Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response
Abstract
1. Introduction
2. Materials and Methods
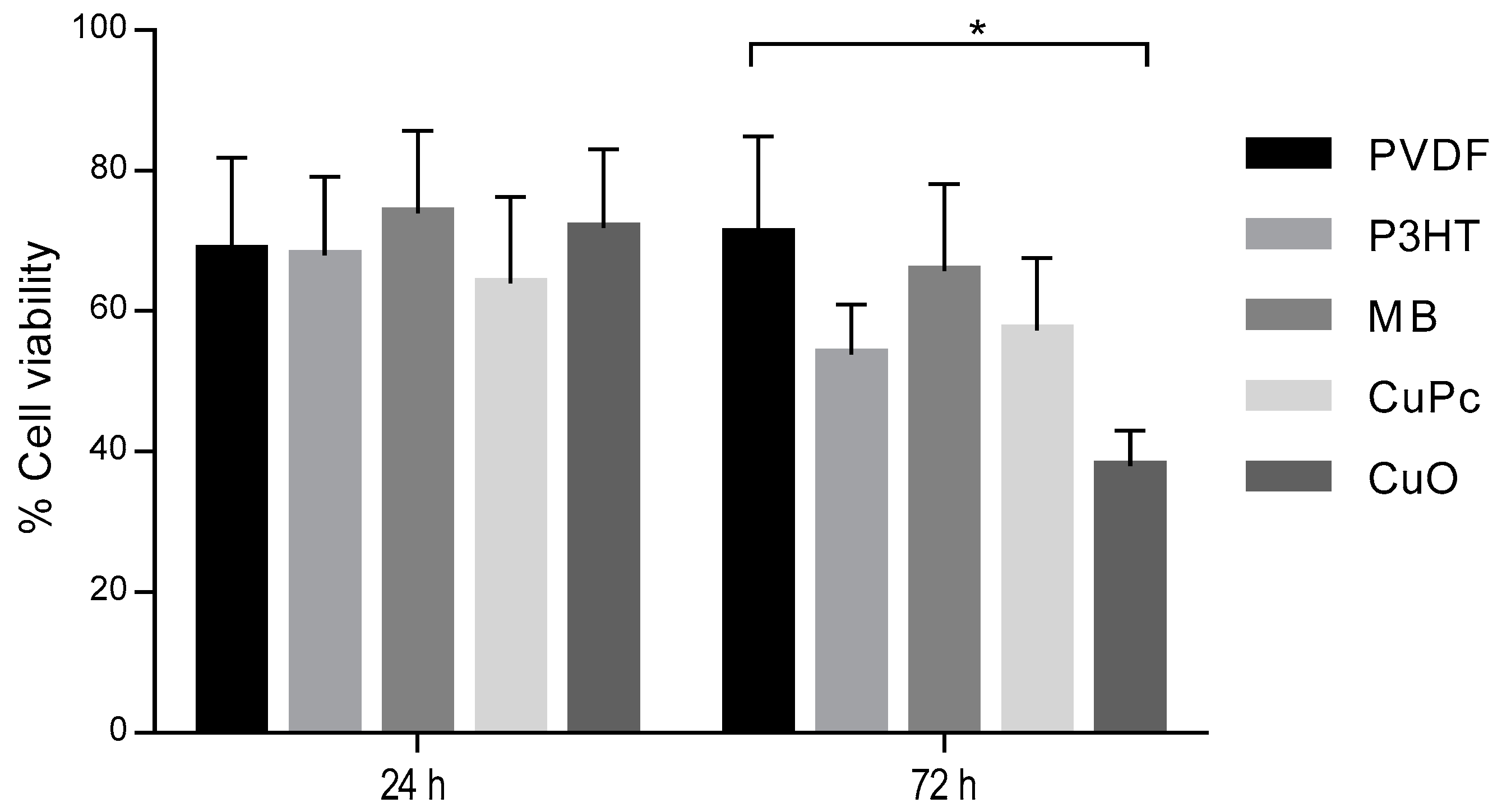
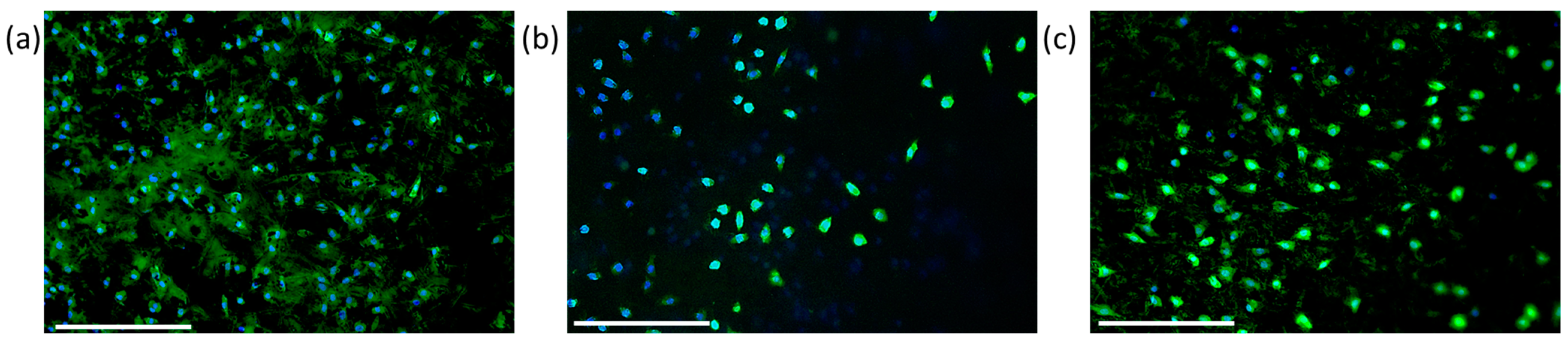
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarino, V.; Zuppolini, S.; Borriello, A.; Ambrosio, L. Electro-active polymers (EAPs): A promising route to design bio-organic/bioinspired platforms with on demand functionalities. Polymers 2016, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garcia, W.; Ramakrishna, S.; Thomas, S.W. Electrospinning technique for fabrication of coaxial nanofibers of semiconducting polymers. Polymers 2022, 14, 5073. [Google Scholar] [CrossRef] [PubMed]
- Serrano, W.; Meléndez, A.; Ramos, I.; Pinto, N.J. Electrospun composite poly(lactic acid)/polyaniline nanofibers from low concentrations in CHCl3: Making a biocompatible polyester electro-active. Polymer 2014, 55, 5727–5733. [Google Scholar] [CrossRef]
- Zuppolini, S.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Optimization of polydopamine coatings onto poly–ε–caprolactone electrospun fibers for the fabrication of bio-electroconductive interfaces. J. Funct. Biomater. 2020, 11, 19. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Zuppolini, S.; Zarrelli, M.; Mazzotta, E.; Borriello, A.; Malitesta, C.; Borriello, A.; Guarino, V. Polydopamine-Coated Alginate Microgels: Process Optimization and In Vitro Validation. J. Funct. Biomater. 2023, 14, 2. [Google Scholar] [CrossRef]
- Saracino, E.; Zuppolini, S.; Guarino, V.; Benfenati, V.; Borriello, A.; Zamboni, R.; Ambrosio, L. Polyaniline nano-needles into electrospun bio active fibres support in vitro astrocyte response. RSC Adv. 2021, 11, 11347–11355. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Li, Y.; Wang, T.; Xu, T.; Chen, J.; Fan, Z.; Wang, Y.; Wang, B. Facile Preparation of Highly Conductive Poly(amide-imide) Composite Films beyond 1000 S m−a through Ternary Blend Strategy. Polymers 2019, 11, 546. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Wu, L.; Jin, Z.; Liu, Y.; Ning, H.; Liu, X.; Hu, N. Recent advances in the preparation of PVDF-based piezoelectric materials. Nanotech. Rev. 2022, 11, 1386–1407. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Tong, S.; Li, X.; Song, W. Three-dimensional network films of electrospun copper oxide nanofibers for glucose determination. Biosens. Bioelectron. 2009, 25, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.G.; Ozarowski, A.; Archibong, E.; Thomas, S.; Serrano-Garcia, W.; Stoian, S.A.; Mateeva, N. Synthesis and characterization of novel polyethylene oxide–dinuclear Cu(II) complex electrospun nanofibers. Mater. Lett. 2019, 238, 58–61. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive–Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Zhang, S.; Liu, C.-T.; Ma, G.; Azoulay, J.D.; Gu, X.; Gangishetty, M.K.; Kundu, S. Effects of Poly(3-hexylthiophene) Molecular Weight and the Aging of Spinning Solution on the Electrospun Fiber Properties. ACS Appl. Polym. Mater. 2022, 4, 8812–8824. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Yang, G.; Xie, Q.; Zhong, S.; Su, X.; Zhang, B. Improvement of Sensing Properties for Copper Phthalocyanine Sensors Based on Polymer Nanofibers Scaffolds. Langmuir 2020, 36, 4532–4539. [Google Scholar] [CrossRef]
- Esokkiya, A.; Sudalaimani, S.; Sanjeev Kumar, K.; Sampathkumar, P.; Suresh, C.; Giribabu, K. Poly(methylene blue)-Based Electrochemical Platform for Label-Free Sensing of Acrylamide. ACS Omega 2021, 6, 9528–9536. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zheng, W.; Yang, J.; Zhang, H.; Wang, C. Electrospun palladium (IV)-doped copper oxide composite nanofibers for non-enzymatic glucose sensors. Electrochem. Commun. 2009, 11, 1811–1814. [Google Scholar] [CrossRef]
- Bakhtiyar, M.J.; Raza, Z.A.; Aslam, M.; Bajwa, S.Z.; Rehman, M.S.; Rafiq, S. Cupric oxide nanoparticles incorporated poly(hydroxybutyrate) nanocomposite for potential biosensing application. Int. J. Biol. Macromol. 2022, 213, 1018–1028. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Hao, L.; Wu, C.; Lu, M.; Zhang, L.; Yu, D. Electrospun cellulose-based conductive polymer nanofibrous mats: Composite scaffolds and their influence on cell behavior with electrical stimulation for nerve tissue engineering. Soft Matter 2020, 16, 6591–6598. [Google Scholar] [CrossRef]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, W.-X.; Gao, Y.-R.; Wei, Y.-N.; Shu, Y.; Wang, J.-H. The State-of-the-Art Advances of Copper-based Nanostructures in the Enhancement on Chemodynamic Therapy. J. Mater. Chem. B 2020, 9, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Bozari, E.; Eavani, S. Copper phthalocyanine fluorapatite nano composite and its pH- and photo- responsive properties. Optik 2021, 232, 166483. [Google Scholar] [CrossRef]
- Ju, H.; Zhou, J.; Cai, C.; Chen, H. The electrochemical behavior of methylene blue at a microcylinder carbon fiber electrode. Electroanalysis 1995, 7, 1165–1170. [Google Scholar] [CrossRef]
- Zohri, A.N.A.; Kassim, R.M.F.; Hassan, S.H.A. Methylene blue as an exogenous electron mediator on bioelectricity from molasses using Meyerozyma guilliermondii as biocatalyst. Biomass Conv. Bioref. 2022, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.; Xi, F.; Zhang, Y.; Lin, X. Reagentless biosensor based on layer-by-layer assembly of functional multiwall carbon nanotubes and enzyme-mediator biocomposite. J. Zhejiang Univ. Sci. B 2011, 12, 468–476. [Google Scholar] [CrossRef]
- Yao, H.; Li, N.; Xu, S.; Xu, J.; Zhu, J.; Chen, H. Electrochemical study of a new methylene blue/silicon oxide nanocomposition mediator and its application for stable biosensor of hydrogen peroxide. Biosens. Bioelectron. 2005, 21, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garcia, W.; Pinto, N.J. Electrospun Fibers of Poly(Vinylidene Fluoride-Trifluoroethylene)/Poly(3-Hexylthiophene) Blends from Tetrahydrofuran. Ferroelectrics 2012, 432, 41–48. [Google Scholar] [CrossRef]
- Serrano, W.; Meléndez, A.; Ramos, I.; Pinto, N.J. Poly(lactic acid)/poly(3-hexylthiophene) composite nanofiber fabrication for electronic applications. Polym. Int. 2016, 65, 503–507. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Tortiglione, C.; Antognazza, M.R.; Tino, A.; Bossio, C.; Marchesano, V.; Bauduin, A.; Zangoli, M.; Vaquero Morata, S.; Lanzani, G. Semiconducting polymers are light nanotransducers in eyeless animals. Sci. Adv. 2017, 3, e1601699. [Google Scholar] [CrossRef]
- Prien, S.D.; Dunn, C.; Messer, R.H. Adhesion-promoting properties of dyes routinely used during fertility surgeries. J. Assist. Reprod. Genet. 1995, 12, 136–140. [Google Scholar] [CrossRef]
- Zia, M.; Naz, S.; Mukhtiar, A. On the Toxicity Copper Oxide Nanoparticles: A Review Study. TIET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic Charged Nanofiber Filter for Filtering Airborne Novel Coronavirus (COVID-19) and Nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shi, J.; Xiao, X.; Hong, Y.; Li, X.; Zhang, W.; Cheng, Y.; Wang, Z.; Li, W.J.; Chen, J.; et al. Self-charging electrostatic face masks leveraging triboelectrification for prolonged air filtration. Nat. Commun. 2022, 13, 7835. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.; Guarino, V. P3HT loaded piezoelectric electrospun fibers for tunable molecular adsorption. Mater. Lett. 2020, 266, 127458. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Styczynski, A.R.; Anwar, K.N.; Sultana, H.; Ghanem, A.; Lurain, N.; Chua, A.; Novak, R.M. In vitro antiretroviral activity and in vivo toxicity of the potential topical microbicide copper phthalocyanine sulfate. Virol. J. 2015, 12, 132. [Google Scholar] [CrossRef]
- Vzorov, A.; Marzilli, L.; Compans, R.; Dixon, D. Prevention of HIV-1 infection by phthalocyanines. Antivir. Res. 2003, 59, 99–109. [Google Scholar] [CrossRef]
- Wu, J.; Xu, H.; Tang, W.; Kopelman, R.; Philbert, M.A.; Xi, C. Eradication of Bacteria in Suspension and Biofilms Using Methylene Blue-Loaded Dynamic Nanoplatforms. Antimicrob. Agents Chemother. 2009, 53, 3042–3048. [Google Scholar] [CrossRef]
- Piccirillo, C.; Perni, S.; Gil-Thomas, J.; Prokopovich, P.; Wilson, M.; Pratten, J.; Parkin, I.P. Antimicrobial activity of methylene blue and toluidine blue O covalently bound to a modified silicone polymer surface. J. Mater. Chem. 2009, 19, 6167. [Google Scholar] [CrossRef]
- Floyd, R.A.; Schneider, J.E.; Dittmer, D.P. Methylene blue photoinactivation of RNA viruses. Antivir. Res. 2004, 61, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Papin, J.F.; Floyd, R.A.; Dittmer, D.P. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antivir. Res. 2005, 68, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Zhou, J.; Zhou, L.; Wei, S. A smart copper-phthalocyanine framework nanoparticle for enhancing photodynamic therapy in hypoxic conditions by weakening cells through ATP depletion. J. Mater. Chem. B 2018, 6, 2078–2088. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.-H.; Zheng, B.-D.; Tang, J.; Zhao, Y.; Zheng, B.-Y.; Huang, J.-D. New application of phthalocyanine molecules: From photodynamic therapy to photo-thermal therapy by means of structural regulation rather than formation of aggregates. Chem. Sci. 2018, 9, 2098–2104. [Google Scholar] [CrossRef]
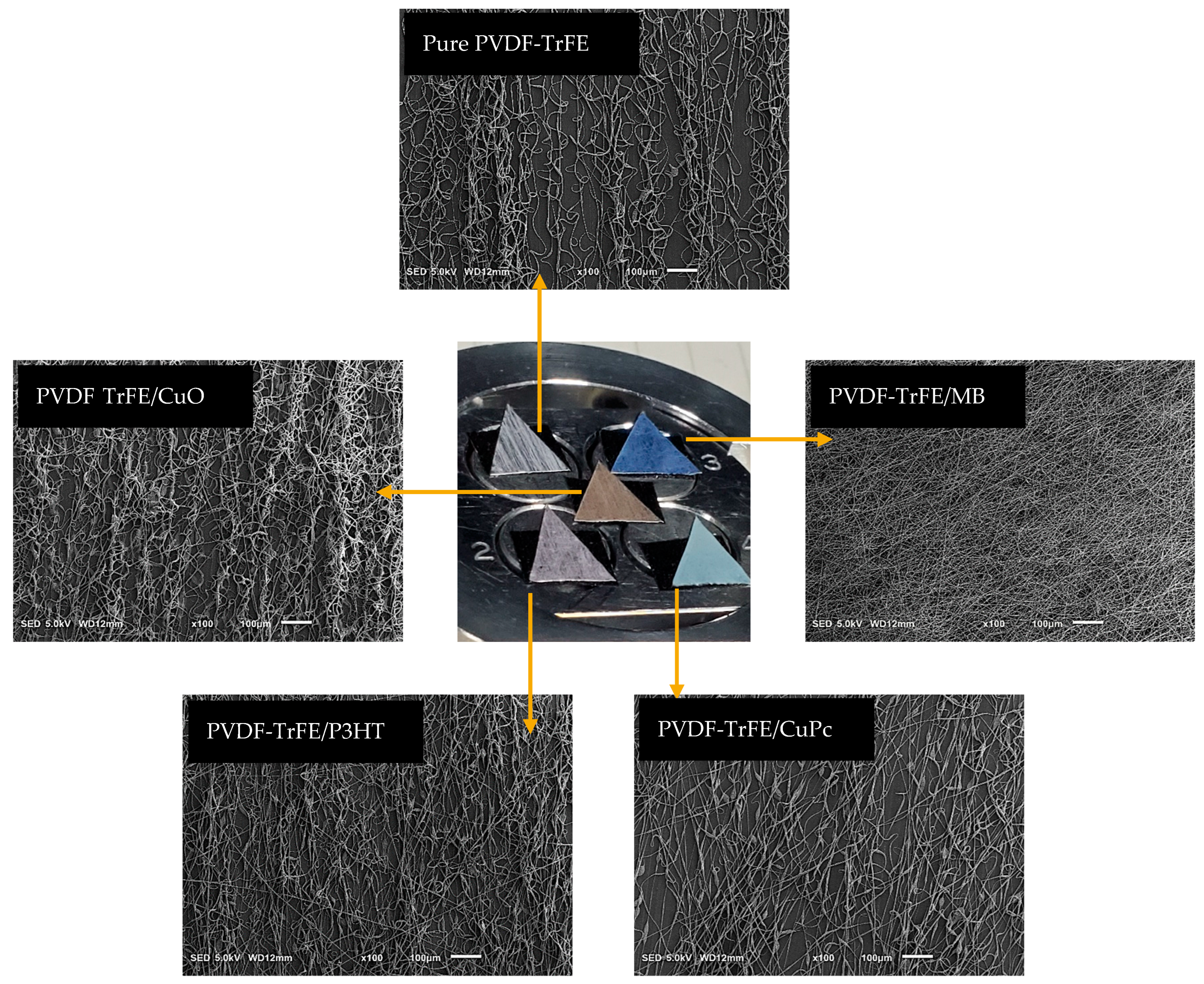
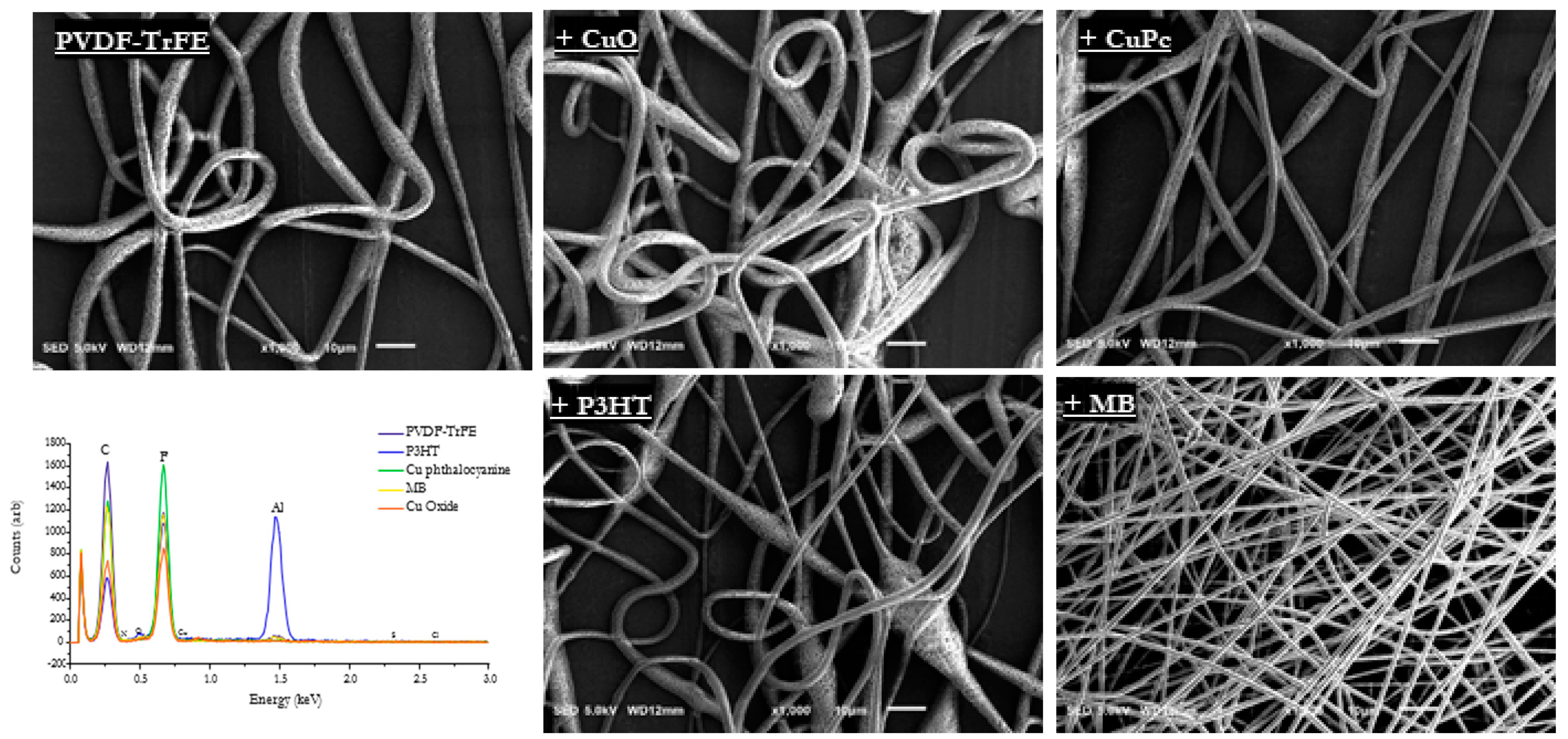
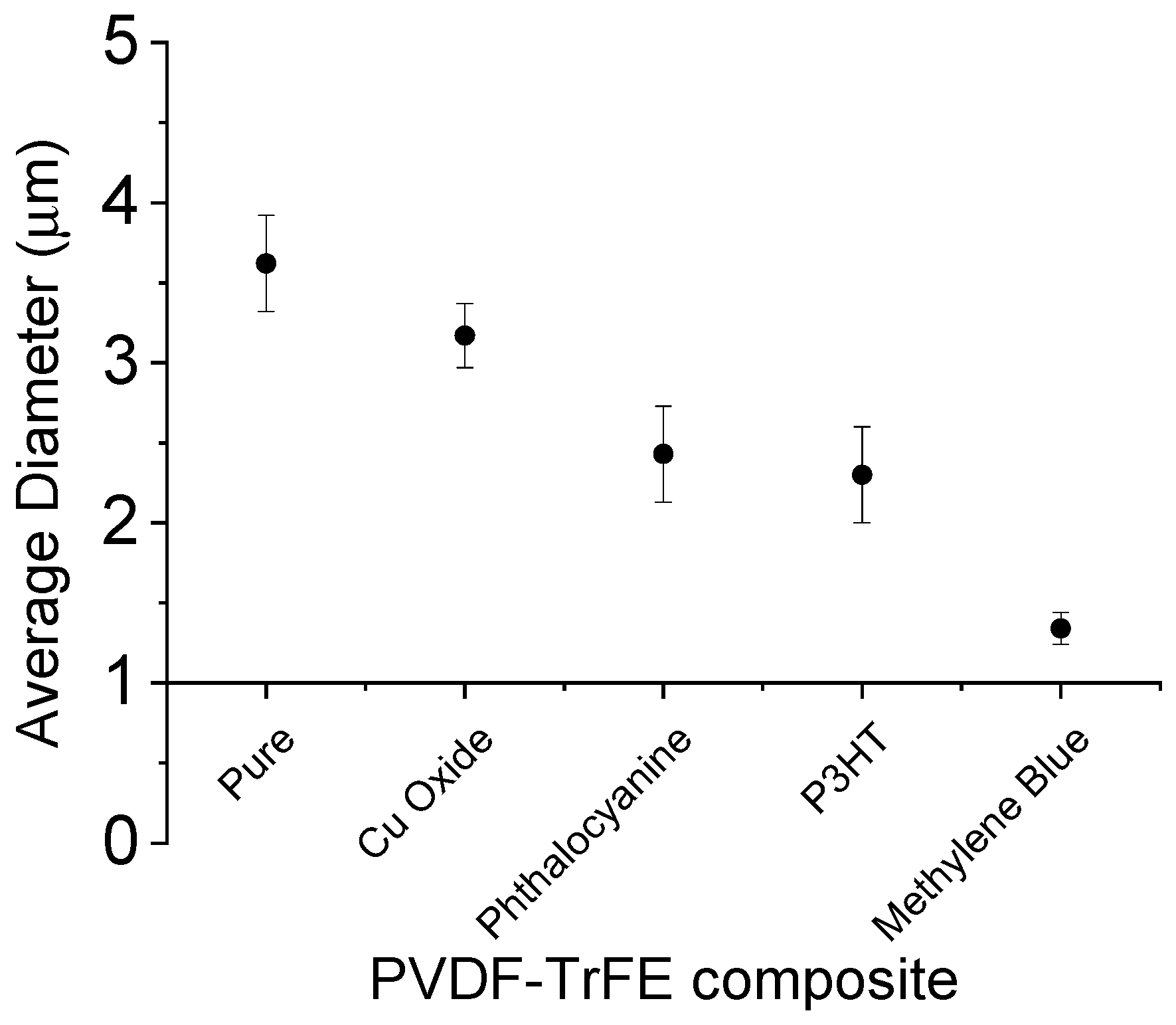
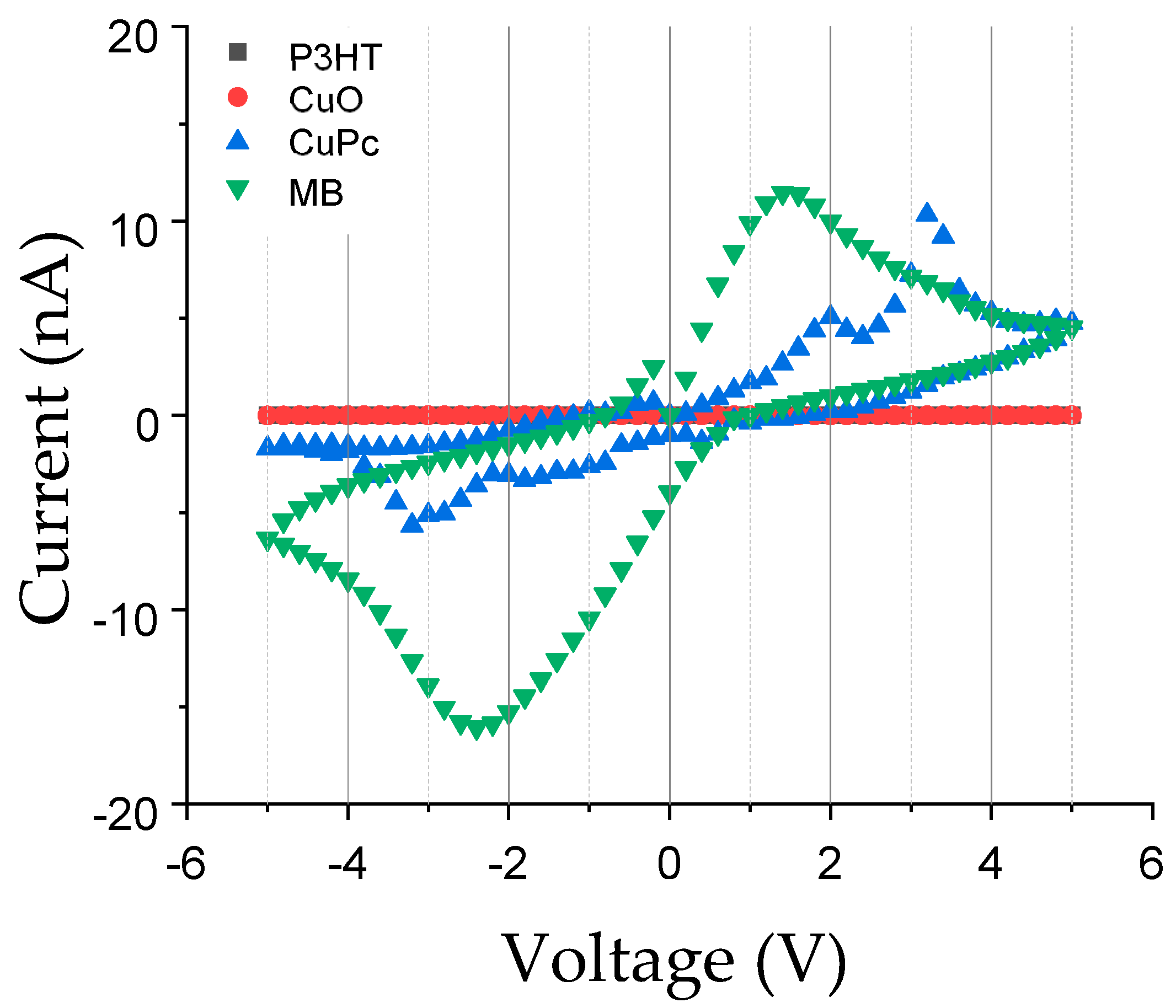

| Active Component | Weight (mg) | Amount of 13 wt% PVDF-TrFE/THF (g) | Composite Solution (wt%) |
|---|---|---|---|
| P3HT 1 | 1.4 | 1.45 | 0.09 |
| Cu Phthalocyanine | 10.9 | 1.5 | 0.721 |
| Methylene Blue | 10.2 | 1.5 | 0.675 |
| CuO | 8.9 | 1.5 | 0.589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Garcia, W.; Cruz-Maya, I.; Melendez-Zambrana, A.; Ramos-Colon, I.; Pinto, N.J.; Thomas, S.W.; Guarino, V. Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response. Materials 2023, 16, 3106. https://doi.org/10.3390/ma16083106
Serrano-Garcia W, Cruz-Maya I, Melendez-Zambrana A, Ramos-Colon I, Pinto NJ, Thomas SW, Guarino V. Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response. Materials. 2023; 16(8):3106. https://doi.org/10.3390/ma16083106
Chicago/Turabian StyleSerrano-Garcia, William, Iriczalli Cruz-Maya, Anamaris Melendez-Zambrana, Idalia Ramos-Colon, Nicholas J. Pinto, Sylvia W. Thomas, and Vincenzo Guarino. 2023. "Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response" Materials 16, no. 8: 3106. https://doi.org/10.3390/ma16083106
APA StyleSerrano-Garcia, W., Cruz-Maya, I., Melendez-Zambrana, A., Ramos-Colon, I., Pinto, N. J., Thomas, S. W., & Guarino, V. (2023). Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response. Materials, 16(8), 3106. https://doi.org/10.3390/ma16083106










