Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reconstruction of Femur Bone Model
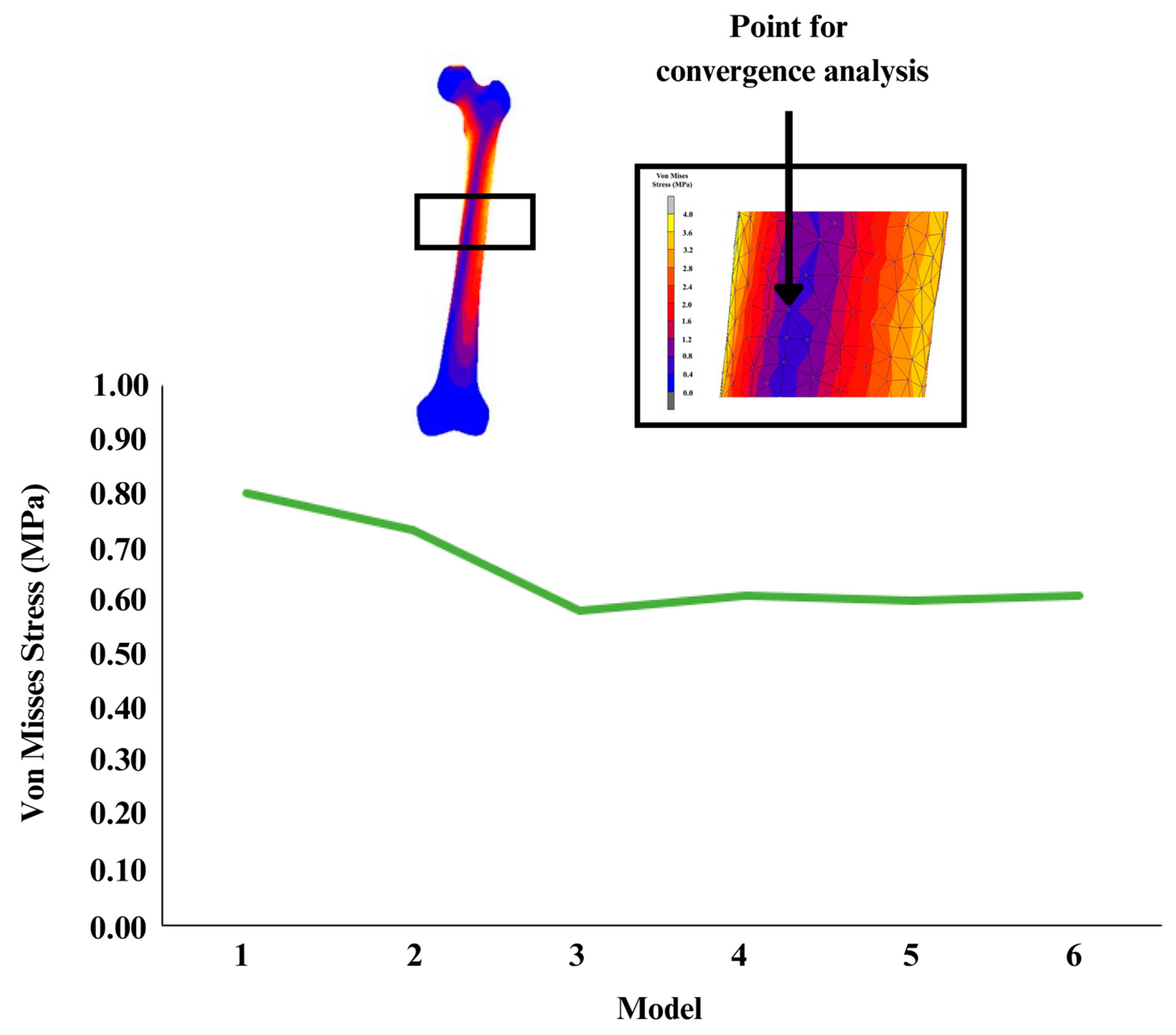
2.2. Mesh Convergence Study
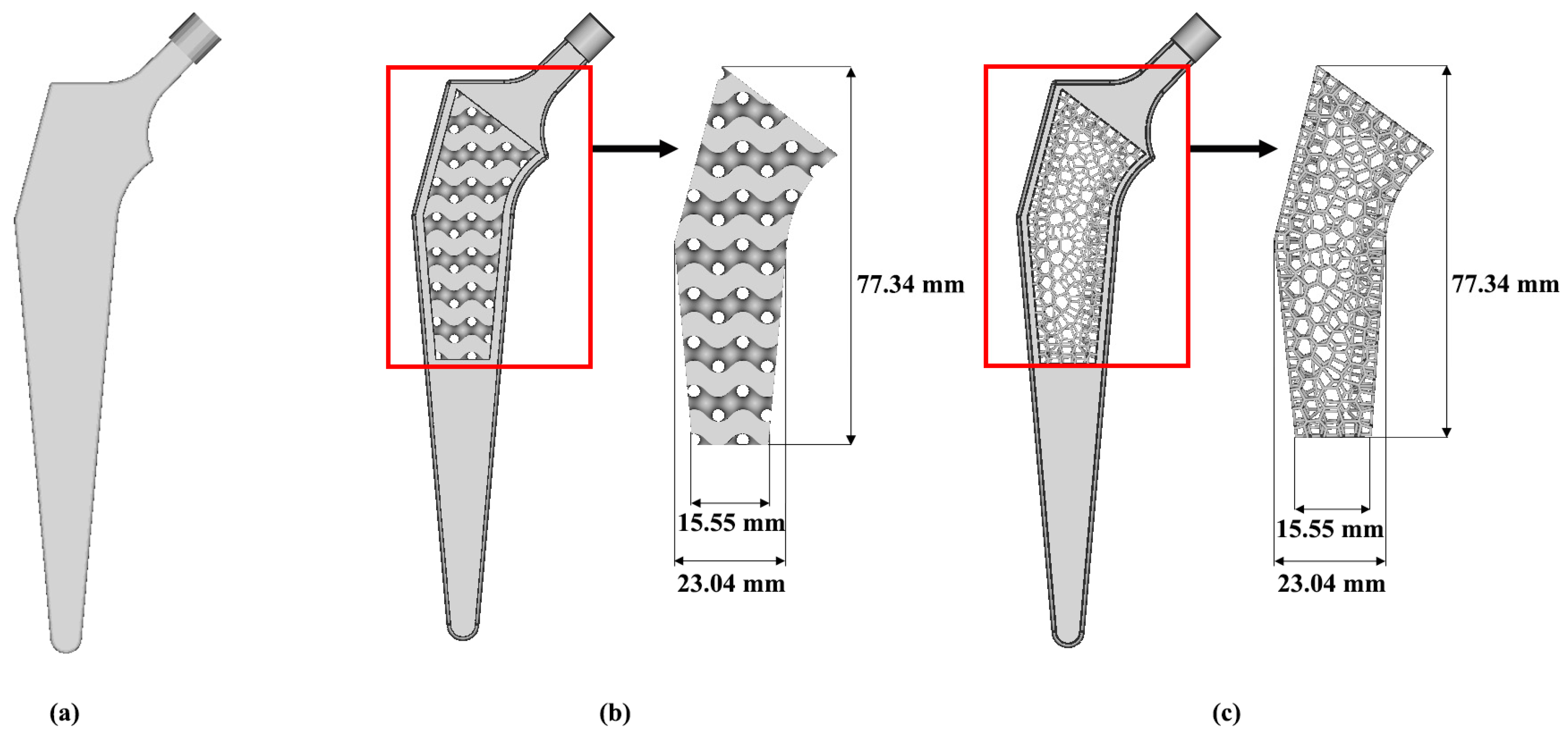
2.3. Porous Hip Implant
2.4. Finite Element Model
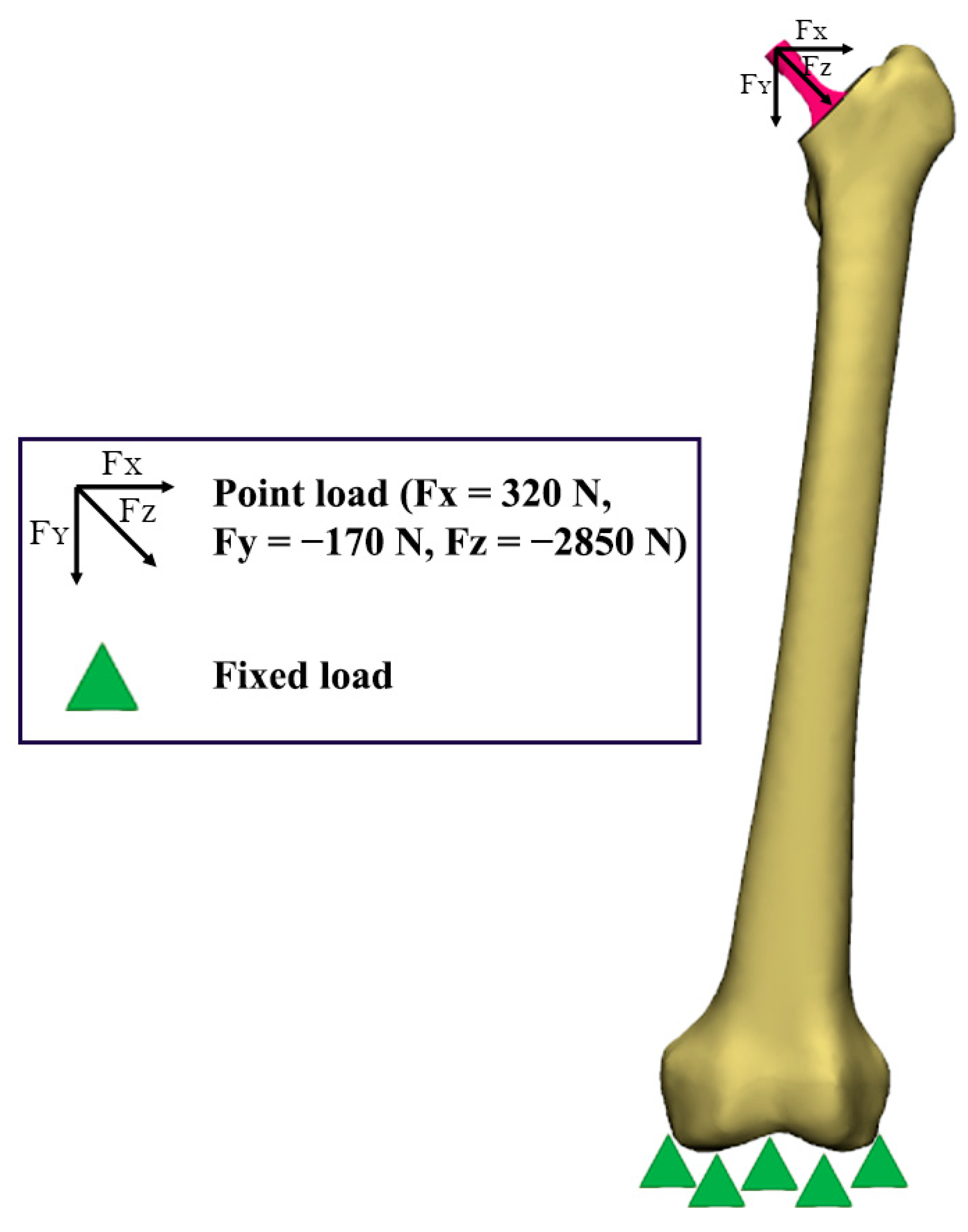
2.5. Boundary Conditions
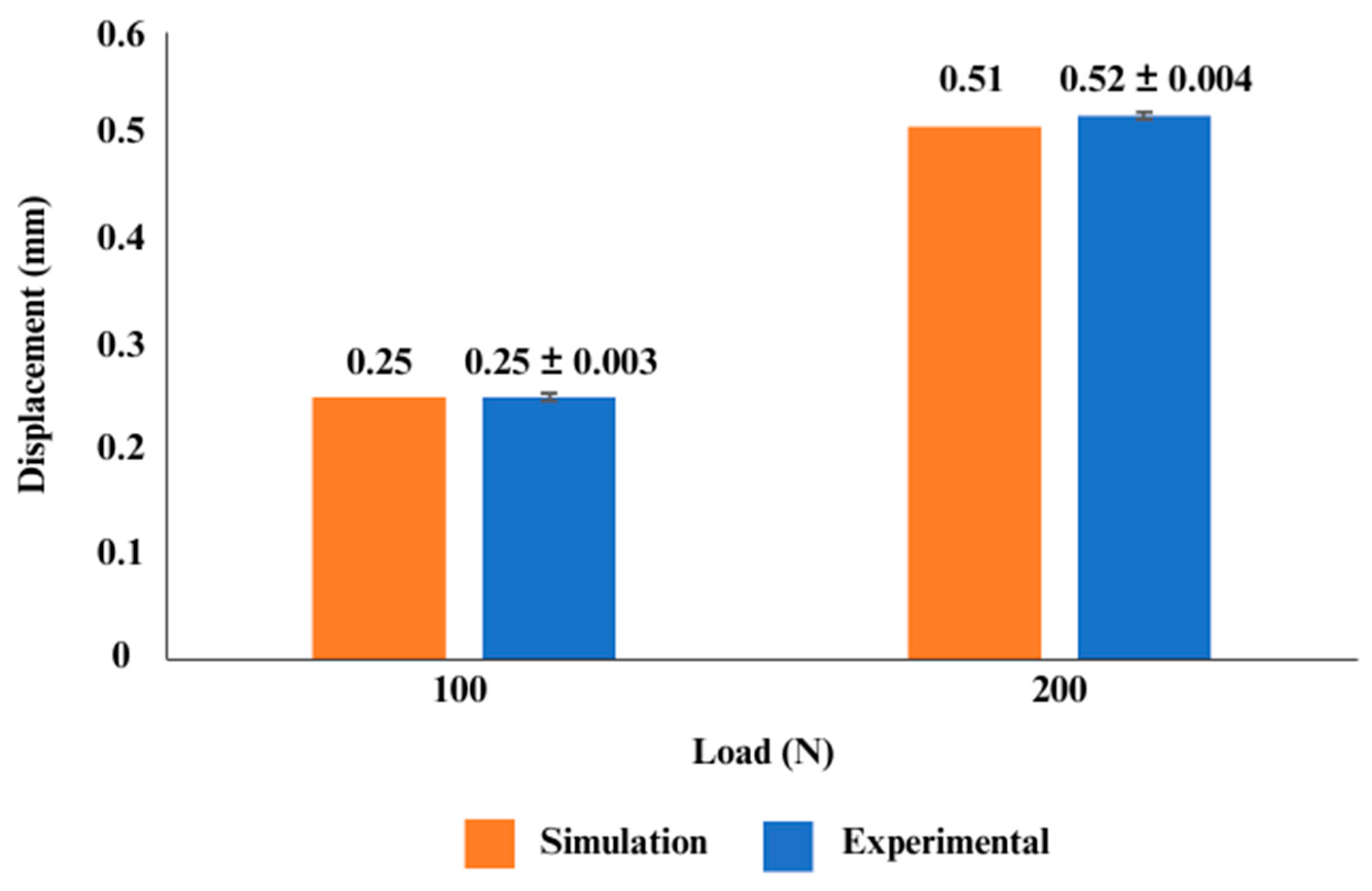
2.6. Model Validation
3. Results
3.1. Weight of Lightweight Hip Implant
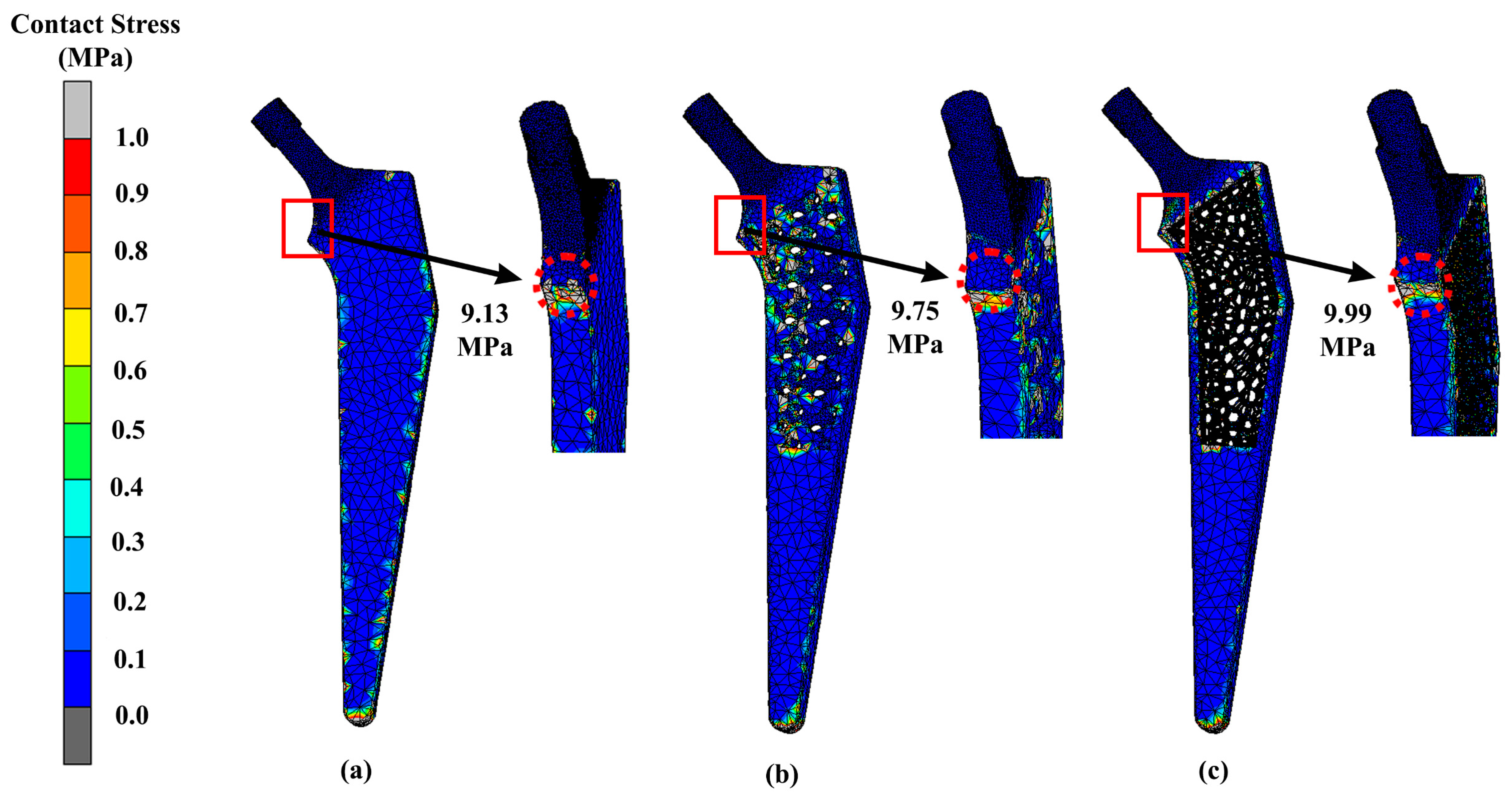
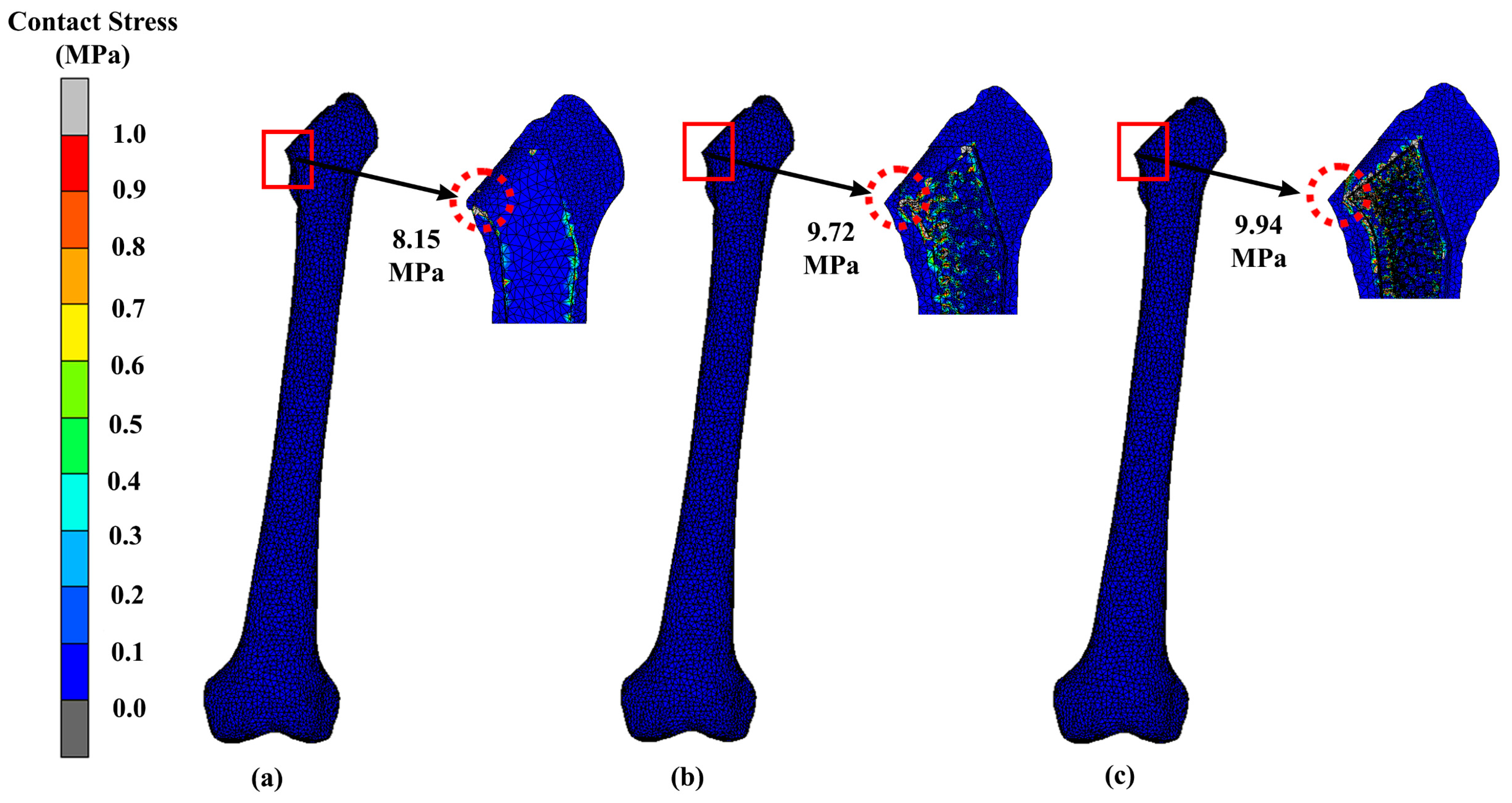
3.2. Stress Distribution
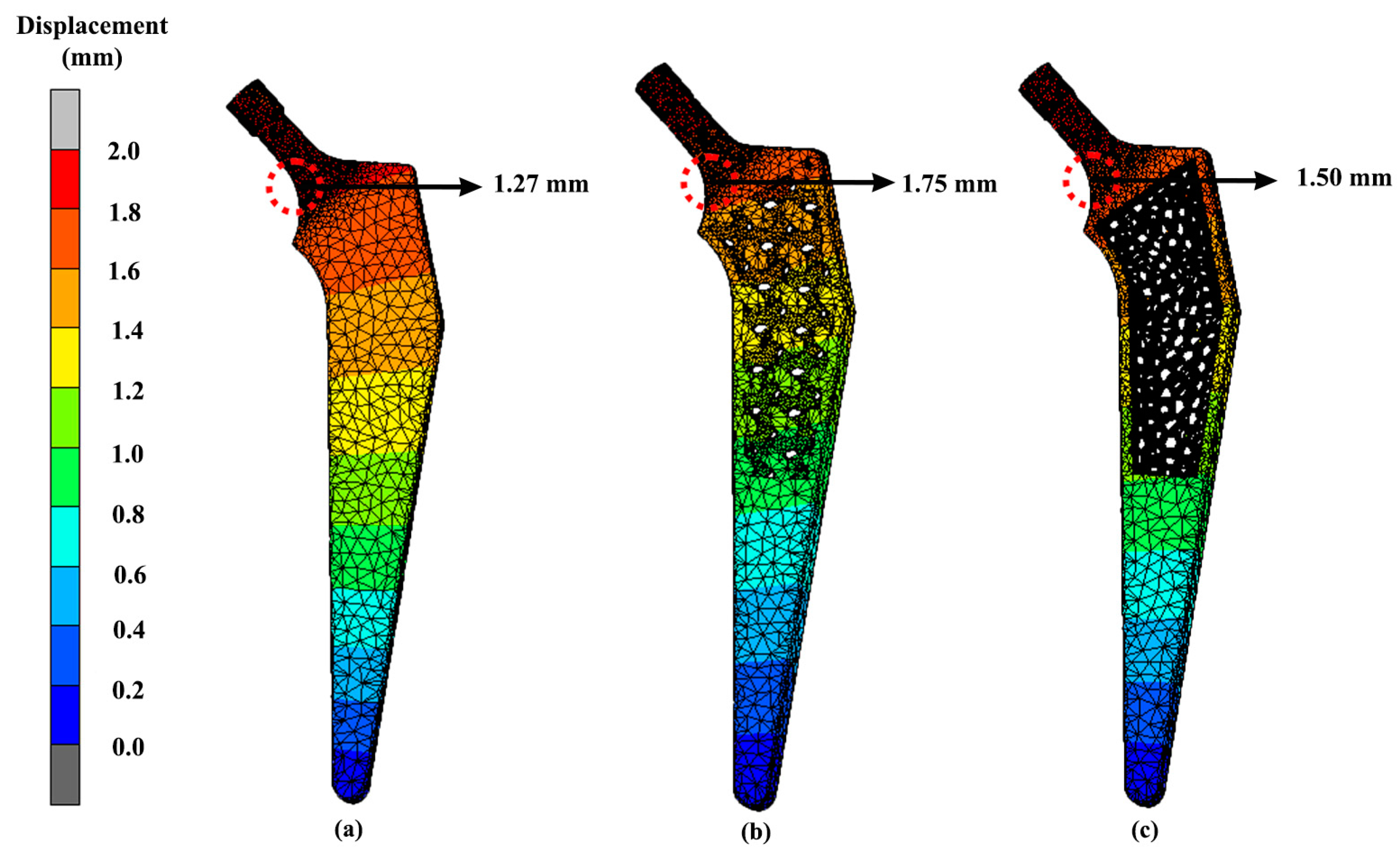
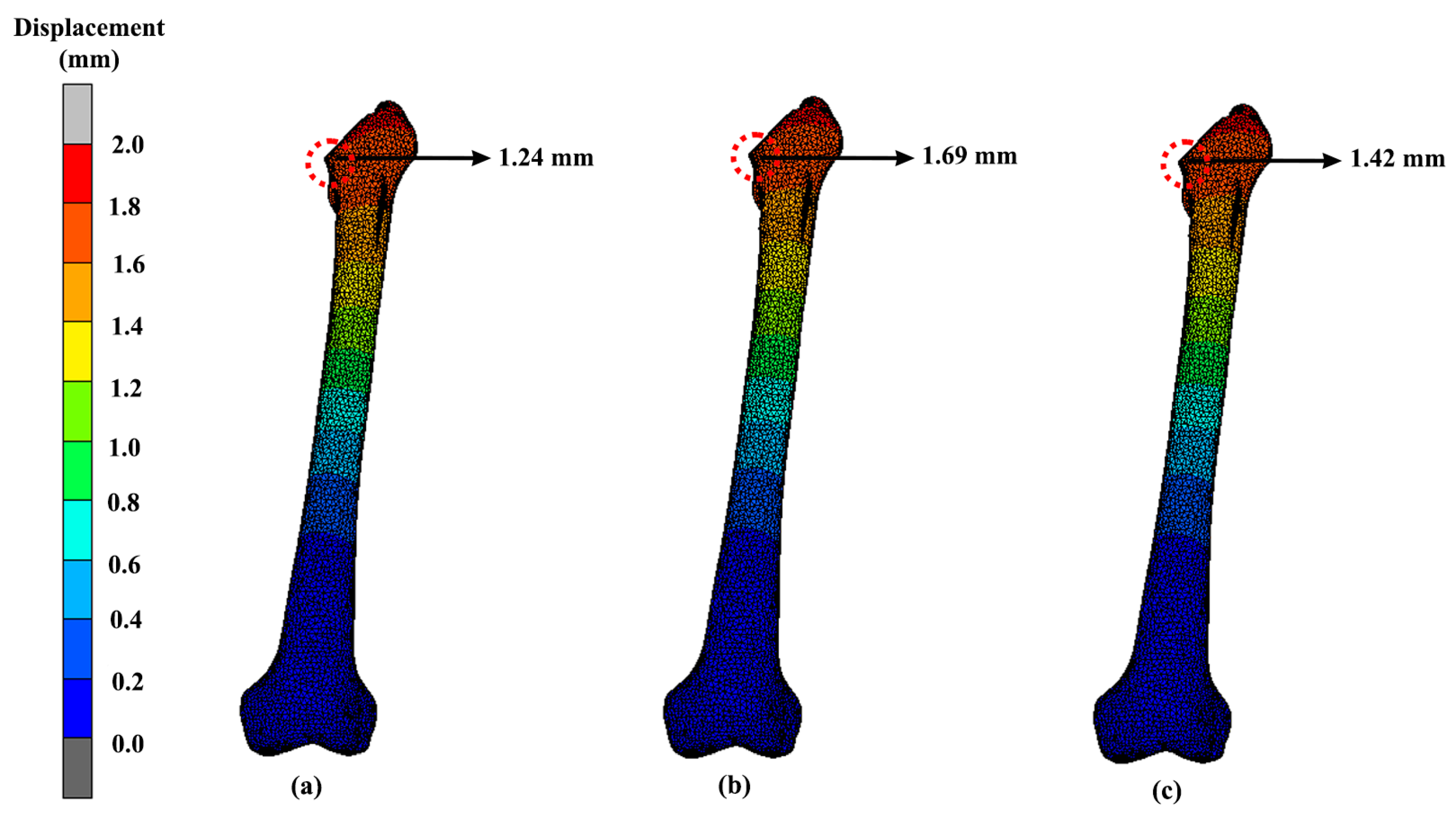
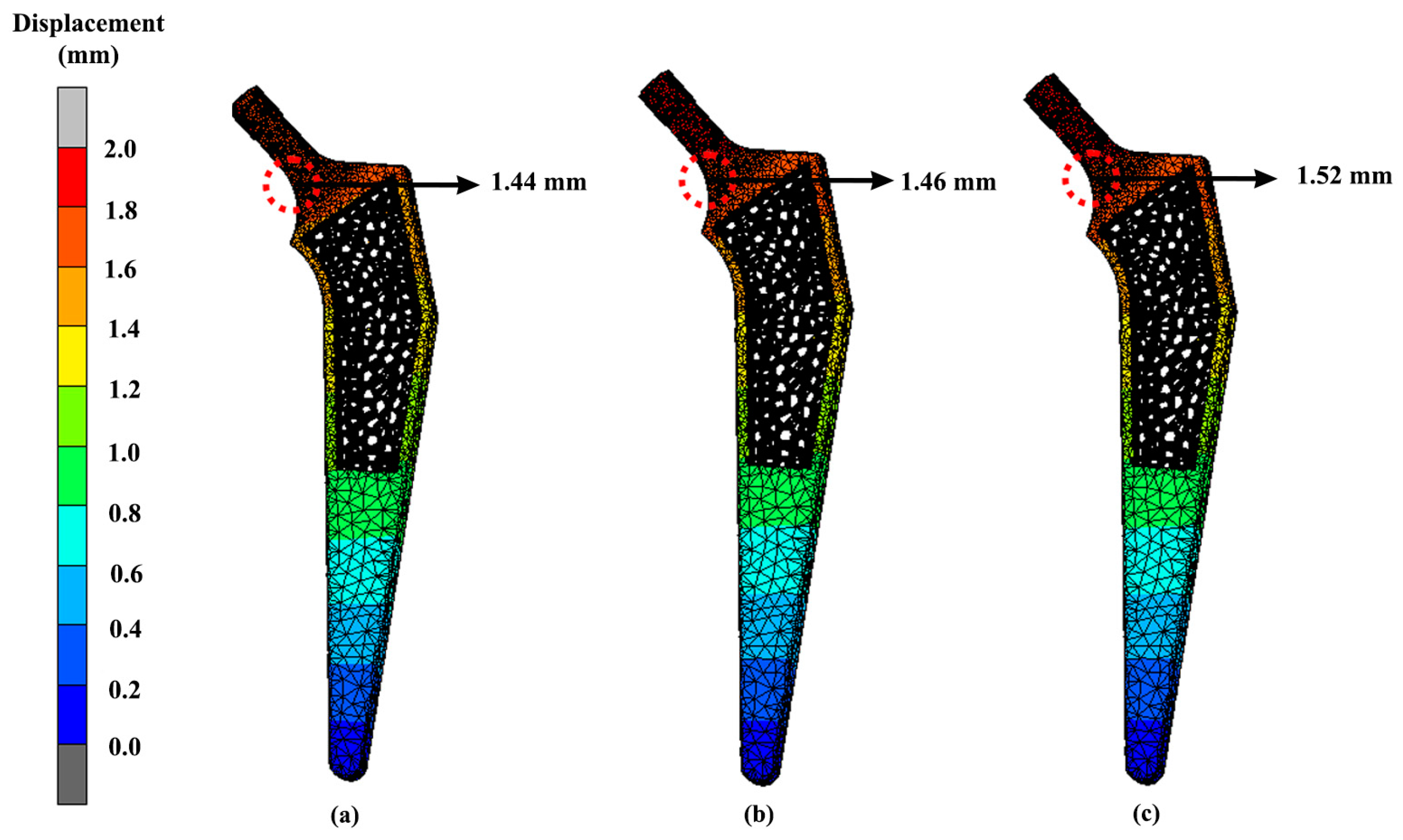
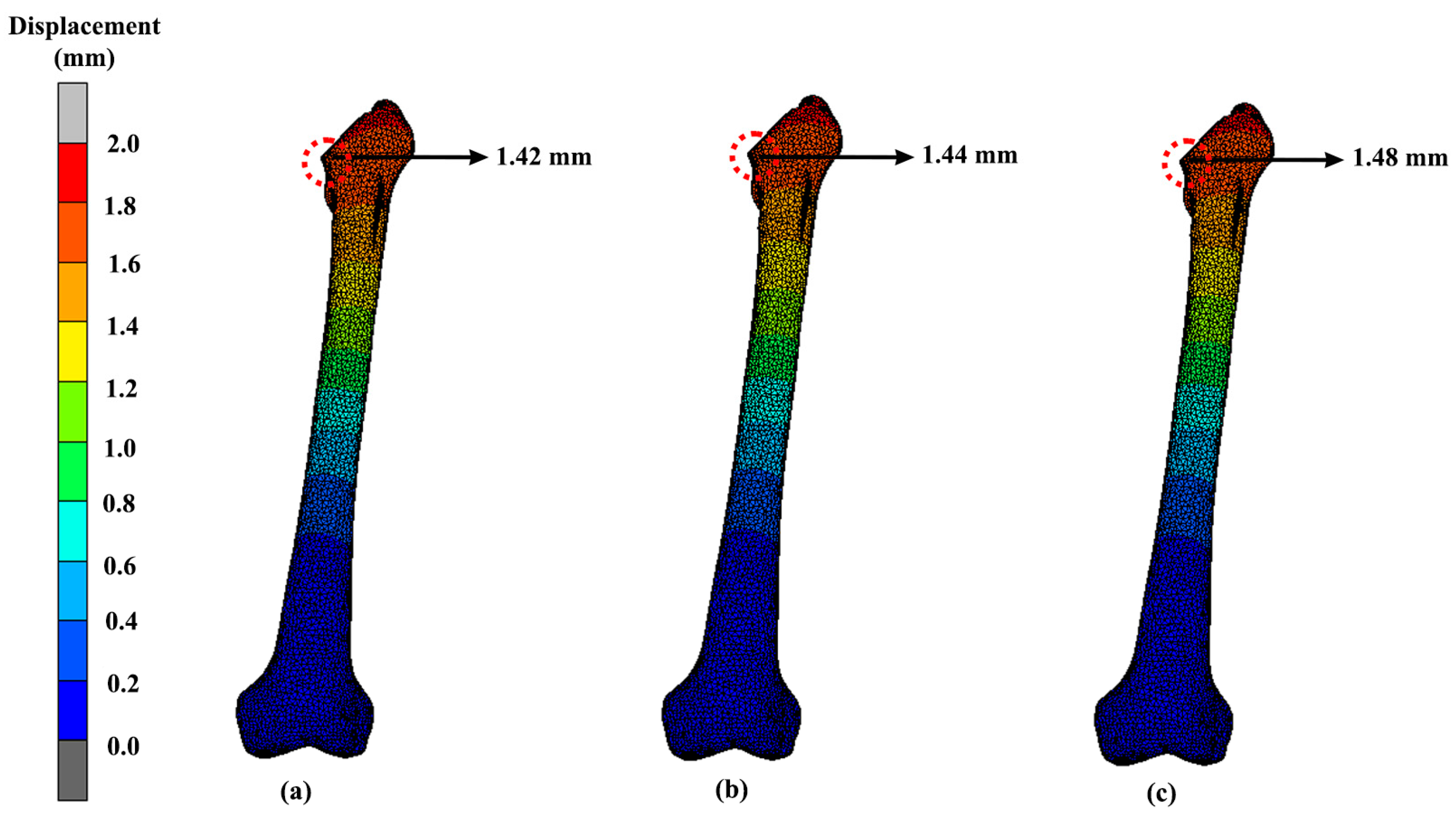
3.3. Displacement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Food and Drug Administration. General Information about Hip Implants. Available online: https://www.fda.gov/medical-devices/metal-metal-hip-implants/general-information-about-hip-implants (accessed on 28 August 2021).
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; Van Der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, E.; Winarni, T.I.; Kurniawan, F.D.; Shiddiq, S.A.; van der Heide, E. Analysis of Contact Pressure in a 3D Model of Dual-Mobility Hip Joint Prosthesis under a Gait Cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted Walking Condition for Computational Simulation Approach on Bearing of Hip Joint Prosthesis: Review over the Past 30 Years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Ammarullah, M.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Delikanli, Y.E.; Kayacan, M.C. Design, Manufacture, and Fatigue Analysis of Lightweight Hip Implants. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001983683. [Google Scholar] [CrossRef]
- Yang, C.-T.; Wei, H.-W.; Kao, H.-C.; Cheng, C.-K. Design and Test of Hip Stem for Medullary Revascularization. Med. Eng. Phys. 2009, 31, 994–1001. [Google Scholar] [CrossRef]
- Polkowski, G.G.; Clohisy, J.C. Hip Biomechanics. Sport. Med. Arthrosc. 2010, 18, 56–62. [Google Scholar] [CrossRef]
- Fiorentino, A.; Zarattini, G.; Pazzaglia, U.; Ceretti, E. Hip Prosthesis Design. Market Analysis, New Perspectives and an Innovative Solution. Procedia CIRP 2013, 5, 310–314. [Google Scholar] [CrossRef]
- Kayacan, M.C.; Baykal, Y.B.; Karaaslan, T.; Özsoy, K.; Alaca, İ.; Duman, B.; Delikanlı, Y.E. Monitoring the Osseointegration Process in Porous Ti6Al4V Implants Produced by Additive Manufacturing: An Experimental Study in Sheep. J. Appl. Biomater. Funct. Mater. 2018, 16, 68–75. [Google Scholar] [CrossRef]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of Surface Roughness of Titanium Implants on Bone Remodeling Activity of Femur in Rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Moulton, D.L.; Lindsey, R.W.; Gugala, Z.; Latta, L. Proximal Femur Size and Geometry in Cementless Total Hip Arthroplasty Patients. F1000Research 2015, 4, 161. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Lee, K.R. Total Hip Arthroplasty with an Uncemented Tapered Femoral Component in Patients Younger than 50 Years of Age: A Minimum 20-Year Follow-up Study. J. Arthroplast. 2016, 31, 1275–1278. [Google Scholar] [CrossRef]
- Alkentar, R.; Kladovasilakis, N.; Tzetzis, D.; Mankovits, T. Effects of Pore Size Parameters of Titanium Additively Manufactured Lattice Structures on the Osseointegration Process in Orthopedic Applications: A Comprehensive Review. Crystals 2023, 13, 113. [Google Scholar] [CrossRef]
- Sivarasu, S.; Beulah, P.; Mathew, L. Light Weight Femoral Stem Optimization Based on Design and Materials. Biomed. Eng. Appl. Basis Commun. 2011, 23, 37–43. [Google Scholar] [CrossRef]
- Alkentar, R.; Máté, F.; Mankovits, T. Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method. Materials 2022, 15, 6335. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Mughal, U.N.; Khawaja, H.A.; Moatamedi, M. Finite Element Analysis of Human Femur Bone. Int. J. Multiphys 2015, 9, 101–108. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca Stress Study of CoCrMo-on-CoCrMo Bearings Based on Body Mass Index Using 2D Computational Model. J. Tribol. 2022, 33, 31–38. [Google Scholar]
- De Sterck, H.; Manteuffel, T.; McCormick, S.; Nolting, J.; Ruge, J.; Tang, L. Efficiency-Basedh-Andhp-Refinement Strategies for Finite Element Methods. Numer. Linear Algebr. Appl. 2008, 15, 89–114. [Google Scholar] [CrossRef]
- Ramlee, M.H.; Abdul Kadir, M.R.; Murali, M.R.; Kamarul, T. Biomechanical Evaluation of Two Commonly Used External Fixators in the Treatment of Open Subtalar Dislocation—A Finite Element Analysis. Med. Eng. Phys. 2014, 36, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pani, M.; Taddei, F.; Mazzà, C.; Li, X.; Viceconti, M. Large-Scale Finite Element Analysis of Human Cancellous Bone Tissue Micro Computer Tomography Data: A Convergence Study. J. Biomech. Eng. 2014, 136, 101013. [Google Scholar] [CrossRef]
- Jetté, B.; Brailovski, V.; Simoneau, C.; Dumas, M.; Terriault, P. Development and in vitro Validation of a Simplified Numerical Model for the Design of a Biomimetic Femoral Stem. J. Mech. Behav. Biomed. Mater. 2018, 77, 539–550. [Google Scholar] [CrossRef]
- Kim, J.-T.; Jung, C.-H.; Shen, Q.H.; Cha, Y.-H.; Park, C.H.; Yoo, J.-I.; Song, H.K.; Jeon, Y.; Won, Y.-Y. Mechanical Effect of Different Implant Caput-Collum-Diaphyseal Angles on the Fracture Surface after Fixation of an Unstable Intertrochanteric Fracture: A Finite Element Analysis. Asian J. Surg. 2019, 42, 947–956. [Google Scholar] [CrossRef]
- CORAIL® HIP SYSTEM; Warsaw, Poland. 2016. Available online: https://tehranarkak.net/files/1245657_DSUSJRC01161350%20Corail%20ST-total%20hip%20system.pdf (accessed on 4 March 2023).
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Azmat, A.; Asrar, S.; Channa, I.A.; Ashfaq, J.; Ali Chandio, I.; Chandio, A.D.; Ali Shar, M.; AlSalhi, M.S.; Devanesan, S. Comparative Study of Biocompatible Titanium Alloys Containing Non-Toxic Elements for Orthopedic Implants. Crystals 2023, 13, 467. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef]
- Abd Aziz, A.U.; Abdul Wahab, A.H.; Abdul Rahim, R.A.; Abdul Kadir, M.R.; Ramlee, M.H. A Finite Element Study: Finding the Best Configuration between Unilateral, Hybrid, and Ilizarov in Terms of Biomechanical Point of View. Injury 2020, 51, 2474–2478. [Google Scholar] [CrossRef]
- Mohammad Kata, N.; Zainal Abidin, N.A.; Abd Aziz, A.U.; Abdullah, A.H.; Wui, N.B.; Nasution, A.K.; Abdul Kadir, M.R.; Ramlee, M.H. Finite Element Analysis of External Fixator for Treating Femur Fracture: Analysis on Stainless Steel and Titanium as Material of External Fixator. Malays. J. Fundam. Appl. Sci. 2021, 17, 274–284. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Cook, G.E.; Samiezadeh, S.; Morison, Z.; Aziz, M.S.R.; Bougherara, H.; Zdero, R.; Schemitsch, E.H. Biomechanical Optimization of the Angle and Position for Surgical Implantation of a Straight Short Stem Hip Implant. Med. Eng. Phys. 2017, 39, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Chowdhury, M.E.H.; Islam, M.T.; Musharavati, F.; Mahmud, S.; Hafizh, M.; Ayari, M.A.; Khandakar, A.; Alam, M.K.; Nezhad, E.Z. Design and Performance Evaluation of a Novel Spiral Head-Stem Trunnion for Hip Implants Using Finite Element Analysis. Materials 2023, 16, 1466. [Google Scholar] [CrossRef] [PubMed]
- Turbucz, M.; Pokorni, A.J.; Szőke, G.; Hoffer, Z.; Kiss, R.M.; Lazary, A.; Eltes, P.E. Development and Validation of Two Intact Lumbar Spine Finite Element Models for in silico Investigations: Comparison of the Bone Modelling Approaches. Appl. Sci. 2022, 12, 10256. [Google Scholar] [CrossRef]
- Zainal Abidin, N.A.; Ramlee, M.H.; Ab Rashid, A.M.; Ng, B.W.; Gan, H.S.; Abdul Kadir, M.R. Biomechanical Effects of Cross-Pin’s Diameter in Reconstruction of Anterior Cruciate Ligament—A Specific Case Study via Finite Element Analysis. Injury 2022, 53, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.; Giner, E.; Larraínzar-Garijo, R.; Caeiro, J.R.; Miguélez, M.H. Numerical Modelling of Femur Fracture and Experimental Validation Using Bone Simulant. Ann. Biomed. Eng. 2017, 45, 2395–2408. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Zhang, N.; Zhang, M.; Cheng, C.K. Femoral Stems with Porous Lattice Structures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 1136. [Google Scholar] [CrossRef]
- Arredondo, A.I.B.; Aguilar, E.S.Z.; Bencomo, M.D.M.; Acosta-Sánchez, L.A. Porous Lattice Structure of Femoral Stem for Total Hip Arthroplasty. Rev. Mex. Ing. Biomédica 2020, 41, 69–79. [Google Scholar] [CrossRef]
- Arabnejad Khanoki, S.; Pasini, D. Fatigue Design of a Mechanically Biocompatible Lattice for a Proof-of-Concept Femoral Stem. J. Mech. Behav. Biomed. Mater. 2013, 22, 65–83. [Google Scholar] [CrossRef]
- Piolanti, N.; Neri, E.; Bonicoli, E.; Parchi, P.D.; Marchetti, S.; Manca, M.; Bonini, L.; Banci, L.; Scaglione, M. Use of a Plasma-Sprayed Titanium-Hydroxyapatite Femoral Stem in Hip Arthroplasty in Patients Older than 70 Years. Is Cementless Fixation a Reliable Option in the Elderly? J. Clin. Med. 2021, 10, 4735. [Google Scholar] [CrossRef] [PubMed]
- Martinho, T.; Stoffel, K. Treatment of Intertrochanteric Femur Fractures with Hip Arthroplasty in Older Patients: A Narrative Review of Indications and Outcomes. Medicina 2021, 57, 763. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef]
- Vinogradov, A.; Merson, E.; Myagkikh, P.; Linderov, M.; Brilevsky, A.; Merson, D. Attaining High Functional Performance in Biodegradable Mg-Alloys: An Overview of Challenges and Prospects for the Mg-Zn-Ca System. Materials 2023, 16, 1324. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Choroszyński, M.; Choroszyński, M.R.; Skrzypek, S.J. Biomaterials for Hip Implants—Important Considerations Relating to the Choice of Materials. Bio-Algorithms Med.-Syst. 2017, 13, 133–145. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Jamari, J. Tresca Stress Evaluation of Metal-on-UHMWPE Total Hip Arthroplasty during Peak Loading from Normal Walking Activity. Mater. Today Proc. 2022, 63, S143–S146. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Umer, U.; Alkhalefah, H.; Ahmed, F.; Hashmi, F.H. Design, Analysis, and 3D Printing of a Patient-Specific Polyetheretherketone Implant for the Reconstruction of Zygomatic Deformities. Polymers 2023, 15, 886. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, N.; Babu, A.G.; Sivasankar, M. Failure Analysis of Cementless Hip Joint Prosthesis. Adv. Mater. Res. 2014, 845, 403–407. [Google Scholar] [CrossRef]
- Chethan, K.N.; Shyamasunder Bhat, N.; Zuber, M.; Satish Shenoy, B. Finite Element Analysis of Different Hip Implant Designs along with Femur under Static Loading Conditions. J. Biomed. Phys. Eng. 2019, 9, 507–516. [Google Scholar] [CrossRef]
- Crego-Vita, D.; Aedo-Martín, D.; Sánchez-Pérez, C. Case Report of Early Aseptic Loosening of Total Hip Arthroplasty in Monostotic Paget Disease, a Diagnostic Challenge. Int. J. Surg. Case Rep. 2016, 24, 215–218. [Google Scholar] [CrossRef]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Wang, D.; Wang, C.; Wang, X.; Liu, A.; Nester, C.J.; Howard, D. An in vivo Experimental Validation of a Computational Model of Human Foot. J. Bionic Eng. 2009, 6, 387–397. [Google Scholar] [CrossRef]
- Cheung, J.T.M.; Zhang, M.; An, K.N. Effect of Achilles Tendon Loading on Plantar Fascia Tension in the Standing Foot. Clin. Biomech. 2006, 21, 194–203. [Google Scholar] [CrossRef]
- McNamara, B.P.; Cristofolini, L.; Toni, A.; Taylor, D. Relationship between Bone-Prosthesis Bonding and Load Transfer in Total Hip Reconstruction. J. Biomech. 1997, 30, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Bougherara, H.; Zdero, R.; Shah, S.; Miric, M.; Papini, M.; Zalzal, P.; Schemitsch, E.H. A Biomechanical Assessment of Modular and Monoblock Revision Hip Implants Using FE Analysis and Strain Gage Measurements. J. Orthop. Surg. Res. 2010, 5, 34. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar Mittal, V.; Gupta, V. Homogeneous Modelling and Analysis of Hip Prosthesis Using FEA. J. Phys. Conf. Ser. 2019, 1240, 12118. [Google Scholar] [CrossRef]


| Model | Mesh Size (mm) | Number of Elements | Number of Nodes |
|---|---|---|---|
| 1 | 6.0 | 56,099 | 10,491 |
| 2 | 5.5 | 90,640 | 16,639 |
| 3 | 5.0 | 160,794 | 29,025 |
| 4 | 4.5 | 220,084 | 39,058 |
| 5 | 4.0 | 308,387 | 53,652 |
| 6 | 3.5 | 458,143 | 78,008 |
| Design | Mass (g) | Percentage Different Compared with Solid Implant (%) |
|---|---|---|
| Solid | 160.68 | |
| Gyroid | 127.86 | 20 |
| Voronoi | 103.97 | 35 |
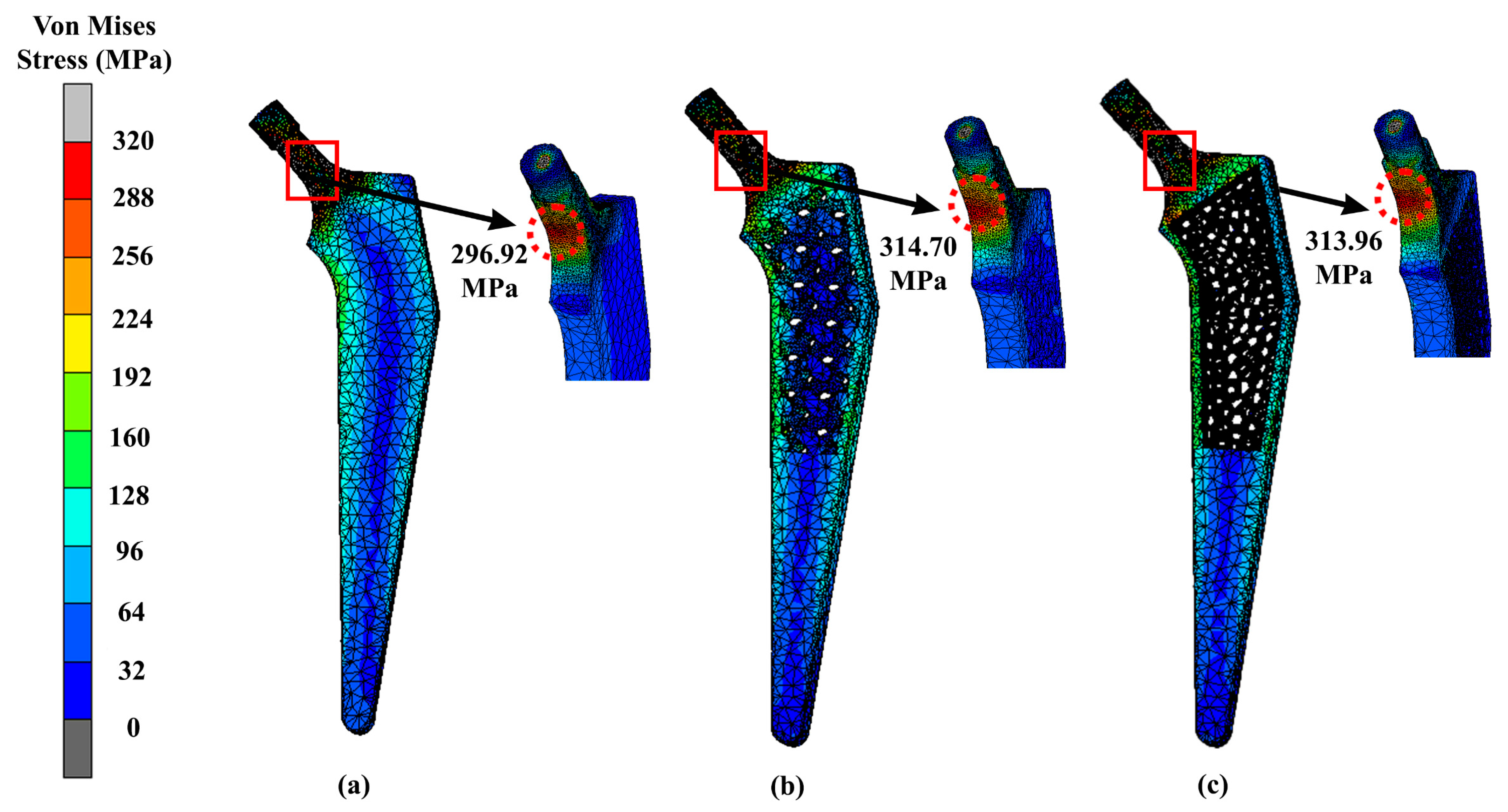
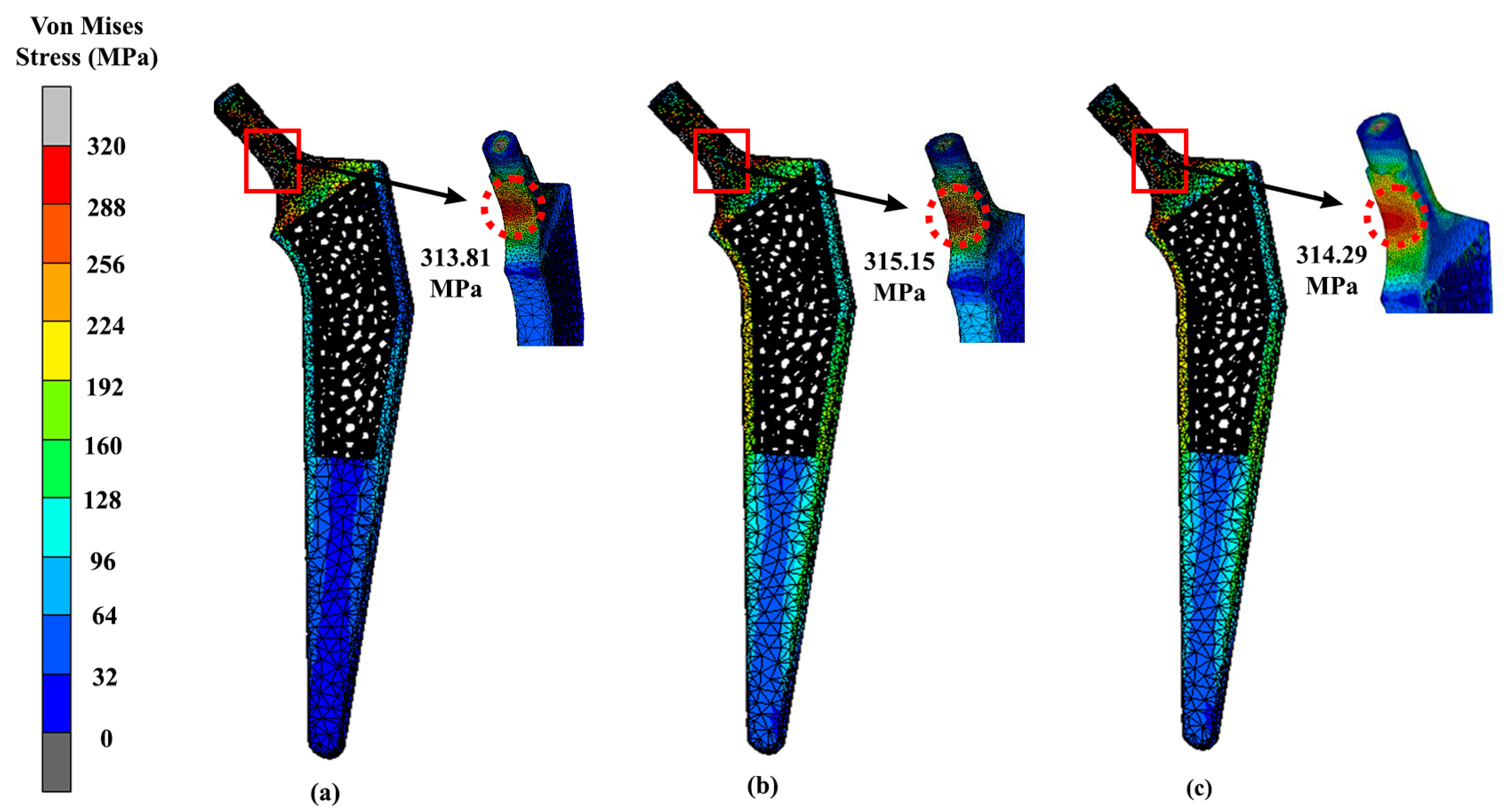
| Design | Maximum Stress (MPa) | Displacement (mm) |
|---|---|---|
| Solid | 296.92 | 1.27 |
| Gyroid | 314.70 | 1.75 |
| Voronoi | 313.96 | 1.50 |
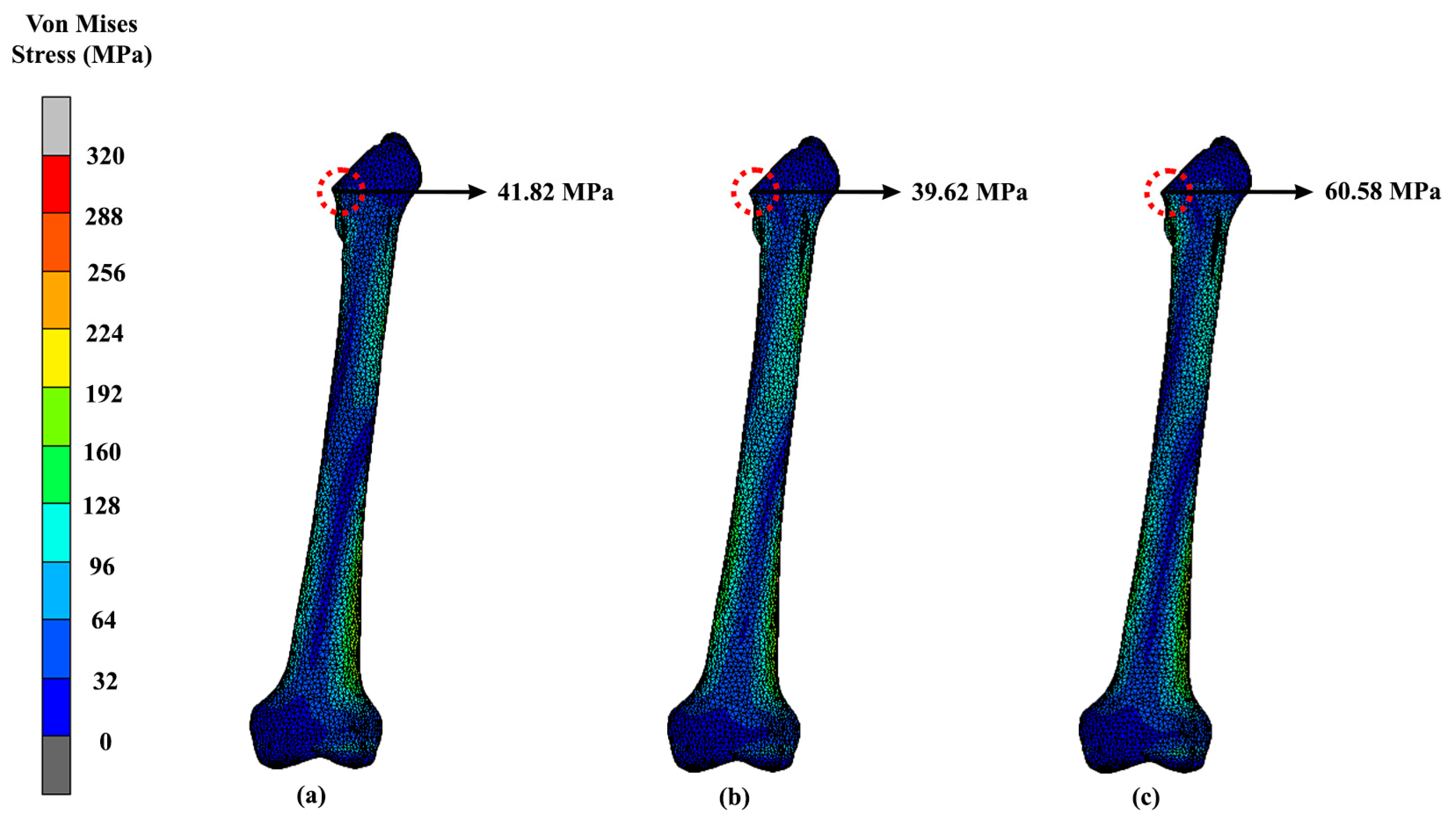
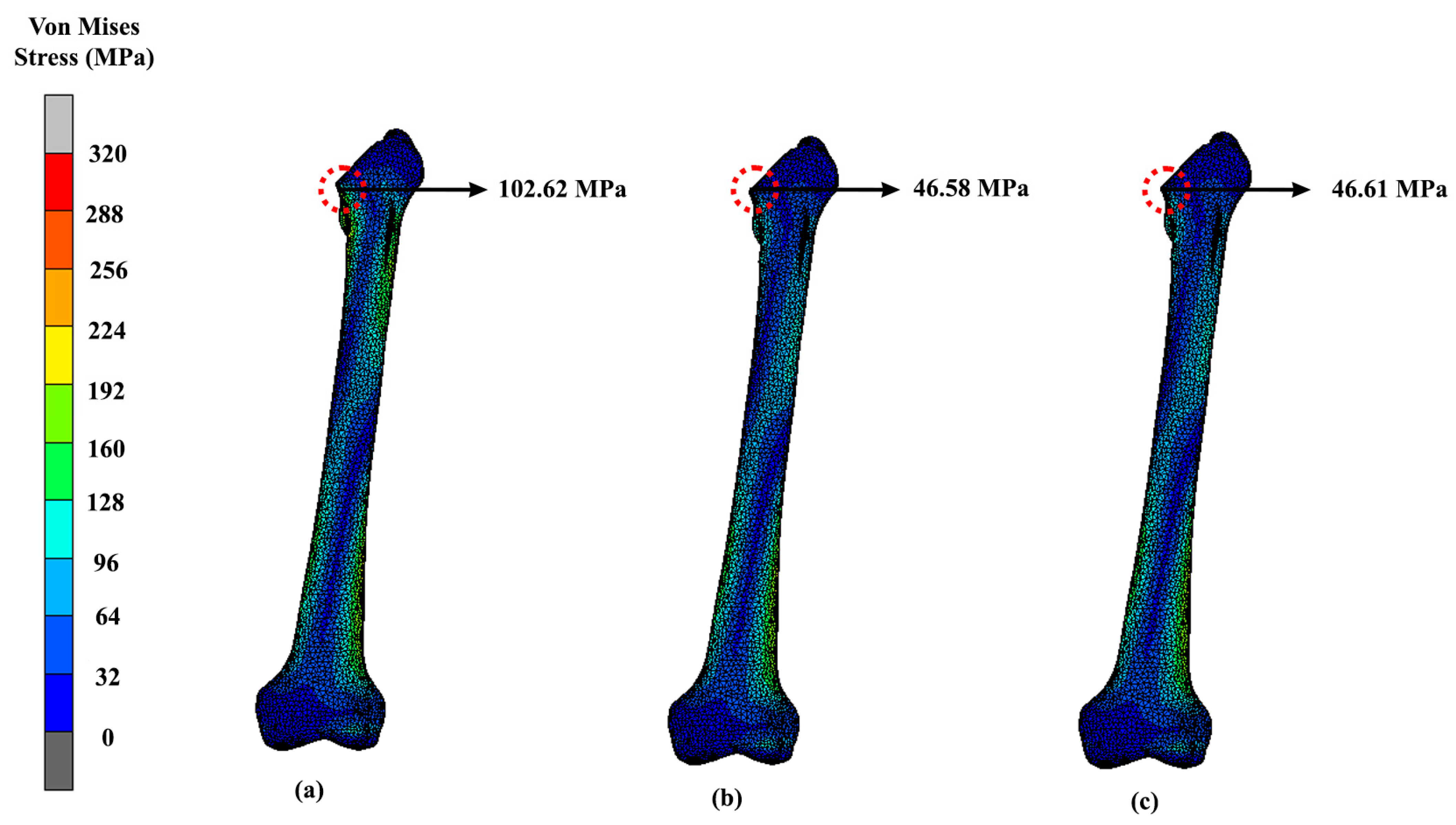
| Design | Maximum Stress (MPa) | Displacement (mm) |
|---|---|---|
| Solid | 41.82 | 1.24 |
| Gyroid | 39.62 | 1.69 |
| Voronoi | 60.58 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Abdul Kadir, M.R.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. https://doi.org/10.3390/ma16093298
Salaha ZFM, Ammarullah MI, Abdullah NNAA, Aziz AUA, Gan H-S, Abdullah AH, Abdul Kadir MR, Ramlee MH. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials. 2023; 16(9):3298. https://doi.org/10.3390/ma16093298
Chicago/Turabian StyleSalaha, Zatul Faqihah Mohd, Muhammad Imam Ammarullah, Nik Nur Ain Azrin Abdullah, Aishah Umairah Abd Aziz, Hong-Seng Gan, Abdul Halim Abdullah, Mohammed Rafiq Abdul Kadir, and Muhammad Hanif Ramlee. 2023. "Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model" Materials 16, no. 9: 3298. https://doi.org/10.3390/ma16093298
APA StyleSalaha, Z. F. M., Ammarullah, M. I., Abdullah, N. N. A. A., Aziz, A. U. A., Gan, H.-S., Abdullah, A. H., Abdul Kadir, M. R., & Ramlee, M. H. (2023). Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials, 16(9), 3298. https://doi.org/10.3390/ma16093298









