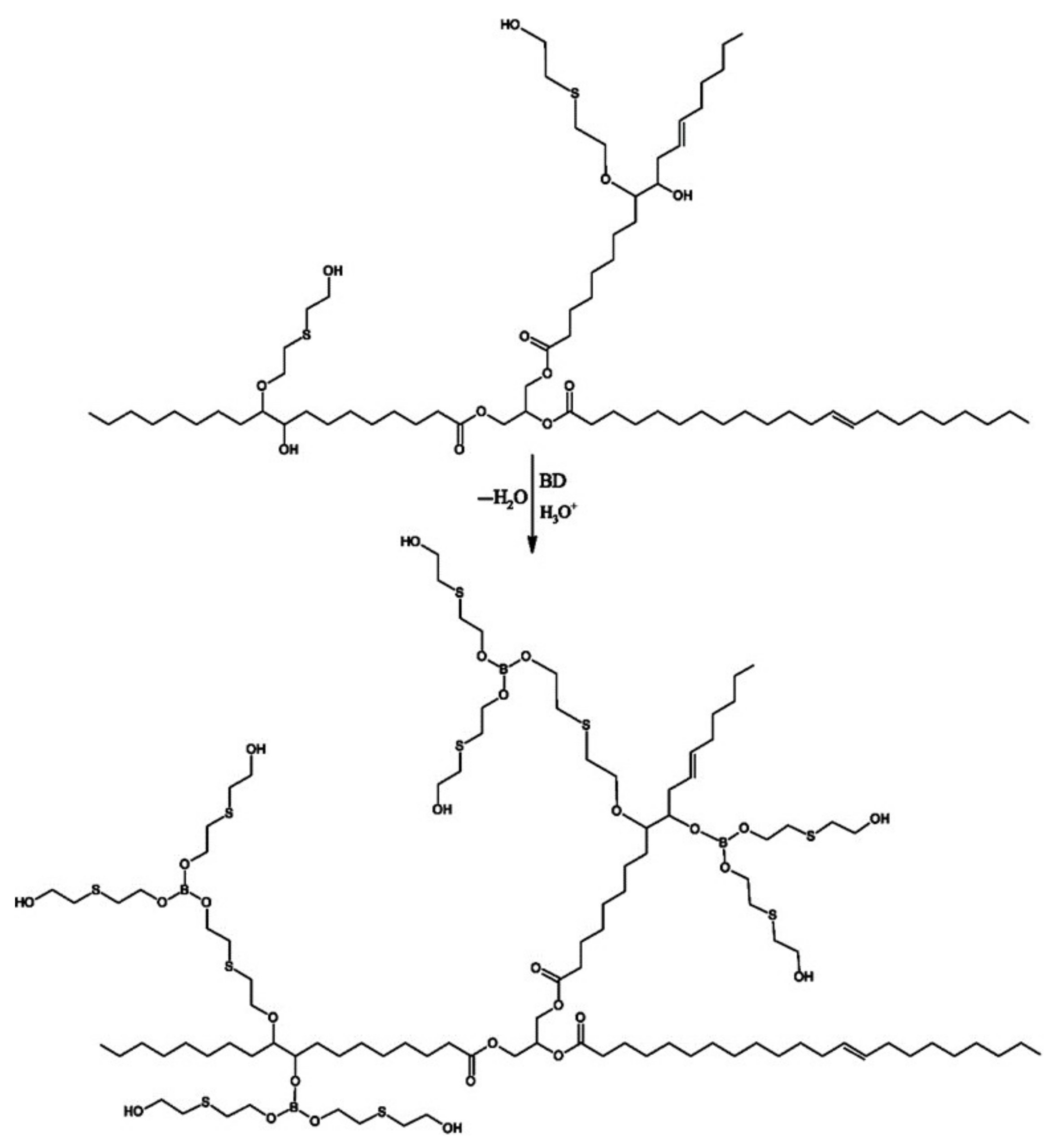
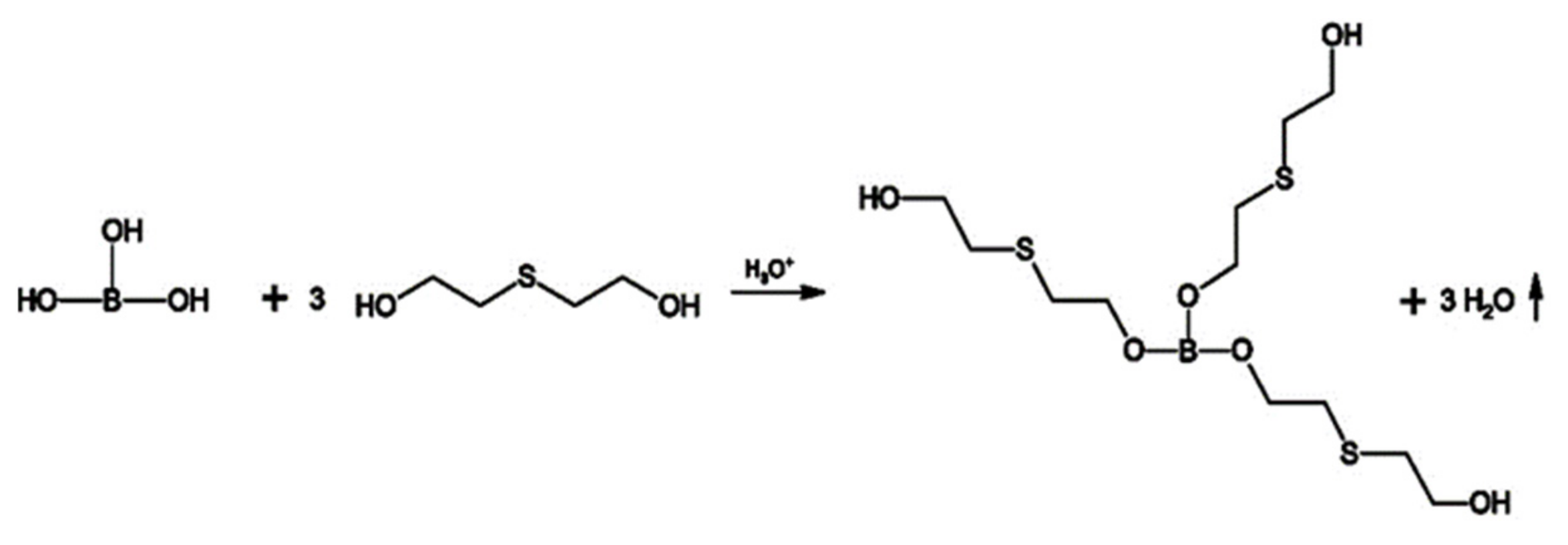
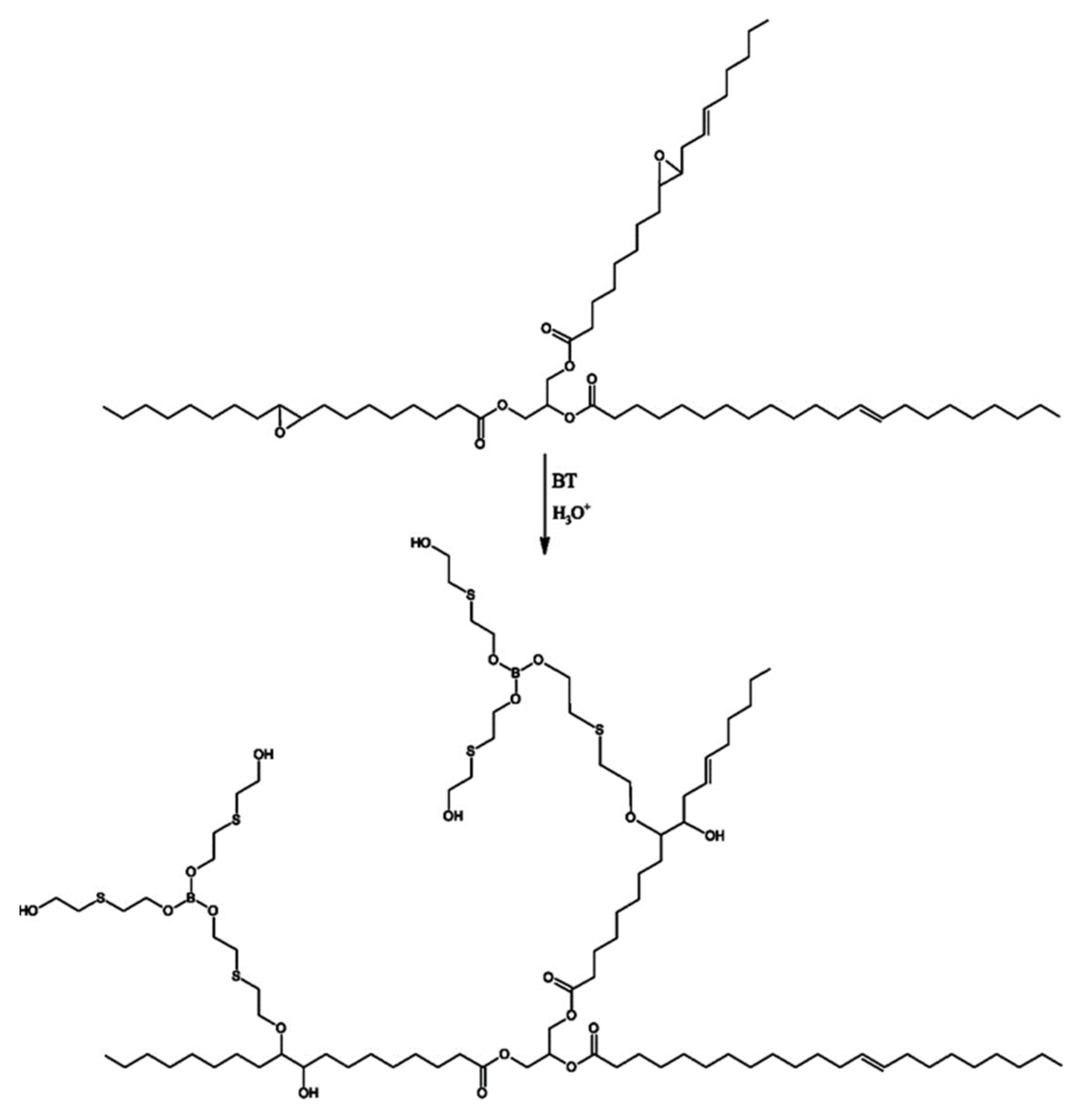
2.2.1. Synthesis of MP1 Bio-Polyol
2.2.2. Synthesis of MP2 Bio-Polyol
2.2.3. Synthesis of MP3 Bio-Polyol
2.3. Assessment of Bio-Polyol Properties
2.3.1. Physicochemical Tests of Bio-Polyols
2.3.2. Analytical Tests of Bio-Polyols
2.3.3. Spectroscopy Tests
2.3.4. Differential Scanning Calorimetry
2.4. Preparation of Rigid Polyurethane/Polyisocyanurate Foams Based on MPs Bio-Polyols
2.5. Flammability Tests of Rigid Polyurethane/Polyisocyanurate Foams Modified by Bio-Polyols Containing Sulfur and Boron Atoms
- TTI—time to ignition, (s);
- THR—total heat release from the surface unit of the analyzed material, (MJ/m2);
- MLR—mass loss rate of sample/burning rate, (g/m2·s);
- HRR—heat release rate from the sample during combustion, (kW/m2);
- tHRRmax—time to reach the maximum value of HRR (HRRmax), (s);
- CO, CO2—emission of CO and CO2, respectively (kg/kg);
- TSR—total smoke release, (m2/m2).
3. Results and Discussion
3.1. Physicochemical Tests of Bio-Polyols
3.2. Analytical Tests of Bio-Polyols
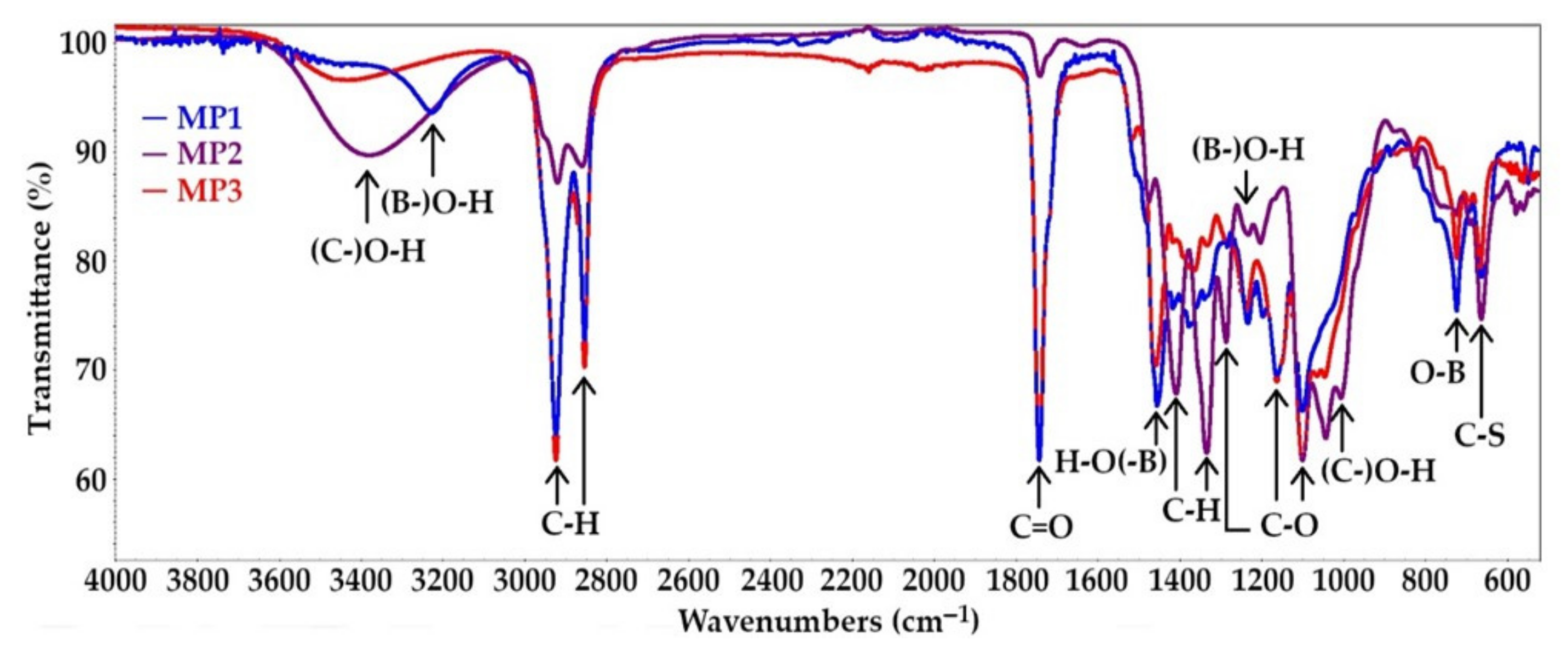
3.3. Spectroscopy Tests of Bio-Polyols
3.4. Differential Scanning Calorimetry of Bio-Polyols
3.5. Flammability Tests of Rigid Polyurethane/Polyisocyanurate Foams Modified by Bio-Polyols Containing Boron and Sulfur Atoms
3.5.1. Basic Flammability Tests
3.5.2. Cone Calorimeter Test
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics—The Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 1 March 2023).
- Market Reports. Available online: https://www.marketsandmarkets.com/Market-Reports/green-and-bio-polyols-market-1175.html (accessed on 1 March 2023).
- Satti, S.M.; Shah, A.A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, U. Towards a Sustainable Future? The EU Policies Concerning Plastics and Their Didactical Potential for Primary and Secondary Teaching. Discourse Commun. Sustain. Educ. 2019, 10, 47–62. [Google Scholar] [CrossRef]
- Rondinelli, D.A.; A Berry, M. Environmental citizenship in multinational corporations: Social responsibility and sustainable development. Eur. Manag. J. 2000, 18, 70–84. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. Synthesis and application of new bio-polyols based on mustard oil for the production of selected polyurethane materials. Ind. Crops Prod. 2020, 155, 112831. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyżowska, M.; Ryszkowska, J. Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crops Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E. Effect of hydroxylated soybean oil and bio-based propanediol on the structure and thermal properties of synthesized bio-polyurethanes. Ind. Crops Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Chen, R.; Kessler, M.R. Polyurethanes from Solvent-Free Vegetable Oil-Based Polyols. ACS Sustain. Chem. Eng. 2014, 2, 2465–2476. [Google Scholar] [CrossRef]
- Natural Oil Polyols Market Size, Share & Trends Analysis Report by Product (Soy Oil, Castor Oil), by End-Use (Construction, Electronics & Appliances), by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/natural-oil-polyols-nop-market (accessed on 31 August 2021).
- Ionescu, M. Polyols for Polyurethanes: Chemistry and Technology, 3rd ed.; De Gruyter: Berlin, Germany, 2019; Volume 1. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Omrani, I.; Farhadian, A.; Babanejad, N.; Shendi, H.K.; Ahmadi, A.; Nabid, M.R. Synthesis of novel high primary hydroxyl functionality polyol from sunflower oil using thiol-yne reaction and their application in polyurethane coating. Eur. Polym. J. 2016, 82, 220–231. [Google Scholar] [CrossRef]
- Cruz-Aldaco, K.; Flores-Loyola, E.; Aguilar-González, C.N.; Burgos, N.; Jiménez, A. Synthesis and Thermal Characterization of Polyurethanes Obtained from Cottonseed and Corn Oil-Based Polyols. J. Renew. Mater. 2016, 4, 178–184. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Sain, M. Synthesis of Soy-Polyol by Two Step Continuous Route and Development of Soy-Based Polyurethane Foam. J. Polym. Environ. 2010, 18, 437–442. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Mosiewicki, M.A.; Corcuera, M.A.; Eceiza, A.; Aranguren, M.I. Linseed Oil-Based Polyurethane Rigid Foams: Synthesis and Characterization. J. Renew. Mater. 2015, 3, 3–13. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Kośny, J.; Strąkowska, A.; Masłowski, M.; Strzelec, K. Linseed oil as a natural modifier of rigid polyurethane foams. Ind. Crops Prod. 2018, 115, 40–51. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Kirpluks, M.; Vanags, E.; Abolins, A.; Michalowski, S.; Fridrihsone, A.; Cabulis, U. High functionality bio-polyols from tall oil and rigid polyurethane foams formulated solely using bio-polyols. Materials 2020, 13, 8, 1985. [Google Scholar] [CrossRef]
- Pawlik, H.; Prociak, A. Influence of Palm Oil-Based Polyol on the Properties of Flexible Polyurethane Foams. J. Polym. Environ. 2012, 20, 438–445. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Crude Oil Market. Available online: https://tradingeconomics.com/commodity/crude-oil (accessed on 1 March 2023).
- Mustard Seed Market. Available online: https://www.helgilibrary.com/charts/which-country-produces-the-most-mustard-seeds (accessed on 1 March 2023).
- Paciorek-Sadowska, J.; Borowicz, M.; Czuprynski, B.; Liszkowska, J. New bio-polyol based on white mustard seed (Sinapis alba) as an alternative raw material for the polyurethane industry. Polimery 2018, 63, 694–699. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J. Studies on Influence of Derivatives of N,N′-Di(Methyleneoxy-Hydoxyalkyl)Urea and Boric Acid on Properties of Rigid Polyurethane-Polyisocyanurate Foams; Wydawnictwo Uczelniane Uniwersytetu Kazimierza Wielkiego: Bydgoszcz, Poland, 2011. (In Polish) [Google Scholar]
- Paciorek-Sadowska, J.; Czupryński, B. New compounds for production of polyurethane foams. J. Appl. Polym. Sci. 2006, 102, 5918–5926. [Google Scholar] [CrossRef]
- Rao, W.-H.; Xu, H.-X.; Xu, Y.-J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.-Z. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Bhakri, S.; Ghozali, M.; Cahyono, E.; Triwulandari, E.; Restu, W.K.; Solihat, N.N.; Iswanto, A.H.; Antov, P.; Savov, V.; Hua, L.S.; et al. Development and Characterization of Eco-Friendly Non-Isocyanate Urethane Monomer from Jatropha curcas Oil for Wood Composite Applications. J. Renew. Mater. 2023, 11, 41–59. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, Y.; Wang, N.; Wang, Y. Flame retardant polyurethane foam prepared from compatible blends of soybean oil-based polyol and phosphorus containing polyol. J. Appl. Polym. Sci. 2018, 135, 45779. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef]
- Chmiel, E.; Barcikowska, M.; Lubczak, J. Pianki poliuretanowe z pierścieniem 1, 3, 5-triazynowym i atomami krzemu. Chem. Technol. Biotechnol. 2017, 1–16. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New bio-polyol based on white mustard seed oil for rigid PUR-PIR foams. Pol. J. Chem. Technol. 2018, 20, 24–31. [Google Scholar] [CrossRef]
- Academic Forum. Technical Mustard Oil. Available online: https://prenumeruj.forumakademickie.pl/fa/2009/01/techniczny-olej-z-gorczycy/ (accessed on 1 March 2023).
- Ionescu, M.; Petrović, Z.S. High Functionality Polyether Polyols Based on Polyglycerol. J. Cell. Plast. 2010, 46, 223–237. [Google Scholar] [CrossRef]
- Prociak, A. New Generation Polyurethane Thermal Insulation Materials; Wydawnictwo Politechniki Krakowskej: Cracow, Poland, 2008. (In Polish) [Google Scholar]
- Lim, H.; Kim, S.H.; Kim, B.K. Effects of the hydroxyl value of polyol in rigid polyurethane foams. Polym. Adv. Technol. 2008, 19, 1729–1734. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Introduction to Bio-Based Polyols and Polyurethanes. In Bio-Based Polyols and Polyurethanes; Springer Briefs in Molecular Science; Springer: Cham, Switzerland, 2015; pp. 1–13. [Google Scholar] [CrossRef]
- Xia, Y.; Quirino, R.L.; Larock, R.C. Bio-based Thermosetting Polymers from Vegetable Oils. J. Renew. Mater. 2013, 1, 3–27. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Grzybowski, Ł.; Czupryński, B. Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material. Polymers 2019, 11, 1963. [Google Scholar] [CrossRef]
- Zarzyka, I. The Modification of Polyurethane Foams Using New Boroorganic Polyols. II. Polyurethane Foams from Boron-Modified Hydroxypropyl Urea Derivatives. Polym. Technol. Eng. 2014, 53, 207–215. [Google Scholar] [CrossRef]
- Kırbaş, I. Improving the structural and physical properties of boric acid-doped rigid polyurethane materials. Compos. Adv. Mater. 2021, 30, 26349833211010819. [Google Scholar] [CrossRef]
- Kattiyaboot, T.; Thongpin, C. Effect of Natural Oil Based Polyols on the Properties of Flexible Polyurethane Foams Blown by Distilled Water. Energy Procedia 2016, 89, 177–185. [Google Scholar] [CrossRef]
- SDBS—Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi (accessed on 4 September 2022).
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Zhan, G.; Zhao, L.; Hu, S.; Gan, W.; Yu, Y.; Tang, X. A novel biobased resin-epoxidized soybean oil modified cyanate ester. Polym. Eng. Sci. 2008, 48, 1322–1328. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, M.; Yu, R.; Huang, J.; Dong, X.; Zhang, R.; Zhu, J. Bio-based shape memory polyurethanes (Bio-SMPUs) with short side chains in the soft segment. J. Mater. Chem. A 2014, 2, 11490–11498. [Google Scholar] [CrossRef]
- Lin, B.; Yang, L.; Dai, H.; Hou, Q.; Zhang, L. Thermal analysis of soybean oil based polyols. J. Therm. Anal. Calorim. 2009, 95, 977–983. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Structure analysis and thermal degradation characteristics of bio-based poly(propylene succinate)s obtained by using different catalyst amounts. J. Therm. Anal. Calorim. 2017, 130, 197–206. [Google Scholar] [CrossRef]
- Pillai, P.K.; Li, S.; Bouzidi, L.; Narine, S.S. Metathesized palm oil polyol for the preparation of improved bio-based rigid and flexible polyurethane foams. Ind. Crops Prod. 2016, 83, 568–576. [Google Scholar] [CrossRef]
- Liszkowska, L. Properties of Rigid PUR-PIR Foams Obtained with the Use of Condensation Products of Citric Acid with Diols and Selected Glycolysates; Wydawnictwo UKW: Bydgoszcz, Poland, 2016. (In Polish) [Google Scholar]
- Czupryński, B. Issues from Chemistry and Technology of Polyurethanes; Wydawnictwo Akademii Bydgoskiej: Bydgoszcz, Poland, 2004. (In Polish) [Google Scholar]
- Rasmussen, C.L.; Glarborg, P.; Marshall, P. Mechanisms of radical removal by SO2. Proc. Combust. Inst. 2007, 31, 339–347. [Google Scholar] [CrossRef]
- ISO Standard 3582:2000/Amd 1:2007; Flexible Cellular Polymeric Materials—Laboratory Assessment of Horizontal Burning Characteristics of Small Specimens Subjected to a Small Flame—Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2007.
- Chmiel, E.; Lubczak, J. Synthesis of oligoetherols from mixtures of melamine and boric acid and polyurethane foams formed from these oligoetherols. Polym. Bull. 2019, 76, 2253–2275. [Google Scholar] [CrossRef]
- Alarie, Y. Toxicity of Fire Smoke. Crit. Rev. Toxicol. 2002, 32, 259–289. [Google Scholar] [CrossRef]




| Foam Symbol | Rokopol RF551 (Eq) (g) | Bio-Polyol (Eq) (g) | Tegostab 8460 (g) | 33% DABCO (g) | 33% Potassium Acetate (g) | Distilled Water (g) | Purocyn B (Eq) (g) |
|---|---|---|---|---|---|---|---|
| Ref. | 1.0 66.79 | 0.0 0.00 | 5.40 | 3.17 | 7.93 | 3.17 | 3.7 250.60 |
| MP1.01 | 0.9 60.11 | 0.1 50.77 | 6.15 | 3.62 | 9.04 | 3.17 | 3.7 250.60 |
| MP2.01 | 0.9 60.11 | 0.1 14.89 | 5.54 | 3.26 | 8.14 | 3.17 | 3.7 250.60 |
| MP2.02 | 0.8 53.43 | 0.2 29.79 | 5.67 | 3.34 | 8.35 | 3.17 | 3.7 250.60 |
| MP2.03 | 0.7 46.75 | 0.3 44.68 | 5.81 | 3.42 | 8.55 | 3.17 | 3.7 250.60 |
| MP2.04 | 0.6 40.07 | 0.4 59.57 | 5.95 | 3.50 | 8.76 | 3.17 | 3.7 250.60 |
| MP2.05 | 0.5 33.39 | 0.5 74.47 | 6.09 | 3.58 | 8.96 | 3.17 | 3.7 250.60 |
| MP3.01 | 0.9 60.11 | 0.1 15.72 | 5.54 | 3.26 | 8.16 | 3.17 | 3.7 250.60 |
| MP3.02 | 0.8 53.43 | 0.2 31.44 | 5.70 | 3.35 | 8.38 | 3.17 | 3.7 250.60 |
| MP3.03 | 0.7 46.75 | 0.3 47.16 | 5.86 | 3.45 | 8.61 | 3.17 | 3.7 250.60 |
| MP3.04 | 0.6 40.07 | 0.4 62.89 | 6.01 | 3.54 | 8.84 | 3.17 | 3.7 250.60 |
| MP3.05 | 0.5 33.39 | 0.5 78.61 | 6.16 | 3.63 | 9.07 | 3.17 | 3.7 250.60 |
| Parameter | MP1 | MP2 | MP3 |
|---|---|---|---|
| State of matter (at 20 °C) | solid | liquid | liquid |
| Color | brown | light-orange | orange |
| Smell | odorless | slightly sulfuric | slightly sulfuric |
| Density (g/cm3) | 1.19 ± 0.01 | 1.09 ± 0.02 | 1.10 ± 0.01 |
| Viscosity at 20 °C (mPa·s) | - | 9400 ± 200 | 8800 ± 250 |
| pH (-) | 4.5 ± 0.2 | 7.0 ± 0.1 | 7.1 ± 0.1 |
| Parameter | MP1 | MP2 | MP3 |
|---|---|---|---|
| Hydroxyl value (mg KOH/g) | 55.25 ± 0.43 | 188.34 ± 2.91 | 178.42 ± 4.34 |
| Acid value (mg KOH/g) | 55.25 ± 0.43 | 2.42 ± 0.22 | 1.13 ± 0.13 |
| Water content (%wt.) | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Element | MP1 | MP2 | MP3 |
|---|---|---|---|
| Carbon (%) | 54.53 | 65.20 | 66.20 |
| Hydrogen (%) | 10.16 | 11.48 | 11.69 |
| Nitrogen (%) | 0.00 | 0.00 | 0.00 |
| Sulfur (%) | 5.08 | 7.44 | 5.49 |
| Oxygen (%) | 26.18 | 14.06 | 15.82 |
| Boron (%) | 4.05 | 1.82 | 0.80 |
| Parameter | MP1 | MP2 | MP3 |
|---|---|---|---|
| Mw (g/mol) | 4131 | 3998 | 2887 |
| Mn (g/mol) | 1265 | 2654 | 2321 |
| D (-) | 3.27 | 1.51 | 1.24 |
| f (-) | 1.24 | 8.90 | 7.38 |
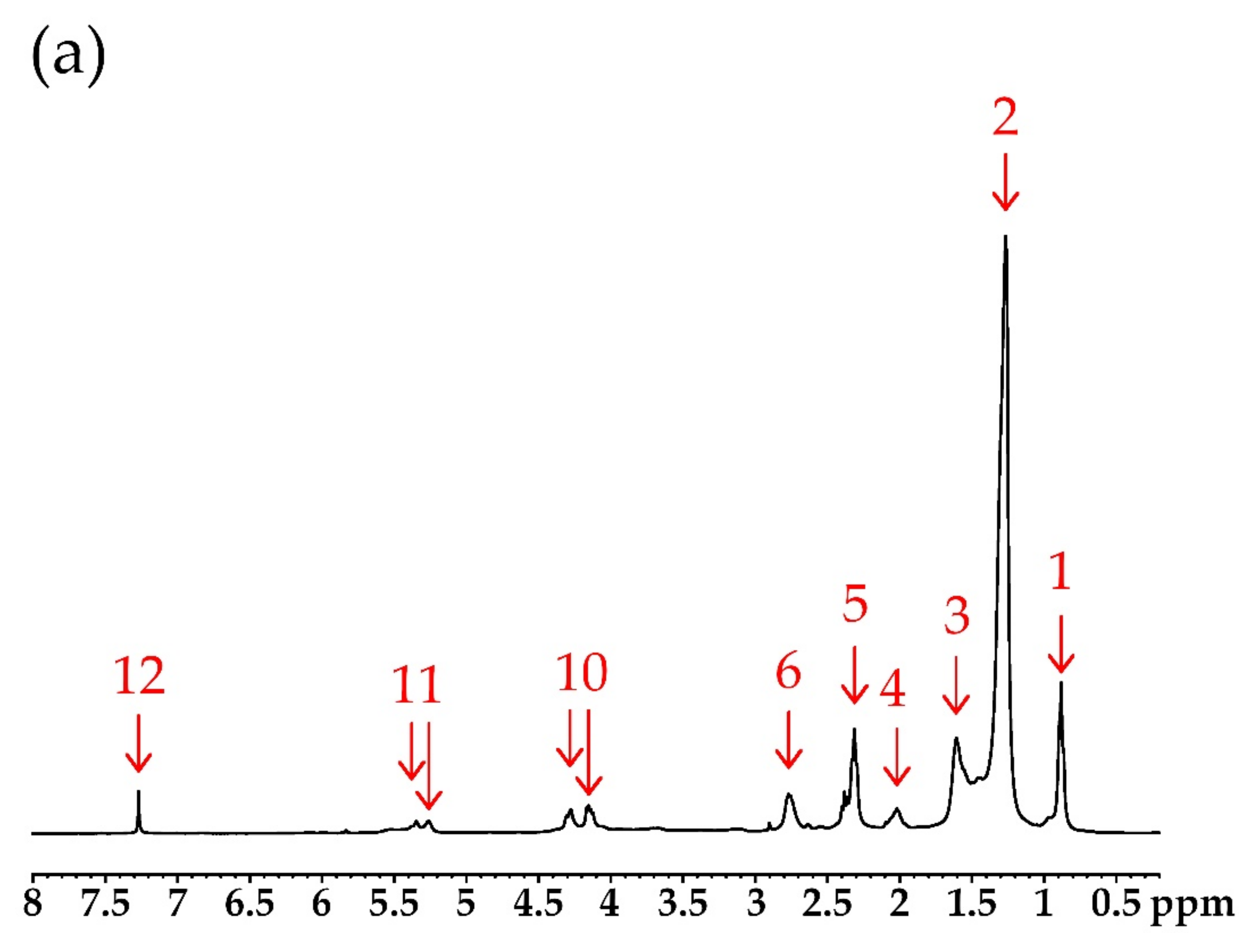
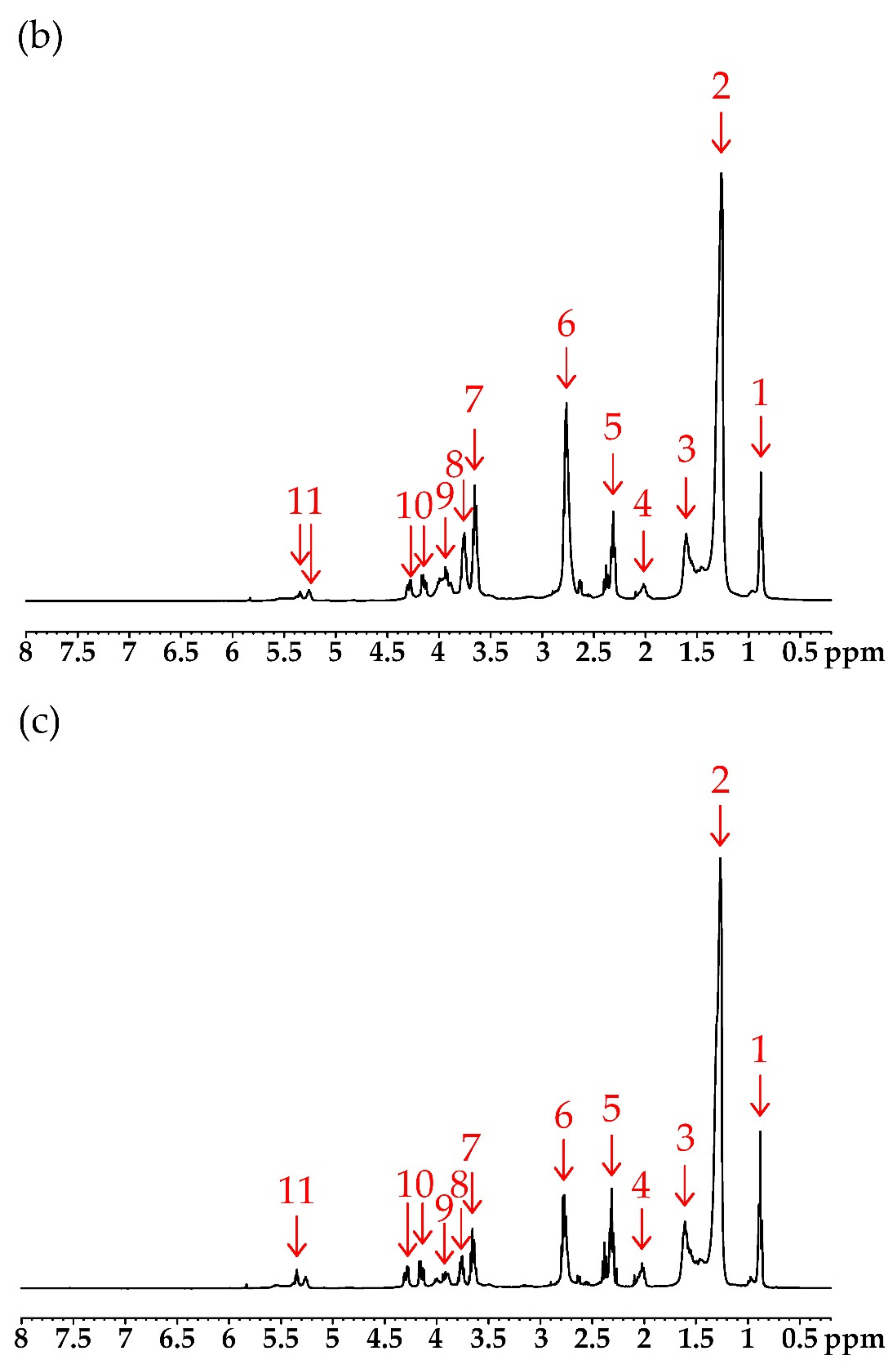
| Number of Peak | Chemical Shift (ppm) | Type of Protons | Structure * |
|---|---|---|---|
| 1 | 0.86–0.88 | protons of ending groups –CH3 | CH3 |
| 2 | 1.25–1.40 | protons of CH2 groups in the fatty acid chain | CH2 |
| 3 | 1.60 | protons of the β-CH2 group to the carbonyl (ester) group | –CH2–CH2–CO– |
| 4 | 2.10–2.25 | protons of α-CH2 groups to the olefin group | –CH2–CH=CH– |
| 5 | 2.29–2.32 | protons of the α-CH2 group to the carbonyl (ester) group | –CH2–CO– |
| 6 | 2.70–2.80 | protons of the α-CH2 group to the sulfide (thioester) group | –CH2–S– |
| 7 | 3.52–3.60 | protons of α-CH2 groups to the hydroxyl group | –CH2–OH |
| 8 | 3.70–3.80 | protons of hydroxyl groups at the end of the chain | –OH |
| 9 | 3.85–4.10 | protons of α-CH2 groups to the borate group | –CH2–OB< |
| 10 | 4.15–4.28 | methylene protons of glyceryl | –CH2–CH–CH2– |
| 11 | 5.25 | methane protons of glyceryl | CH2–CH–CH2– |
| 12 | 7.27 | protons of the hydroxyl group in boric acid | –B–OH |
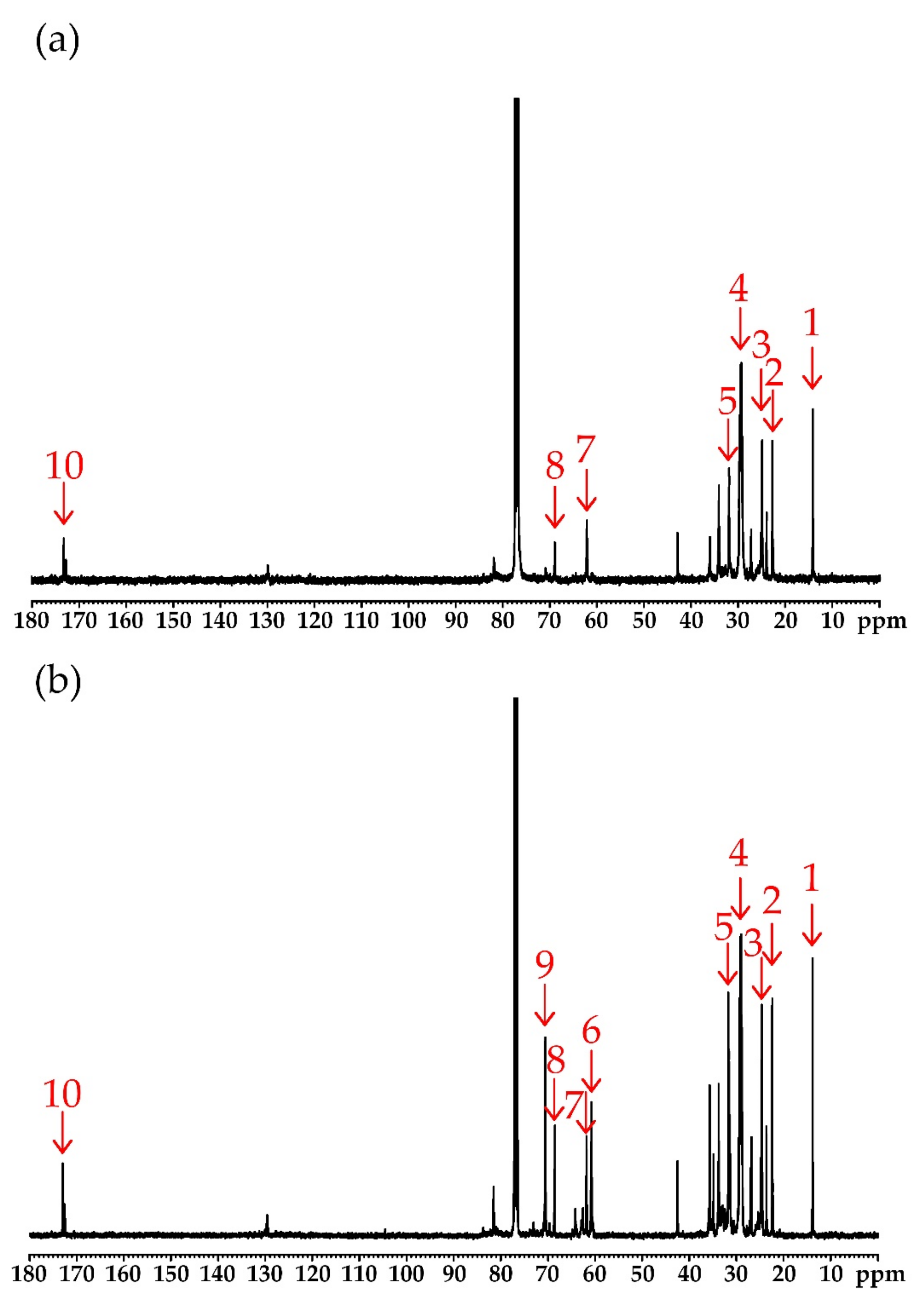
| Number of Peak | Chemical Shift (ppm) | Type of Carbon | Structure * |
|---|---|---|---|
| 1 | 14.30 | carbons of ending groups | –CH3 |
| 2 | 22.70 | carbons of penultimate groups | –CH2–CH3 |
| 3 | 27.20–29.80 | carbons of CH2 groups in the fatty acid chains | –CH2– |
| 4 | 31.90 | carbons of α-CH2 groups to the carbonyl group | –CH2–OOC–CH2– |
| 5 | 33.00–35.00 | carbons of α-CH2 groups to sulfide group | –S–CH2– |
| 6 | 61.10 | carbons of β-CH2 groups to sulfide group | –S–CH2–CH2– |
| 7 | 62.10 | methylene carbons of glyceryl | –CH2–CH–CH2– |
| 8 | 68.90 | methane carbons of glyceryl | –CH2–CH–CH2– |
| 9 | 73.86 | carbons of α-CH groups linked to a hydroxyl group within the chain | >CH–OH |
| 10 | 172.80–173.25 | carbons of carbonyl groups | >C=O |
| Sample | Tg (°C) | Tm (°C) | Td (°C) |
|---|---|---|---|
| MO | - | −21.3 ± 0.4 | - |
| MP1 | 18.3 ± 0.3 | 70.2 ± 0.7 | 150.2 ± 0.6 |
| MP2 | −30.3 ± 0.5 | 14.9 ± 0.5 | 75.9 ± 0.6 |
| MP3 | −32.1 ± 0.4 | 11.2 ± 0.3 | - |
| Parameter | TTI | THR | MLR | HRR | tHRRmax | Released CO | Released CO2 | TSR |
|---|---|---|---|---|---|---|---|---|
| Unit | (s) | (MJ/m2) | (g/m2·s) | (kW/m2) | (s) | (kg/kg) | (kg/kg) | (m2/m2) |
| Ref. | 3 | 24.3 | 10.22 | 278.90 | 10 | 0.8201 | 6.20 | 912.4 |
| MP1.01 | 4 | 23.6 | 8.34 | 138.94 | 10 | 0.0437 | 1.32 | 752.5 |
| MP2.01 | 5 | 21.6 | 6.38 | 107.34 | 20 | 0.0322 | 1.22 | 542.6 |
| MP2.02 | 5 | 21.3 | 6.02 | 102.86 | 30 | 0.0274 | 1.11 | 499.4 |
| MP2.03 | 7 | 21.1 | 5.42 | 98.02 | 40 | 0.0226 | 1.07 | 473.8 |
| MP2.04 | 7 | 20.3 | 5.21 | 92.76 | 45 | 0.0178 | 1.06 | 439.7 |
| MP2.05 | 8 | 19.5 | 5.11 | 83.79 | 45 | 0.0163 | 1.05 | 426.3 |
| MP3.01 | 4 | 22.0 | 6.79 | 115.33 | 20 | 0.0321 | 1.27 | 535.7 |
| MP3.02 | 5 | 21.7 | 6.19 | 108.36 | 35 | 0.0246 | 1.18 | 493.3 |
| MP3.03 | 6 | 21.4 | 5.55 | 104.32 | 40 | 0.0216 | 1.11 | 482.0 |
| MP3.04 | 7 | 20.5 | 5.39 | 93.76 | 40 | 0.0190 | 1.10 | 447.3 |
| MP3.05 | 7 | 19.7 | 5.35 | 86.90 | 43 | 0.0186 | 1.09 | 436.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowicz, M.; Isbrandt, M.; Paciorek-Sadowska, J.; Sander, P. Comparing the Properties of Bio-Polyols Based on White Mustard (Sinapis alba) Oil Containing Boron and Sulfur Atoms Obtained by Various Methods and Checking Their Influence on the Flammability of Rigid Polyurethane/Polyisocyanurate Foams. Materials 2023, 16, 3401. https://doi.org/10.3390/ma16093401
Borowicz M, Isbrandt M, Paciorek-Sadowska J, Sander P. Comparing the Properties of Bio-Polyols Based on White Mustard (Sinapis alba) Oil Containing Boron and Sulfur Atoms Obtained by Various Methods and Checking Their Influence on the Flammability of Rigid Polyurethane/Polyisocyanurate Foams. Materials. 2023; 16(9):3401. https://doi.org/10.3390/ma16093401
Chicago/Turabian StyleBorowicz, Marcin, Marek Isbrandt, Joanna Paciorek-Sadowska, and Paweł Sander. 2023. "Comparing the Properties of Bio-Polyols Based on White Mustard (Sinapis alba) Oil Containing Boron and Sulfur Atoms Obtained by Various Methods and Checking Their Influence on the Flammability of Rigid Polyurethane/Polyisocyanurate Foams" Materials 16, no. 9: 3401. https://doi.org/10.3390/ma16093401
APA StyleBorowicz, M., Isbrandt, M., Paciorek-Sadowska, J., & Sander, P. (2023). Comparing the Properties of Bio-Polyols Based on White Mustard (Sinapis alba) Oil Containing Boron and Sulfur Atoms Obtained by Various Methods and Checking Their Influence on the Flammability of Rigid Polyurethane/Polyisocyanurate Foams. Materials, 16(9), 3401. https://doi.org/10.3390/ma16093401










