Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys
Abstract
1. Introduction
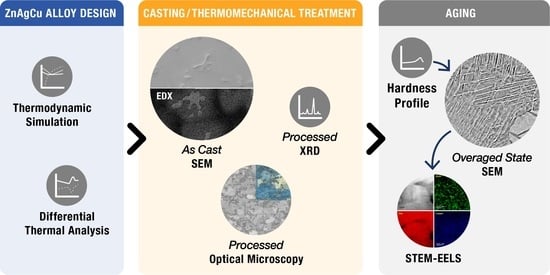
2. Materials and Methods
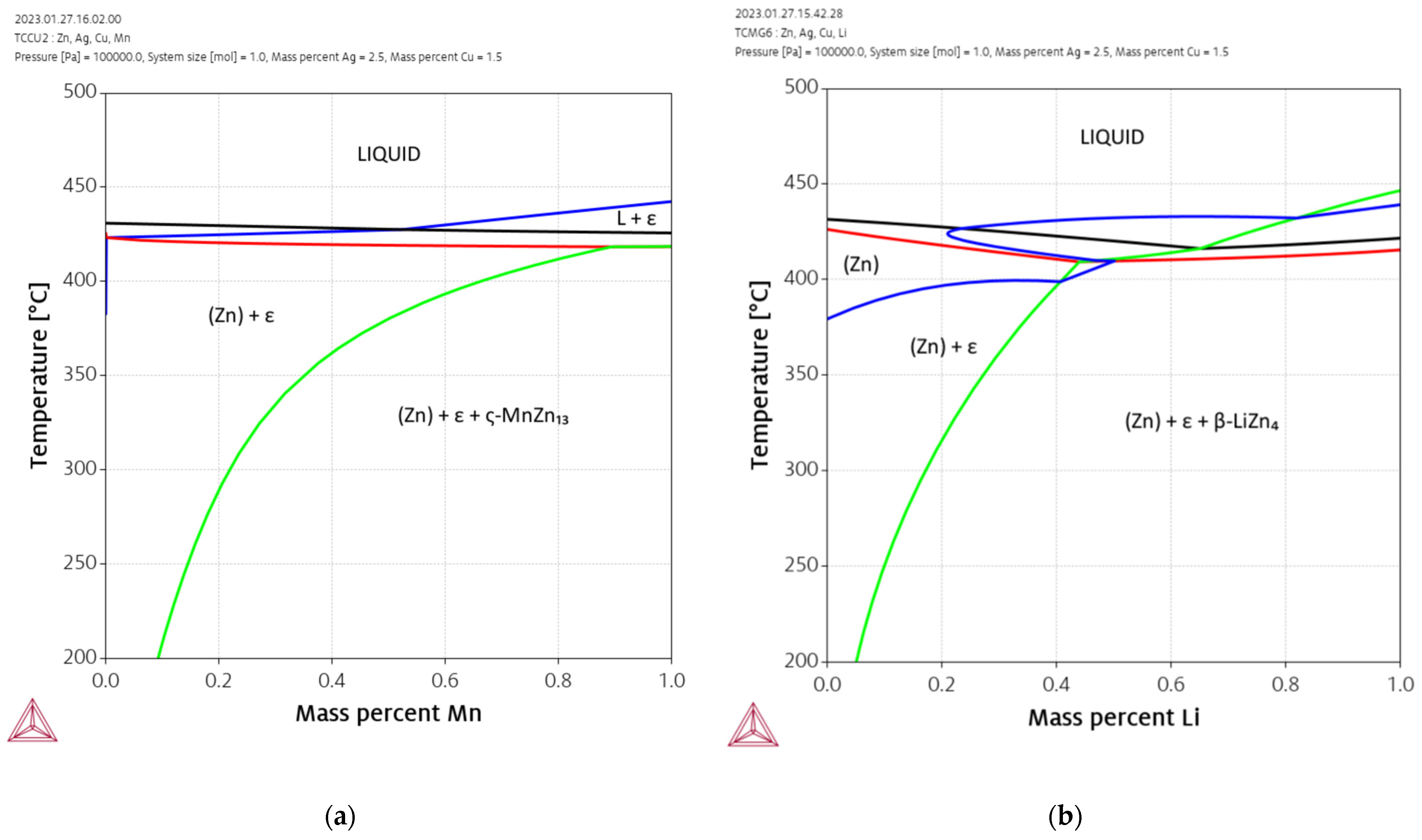
2.1. Thermodynamic Calculations
2.2. Casting and Thermomechanical Treatment
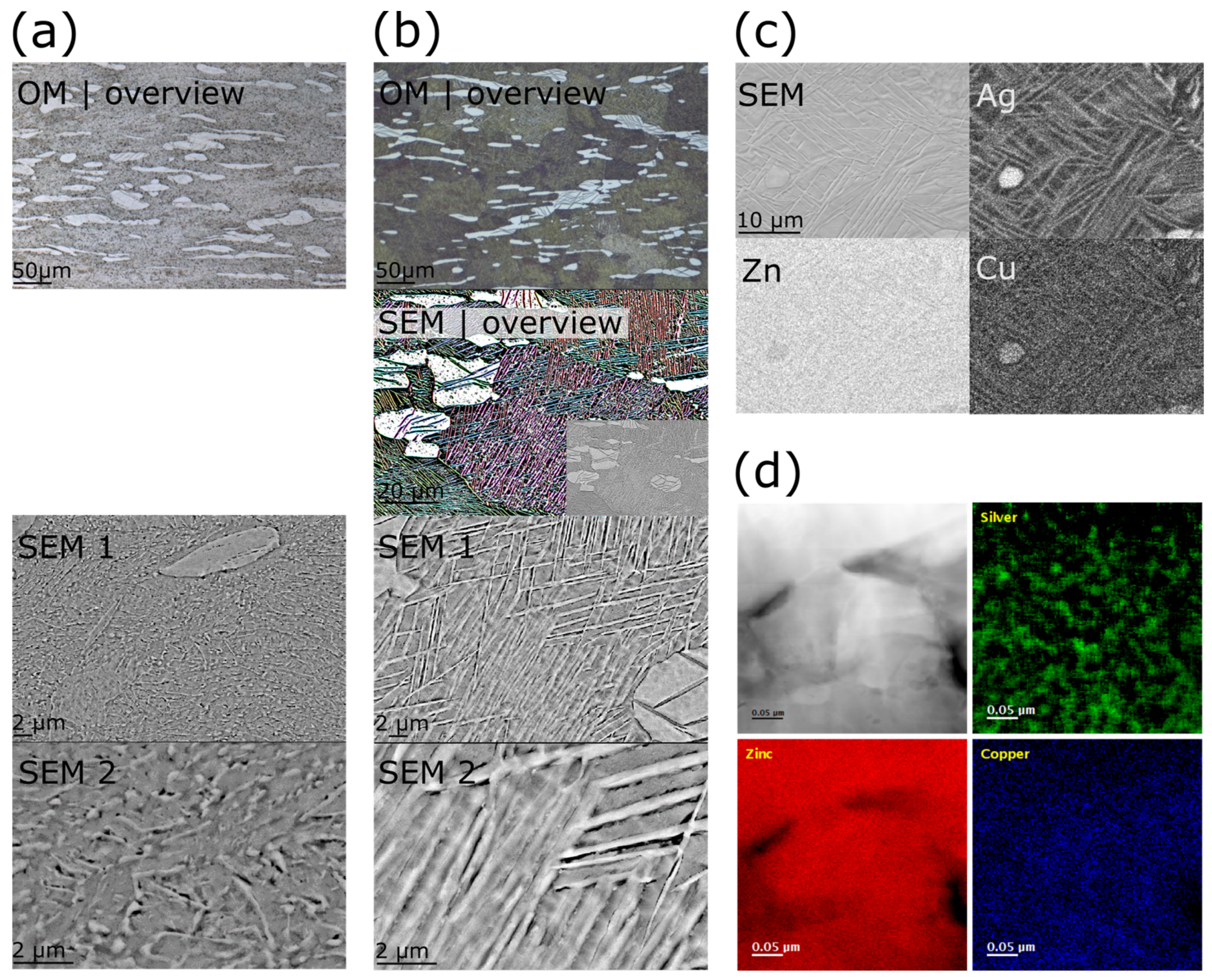
2.3. Microstructure Characterization
2.4. Thermal Analysis
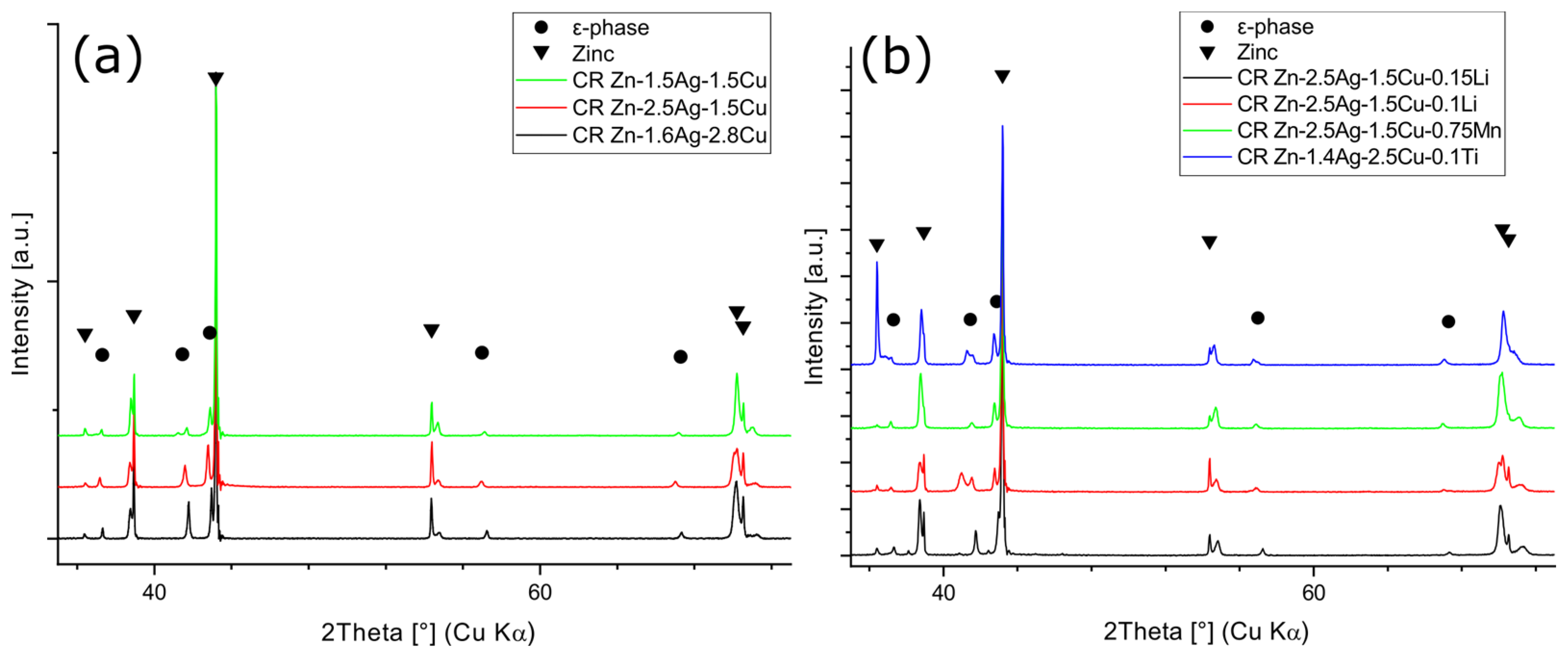
2.5. X-ray Diffraction
3. Results
3.1. Alloy Development
3.2. Alloy Processing
3.3. As Cast Alloys
3.4. Microstructure Refinement by Thermomechanical Treatment
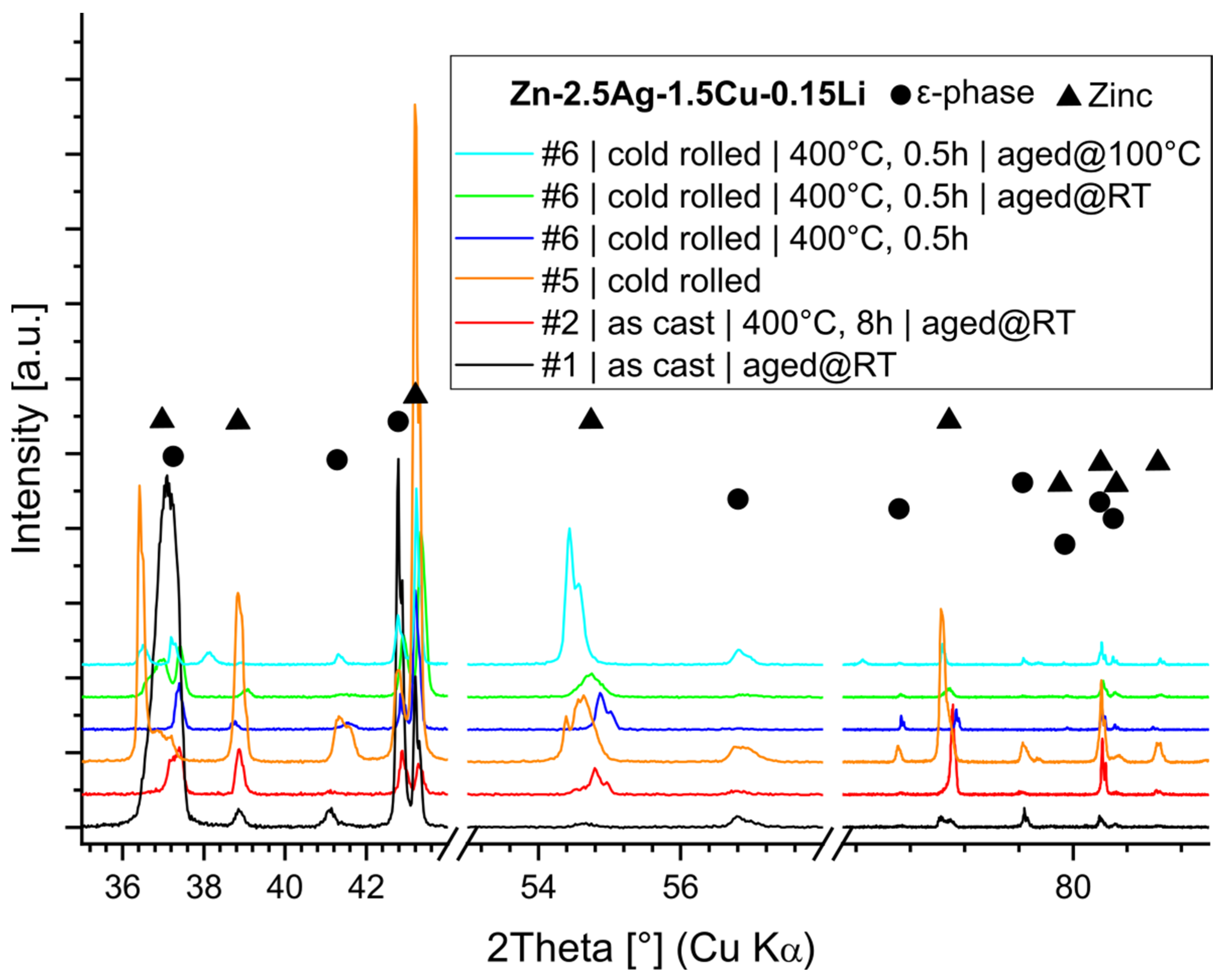
3.5. Phase Analysis
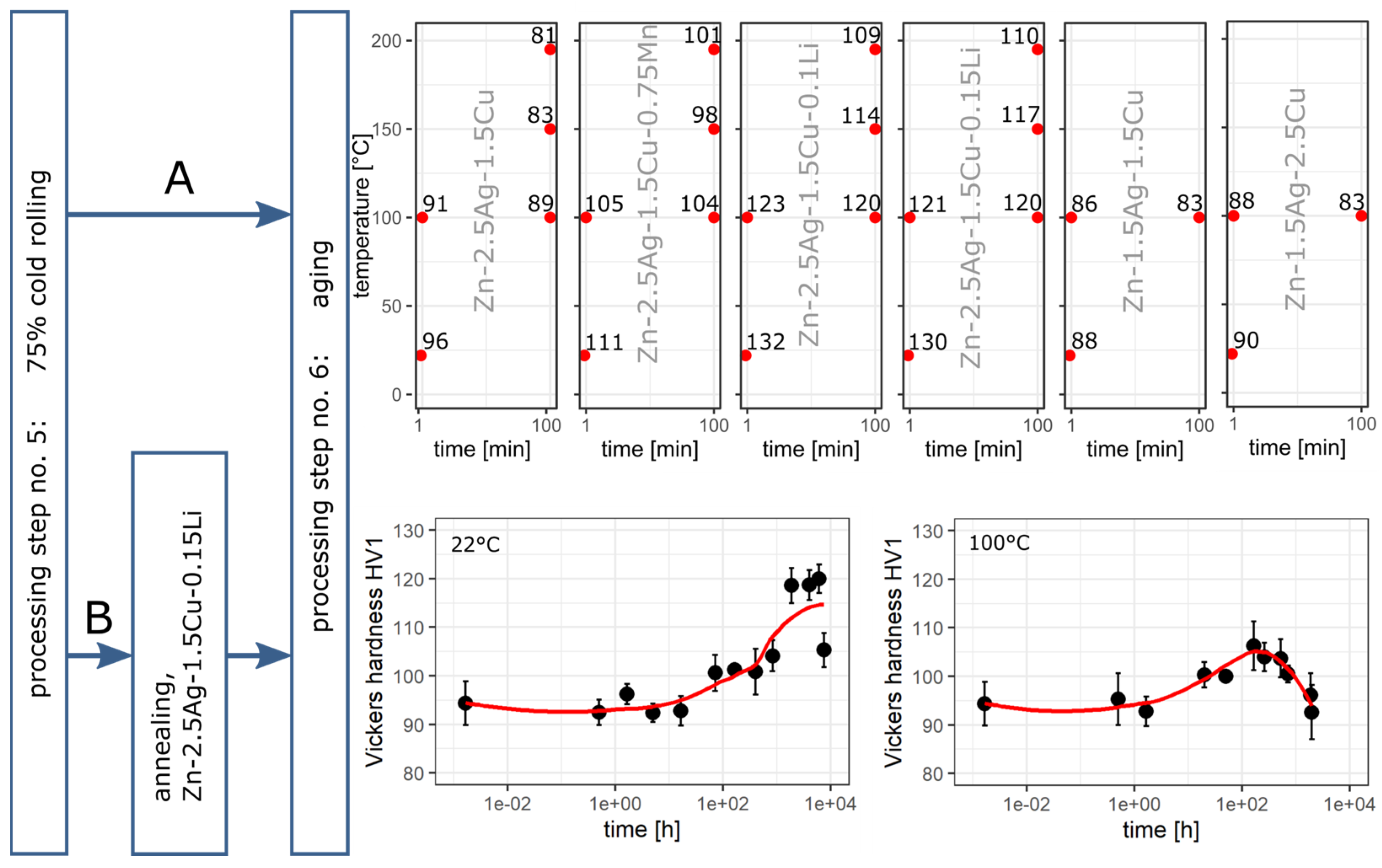
3.6. Aging Behavior
4. Discussion
4.1. Biodegradable Zn Alloys
4.2. Alloy Design and Phases
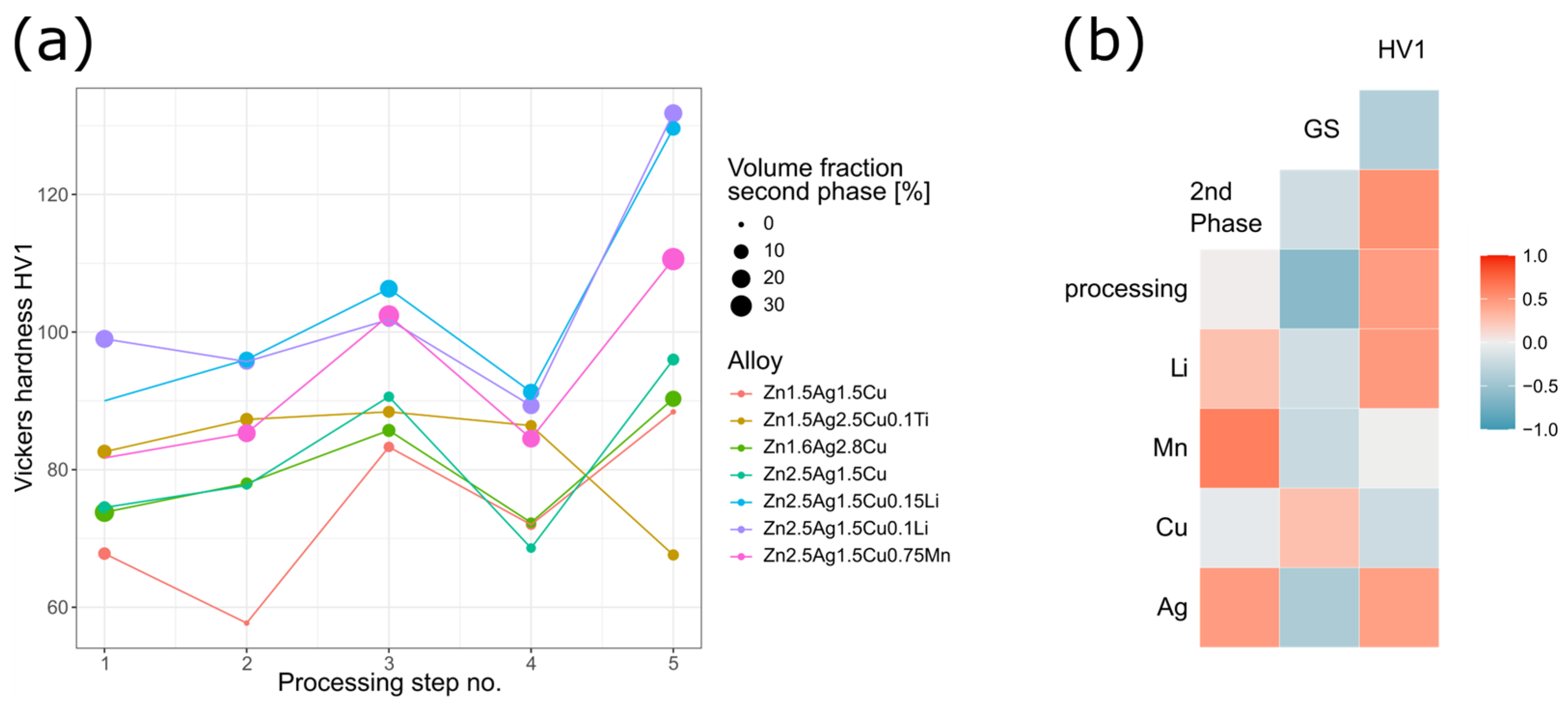
4.3. Processing—Microstructure—Hardness Relationship
- Similar to Hall–Petch strengthening where strength and grain size are inversely correlated, smaller matrix grains (GS) resulted in a higher Vickers hardness (HV1).
- The solubility of the alloying elements in Zn can be derived from phase diagrams: Ag > Cu > Mn ≈ Li, which tends to be inversely correlated with the volume fraction of the corresponding secondary phase: Mn > Li > Cu > Ag [22]. Especially Mn led to a pronounced increase in the volume fraction of the second phase.
- Even though processing disrupted the dendrites and affected the morphology of the secondary phase, it had only a negligible impact on the volume fraction. Processing led to a refined microstructure and to an increase in hardness.
- Solid solution strengthening certainly plays a role, but the quantification is beyond the scope of this study.
- During solidification, the Ag-Cu enriched, higher melting secondary phase acts as a barrier limiting the diffusion and thus affecting the grain formation of the matrix. Accordingly, a high-volume fraction of incoherent secondary phase particles and smaller matrix grains result in increased hardness.
- Alloying with Li and Mn, respectively, resulted in an increase in the volume fraction of the ε-phase and smaller matrix grain, both contributing to a higher hardness.
4.4. Aging
5. Conclusions
- Potential alloying elements were selected based on a comprehensive literature review. Compositions of promising alloys were optimized relying on thermodynamic simulations.
- Plastic deformation by rolling caused dynamic precipitation from solid solution.
- The Rietveld analysis indicated that cold rolling induced variations in the crystallographic c-axis of the Zn phase. This may be caused by precipitation-related heterogeneity in the matrix or by the formation of stacking faults. A subsequent heat treatment obliterated that effect.
- Due to the low solubility and the ε-phase stabilizing effect, alloying with Mn resulted in an increase in volume fraction and a matrix grain refinement. Consequently, hardness increased. According to the EDX analysis, Mn was dissolved in the secondary phase, but no distinct phase could be identified by XRD analysis.
- Alloying with Li resulted in a slight increase in the volume fraction of the second phase. However, a noticeable change in the morphology of the matrix microstructure was observed. Furthermore, alloying with Li led to exceptionally fine precipitates. Li could be detected by ICP-OES but not by any structural analysis method employed in this investigation, such as XRD and EELS, so far.
- Quaternary alloys showed improved properties compared to ternary alloys. A comparison of the atomic concentrations revealed that Li had a stronger impact on hardness than Mn.
- Meeting the required mechanical benchmarks after TMT is a crucial step in the biodegradable Zn alloy development of, but this is not sufficient. Scientists face a multilemma that remains to be solved.
- Nowadays the ability to make a comparative assessment of such biodegradable alloys is rather limited due to the various compositions and TMTs. Therefore, it may be advisable to establish more standardized tests to evaluate the tradeoffs between performance, stability, corrosion, and cytotoxicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Quantitative Analysis of Alloys [wt%] | Nominal Composition |
|---|---|
| Zn-1.5Ag-1.5Cu (ICP) | Zn-1.5Ag-1.5Cu |
| Zn-1.6Ag-2.8Cu (ICP) | Zn-1.5Ag-2.5Cu |
| Zn-1.4Ag-2.5Cu-0.13Ti (XRF) | Zn-1.5Ag-2.5Cu-0.1Ti |
| Zn-2.5Ag-1.5Cu (ICP) | Zn-2.5Ag-1.5Cu |
| Zn-2.5Ag-1.5Cu-0.3Mn (ICP) | Zn-2.5Ag-1.5Cu-0.3Mn |
| Zn-2.5Ag-1.5Cu-0.75Mn (ICP) | Zn-2.5Ag-1.5Cu-0.75Mn |
| Zn-2.5Ag-1.5Cu-0.06Li (ICP) | Zn-2.5Ag-1.5Cu-0.06Li |
| Zn-2.4Ag-1.5Cu-0.1Li (ICP) | Zn-2.5Ag-1.5Cu-0.1Li |
| Zn-2.5Ag-1.5Cu-0.15Li (ICP) | Zn-2.5Ag-1.5Cu-0.15Li |
| Alloy | Grain Size [µm] | Sec. Phase [%] | Nano-Sized Precipitates | HV1 |
|---|---|---|---|---|
| Processing step #1: As Cast | ||||
| Zn-1.5Ag-1.5Cu | 195 | 6 | - | 68 |
| Zn-1.5Ag-2.5Cu | 272 | 23 | - | 74 |
| Zn-1.4Ag-2.5Cu-0.1Ti | 216 | 9 | NA *** | 84 |
| Zn-2.5Ag-1.5Cu | 167 | NA | NA *** | 72 |
| Zn-2.5Ag-1.5Cu-0.3Mn | 290 * | NA | NA *** | 74 |
| Zn-2.5Ag-1.5Cu-0.75Mn | 118 * | NA | NA *** | 82 |
| Zn-2.5Ag-1.5Cu-0.06Li | 164 * | NA | NA *** | 85 |
| Zn-2.5Ag-1.5Cu-0.1Li | 189 * | 19 | NA *** | 99 |
| Zn-2.5Ag-1.5Cu-0.15Li | NA * | NA | NA *** | 90 |
| Processing step #2: Homogenization (400 °C, 8 h) | ||||
| Zn-1.5Ag-1.5Cu | 267 ** | 0 | NA *** | 58 |
| Zn-1.5Ag-2.5Cu | 298 ** | 5 | - | 78 |
| Zn-1.4Ag-2.5Cu-0.1Ti | 211 ** | 7 | NA *** | 91 |
| Zn-2.5Ag-1.5Cu | 271 ** | 1 | - | 77 |
| Zn-2.5Ag-1.5Cu-0.3Mn | 310 ** | NA | NA *** | 73 |
| Zn-2.5Ag-1.5Cu-0.75Mn | 88 | 19 | NA *** | 85 |
| Zn-2.5Ag-1.5Cu-0.06Li | 129 ** | NA | NA *** | 92 |
| Zn-2.5Ag-1.5Cu-0.1Li | 152 ** | 15 | NA *** | 96 |
| Zn-2.5Ag-1.5Cu-0.15Li | 149 ** | 13 | NA *** | 96 |
| Processing step #3: Hot Rolling (200 °C, 50%) | ||||
| Zn-1.5Ag-1.5Cu | NA * | 3 | + | 84 |
| Zn-1.5Ag-2.5Cu | NA * | 7 | + | 86 |
| Zn-1.4Ag-2.5Cu-0.1Ti | NA * | 5 | NA *** | 86 |
| Zn-2.5Ag-1.5Cu | NA * | 4 | + | 91 |
| Zn-2.5Ag-1.5Cu-0.3Mn | NA * | NA | NA *** | 88 |
| Zn-2.5Ag-1.5Cu-0.75Mn | NA * | 27 | NA *** | 103 |
| Zn-2.5Ag-1.5Cu-0.06Li | NA * | NA | NA *** | 108 |
| Zn-2.5Ag-1.5Cu-0.1Li | NA * | 10 | NA *** | 102 |
| Zn-2.5Ag-1.5Cu-0.15Li | NA * | 18 | NA *** | 106 |
| Processing step #4: Annealing (400 °C, 0.5 h) | ||||
| Zn-1.5Ag-1.5Cu | 189 | 3 | NA *** | 72 |
| Zn-1.5Ag-2.5Cu | 74 | 3 | - | 72 |
| Zn-1.4Ag-2.5Cu-0.1Ti | 42 | 4 | NA *** | 87 |
| Zn-2.5Ag-1.5Cu | 75 | 2 | - | 69 |
| Zn-2.5Ag-1.5Cu-0.3Mn | 90 | NA | NA *** | 74 |
| Zn-2.5Ag-1.5Cu-0.75Mn | 35 | 18 | NA *** | 85 |
| Zn-2.5Ag-1.5Cu-0.06Li | 79 | NA | NA *** | 88 |
| Zn-2.5Ag-1.5Cu-0.1Li | 91 | 17 | NA *** | 89 |
| Zn-2.5Ag-1.5Cu-0.15Li | 99 | 14 | NA *** | 91 |
| Processing step #5: Cold Rolling (22 °C, 50%) | ||||
| Zn-1.5Ag-1.5Cu | NA * | 0 | NA *** | 88 |
| Zn-1.5Ag-2.5Cu | NA * | 14 | + | 90 |
| Zn-1.4Ag-2.5Cu-0.1Ti | NA * | 4 | NA *** | 69 |
| Zn-2.5Ag-1.5Cu | NA * | 5 | + | 96 |
| Zn-2.5Ag-1.5Cu-0.3Mn | NA * | NA | NA *** | 95 |
| Zn-2.5Ag-1.5Cu-0.75Mn | NA * | 34 | + | 111 |
| Zn-2.5Ag-1.5Cu-0.06Li | NA * | NA | NA *** | 124 |
| Zn-2.5Ag-1.5Cu-0.1Li | NA * | 19 | + | 132 |
| Zn-2.5Ag-1.5Cu-0.15Li | NA * | 10 | + | 129 |
References
- Milazzo, G.; Caroli, S.; Braun, R.D. Tables of Standard Electrode Potentials. J. Electrochem. Soc. 1978, 125, 261C. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, Ș.-C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. Addressing the Slow Corrosion Rate of Biodegradable Fe-Mn: Current Approaches and Future Trends. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100822. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-Term Surveillance of Zinc Implant in Murine Artery: Surprisingly Steady Biocorrosion Rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, X.; Lin, W.; Chen, D.; Zhu, D.; Dai, K.; Zheng, Y. Enhanced Osseointegration of Zn-Mg Composites by Tuning the Release of Zn Ions with Sacrificial Mg-Rich Anode Design. ACS Biomater. Sci. Eng. 2019, 5, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; von zur Mühlen, C.; Bode, C.; Epting, T.; Haberstroh, J.; Mey, L.; Schwarzbach, H.; et al. Zn-Alloy Provides a Novel Platform for Mechanically Stable Bioresorbable Vascular Stents. PLoS ONE 2019, 14, e0209111. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-X.; Zhou, C.; Shi, Y.-P.; Shi, Z.-Z.; Lu, T.-H.; Hao, Y.; Liu, C.-H.; Wang, X.; Zhang, H.-J.; Wang, L.-N. Hemocompatibility of Biodegradable Zn-0.8 wt% (Cu, Mn, Li) Alloys. Mater. Sci. Eng. C 2019, 104, 109896. [Google Scholar] [CrossRef]
- Zhao, S.; Seitz, J.-M.; Eifler, R.; Maier, H.J.; Guillory, R.J.; Earley, E.J.; Drelich, A.; Goldman, J.; Drelich, J.W. Zn-Li Alloy after Extrusion and Drawing: Structural, Mechanical Characterization, and Biodegradation in Abdominal Aorta of Rat. Mater. Sci. Eng. C 2017, 76, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Z.-Z.; Hao, Y.; Li, H.-F.; Liu, X.-F.; Volinsky, A.A.; Zhang, H.-J.; Wang, L.-N. High-Performance Hot-Warm Rolled Zn-0.8Li Alloy with Nano-Sized Metastable Precipitates and Sub-Micron Grains for Biodegradable Stents. J. Mater. Sci. Technol. 2019, 35, 2618–2624. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Liu, H.; Fang, H.; Li, D.; Xu, X.; Yan, Y.; Chen, L.; Lu, Y.; Yu, K. Mechanical Strengthening Mechanism of Zn-Li Alloy and Its Mini Tube as Potential Absorbable Stent Material. Mater. Lett. 2019, 235, 220–223. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Zheng, Y.; Qiao, L.; Yan, Y. In Vitro Degradation Behavior of Novel Zn–Cu–Li Alloys: Roles of Alloy Composition and Rolling Processing. Mater. Des. 2021, 212, 110288. [Google Scholar] [CrossRef]
- Su, Y.; Fu, J.; Lee, W.; Du, S.; Qin, Y.-X.; Zheng, Y.; Wang, Y.; Zhu, D. Improved Mechanical, Degradation, and Biological Performances of Zn–Fe Alloys as Bioresorbable Implants. Bioact. Mater. 2022, 17, 334–343. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Mech, K.; Marciszko, M.; Boelter, G.; Banzhaf, M.; Bała, P. Design of Novel Zn-Ag-Zr Alloy with Enhanced Strength as a Potential Biodegradable Implant Material. Mater. Des. 2019, 183, 108154. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical Characteristics, In Vitro Degradation, Cytotoxicity, and Antibacterial Evaluation of Zn-4.0Ag Alloy as a Biodegradable Material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, Mechanical Properties and in Vitro Degradation Behavior of Newly Developed ZnAg Alloys for Degradable Implant Applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Han, X.; Heiss, A.; Richter, A.; Legner, C.; Klotz, U.E.; et al. Evaluation of a Zn–2Ag–1.8Au–0.2V Alloy for Absorbable Biocompatible Materials. Materials 2020, 13, 56. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, D.; Lin, J.; Dai, Y.; Luan, Y.; Sun, Q.; Shi, Z.; Wang, K.; Gao, Y.; Lin, J.; et al. Development of Biodegradable Zn–1Mg–0.1RE (RE = Er, Dy, and Ho) Alloys for Biomedical Applications. Acta Biomater. 2020, 117, 384–399. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Ardakani, M.S.; Mostaed, A.; Reaney, I.M.; Goldman, J.; Drelich, J.W. Towards Revealing Key Factors in Mechanical Instability of Bioabsorbable Zn-Based Alloys for Intended Vascular Stenting. Acta Biomater. 2020, 105, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Z.; Gao, X.-X.; Zhang, H.-J.; Liu, X.-F.; Li, H.-Y.; Zhou, C.; Yin, Y.-X.; Wang, L.-N. Design Biodegradable Zn Alloys: Second Phases and Their Significant Influences on Alloy Properties. Bioact. Mater. 2020, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Lech, S.; Wieczerzak, K.; Langdon, T.G.; Bała, P. A Novel High-Strength Zn-3Ag-0.5Mg Alloy Processed by Hot Extrusion, Cold Rolling, or High-Pressure Torsion. Metall. Mater. Trans. A 2020, 51, 3335–3348. [Google Scholar] [CrossRef]
- Haghshenas, M. Mechanical Characteristics of Biodegradable Magnesium Matrix Composites: A Review. J. Magnes. Alloys 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 1. [Google Scholar]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of Alloying Elements on the Corrosion Behavior and Biocompatibility of Biodegradable Magnesium Alloys: A Review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-Bearing Applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Significance of Homologous Temperature in Softening Behavior and Grain Size of Pure Metals Processed by High-Pressure Torsion. Mater. Sci. Eng. A 2011, 528, 7514–7523. [Google Scholar] [CrossRef]
- Liu, J.H.; Huang, C.X.; Wu, S.D.; Zhang, Z.F. Tensile Deformation and Fracture Behaviors of High Purity Polycrystalline Zinc. Mater. Sci. Eng. A 2008, 490, 117–125. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The Influence of Alloying and Fabrication Techniques on the Mechanical Properties, Biodegradability and Biocompatibility of Zinc: A Comprehensive Review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; Jia, Q.; Bian, D.; Guan, S.; Kulyasova, O.; Valiev, R.Z.; Rau, J.V.; Zheng, Y. Recent Advances on the Mechanical Behavior of Zinc Based Biodegradable Metals Focusing on the Strain Softening Phenomenon. Acta Biomater. 2022, 152, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yue, R.; Zhang, J.; Huang, H.; Niu, J.; Yuan, G. Biodegradable Zn-1.5Cu-1.5Ag Alloy with Anti-Aging Ability and Strain Hardening Behavior for Cardiovascular Stents. Mater. Sci. Eng. C 2020, 116, 111172. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Bals, S.; Tirry, W.; Geurts, R.; Yang, Z.; Schryvers, D. High-Quality Sample Preparation by Low KV FIB Thinning for Analytical TEM Measurements. Microsc. Microanal. 2007, 13, 80–86. [Google Scholar] [CrossRef]
- Schmid-Fetzer, R.; Hallstedt, B. Is Zinc HCP_ZN or HCP_A3? Calphad 2012, 37, 34–36. [Google Scholar] [CrossRef]
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. (Eds.) Alloy Phase Diagrams. In ASM Handbook; ASM International: Novelty, OH, USA, 2016; Volume 3. [Google Scholar]
- Petzow, G.; Effenberg, G. (Eds.) Ternary Alloys; VCH Verlagsgesellschaft: Weinheim, Germany, 1988; Volume 2. [Google Scholar]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Bała, P. Fine-Tuning of Mechanical Properties in a Zn–Ag–Mg Alloy via Cold Plastic Deformation Process and Post-Deformation Annealing. Bioact. Mater. 2021, 6, 3424–3436. [Google Scholar] [CrossRef]
- Jiang, J.; Qian, Y.; Huang, H.; Niu, J.; Yuan, G. Biodegradable Zn-Cu-Mn Alloy with Suitable Mechanical Performance and in Vitro Degradation Behavior as a Promising Candidate for Vascular Stents. Mater. Sci. Eng. C 2022, 133, 112652. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Selection of Extraction Medium Influences Cytotoxicity of Zinc and Its Alloys. Acta Biomater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu Alloy as a Biodegradable Material for Potential Vascular Stents Application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Szewczyk, P.K.; Kawałko, J.; Mech, K.; Grünewald, A.; Unalan, I.; Taccardi, N.; Boelter, G.; Banzhaf, M.; et al. In Vitro Cytocompatibility and Antibacterial Studies on Biodegradable Zn Alloys Supplemented by a Critical Assessment of Direct Contact Cytotoxicity Assay. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting Medium for Corrosion Testing of Bioabsorbable Magnesium and Other Metals—A Critical Review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Li, P.; Dai, J.; Schweizer, E.; Rupp, F.; Heiss, A.; Richter, A.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L.; Alexander, D. Response of Human Periosteal Cells to Degradation Products of Zinc and Its Alloy. Mater. Sci. Eng. C 2020, 108, 110208. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Jun, I.; Seok, H.-K.; Lee, K.-S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.-C.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, Z.; Jiang, Y.; Zhang, K.; Li, X.; Ma, M.; Li, Y.; Ma, B. Biodegradable Metals for Bone Fracture Repair in Animal Models: A Systematic Review. Regen. Biomater. 2021, 8, rbaa047. [Google Scholar] [CrossRef] [PubMed]
- García-Mintegui, C.; Córdoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jiménez-Piqué, E.; Ginebra, M.-P.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu Alloys for Stenting Applications: From Nanoscale Mechanical Characterization to In Vitro Degradation and Biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and Characterizations of Novel Biodegradable Zn-Cu-Mg Alloys for Potential Biodegradable Implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Guillory, R.J.; Mostaed, E.; Oliver, A.A.; Morath, L.M.; Earley, E.J.; Flom, K.L.; Kolesar, T.M.; Mostaed, A.; Kwesiga, M.P.; Drelich, J.W.; et al. Improved Biocompatibility of Zn-Ag-Based Stent Materials by Microstructure Refinement. Acta Biomater. 2022, 145, 416–426. [Google Scholar] [CrossRef]
- Li, P.; Qian, J.; Zhang, W.; Schille, C.; Schweizer, E.; Heiss, A.; Klotz, U.E.; Scheideler, L.; Wan, G.; Geis-Gerstorfer, J. Improved Biodegradability of Zinc and Its Alloys by Sandblasting Treatment. Surf. Coat. Technol. 2021, 405, 126678. [Google Scholar] [CrossRef]
- Hersent, E.; Marthinsen, K.; Nes, E. On the Effect of Atoms in Solid Solution on Grain Growth Kinetics. Metall. Mater. Trans. A 2014, 45, 4882–4890. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the Use of Zinc and Its Alloys as Biodegradable Metals: Perspective from Biomechanical Compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly Extruded Biodegradable Zn-0.05wt%Mg Alloy with Improved Strength and Ductility: In Vitro and in Vivo Studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]

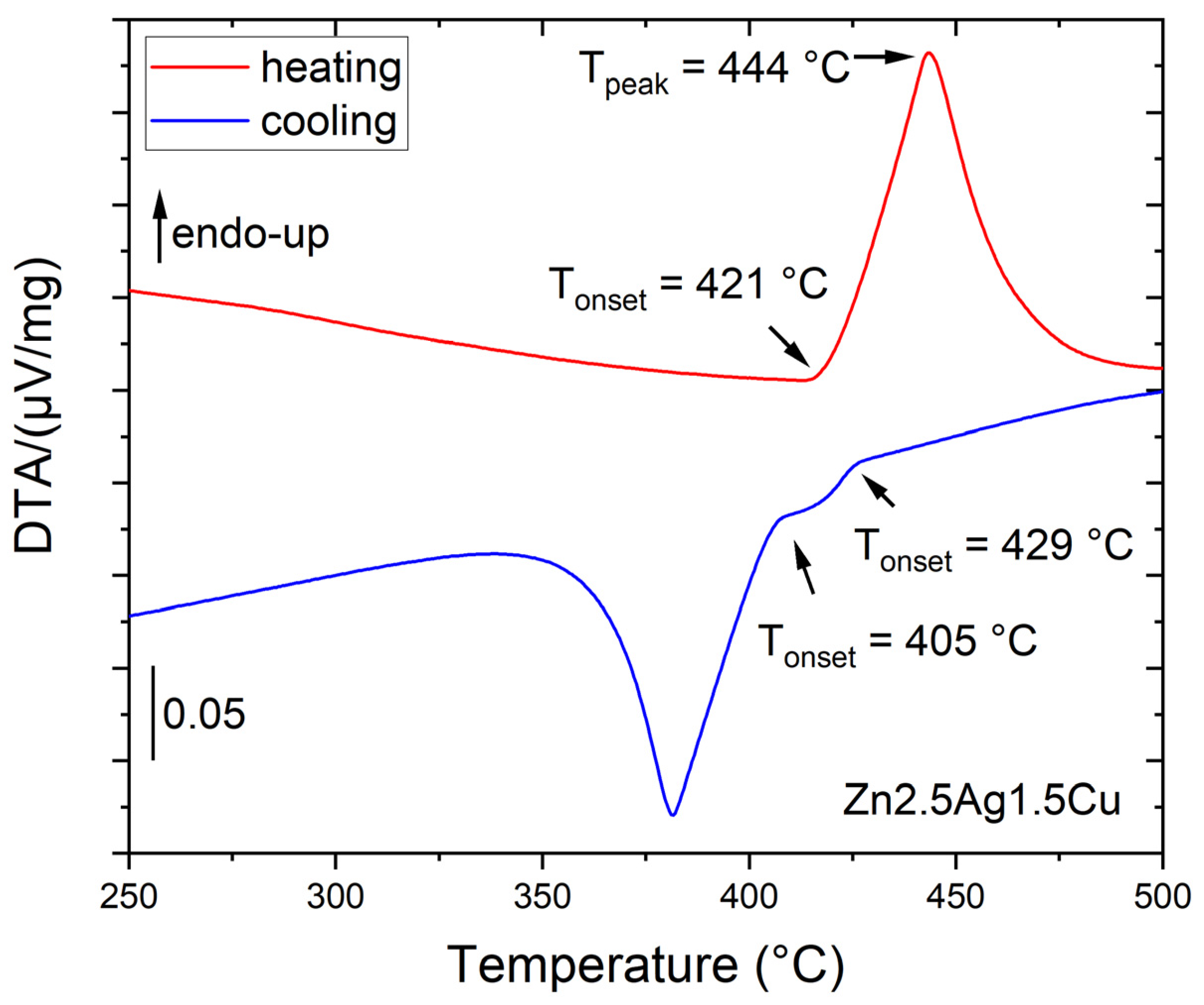



| Alloy | ThermoCalc TCMG6 | DTA Measurement | ||||
|---|---|---|---|---|---|---|
| Tsol [°C] | Tliq [°C] | Tonset ↑ =Tsol | Tpeak ↑ =Tliq | Tonset1 ↓ =Tliq supercooled | Tonset2 ↓ =Tperitectic | |
| Zn-2.5Ag-1.5Cu | 426 | 431 | 421 | 444 | 429 | 405 |
| Zn-2.5Ag-1.5Cu-0.1Li | 397 | 427 | 414 | 438 | 422 | 403 |
| Zn-2.5Ag-1.5Cu-0.75Mn | 419 | 460 | 420 | 444 | 435 | 400 |
| Phase | Sample | a [Å] | c [Å] |
|---|---|---|---|
| Zinc | ICDD-PDF No 65-3358 P63/mmc | 2.66(5) | 4.94(7) |
| Zn-1.5Ag-1.5Cu | 2.67(9) | 4.92(9) | |
| Zn-1.5Ag-2.5Cu | 2.68(0) | 4.89(3) | |
| Zn-2.5Ag-1.5Cu | 2.68(2) | 4.92(8) | |
| Zn-2.5Ag-1.5Cu-0.75Mn | 2.68(5) | 4.92(9) | |
| Zn-2.5Ag-1.5Cu-0.1Li | 2.68(3) | 4.91(9) | |
| Zn-2.5Ag-1.5Cu-0.15Li | 2.67(6) | 4.92(7) | |
| Zinc II | Zn-1.5Ag-1.5Cu | 2.66(8) | 4.84(9) |
| Zn-1.5Ag-2.5Cu | 2.66(8) | 4.83(6) | |
| Zn-2.5Ag-1.5Cu | 2.66(8) | 4.83(9) | |
| Zn-2.5Ag-1.5Cu-0.75Mn | 2.66(8) | 4.83(4) | |
| Zn-2.5Ag-1.5Cu-0.1Li | 2.67(0) | 4.83(5) | |
| Zn-2.5Ag-1.5Cu-0.15Li | 2.66(8) | 4.87(0) | |
| ε-phase | ICDD-PDF No 51-0642 | 2.78(1) | 4.35(3) |
| Zn-1.5Ag-1.5Cu | 2.78(5) | 4.33(2) | |
| Zn-1.5Ag-2.5Cu | 2.77(9) | 4.32(0) | |
| Zn-2.5Ag-1.5Cu | 2.79(1) | 4.34(1) | |
| Zn-2.5Ag-1.5Cu-0.75Mn | 2.79(4) | 4.34(5) | |
| Zn-2.5Ag-1.5Cu-0.1Li | 2.79(3) | 4.34(9) | |
| Zn-2.5Ag-1.5Cu-0.15Li | 2.79(1) | 4.35(0) |
| Zn-2.5Ag-1.5Cu-0.15Li TMT Step|Heat Treatment|Aging | Zn ICSD-247147-P63 mmc | ε-(Ag,Cu)Zn ICSD-103157-P63 mmc | HV1 (Day 0) | ||
|---|---|---|---|---|---|
| a [Å] | c [Å] | a [Å] | c [Å] | ||
| #1 (AC)|–|398 days, 22 °C | 2.68 | 4.85 | 2.79 | 4.37 | 102 (90) |
| #2 (HT)|–|397 days, 22 °C | 2.68 | 4.84 | 2.78 | 4.39 | 111 (96) |
| #5 (CR)|–|0 days | Table 2 | Table 2 | 2.79 | 4.35 | 130 |
| #6 (CR)|0.5 h, 400 °C|0 days | 2.69 | 4.82 | 2.78 | 4.38 | 94 |
| #6 (CR)|0.5 h, 400 °C|322 days, 22 °C | 2.67 | 4.92 | 2.79 | 4.37 | 105+ |
| #6 (CR)|0.5 h, 400 °C|83 days, 100 °C | 2.68 | 4.88 | 2.79 | 4.38 | 93+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiss, A.; Thatikonda, V.S.; Richter, A.; Schmitt, L.-Y.; Park, D.; Klotz, U.E. Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys. Materials 2023, 16, 3198. https://doi.org/10.3390/ma16083198
Heiss A, Thatikonda VS, Richter A, Schmitt L-Y, Park D, Klotz UE. Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys. Materials. 2023; 16(8):3198. https://doi.org/10.3390/ma16083198
Chicago/Turabian StyleHeiss, Alexander, Venkat Sai Thatikonda, Andreas Richter, Lisa-Yvonn Schmitt, Daesung Park, and Ulrich E. Klotz. 2023. "Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys" Materials 16, no. 8: 3198. https://doi.org/10.3390/ma16083198
APA StyleHeiss, A., Thatikonda, V. S., Richter, A., Schmitt, L.-Y., Park, D., & Klotz, U. E. (2023). Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys. Materials, 16(8), 3198. https://doi.org/10.3390/ma16083198