The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics
Abstract
1. Introduction
2. Materials and Methods
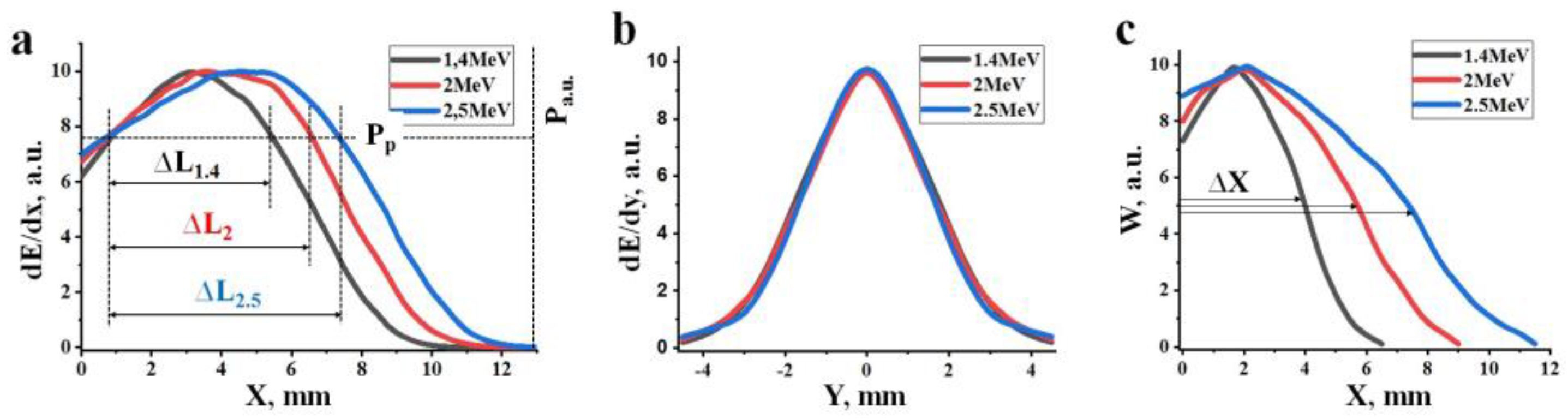
2.1. Energy Losses of the Electron Beam in the Material
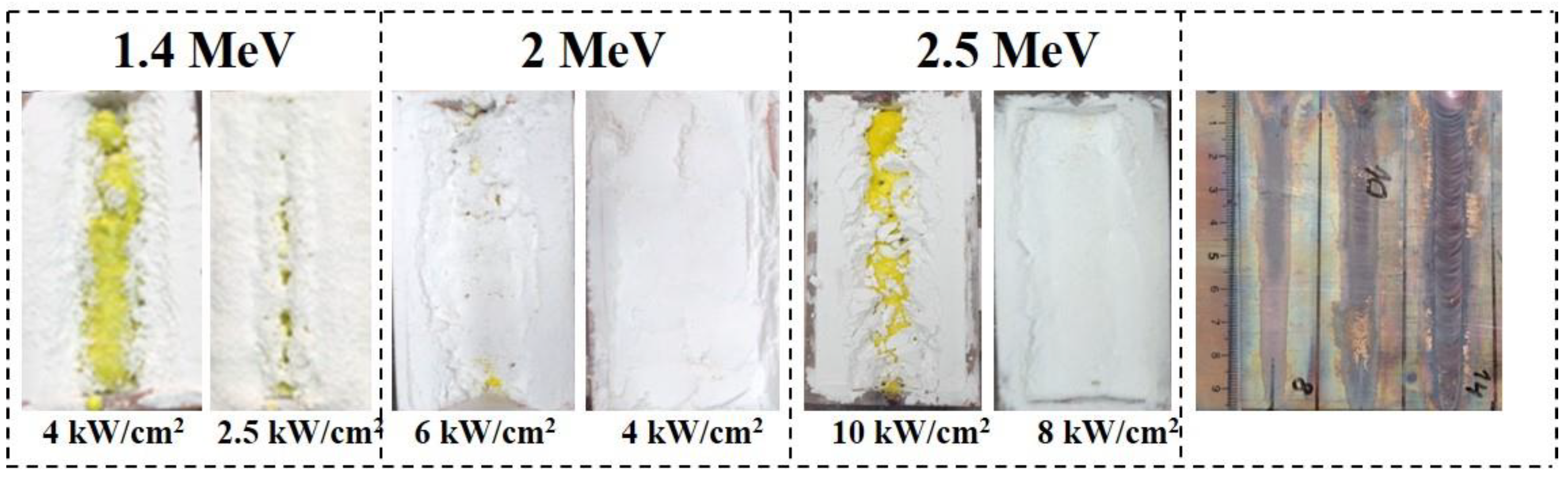
2.2. Synthesis of YAG Ceramics
3. Results
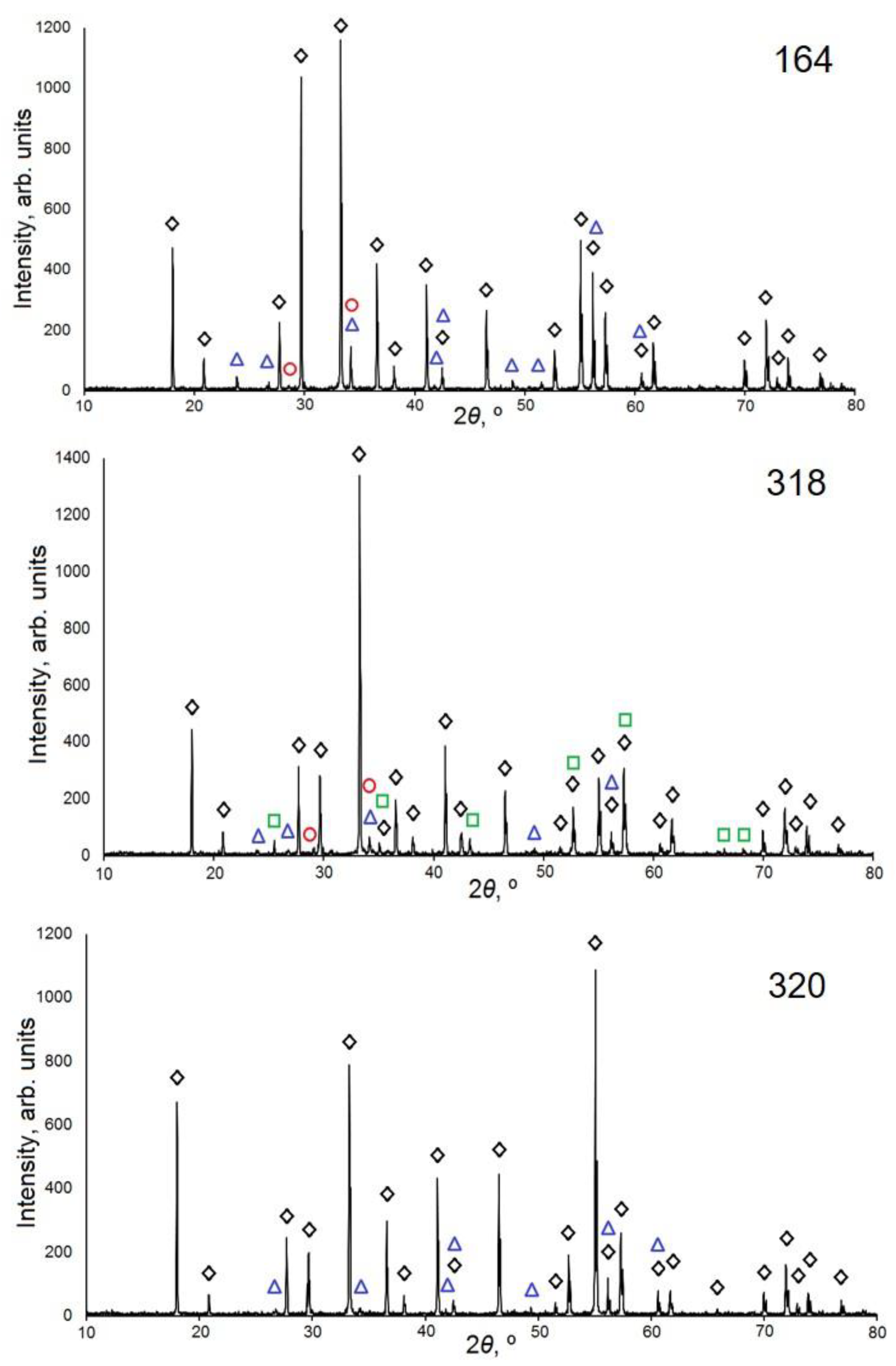
3.1. Structure of Synthesized Ceramics
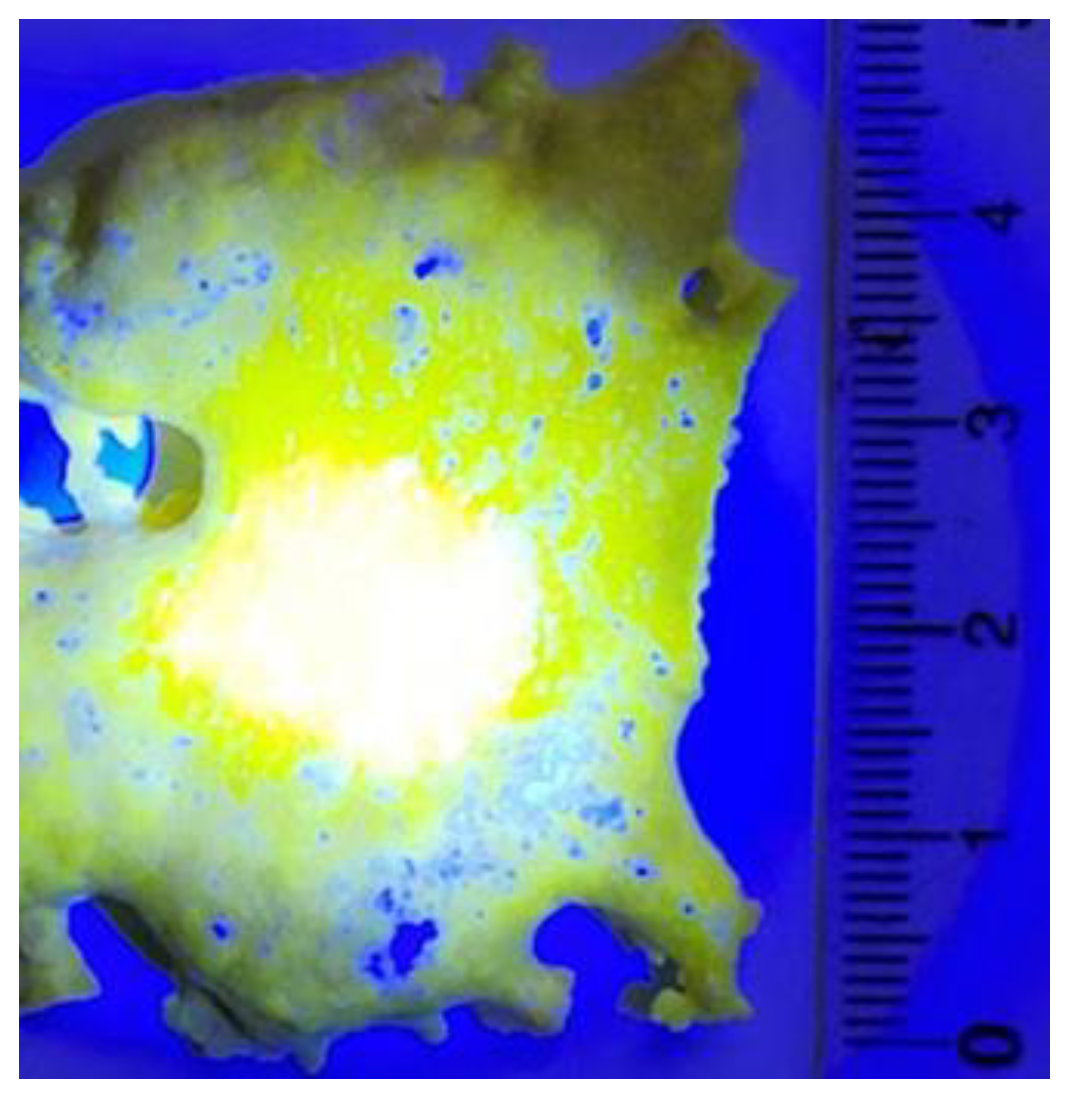
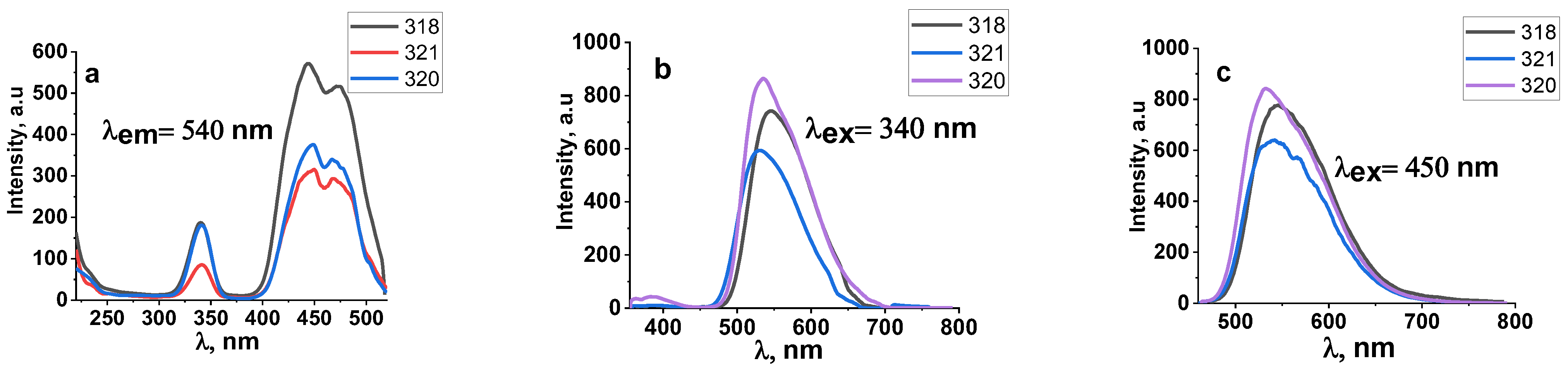
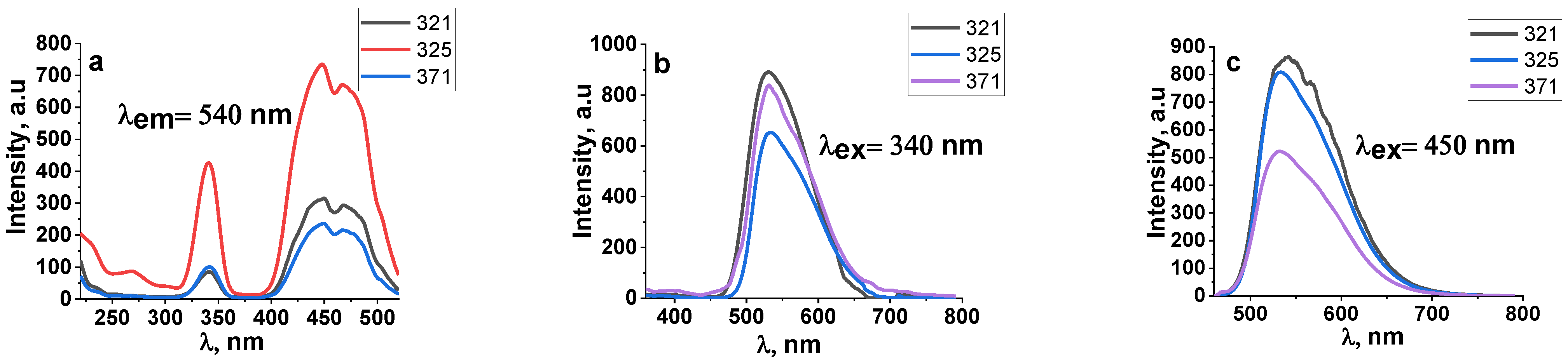
3.2. Luminescence of Synthesized Ceramics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Z.; Yu, S.; Li, Y.; Ruan, S.; Kong, L.B.; Huang, Q.; Huang, Z.; Zhou, K.; Su, H.; Yao, Z.; et al. Materials Development and Potential Applications of Transparent Ceramics: A Review. Mater. Sci. Eng. R Rep. 2020, 139, 100518. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped Garnet Phosphors: Composition Modification, Luminescence Properties and Applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Smet, P.; Parmentier, A.; Poelman, D. Selecting Conversion Phosphors for White Light-Emtting Diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in Phosphor-Converted White Light-Emitting Diodes: Recent Advances in Materials, Techniques and Properties. Mater. Sci. Eng. R Rep. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, M.; Su, Q. Comparative Investigation on Synthesis and Photoluminescence of YAG:Ce Phosphor. Mater. Sci. Eng. B 2004, 106, 251. [Google Scholar] [CrossRef]
- Sharma, S.K.; James, J.; Gupta, S.K.; Hussain, S. UV-A,B,C Emitting Persistent Luminescent Materials. Materials 2022, 16, 236. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Shen, Q.; Shi, P. Flux-Grown Y3Al5O12:Ce3+ Phosphors with Improved Crystallinity and Dispersibility. Ceram. Int. 2014, 40, 15313–15317. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, C.; Huang, J.; Chen, J.; Chen, D.; Wang, W.; Wang, F.; Cao, Y.; Yuan, X. Luminescence Properties of YAG:Ce, Gd Phosphors Synthesized under Vacuum Condition and Their White LED Performances. Opt. Mater. Express OME 2014, 4, 649–655. [Google Scholar] [CrossRef]
- Yang, Y.-G.; Wang, X.-P.; Liu, B.; Zhang, Y.-Y.; Lv, X.-S.; Li, J.; Wei, L.; Yu, H.-J.; Hu, Y.; Zhang, H.-D. Molten Salt Synthesis and Luminescence of Dy3+-Doped Y3Al5O12 Phosphors. Luminescence 2020, 35, 580–585. [Google Scholar] [CrossRef]
- Murai, S.; Fujita, K.; Iwata, K.; Tanaka, K. Scattering-Based Hole Burning in Y3Al5O12:Ce3+ Monoliths with Hierarchical Porous Structures Prepared via the Sol–Gel Route. J. Phys. Chem. C 2011, 115, 17676–17681. [Google Scholar] [CrossRef]
- Dippong, T.; Andrea Levei, E.; Cadar, O.; Grigore Deac, I.; Lazar, M.; Borodi, G.; Petean, I. Effect of Amorphous SiO2 Matrix on Structural and Magnetic Properties of Cu0.6Co0.4Fe2O4/SiO2 Nanocomposites. J. Alloys Compd. 2020, 849, 156695. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Kemel’bekova, A.E.; Lisitsyn, V.M.; Mukhamedshina, D.M.; Nemkaeva, R.R.; Tulegenova, A.T. Aerosol Synthesis of Highly Dispersed Y3Al5O12:Ce3+ Phosphor with Intense Photoluminescence. Phys. Solid State 2019, 61, 1840–1845. [Google Scholar] [CrossRef]
- Dai, P.; Ji, C.; Shen, L.; Qian, Q.; Guo, G.; Zhang, X.; Bao, N. Photoluminescence Properties of YAG:Ce3+,Pr3+ Nano-Sized Phosphors Synthesized by a Modified Co-Precipitation Method. J. Rare Earths 2017, 35, 341–346. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Hu, D.; Dobrotvorska, M.; Yavetskiy, R.; Dai, Z.; Xie, T.; Yuan, Q.; Chen, H.; Liu, Q.; et al. Fine-Grained Tb3Al5O12 Transparent Ceramics Prepared by Co-Precipitation Synthesis and Two-Step Sintering. Magnetochemistry 2023, 9, 47. [Google Scholar] [CrossRef]
- Serrano-Bayona, R.; Chu, C.; Liu, P.; Roberts, W.L. Flame Synthesis of Carbon and Metal-Oxide Nanoparticles: Flame Types, Effects of Combustion Parameters on Properties and Measurement Methods. Materials 2023, 16, 1192. [Google Scholar] [CrossRef]
- Huczko, A.; Kurcz, M.; Baranowski, P.; Bystrzejewski, M.; Bhattarai, A.; Dyjak, S.; Bhatta, R.; Pokhrel, B.; Kafle, B.P. Fast Combustion Synthesis and Characterization of YAG:Ce3+ Garnet Nanopowders. Phys. Status Solidi (B) 2013, 250, 2702–2708. [Google Scholar] [CrossRef]
- Le Godec, Y.; Le Floch, S. Recent Developments of High-Pressure Spark Plasma Sintering: An Overview of Current Applications, Challenges and Future Directions. Materials 2023, 16, 997. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Tulegenova, A.; Kaneva, E.; Mussakhanov, D.; Gritsenko, B. Express Synthesis of YAG:Ce Ceramics in the High-Energy Electrons Flow Field. Materials 2023, 16, 1057. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Golkovsky, M.G.; Musakhanov, D.A.; Tulegenova, A.T.; Abdullin, K.A.; Aitzhanov, M.B. YAG Based Phosphors, Synthesized in a Field of Radiation. J. Phys. Conf. Ser. 2018, 1115, 052007. [Google Scholar] [CrossRef]
- Volchenko, T.S.; Yalovets, A.P. Calculation of the Effective Thermal Conductivity of Powders Formed by Spherical Particles in a Gaseous Atmosphere. Tech. Phys. 2016, 61, 324–336. [Google Scholar] [CrossRef]
- Dorenbos, P. 5d-Level Energies of Ce3+ and the Crystalline Environment. IV. Aluminates and “Simple” Oxides. J. Lumin. 2002, 99, 283–299. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. Review of Luminescent Properties of Ce3+-Doped Garnet Phosphors: New Insight into the Effect of Crystal and Electronic Structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Korepanov, V.I.; Yakovlev, V.Y. Evolution of Primary Radiation Defects in Ionic Crystals. Russ. Phys. J. 1996, 39, 1009–1028. [Google Scholar] [CrossRef]
- Shin, J.K.; Jo, S.-H.; Kim, T.-H.; Oh, Y.-H.; Yu, S.; Son, Y.-S.; Kim, T.-H. Removal of NOx Using Electron Beam Process with NaOH Spraying. Nucl. Eng. Technol. 2022, 54, 486–492. [Google Scholar] [CrossRef]
- Yushkov, Y.G.; Tyunkov, A.V.; Oks, E.M.; Zolotukhin, D.B. Electron Beam Evaporation of Boron at Forevacuum Pressures for Plasma-Assisted Deposition of Boron-Containing Coatings. J. Appl. Phys. 2016, 120, 233302. Available online: https://aip.scitation.org/doi/10.1063/1.4972268 (accessed on 11 April 2023). [CrossRef]
- Klimov, A.S.; Bakeev, I.Y.; Dvilis, E.S.; Oks, E.M.; Zenin, A.A. Electron Beam Sintering of Ceramics for Additive Manufacturing. Vacuum 2019, 169, 108933. [Google Scholar] [CrossRef]
- Gao, Y. Surface Modification of TA2 Pure Titanium by Low Energy High Current Pulsed Electron Beam Treatments. Appl. Surf. Sci. 2011, 257, 7455–7460. [Google Scholar] [CrossRef]
- Hegelmann, E.; Jung, A.; Hengst, P.; Zenker, R.; Buchwalder, A. Investigations Regarding Electron Beam Surface Remelting of Plasma Nitrided Spray-Formed Hypereutectic Al–Si Alloy. Adv. Eng. Mater. 2018, 20, 1800244. [Google Scholar] [CrossRef]
- Valkov, S.; Ormanova, M.; Petrov, P. Electron-Beam Surface Treatment of Metals and Alloys: Techniques and Trends. Metals 2020, 10, 1219. [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; Kazakov, A.V.; Oks, E.M.; Tyunkov, A.V.; Yushkov, Y.G. Electron Beam Synthesis of Silicon-Carbon Coatings in the Forevacuum Pressure Range. Ceram. Int. 2022, 48, 13890–13894. [Google Scholar] [CrossRef]
- Kim, J.-U.; Cha, S.-H.; Shin, K.; Jho, J.Y.; Lee, J.-C. Synthesis of Gold Nanoparticles from Gold(I)−Alkanethiolate Complexes with Supramolecular Structures through Electron Beam Irradiation in TEM. J. Am. Chem. Soc. 2005, 127, 9962–9963. [Google Scholar] [CrossRef]




| Main Phase | Related Phase | Rwp(%) | |
|---|---|---|---|
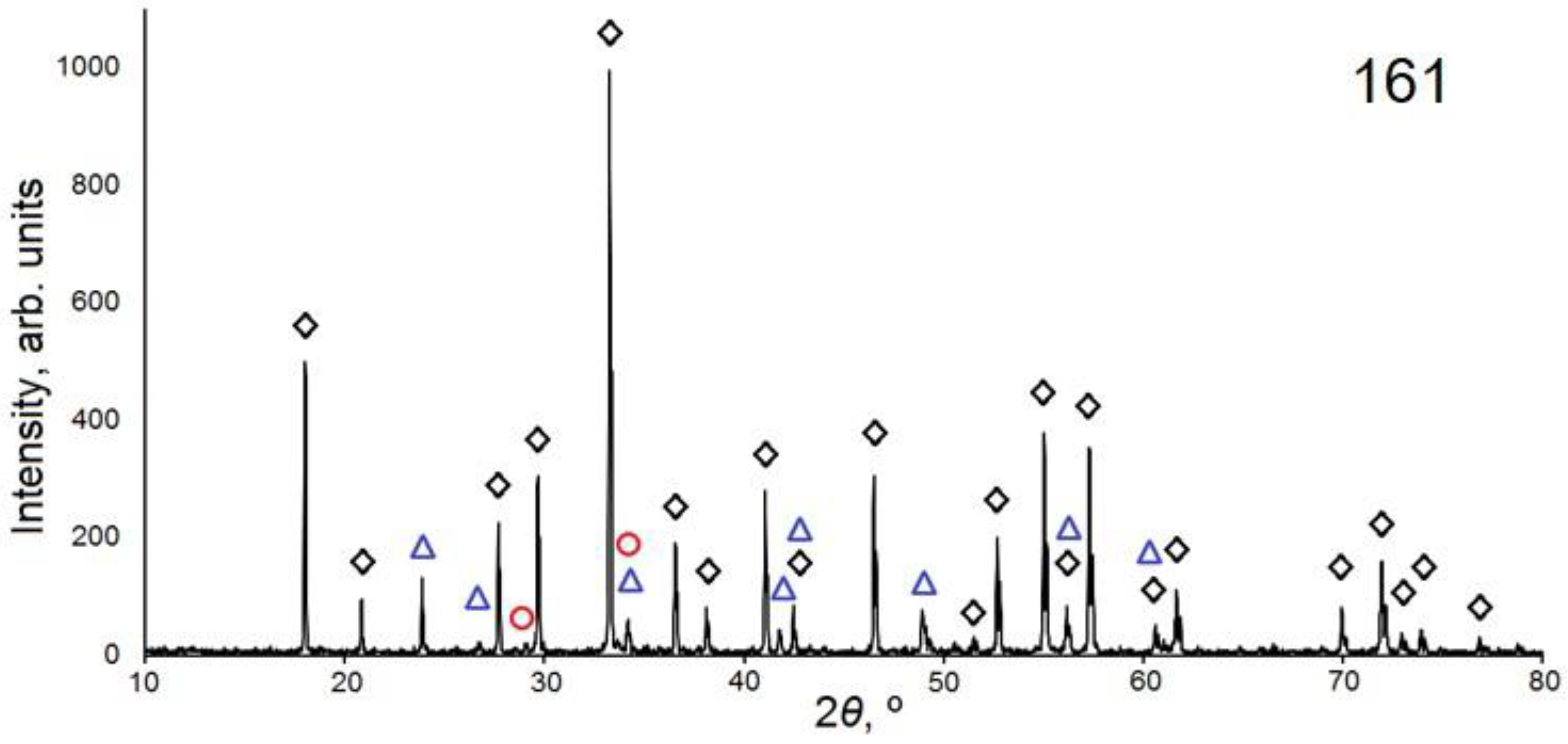
| 161 | Y3Al5O12 (~91%) Ia–3d; a = 12.005(2) Å; V = 1730.3(3) Å3 | YAlO3 (~7%) Y2O3 (~2%) | 5.0 |
| 164 | Y3Al5O12 (~92%) Ia–3d; a = 12.009(4) Å; V = 1732.1(3) Å3 | YAlO3 (~6%) Y2O3 (~2%) | 5.6 |
| 318 | Y3Al5O12 (~91%) Ia–3d; a = 11.999(2) Å; V = 1727.4(3) Å3 | YAlO3 (~4%) Al2O3 (~3%) Y2O3 (~2%) | 4.3 |
| 320 | Y3Al5O12 (~97%) Ia–3d; a = 12.002(2) Å; V = 1728.9(3) Å3 | YAlO3 (~3%) | 5.0 |
| 323 | Y3Al5O12 (~90%) Ia–3d; a = 12.005(2) Å; V = 1730.1(3) Å3 | YAlO3 (~5%) Al2O3 (~3%) Y2O3 (~2%) | 5.3 |
| No. | E, MeV | P, kW | |
|---|---|---|---|
| 318 | 2.5 MeV | 8 kW | Without Scan |
| 320 | 2.5 MeV | 10 kW | Without Scan |
| 321 | 2.5 MeV | 37 kW | With Scan |
| 325 | 2.0 MeV | 30 kW | With Scan |
| 371 | 1.4 MeV | 25 kW | With Scan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisitsyn, V.; Mussakhanov, D.; Tulegenova, A.; Kaneva, E.; Lisitsyna, L.; Golkovski, M.; Zhunusbekov, A. The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics. Materials 2023, 16, 3158. https://doi.org/10.3390/ma16083158
Lisitsyn V, Mussakhanov D, Tulegenova A, Kaneva E, Lisitsyna L, Golkovski M, Zhunusbekov A. The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics. Materials. 2023; 16(8):3158. https://doi.org/10.3390/ma16083158
Chicago/Turabian StyleLisitsyn, Victor, Dossymkhan Mussakhanov, Aida Tulegenova, Ekaterina Kaneva, Liudmila Lisitsyna, Mikhail Golkovski, and Amangeldy Zhunusbekov. 2023. "The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics" Materials 16, no. 8: 3158. https://doi.org/10.3390/ma16083158
APA StyleLisitsyn, V., Mussakhanov, D., Tulegenova, A., Kaneva, E., Lisitsyna, L., Golkovski, M., & Zhunusbekov, A. (2023). The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics. Materials, 16(8), 3158. https://doi.org/10.3390/ma16083158








