Bismuth Vanadate Decked Polyaniline Polymeric Nanocomposites: The Robust Photocatalytic Destruction of Microbial and Chemical Toxicants
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of the PANI Nanotube (NTs)
2.2. The Synthesis of the BiVO4@PANI Nanocomposite
2.3. Characterization
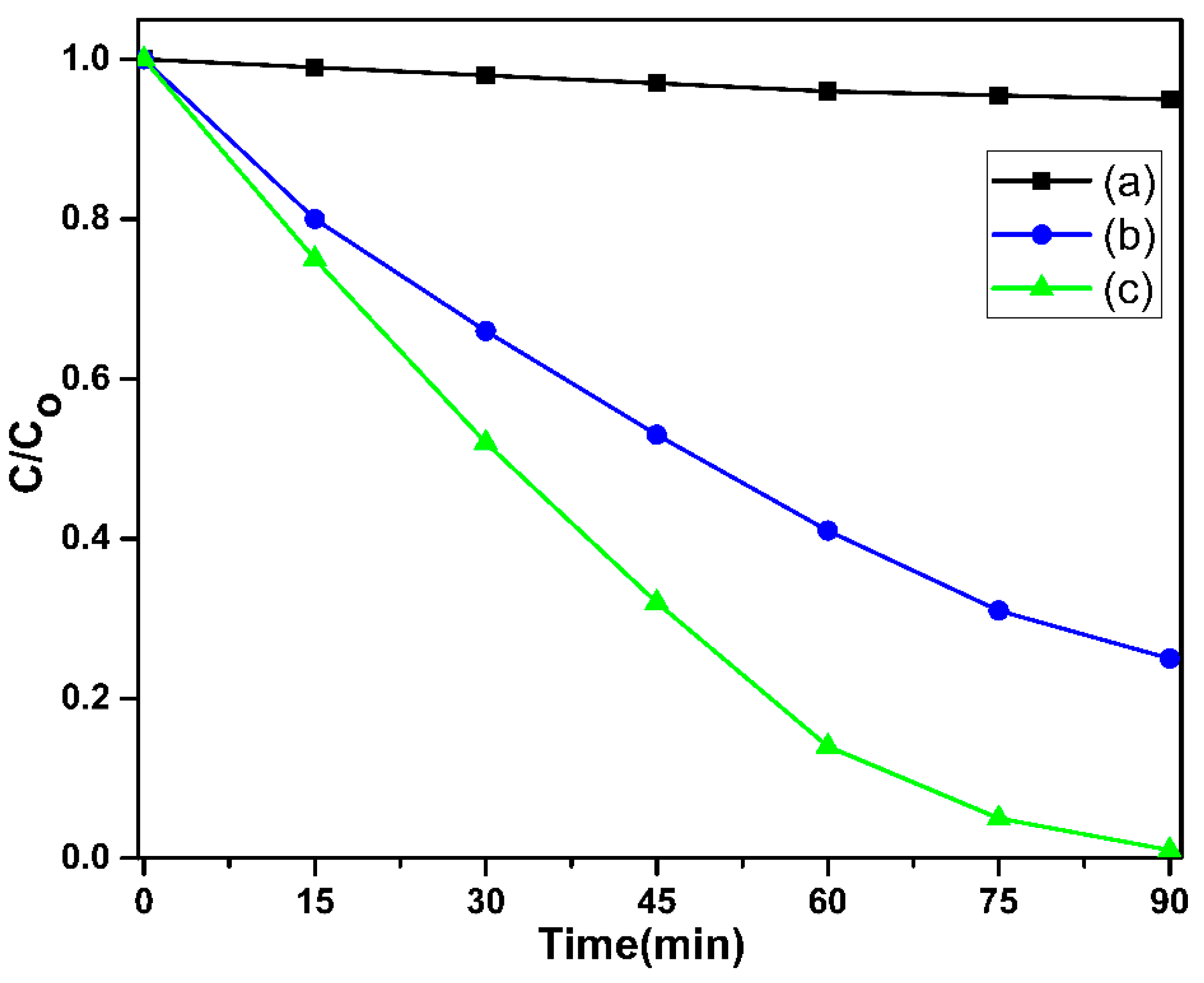
2.4. Photocatalytic Activity
2.5. Antibacterial Activity
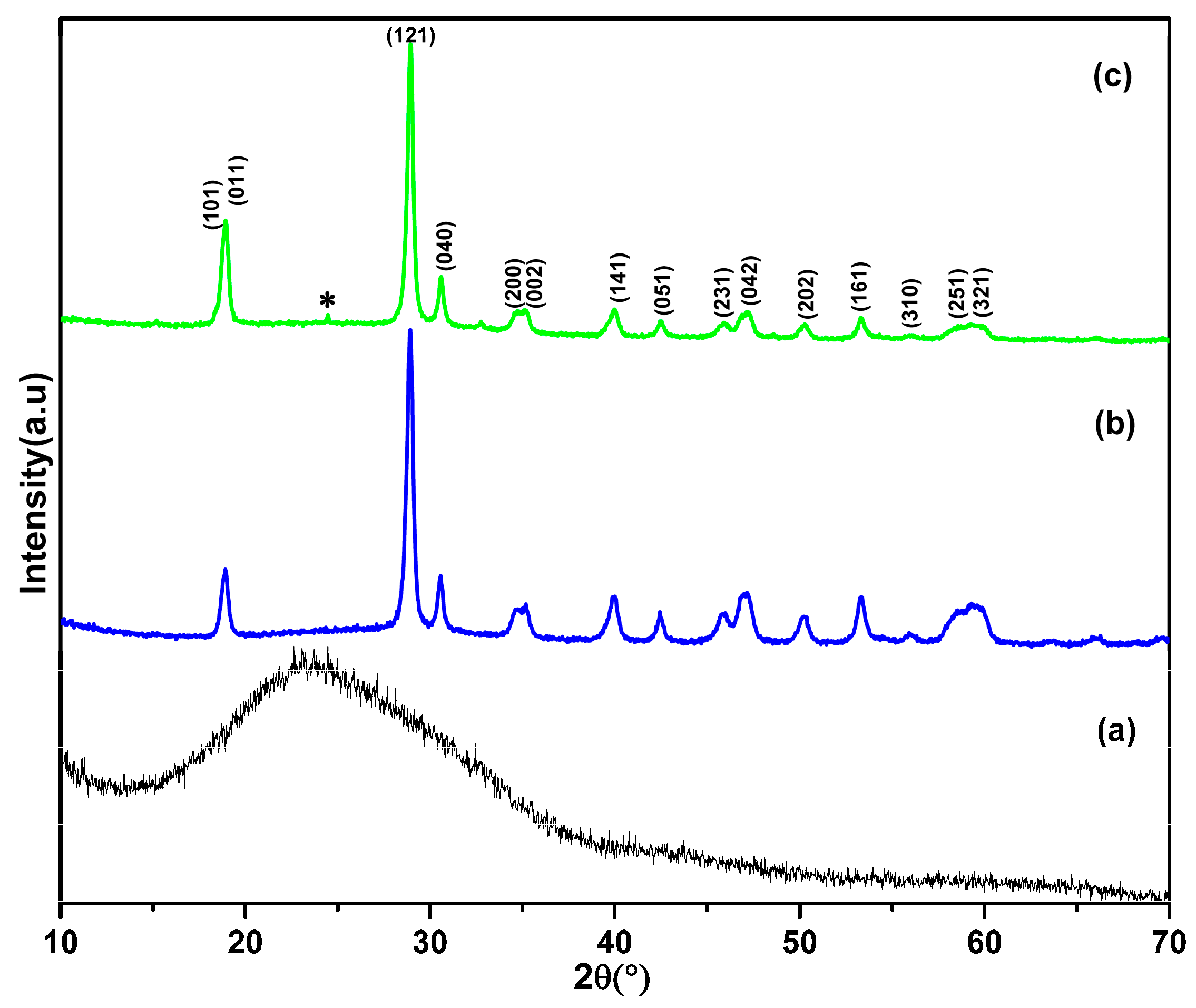
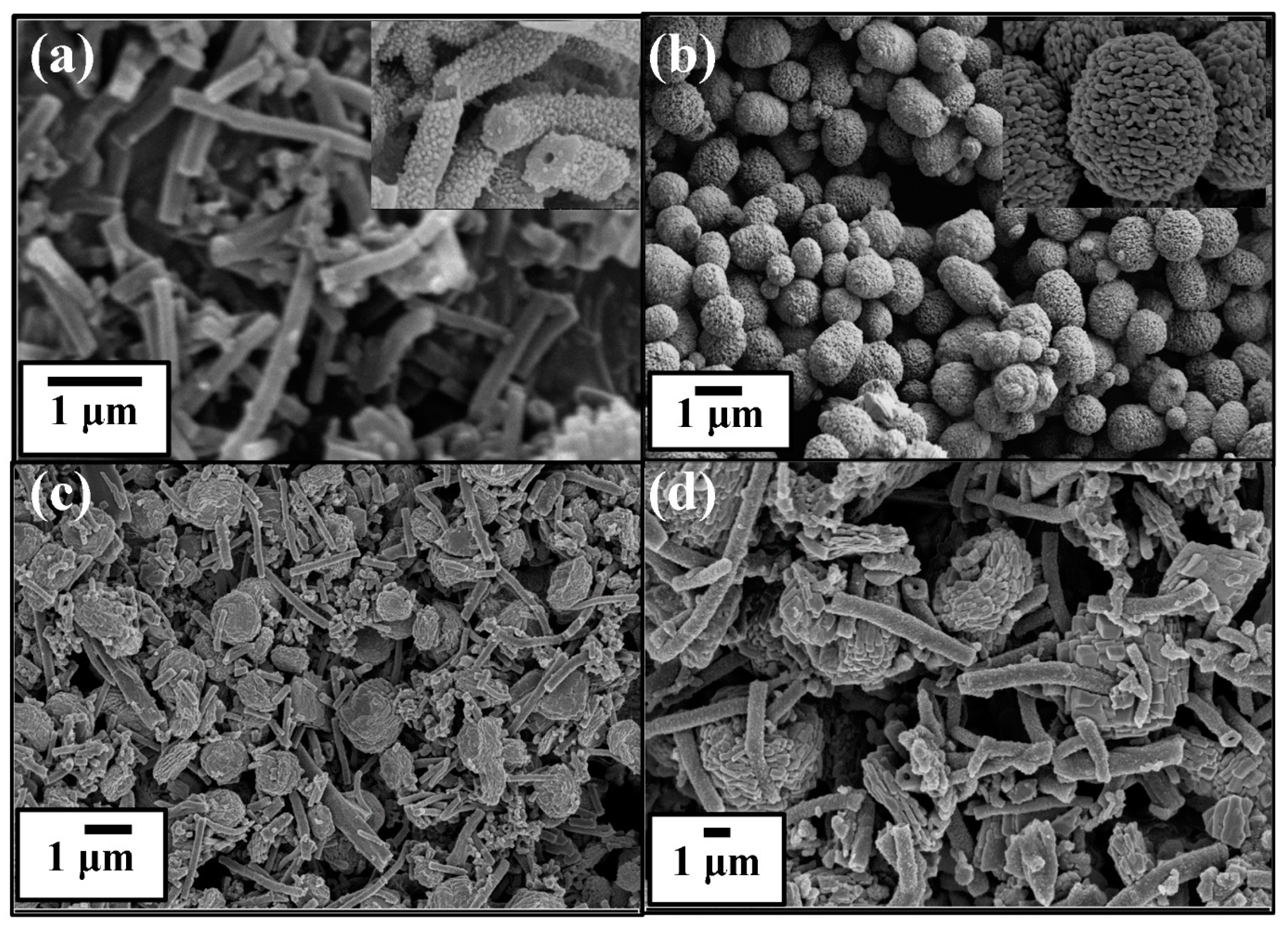
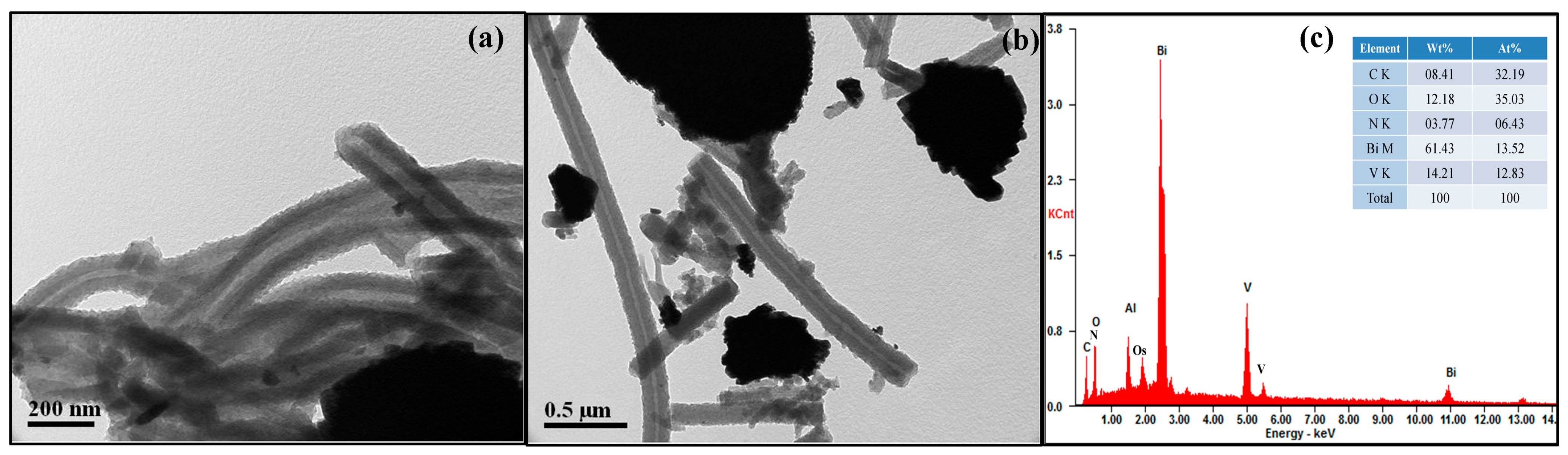
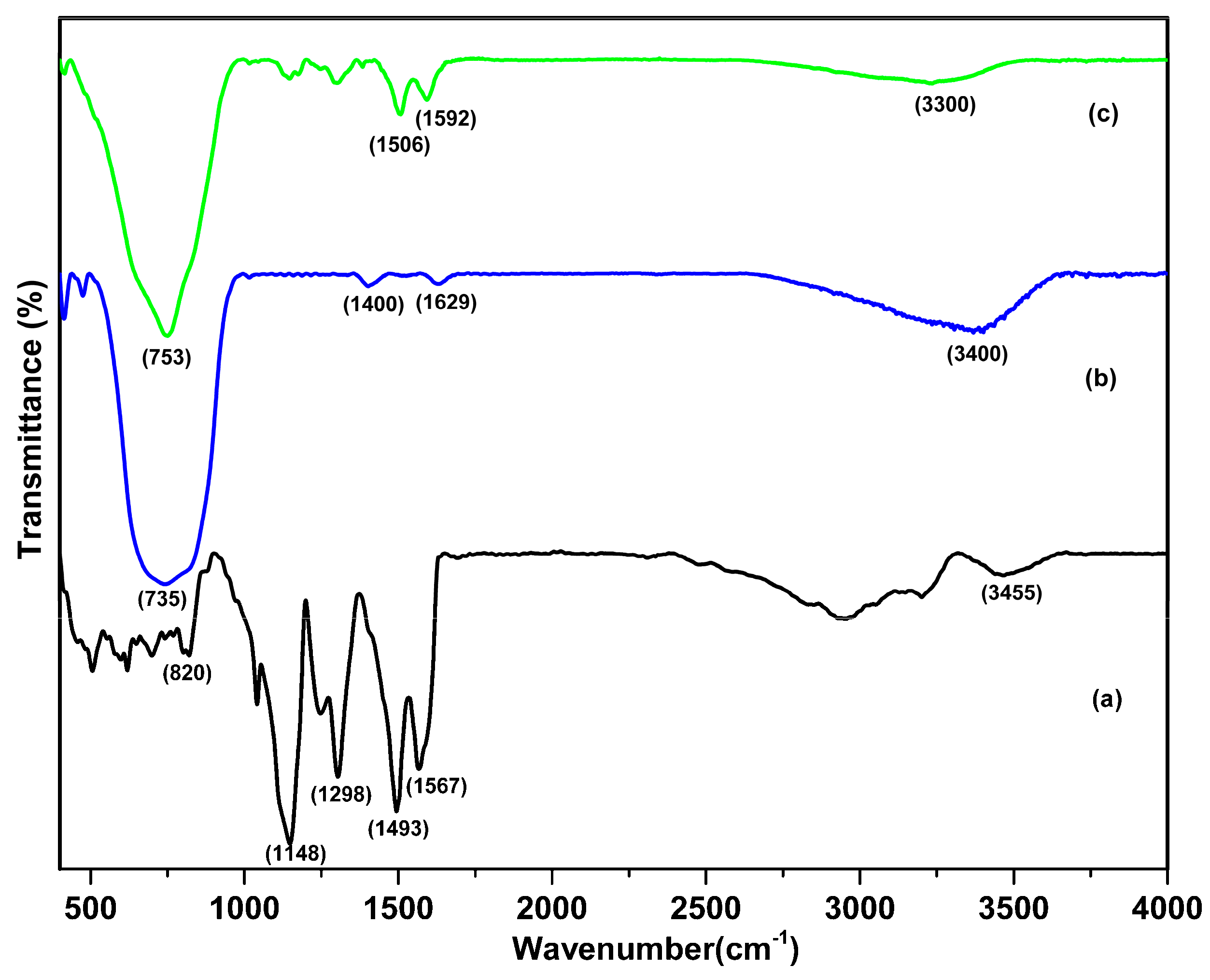
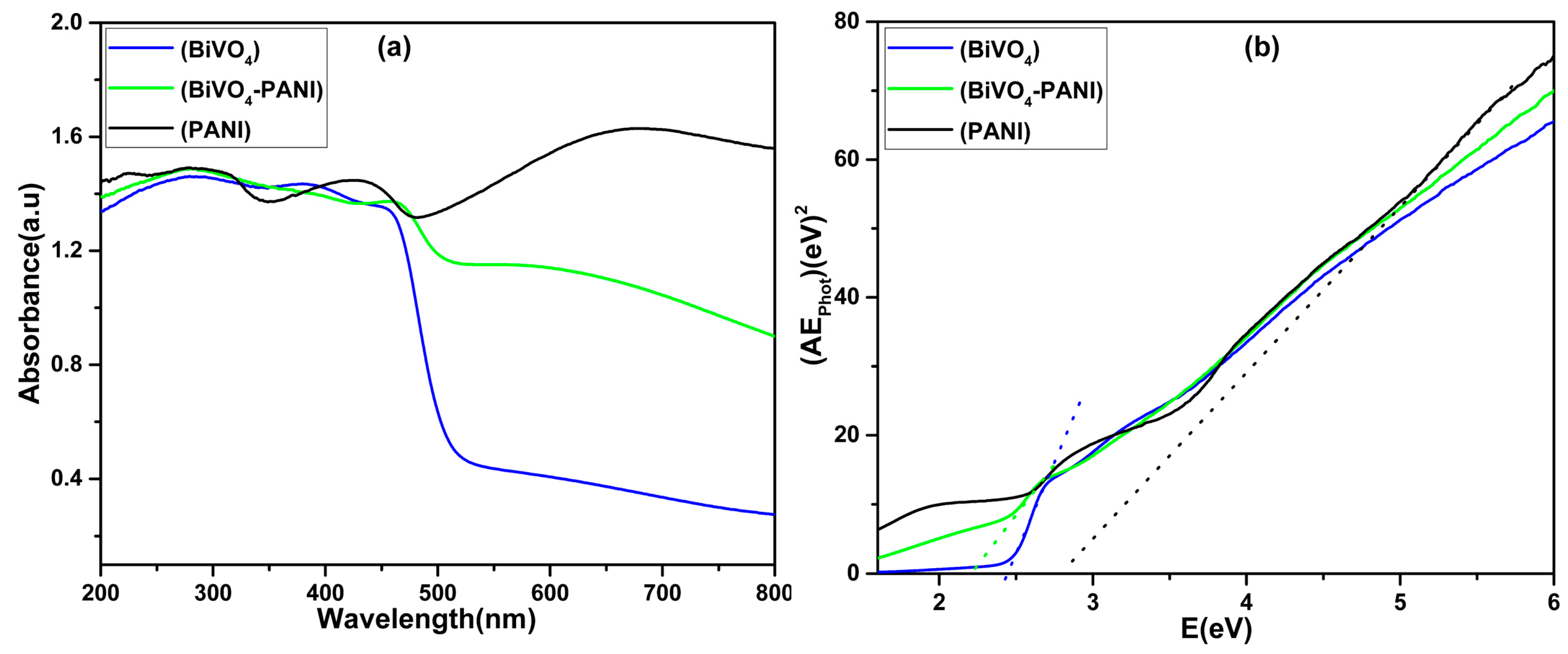
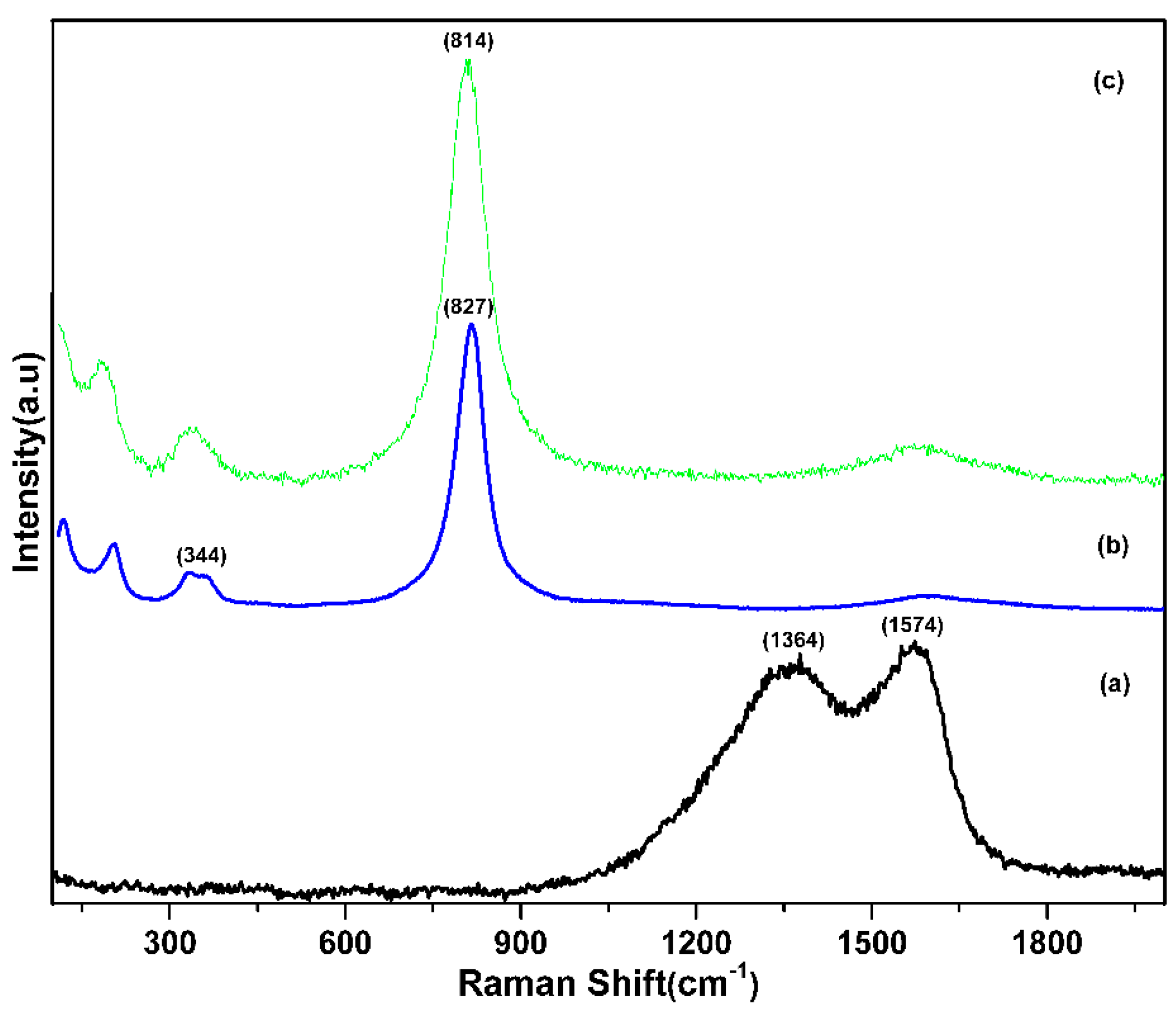
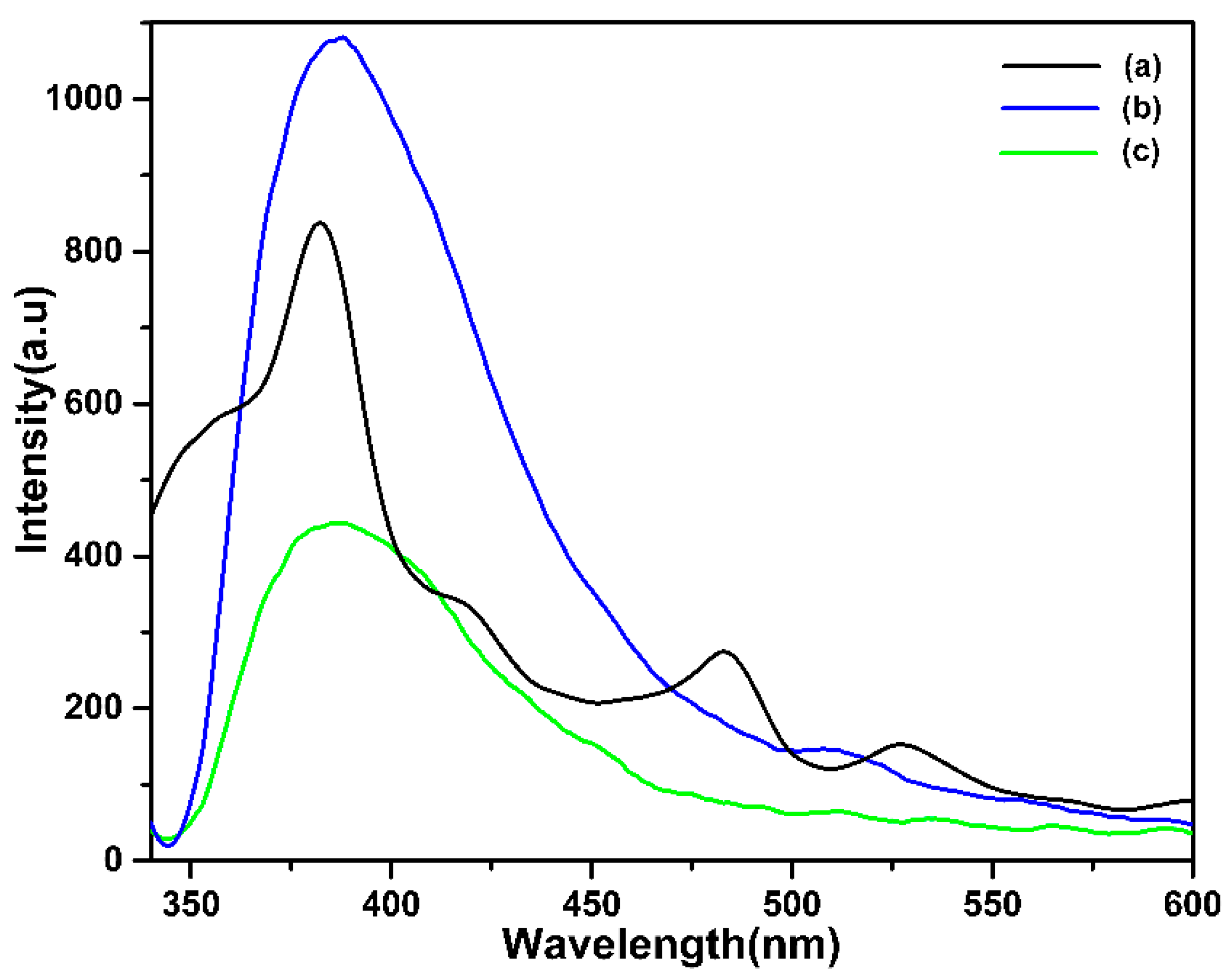
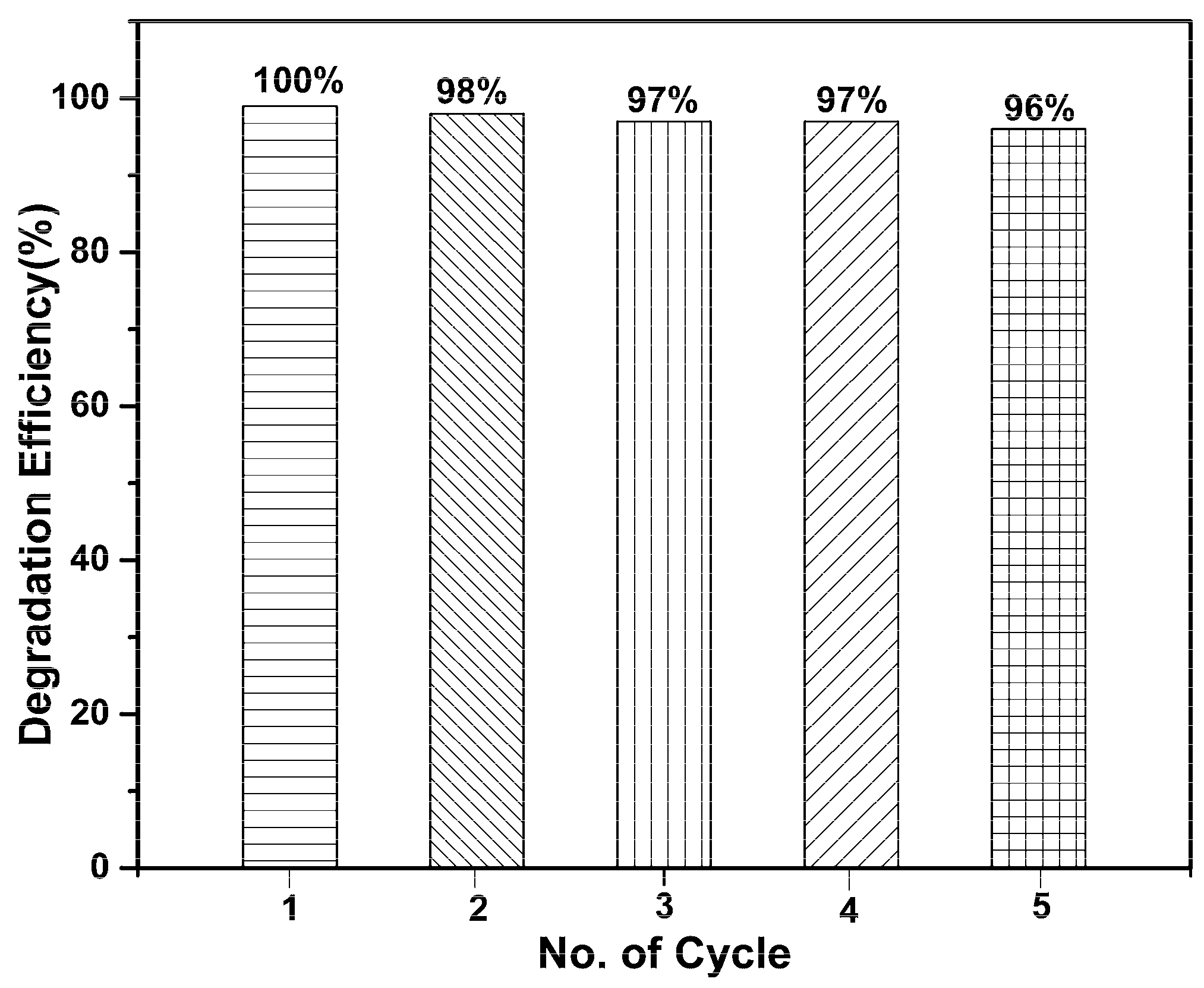
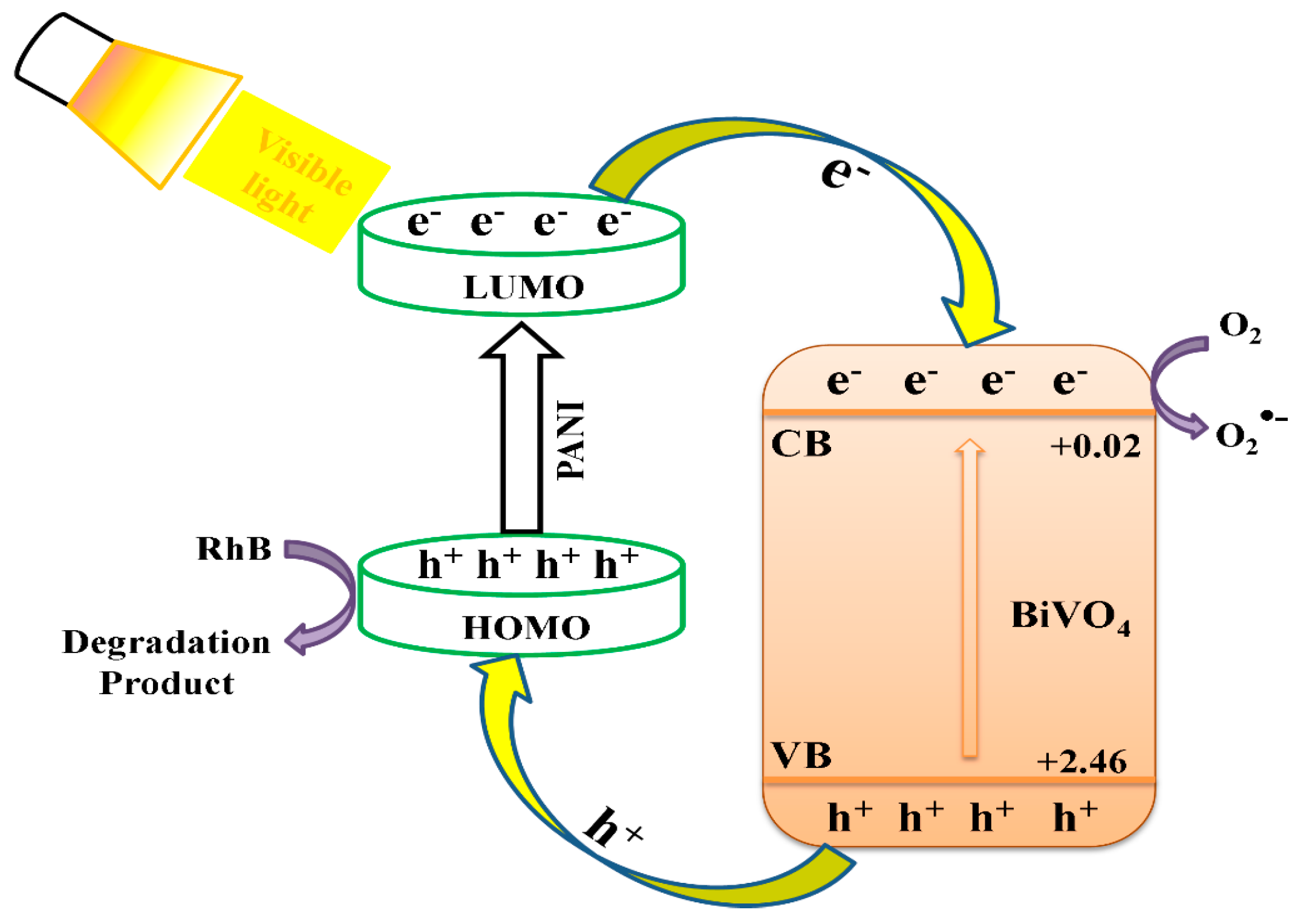
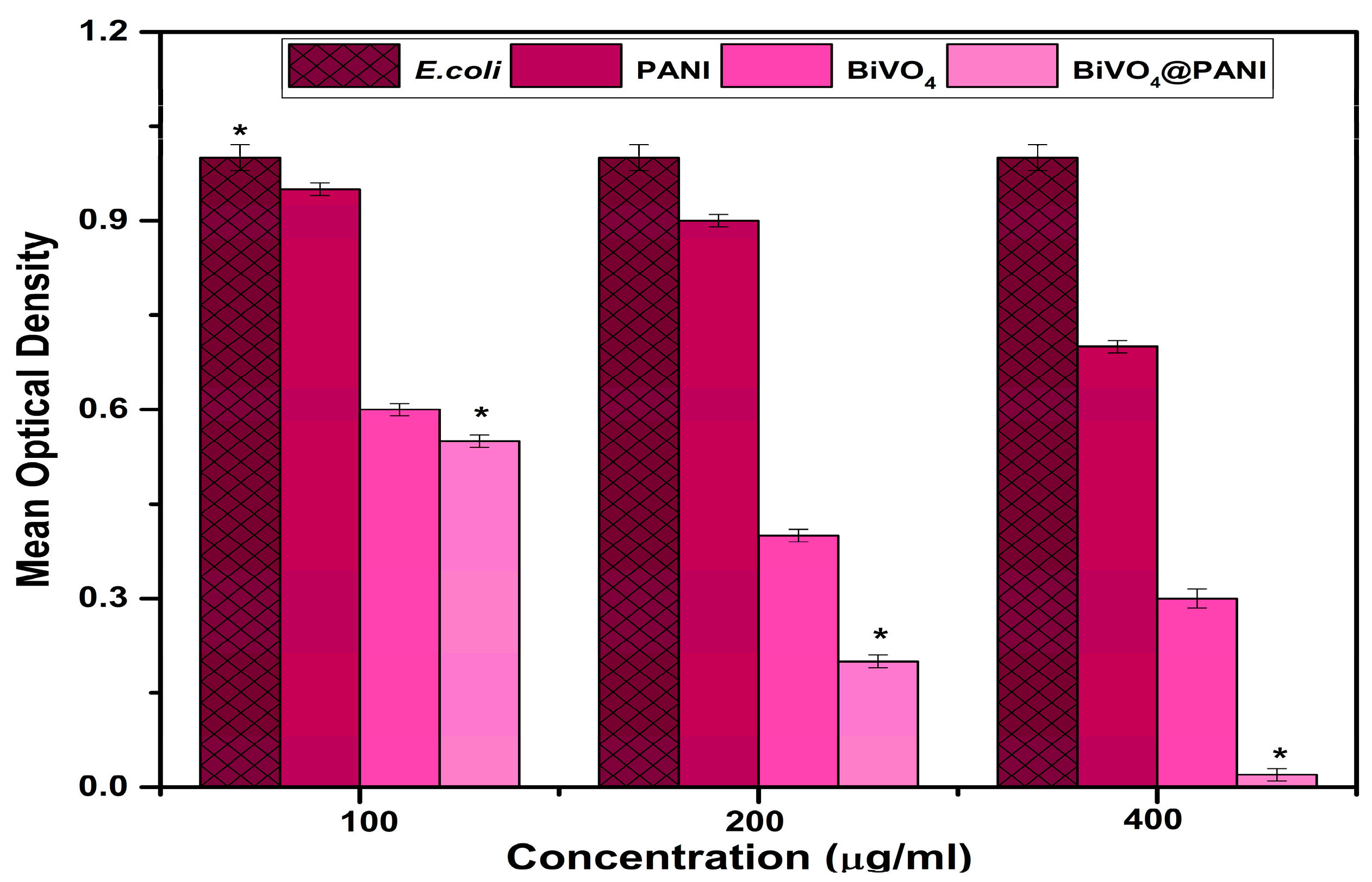
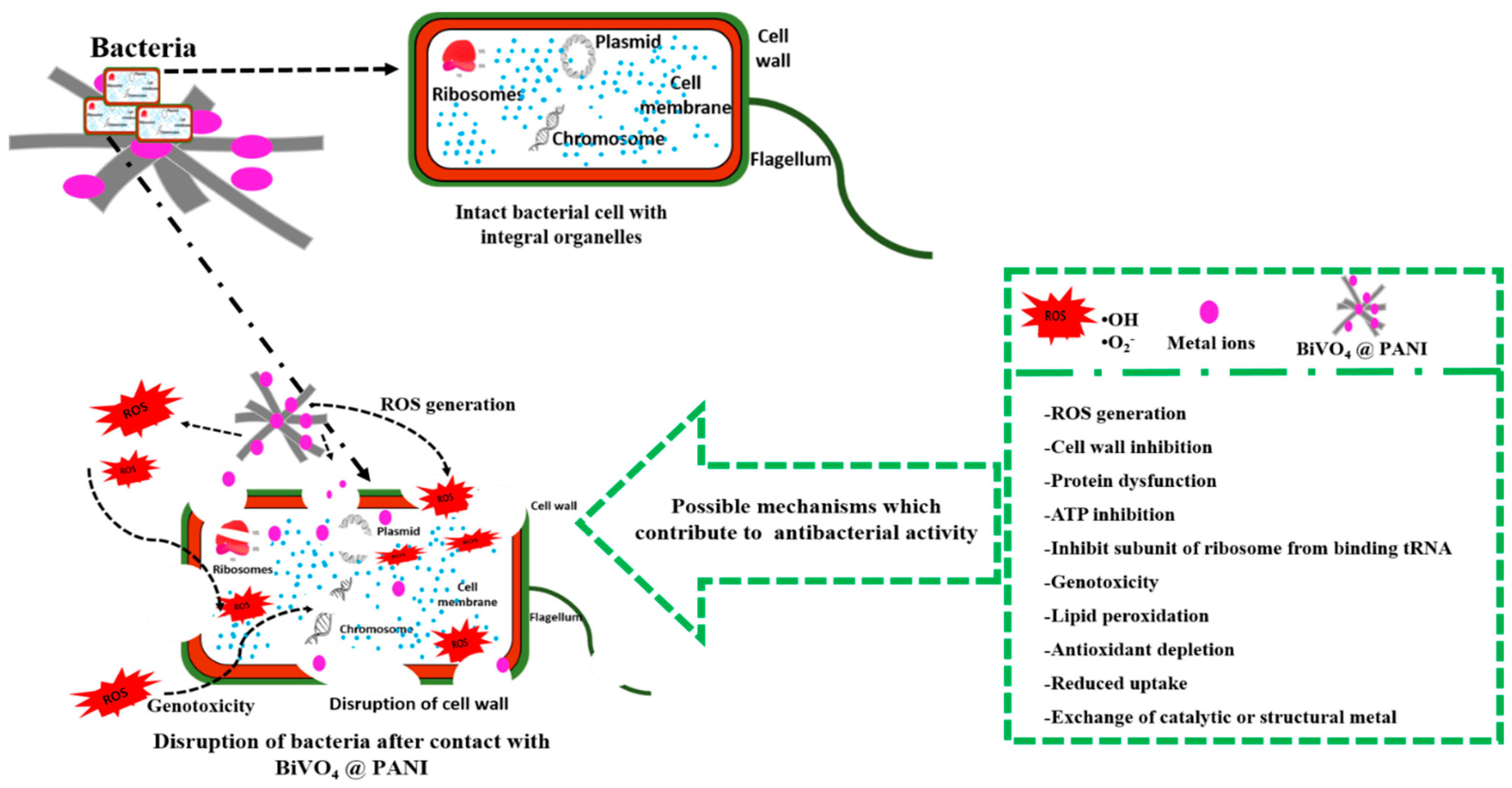
3. Results and Discussion
| Polyaniline Polymeric Nanocomposites | Application | Reference |
|---|---|---|
| PANI/TiO2 nanocomposite | Photocatalysis | [57] |
| PANI/CdO nanocomposite | Photocatalysis | [58] |
| PANI/ZnO nanocomposite | Photocatalysis | [59] |
| Fe3O4 @PANI/TiO2 | Photocatalysis | [60] |
| Al-doped ZnO-PANI | Photocatalysis | [61] |
| PANI/PVA (polyvinyl alcohol)/Ag | Antimicrobial | [62] |
| PANI@ZnO | Antimicrobial | [63] |
| PANI/Pt-Pd | Antimicrobial | [64] |
| PANI/Ag–Pt | Antibacterial | [65] |
| PANI-Ag-Au | Antibacterial | [66] |
| PANI-Zn@CuO | Antibacterial | [67] |
| BiVO4@PANI nanocomposite | Antimicrobial and Photocatalysis | This study |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, J.; Xie, Y.; Cao, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Ho, B.S.; Oh, W.-C. Eco-friendly conductive polymer-based nanocomposites, BiVO4/graphene oxide/polyaniline for excellent photocatalytic performance. Polym. Bull. 2020, 77, 4381–4400. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Amna, T. Bactericide gold decorated mulberry fibers for therapeutic and non-therapeutic tenacities. J. Umm Al-Qura Univ. Appl. Sci. 2023. [Google Scholar] [CrossRef]
- Amna, T.; Alghamdi, A.A.; Shang, K.; Hassan, M.S. Nigella Sativa-Coated Hydroxyapatite Scaffolds: Synergetic Cues to Stimulate Myoblasts Differentiation and Offset Infections. Tissue Eng. Regen. Med. 2021, 18, 787–795. [Google Scholar] [CrossRef]
- Hassan, M.S.; Khan, R.; Amna, T.; Yang, J.; Lee, I.-H.; Sun, M.-Y.; EL-Newehy, M.H.; Al-Deyab, S.S.; Khil, M.-S. The influence of synthesis method on size and toxicity of CeO2 quantum dots: Potential in the environmental remediation. Ceram. Int. 2016, 42, 576–582. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Sheikh, F.A.; Seo, H.C.; Kim, H.-C.; Alotaibi, N.; Alshahrani, T.; Khil, M.-S. Natural mulberry biomass fibers doped with silver as an antimicrobial textile: A new generation fabric. Text. Res. J. 2021, 91, 2581–2587. [Google Scholar] [CrossRef]
- Chae, B.W.; Amna, T.; Hassan, M.S.; Al-Deyab, S.S.; Khil, M.-S. CeO2-Cu2O composite nanofibers: Synthesis, characterization photocatalytic and electrochemical application. Adv. Powder Technol. 2017, 28, 230–235. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Babamale, H.F. A short review on the removal of rhodamine B dye using agricultural waste-based adsorbents. Asian J. Chem. Sci 2020, 7, 25–37. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Bhatt, U.; Soni, V. Toxic effects of Rhodamine B on antioxidant system and photosynthesis of Hydrilla verticillata. J. Hazard. Mater. Lett. 2022, 3, 100069. [Google Scholar] [CrossRef]
- Ahmed, M.; Al-Zaqri, N.; Alsalme, A.; Glal, A.; Esa, M. Rapid photocatalytic degradation of RhB dye and photocatalytic hydrogen production on novel curcumin/SnO 2 nanocomposites through direct Z-scheme mechanism. J. Mater. Sci. Mater. Electron. 2020, 31, 19188–19203. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Hassan, M.S. Facile synthesis of unique bismuth vanadate nano-knitted hollow cage and its application in environmental remediation. Z. Für Nat. A 2019, 74, 259–263. [Google Scholar] [CrossRef]
- Mali, S.S.; Park, G.R.; Kim, H.; Kim, H.H.; Patil, J.V.; Hong, C.K. Synthesis of nanoporous Mo: BiVO4 thin film photoanodes using the ultrasonic spray technique for visible-light water splitting. Nanoscale Adv. 2019, 1, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wang, W.; Sun, S.; Ren, J.; Zhou, L.; Zhang, L. Efficient visible light-induced photocatalytic degradation of contaminant by spindle-like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Shang, M.; Yin, W. Photocatalytic degradation of rhodamine B and phenol by solution combustion synthesized BiVO4 photocatalyst. Catal. Commun. 2010, 11, 982–986. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.C.; Ho, C.; Hou, Y.; Fu, X. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 2005, 21, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Gallarato, L.A.; Mulko, L.E.; Dardanelli, M.S.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Synergistic effect of polyaniline coverage and surface microstructure on the inhibition of Pseudomonas aeruginosa biofilm formation. Colloids Surf. B Biointerfaces 2017, 150, 1–7. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- Falak, S.; Shin, B.K.; Huh, D.S. Antibacterial Activity of Polyaniline Coated in the Patterned Film Depending on the Surface Morphology and Acidic Dopant. Nanomaterials 2022, 12, 1085. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci. 2011, 12, 4872–4884. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.-H.; Yuan, C.-S.; Li, H.-W. Preparation and application of immobilized surfactant-modified PANi-CNT/TiO2 under visible-light irradiation. Materials 2017, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wu, J.; Wang, C.; Zhu, F.; Yan, X.; Gu, Y. Mo2CF2/WS2: Two-Dimensional Van Der Waals Heterostructure for Overall Water Splitting Photocatalyst from Five-Step Screening. J. Phys. Chem. Lett. 2023, 14, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Bryaskova, R.; Pencheva, D.; Nikolov, S.; Kantardjiev, T. Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J. Chem. Biol. 2011, 4, 185–191. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. Preparation and characterization of amphiphilic copolymer PVDF-g-PMABS and its application in improving hydrophilicity and protein fouling resistance of PVDF membrane. Appl. Surf. Sci. 2018, 427, 787–797. [Google Scholar] [CrossRef]
- Saraswathi, M.S.S.A.; Rana, D.; Divya, K.; Gowrishankar, S.; Nagendran, A. Versatility of hydrophilic and antifouling PVDF ultrafiltration membranes tailored with polyhexanide coated copper oxide nanoparticles. Polym. Test. 2020, 84, 106367. [Google Scholar] [CrossRef]
- Falak, S.; Shin, B.K.; Huh, D.S. Single-Step Pore-Selective Silver-Functionalized Honeycomb-Patterned Porous Polystyrene Film Using a Modified Breath Figure Method. Macromol. Res. 2021, 29, 519–523. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Sun, S.; Zhang, L.; Chang, J. Enhanced photocatalytic activity of Bi2WO6 loaded with Ag nanoparticles under visible light irradiation. Appl. Catal. B Environ. 2009, 92, 50–55. [Google Scholar] [CrossRef]
- Chen, C.; Cao, S.; Yu, W.; Xie, X.; Liu, Q.; Tsang, Y.; Xiao, Y. Adsorption, photocatalytic and sunlight-driven antibacterial activity of Bi2WO6/graphene oxide nanoflakes. Vacuum 2015, 116, 48–53. [Google Scholar] [CrossRef]
- Hassan, M.S.; Tirth, V.; Alorabi, A.Q.; Khan, F.; Algahtani, A.; Amna, T. Bi2WO6 nanoflakes incorporated carbon nanofibers to control biological and chemical pollutants: Bifunctional application. Chem. Eng. Commun. 2022, 209, 844–851. [Google Scholar] [CrossRef]
- Kohtani, S.; Tomohiro, M.; Tokumura, K.; Nakagaki, R. Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts. Appl. Catal. B Environ. 2005, 58, 265–272. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Alqarni, L.S.; Alqahtani, H.S.; Alnaam, Y.A.; Almusabi, S.; Alzharani, A.A. High aspect ratio TiO2–Mn3O4 heterostructure: Proficient nanorods for pathogen inhibition and supercapacitor application. Mater. Sci. Technol. 2023, 1–10. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alorabi, A.Q.; Hassan, M.S.; Amna, T.; Azizi, M. Chitosan-Functionalized Hydroxyapatite-Cerium Oxide Heterostructure: An Efficient Adsorbent for Dyes Removal and Antimicrobial Agent. Nanomaterials 2022, 12, 2713. [Google Scholar] [CrossRef] [PubMed]
- Algethami, J.S.; Hassan, M.S.; Alorabi, A.Q.; Alhemiary, N.A.; Fallatah, A.M.; Alnaam, Y.; Almusabi, S.; Amna, T. Manganese Ferrite–Hydroxyapatite Nanocomposite Synthesis: Biogenic Waste Remodeling for Water Decontamination. Nanomaterials 2022, 12, 1631. [Google Scholar] [CrossRef]
- Jeong, W.-H.; Amna, T.; Ha, Y.-M.; Hassan, M.S.; Kim, H.-C.; Khil, M.-S. Novel PANI nanotube@ TiO2 composite as efficient chemical and biological disinfectant. Chem. Eng. J. 2014, 246, 204–210. [Google Scholar] [CrossRef]
- Bibi, A.; Shakoor, A. Charge transport mechanism in dodecylbenzenesulfonic acid doped polyaniline/carbon black composites. Polym. Polym. Compos. 2021, 29 (Suppl. S9), S1044–S1051. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Q.; Yuan, Y.; Ji, F.; Fan, Z.; Zhou, B. Preparation of BiVO4-graphene nanocomposites and their photocatalytic activity. J. Nanomater. 2014, 2014, 77. [Google Scholar] [CrossRef]
- Singu, B.S.; Srinivasan, P.; Pabba, S. Benzoyl peroxide oxidation route to nano form polyaniline salt containing dual dopants for pseudocapacitor. J. Electrochem. Soc. 2011, 159, A6. [Google Scholar] [CrossRef]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. Int. J. Hydrogen Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Khairy, M. Synthesis, characterization, magnetic and electrical properties of polyaniline/NiFe2O4 nanocomposite. Synth. Met. 2014, 189, 34–41. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.-B.; El-Newehy, M.H.; Al-Deyab, S.S.; Khil, M.-S. Smart copper oxide nanocrystals: Synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf. B Biointerfaces 2012, 97, 201–206. [Google Scholar] [CrossRef]
- Abdalsalam, A.H.; Ati, A.A.; Abduljabbar, A.; Hussein, T.A. Structural, optical, electrical and magnetic studies of PANI/ferrite nanocomposites synthesized by PLD technique. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1084–1093. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Tian, D. The structure, vibrational spectra, and thermal expansion study of AVO4 (A = Bi, Fe, Cr) and Co2V2O7. Materials 2020, 13, 1628. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.; El-Azabawy, R.E. Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Xiong, P.; Chen, Q.; He, M.; Sun, X.; Wang, X. Cobalt ferrite–polyaniline heteroarchitecture: A magnetically recyclable photocatalyst with highly enhanced performances. J. Mater. Chem. 2012, 22, 17485–17493. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, N. Sensitization of narrow band gap Bi2S3 hierarchical nanostructures with polyaniline for its enhanced visible-light photocatalytic performance. Colloid Polym. Sci. 2018, 296, 1479–1489. [Google Scholar] [CrossRef]
- Lotfi, S.; Ouardi, M.E.; Ahsaine, H.A.; Assani, A. Recent progress on the synthesis, morphology and photocatalytic dye degradation of BiVO4 photocatalysts: A review. Catal. Rev. 2022, 1–45. [Google Scholar] [CrossRef]
- Acharya, R.; Pati, S.; Parida, K. A review on visible light driven spinel ferrite-g-C3N4 photocatalytic systems with enhanced solar light utilization. J. Mol. Liq. 2022, 357, 119105. [Google Scholar] [CrossRef]
- Acharya, L.; Mishra, B.P.; Pattnaik, S.P.; Acharya, R.; Parida, K. Incorporating nitrogen vacancies in exfoliated B-doped gC3N4 towards improved photocatalytic ciprofloxacin degradation and hydrogen evolution. New J. Chem. 2022, 46, 3493–3503. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Gulce, H.; Eskizeybek, V.; Haspulat, B.; Sarı, F.; Gülce, A.; Avcı, A. Preparation of a new polyaniline/CdO nanocomposite and investigation of its photocatalytic activity: Comparative study under UV light and natural sunlight irradiation. Ind. Eng. Chem. Res. 2013, 52, 10924–10934. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sarı, F.; Gülce, H.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119, 197–206. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Zhao, C.; Zhang, Q.; Geng, W. Synthesis of magnetically separable Fe3O4@ PANI/TiO2 photocatalyst with fast charge migration for photodegradation of EDTA under visible-light irradiation. Chem. Eng. J. 2016, 303, 282–291. [Google Scholar] [CrossRef]
- Mitra, M.; Ghosh, A.; Mondal, A.; Kargupta, K.; Ganguly, S.; Banerjee, D. Facile synthesis of aluminium doped zinc oxide-polyaniline hybrids for photoluminescence and enhanced visible-light assisted photo-degradation of organic contaminants. Appl. Surf. Sci. 2017, 402, 418–428. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Eslahi, H. Synthesis, characterization and antibacterial properties of a novel nanocomposite based on polyaniline/polyvinyl alcohol/Ag. Arab. J. Chem. 2014, 7, 846–855. [Google Scholar] [CrossRef]
- Patil, S.; Pawar, S.; Chougule, M.; Raut, B.; Godse, P.; Sen, S.; Patil, V. Structural, morphological, optical, and electrical properties of PANi-ZnO nanocomposites. Int. J. Polym. Mater. 2012, 61, 809–820. [Google Scholar] [CrossRef]
- Boomi, P.; Prabu, H.G.; Mathiyarasu, J. Synthesis, characterization and antibacterial activity of polyaniline/Pt–Pd nanocomposite. Eur. J. Med. Chem. 2014, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Boomi, P.; Prabu, H.G.; Mathiyarasu, J. Synthesis and characterization of polyaniline/Ag–Pt nanocomposite for improved antibacterial activity. Colloids Surf. B Biointerfaces 2013, 103, 9–14. [Google Scholar] [CrossRef]
- Boomi, P.; Prabu, H.G.; Manisankar, P.; Ravikumar, S. Study on antibacterial activity of chemically synthesized PANI-Ag-Au nanocomposite. Appl. Surf. Sci. 2014, 300, 66–72. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Luong, J.H.; Gedanken, A. Antimicrobial properties of polyaniline and polypyrrole decorated with zinc-doped copper oxide microparticles. Polymers 2020, 12, 1286. [Google Scholar] [CrossRef]
- Algethami, J.S.; Hassan, M.S.; Amna, T.; Sheikh, F.A.; Alhamami, M.A.M.; Seliem, A.F.; Faisal, M.; Kim, H.Y. Nanotextured CeO2−SnO2 Composite: Efficient Photocatalytic, Antibacterial, and Energy Storage Fibers. Nanomaterials 2023, 13, 1001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algethami, J.S.; Hassan, M.S.; Amna, T.; Alqarni, L.S.; Alhamami, M.A.M.; Seliem, A.F. Bismuth Vanadate Decked Polyaniline Polymeric Nanocomposites: The Robust Photocatalytic Destruction of Microbial and Chemical Toxicants. Materials 2023, 16, 3314. https://doi.org/10.3390/ma16093314
Algethami JS, Hassan MS, Amna T, Alqarni LS, Alhamami MAM, Seliem AF. Bismuth Vanadate Decked Polyaniline Polymeric Nanocomposites: The Robust Photocatalytic Destruction of Microbial and Chemical Toxicants. Materials. 2023; 16(9):3314. https://doi.org/10.3390/ma16093314
Chicago/Turabian StyleAlgethami, Jari S., M. Shamshi Hassan, Touseef Amna, Laila S. Alqarni, Mohsen A. M. Alhamami, and Amal F. Seliem. 2023. "Bismuth Vanadate Decked Polyaniline Polymeric Nanocomposites: The Robust Photocatalytic Destruction of Microbial and Chemical Toxicants" Materials 16, no. 9: 3314. https://doi.org/10.3390/ma16093314
APA StyleAlgethami, J. S., Hassan, M. S., Amna, T., Alqarni, L. S., Alhamami, M. A. M., & Seliem, A. F. (2023). Bismuth Vanadate Decked Polyaniline Polymeric Nanocomposites: The Robust Photocatalytic Destruction of Microbial and Chemical Toxicants. Materials, 16(9), 3314. https://doi.org/10.3390/ma16093314







