Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root
Abstract
1. Introduction
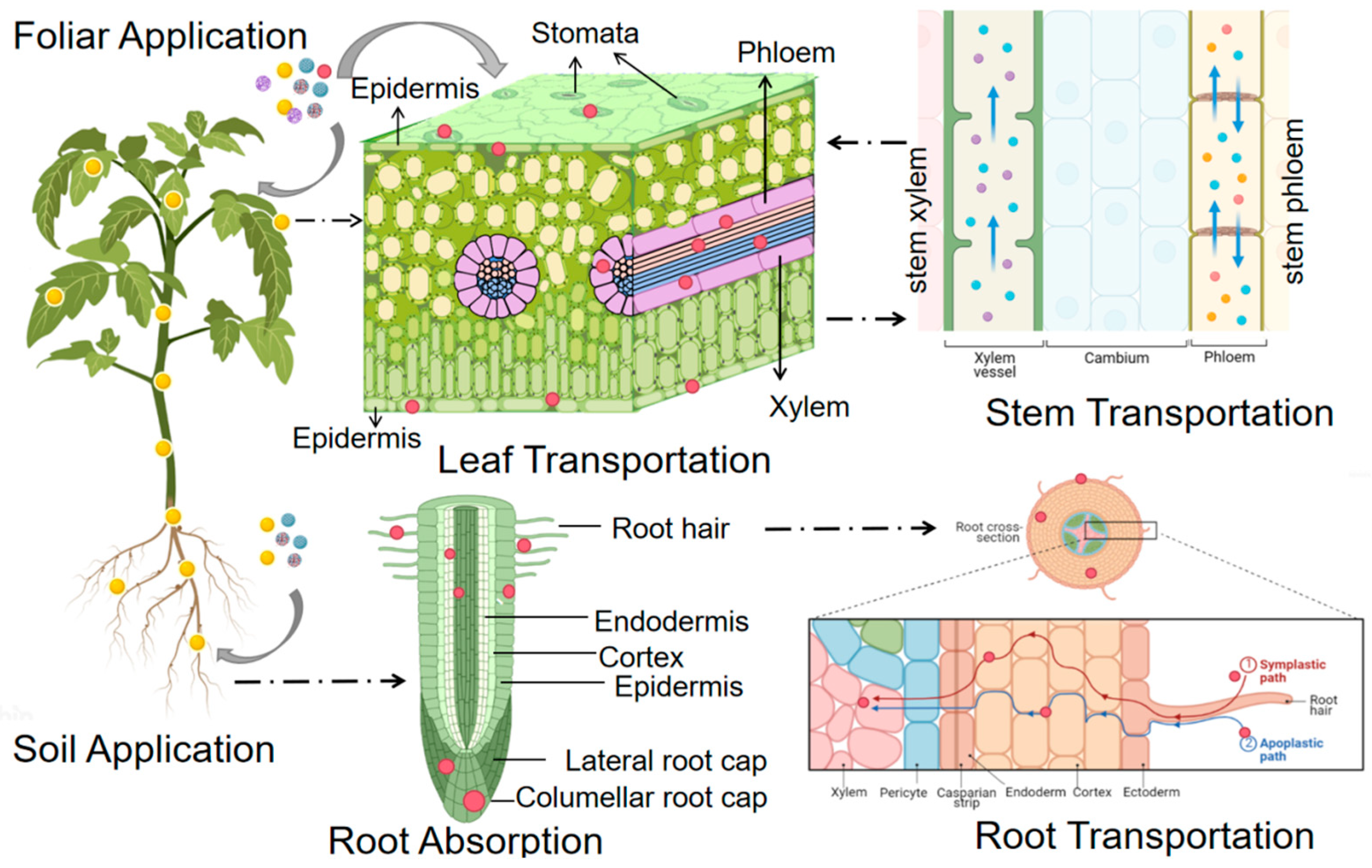
2. Uptake of Nanoparticles in Plant Leaves
2.1. Pathways for Foliar Uptake
2.2. Key Factors That Affecting the Uptake of Nanoparticles via Leaves
2.2.1. Effect of Size and Chemical Composition
2.2.2. Effect of Shape and Surface Charge
2.2.3. Effect of Plant Species
3. Absorption of Nanoparticles in Plant Roots
3.1. Pathways for Root Uptake
3.2. Key Factors That Affecting the Uptake of Nanoparticles via Roots
3.2.1. Effect of Size and Chemical Composition
3.2.2. Effect of Surface Charge
4. Transport and Distribution of Nanoparticles in Leaves
4.1. Transport Pathway of Nanoparticles in Leaves
4.2. Distribution of Nanoparticles Absorbed via Leaves
4.3. Key Factors That Affect the Transport of Nanoparticles Absorbed via Leaves
4.3.1. Effect of Size and Chemical Composition
4.3.2. Effect of Surface Charge
5. Transport of Nanoparticles at the Root
5.1. Transport Pathway
5.2. Key Factors That Affect the Transport of Nanoparticles Absorbed via Roots
5.2.1. Effect of Chemical Composition
5.2.2. Effect of Surface Charge
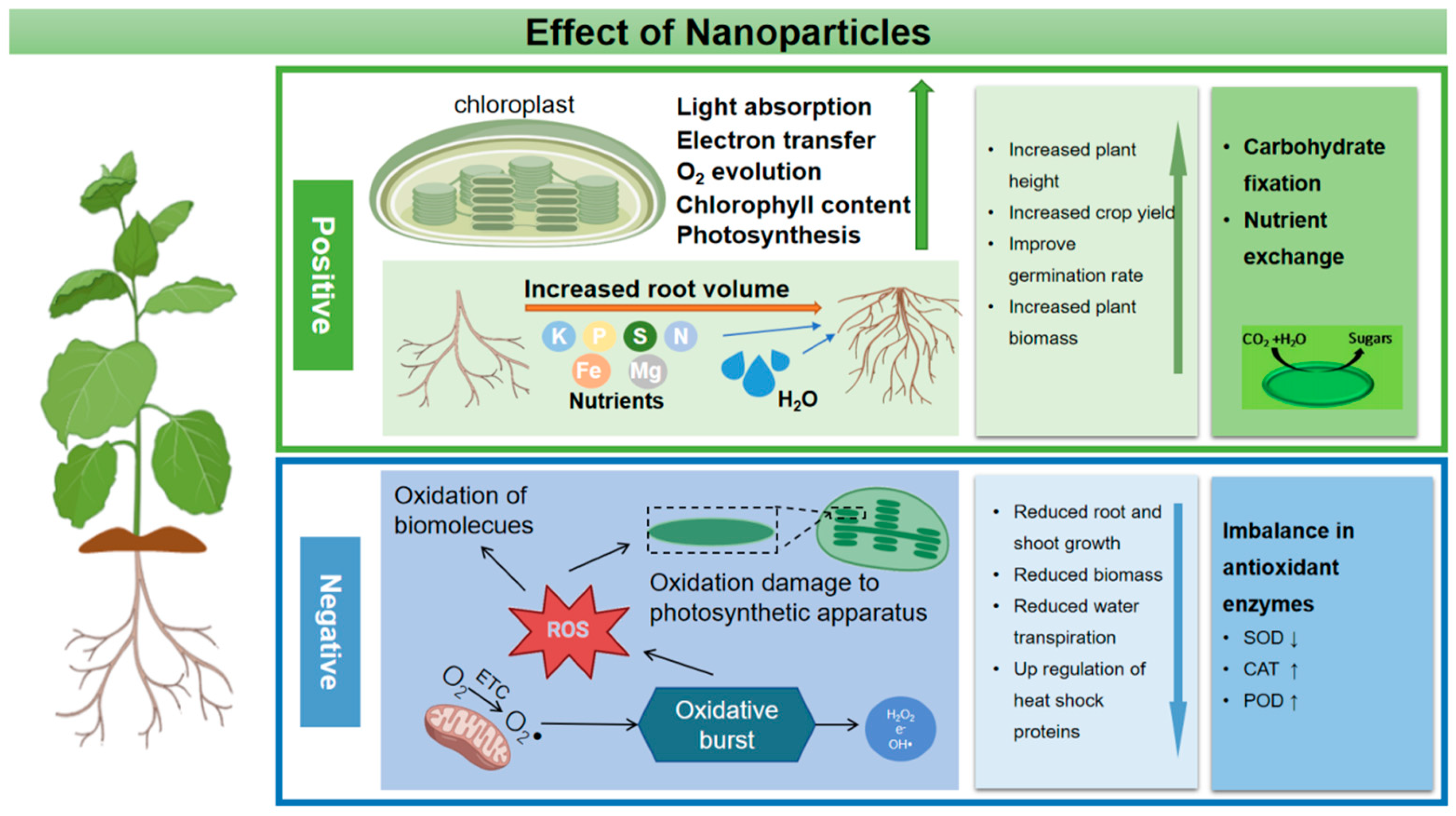
6. Effect of Nanoparticles on Plant Physiological Activity
6.1. Nanoparticles Promote Plant Development and Yield
6.2. Nanoparticles Alleviate Plant Abiotic Stress
6.3. Toxicity of Nanoparticles to Plants
6.3.1. Inhibitory Effect of Nanoparticles on Plant Growth
6.3.2. Genotoxicity and Oxidative Stress Damage of Nanoparticles in Plants
7. Conclusions and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Mattos, B.D.; Antunes, D.R.; Forini, M.M.L.; Monikh, F.A.; Rojas, O.J. Foliage adhesion and interactions with particulate delivery systems for plant nanobionics and intelligent agriculture. Nano Today 2021, 37, 101078. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Singla, R.; Kumari, A.; Yadav, S.K. Impact of Nanomaterials on Plant Physiology and Functions. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 349–377. [Google Scholar]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Yang, C.; Powell, C.A.; Duan, Y.; Shatters, R.; Zhang, M. Antimicrobial Nanoemulsion Formulation with Improved Penetration of Foliar Spray through Citrus Leaf Cuticles to Control Citrus Huanglongbing. PLoS ONE 2015, 10, e0133826. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Banerjee, K.; Pramanik, P.; Maity, A.; Joshi, D.C.; Wani, S.H.; Krishnan, P. Chapter 4—Methods of Using Nanomaterials to Plant Systems and Their Delivery to Plants (Mode of Entry, Uptake, Translocation, Accumulation, Biotransformation and Barriers). In Advances in Phytonanotechnology; Ghorbanpour, M., Wani, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 123–152. [Google Scholar]
- Bussières, P. Estimating the number and size of phloem sieve plate pores using longitudinal views and geometric reconstruction. Sci. Rep. 2014, 4, 4929. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Ha, N.; Seo, E.; Kim, S.; Lee, S.J. Adsorption of nanoparticles suspended in a drop on a leaf surface of Perilla frutescens and their infiltration through stomatal pathway. Sci. Rep. 2021, 11, 11556. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci.-Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Jia-Yi, Y.; Meng-Qiang, S.; Zhi-Liang, C.; Yu-Tang, X.; Hang, W.; Jian-Qiang, Z.; Ling, H.; Qi, Z. Effect of foliage applied chitosan-based silicon nanoparticles on arsenic uptake and translocation in rice (Oryza sativa L.). J. Hazard. Mater. 2022, 433, 128781. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Zhan, X.; Li, A.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Role of Charge and Size in the Translocation and Distribution of Zinc Oxide Particles in Wheat Cells. Acs Sustain. Chem. Eng. 2021, 9, 11556–11564. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, M.; Wang, J.; Wang, W. Surface charge affects foliar uptake, transport and physiological effects of functionalized graphene quantum dots in plants. Sci. Total Environ. 2022, 812, 151506. [Google Scholar] [CrossRef]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Trcera, N.; Sorieul, S.; Cécillon, L.; Ouerdane, L.; Legros, S.; Sarret, G. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard. Mater. 2014, 273, 17–26. [Google Scholar] [CrossRef]
- Driscoll, S.P.; Prins, A.; Olmos, E.; Kunert, K.J.; Foyer, C.H. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. J. Exp. Bot. 2006, 57, 381–390. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.-J.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina, C.; Ibarra, M.R.; de la Fuente, J.M.; Rubiales, D.; Pérez-de-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situsubcellular identification. BMC Plant Biol. 2009, 9, 45. [Google Scholar] [CrossRef]
- Willmer, C.M.; Fricker, M.D. Stomatal Responses to Environmental Factors; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Fan, X.; Cao, X.; Zhou, H.; Hao, L.; Dong, W.; He, C.; Xu, M.; Wu, H.; Wang, L.; Chang, Z.; et al. Carbon dioxide fertilization effect on plant growth under soil water stress associates with changes in stomatal traits, leaf photosynthesis, and foliar nitrogen of bell pepper (Capsicum annuum L.). Environ. Exp. Bot. 2020, 179, 104203. [Google Scholar] [CrossRef]
- Schwabe, F.; Schulin, R.; Limbach, L.K.; Stark, W.; Bürge, D.; Nowack, B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 2013, 91, 512–520. [Google Scholar] [CrossRef]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that Soil Properties and Organic Coating Drive the Phytoavailability of Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci.-Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Ren, M.; Varela-Ramirez, A.; Li, C.; Hernandez-Viezcas, J.A.; Aguilera, R.J.; Gardea-Torresdey, J.L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. [Google Scholar] [CrossRef]
- Peng, C.; Duan, D.; Xu, C.; Chen, Y.; Sun, L.; Zhang, H.; Yuan, X.; Zheng, L.; Yang, Y.; Yang, J.; et al. Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants. Environ. Pollut. 2015, 197, 99–107. [Google Scholar] [CrossRef]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, Translocation, and Consequences of Nanomaterials on Plant Growth and Stress Adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Ma, X.; Yan, J. Plant uptake and accumulation of engineered metallic nanoparticles from lab to field conditions. Curr. Opin. Environ. Sci. Health 2018, 6, 16–20. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Rani, S.; Kumari, N.; Sharma, V. Uptake, translocation, transformation and physiological effects of nanoparticles in plants. Arch. Agron. Soil Sci. 2022, 1–21. [Google Scholar] [CrossRef]
- Hu, D.; Bai, Y.; Li, L.; Ai, M.; Jin, J.; Song, K.; Liu, D. Phytotoxicity assessment study of gold nanocluster on broad bean (Vicia faba L.) seedling. Environ. Pollut. Bioavailab. 2022, 34, 284–296. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Varela-Ramirez, A.; Castillo-Michel, H.; Li, C.; Zhang, J.; Aguilera, R.J.; Keller, A.A.; Gardea-Torresdey, J.L. Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: Insight into the uptake mechanism. J. Hazard. Mater. 2012, 225, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Veronesi, G.; Flank, A.-M.; Surble, S.; Herlin-Boime, N.; Carriere, M. Comparative Uptake and Impact of TiO2 Nanoparticles in Wheat and Rapeseed. J. Toxicol. Environ. Health-Part A-Curr. Issues 2012, 75, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Schoenfisch, M.H. Silica Nanoparticle Phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Astete, C.E.; Bodoki, A.E.; Windham, M.; Bodoki, E.; Sabliov, C.M. Zein Nanoparticles Uptake and Translocation in Hydroponically Grown Sugar Cane Plants. J. Agric. Food Chem. 2018, 66, 6544–6551. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G.V. Impact of Surface Charge on Cerium Oxide Nanoparticle Uptake and Translocation by Wheat (Triticum aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Parkinson, S.J.; Tungsirisurp, S.; Joshi, C.; Richmond, B.L.; Gifford, M.L.; Sikder, A.; Lynch, I.; O’Reilly, R.K.; Napier, R.M. Polymer nanoparticles pass the plant interface. Nat. Commun. 2022, 13, 7385. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, Uptake, and Translocation of Engineered Nanomaterials in Vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.S.; Ke, P.C. Differential Uptake of Carbon Nanoparticles by Plant and Mammalian Cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.Y.; Bussiere, S.; Coriou, C.; Robert, T.; Maucourt, M.; Deborde, C.; Moing, A.; Nguyen, C. Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2020, 205, 111145. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ma, Y.; Yang, J.; Zhang, B.; Luo, W.; Feng, S.; Zhang, J.; Wang, G.; He, X.; Zhang, Z. Effects of foliar applications of ceria nanoparticles and CeCl3 on common bean (Phaseolus vulgaris). Environ. Pollut. 2019, 250, 530–536. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, T.; Xian, Y.; Kang, Z.; Zhang, S.; Dumat, C.; Shahid, M.; Li, S. Foliar uptake, biotransformation, and impact of CuO nanoparticles in Lactuca sativa L. var.ramosaHort. Environ. Geochem. Health 2021, 43, 423–439. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Zhu, X.; Liu, S.; Liu, F.; Wang, Y.; Li, X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021, 67, 245–259. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Biswas, A.K.; Tarafdar, J.C.; Rao, A.S. Characterization of Zinc Oxide Nano Particles and Their Effect on Growth of Maize (Zea mays L.) Plant. J. Plant Nutr. 2015, 38, 1505–1515. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative Understanding of Nanoparticle Uptake in Watermelon Plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef]
- Hong, J.; Peralta-Videa, J.R.; Rico, C.; Sahi, S.; Viveros, M.N.; Bartonjo, J.; Zhao, L.; Gardea-Torresdey, J.L. Evidence of Trans location and Physiological Impacts of Foliar Applied CeO2 Nanoparticles on Cucumber (Cucumis sativus) Plants. Environ. Sci. Technol. 2014, 48, 4376–4385. [Google Scholar] [CrossRef]
- Bueno, V.; Gao, X.; Rahim, A.A.; Wang, P.; Bayen, S.; Ghoshal, S. Uptake and Translocation of a Silica Nanocarrier and an Encapsulated Organic Pesticide Following Foliar Application in Tomato Plants. Environ. Sci. Technol. 2022, 56, 6722–6732. [Google Scholar] [CrossRef]
- Wang, W.-N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- de la Rosa, G.; Vázquez-Núñez, E.; Molina-Guerrero, C.; Serafín-Muñoz, A.H.; Vera-Reyes, I. Interactions of nanomaterials and plants at the cellular level: Current knowledge and relevant gaps. Nanotechnol. Environ. Eng. 2021, 6, 7. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, W.; Xu, C.; Li, F.; Cao, L.; Huang, Q. Enhancement of Spirotetramat Transfer in Cucumber Plant Using Mesoporous Silica Nanoparticles as Carriers. J. Agric. Food Chem. 2018, 66, 11592–11600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Chen, R.; Feng, Q.; Zhan, X. Cellular Process of Polystyrene Nanoparticles Entry into Wheat Roots. Environ. Sci. Technol. 2022, 56, 6436–6444. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.-G.; Omer, A.M. Foliar application of nano chitosan NPK fertilizer improves the yield of wheat plants grown on two different soils. Egypt. J. Exp. Biol. 2018, 14, 63–72. [Google Scholar]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci.-Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Micro-XRE and Micro-XANES Confirmation of the Uptake and Translocation of TiO2 Nanoparticles in Cucumber (Cucumis sativus) Plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wu, Y.; Zhao, C.; Xu, Y.; Lu, J.; Xiang, S.; Zong, F.; Wu, X. Polymeric Nanoparticles as a Metolachlor Carrier: Water-Based Formulation for Hydrophobic Pesticides and Absorption by Plants. J. Agric. Food Chem. 2017, 65, 7371–7378. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle Uptake in Plants: Gold Nanomaterial Localized in Roots of Arabidopsis thaliana by X-ray Computed Nanotomography and Hyperspectral Imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019, 3476347. [Google Scholar] [CrossRef]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Vaghar, M.S.; Sayfzadeh, S.; Zakerin, H.R.; Kobraee, S.; Valadabadi, S.A. Foliar application of iron, zinc, and manganese nano-chelates improves physiological indicators and soybean yield under water deficit stress. J. Plant Nutr. 2020, 43, 2740–2756. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 132–140. [Google Scholar] [CrossRef]
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn application through seed priming improves productivity and grain nutritional quality of silage corn. Saudi J. Biol. Sci. 2022, 29, 103456. [Google Scholar] [CrossRef] [PubMed]
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronomy 2021, 11, 729. [Google Scholar] [CrossRef]
- Armin, M.; Akbari, S.; Mashhadi, S. Effect of time and concentration of nano-Fe foliar application on yield and yield components of wheat. Int. J. Biosci. 2014, 4, 69–75. [Google Scholar]
- Gao, G.; Tester, M.A.; Julkowska, M.M. The Use of High-Throughput Phenotyping for Assessment of Heat Stress-Induced Changes in Arabidopsis. Plant Phenomics 2020, 2020, 3723916. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Ul Mabood, Z.; Hano, C.; Abbasi, B.H. Green Synthesis of Cerium Oxide Nanoparticles (CeO2 NPs) and Their Antimicrobial Applications: A Review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, H.; Liu, C.; Qu, C.; Zheng, L.; Hong, F. Promotion of nano-anatase TiO2 on the spectral responses and photochemical activities of D1/D2/Cyt b559 complex of spinach. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2009, 72, 1112–1116. [Google Scholar] [CrossRef]
- Thabet, S.G.; Sallam, A.; Moursi, Y.S.; Karam, M.A.; Alqudah, A.M. Genetic factors controlling nTiO2 nanoparticles stress tolerance in barley (Hordeum vulgare) during seed germination and seedling development. Funct. Plant Biol. 2021, 48, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Exposure to Weathered and Fresh Nanoparticle and Ionic Zn in Soil Promotes Grain Yield and Modulates Nutrient Acquisition in Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2018, 66, 9645–9656. [Google Scholar] [CrossRef]
- Garcia-Gomez, C.; Obrador, A.; Gonzalez, D.; Babin, M.; Dolores Fernandez, M. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Garcia-Lopez, J.I.; Nino-Medina, G.; Olivares-Saenz, E.; Lira-Saldivar, R.H.; Diaz Barriga-Castro, E.; Vazquez-Alvarado, R.; Rodriguez-Salinas, P.A.; Zavala-Garcia, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M. Growth and Metabolic Responses of Rice (Oryza sativa L.) Cultivated in Phosphorus-Deficient Soil Amended with TiO2 Nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Y.; Xie, C.; Guo, Z.; He, X.; Valsami-Jones, E.; Lynch, I.; Luo, W.; Zheng, L.; Zhang, Z. Plant species-dependent transformation and translocation of ceria nanoparticles. Environ. Sci.-Nano 2019, 6, 60–67. [Google Scholar] [CrossRef]
- Lopez-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Romenus, K.D.; de la Fuente, M.C.; Benavides-Mendoza, A.; Juarez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Liu, C.P.; Li, F.B.; Luo, C.L.; Liu, X.M.; Wang, S.H.; Liu, T.X.; Li, X.D. Foliar application of two silica sols reduced cadmium accumulation in rice grains. J. Hazard. Mater. 2009, 161, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ansari, M.Y.K. Impact of Engineered Si Nanoparticles on Seed Germination, Vigour Index and Genotoxicity Assessment via DNA Damage of Root Tip Cells in Lens culinaris. J. Plant Biochem. Physiol. 2018, 6. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Wang, P.; Zhao, F.-J. Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J. Hazard. Mater. 2017, 322, 292–300. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Si, J.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; et al. Nano-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil Sci. 2020, 66, 1259–1273. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794. [Google Scholar] [CrossRef]
- Sharifan, H.; Ma, X.; Moore, J.M.; Habib, M.R.; Evans, C. Zinc Oxide Nanoparticles Alleviated the Bioavailability of Cadmium and Lead and Changed the Uptake of Iron in Hydroponically Grown Lettuce (Lactuca sativa L. var. Longifolia). ACS Sustain. Chem. Eng. 2019, 7, 16401–16409. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Dou, F.; Sun, W.; Ma, X. Simultaneous mitigation of arsenic and cadmium accumulation in rice (Oryza sativa L.) seedlings by silicon oxide nanoparticles under different water management schemes. Paddy Water Environ. 2020, 19, 569–584. [Google Scholar] [CrossRef]
- Fedorenko, A.G.; Minkina, T.M.; Chernikova, N.P.; Fedorenko, G.M.; Mandzhieva, S.S.; Rajput, V.D.; Burachevskaya, M.V.; Chaplygin, V.A.; Bauer, T.V.; Sushkova, S.N.; et al. The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum). Environ. Geochem. Health 2021, 43, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Spano, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Kalcikova, G.; Gotvajn, A.Z.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef]
- Kalcikova, G. Aquatic vascular plants—A forgotten piece of nature in microplastic research. Environ. Pollut. 2020, 262, 114354. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Sakata, K.; Komatsu, S. Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J. Hazard. Mater. 2016, 304, 291–305. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Fellmann, S.; Eichert, T. Acute Effects of Engineered Nanoparticles on the Growth and Gas Exchange of Zea mays L.—What are the Underlying Causes? Water Air Soil Pollut. 2017, 228, 176. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; José-Yacamán, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515-516, 60–69. [Google Scholar] [CrossRef]
- Jahan, S.; Alias, Y.B.; Bakar, A.F.B.A.; Yusoff, I.B. Toxicity evaluation of ZnO and TiO2 nanomaterials in hydroponic red bean (Vigna angularis) plant: Physiology, biochemistry and kinetic transport. J. Environ. Sci. 2018, 72, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Pittol, M.; Tomacheski, D.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Macroscopic effects of silver nanoparticles and titanium dioxide on edible plant growth. Environ. Nanotechnol. Monit. Manag. 2017, 8, 127–133. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. of Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rekha, K.; Venkidasamy, B.; Thiruvengadam, M. Effect of Copper Oxide Nanoparticles on the Physiology, Bioactive Molecules, and Transcriptional Changes in Brassica rapa ssp. rapa Seedlings. Water Air Soil Pollut. 2019, 230, 48. [Google Scholar] [CrossRef]
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and Transformation of CuO Nanoparticles in the Soil–Rice System during the Life Cycle of Rice Plants. Environ. Sci. Technol. 2017, 51, 4907–4917. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Xie, Y.; Yin, Y.; Zhu, J.; Zhang, X.; Ji, R.; Gu, K.; Peralta-Videa, J.R.; Guo, H. Elevated CO2 levels modify TiO2 nanoparticle effects on rice and soil microbial communities. Sci. Total Environ. 2017, 578, 408–416. [Google Scholar] [CrossRef]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Landa, P.; Cyrusova, T.; Jerabkova, J.; Drabek, O.; Vanek, T.; Podlipna, R. Effect of Metal Oxides on Plant Germination: Phytotoxicity of Nanoparticles, Bulk Materials, and Metal Ions. Water Air Soil Pollut. 2016, 227, 448. [Google Scholar] [CrossRef]
- Yanık, F.; Vardar, F. Toxic Effects of Aluminum Oxide (Al2O3) Nanoparticles on Root Growth and Development in Triticum aestivum. Water Air Soil Pollut. 2015, 226, 296. [Google Scholar] [CrossRef]
- Feichtmeier, N.S.; Walther, P.; Leopold, K. Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 8549–8558. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Ghosh, I.; Sadhu, A.; Moriyasu, Y.; Bandyopadhyay, M.; Mukherjee, A. Manganese oxide nanoparticles induce genotoxicity and DNA hypomethylation in the moss Physcomitrella patens. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 146–157. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free. Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Biczak, R.; Telesiński, A.; Pawłowska, B. Oxidative stress in spring barley and common radish exposed to quaternary ammonium salts with hexafluorophosphate anion. Plant Physiol. Biochem. 2016, 107, 248–256. [Google Scholar] [CrossRef]
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Jurkow, R.; Pokluda, R.; Sekara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef] [PubMed]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Le Van, N.; Ma, C.; Rui, Y.; Cao, W.; Deng, Y.; Liu, L.; Xing, B. The Effects of Fe2O3 Nanoparticles on Physiology and Insecticide Activity in Non-Transgenic and Bt-Transgenic Cotton. Front. Plant Sci. 2016, 6, 1263. [Google Scholar] [CrossRef]
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant. 2020, 170, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.S.; Elamawi, R.M. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia Faba. Environ. Sci. Pollut. Res. 2020, 27, 18972–18984. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Nguyen Van, S.; Dinh Minh, H.; Nguyen Anh, D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar] [CrossRef]
- Behboudi, F.; Sarvestani, Z.T.; Kassaee, M.Z.; Sanavi, S.A.M.M.; Sorooshzadeh, A. Phytotoxicity of Chitosan and SiO2 Nanoparticles to Seed Germination of Wheat (Triticum aestivum L.) and Barley (Hordeum vulgare L.) Plants. Not. Sci. Biol. 2017, 9, 242–249. [Google Scholar] [CrossRef]
| Nanoparticle | Size (nm) | Concentration | Plants | Positive Effect | Reference |
|---|---|---|---|---|---|
| ZnO | 18 | Fresh soil with 6 mg/kg soil | Triticum aestivum Landmark | Leaf chlorophyll levels and shoot height increased | [90] |
| 30 | 0 and 500 mg/kg soil with and without organic P (0, 20 and 50 mg/kg) | Maize | ZnO NMs increased root dry weight | [91] | |
| <100 | 50, 100 and 150 mg/L | Mangifera indica Linn | Improved the total yield (fruit number and weight per tree) the combined application of NPs resulted in an increase in fruit yield, average fruit weight, length, width, TSS, sugars and displayed the lowest acidity percentage | [89] | |
| 12–24 | 1000 and 2000 mg/L | Capsicum chinense | NPs promoted plant growth, increased number and average weight of the fruits, fruit quality, capsaicin and dihydrocapsaicin content at low doses | [92] | |
| TiO2 | 20 | 25–750 mg/L | Oryza sativa | Enhanced accumulation of palmitic acid, amino acids and glycerol in rice grain, improved shoot growth and phosphorus concentration in whole plant and grains | [93] |
| 32 | 10, 100,1000 mg/L | Triticum aseivum | Enhanced growth of lateral roots and biomass with concurrent uptake of titanium | [94] | |
| 27 | 250, 500, 750 mg/L | Cannabls sativa | Increased potassium and phosporus in cucumber fruits | [73] | |
| Cu | 50 | 50–500 mg/L | Solanum lycopersicum | Enhanced lycopene, vitamin-C in tomato fruits, number of fruits and fruit firmness | [95] |
| SiO2 | 4–10 | 5 mM | Oryza sativa | Increased grain yield and weight | [96] |
| Si | 25, 50 mg/L | lentil | Promote seed germination, seedling vigor and biomass | [97] | |
| CeO2 | 15–30 | 200, 500 mg/L | Arabidopsis thaliana | Increased the root elongation, root and shoot growth | [98] |
| Nanoparticle | Size (nm) | Concentration | Plants | Negative Impact | Reference |
|---|---|---|---|---|---|
| ZnO | <50 | 500 ppm | Glycine max | Inhibition of root elongation, cell viability and biomass, generation of superoxides, reduced biomass of foliage, alteration of gene expression | [111] |
| 90 | 400–3200 mg/kg | Zea mays | Increased production of superoxide anions and superoxide dismutase activity decreased mineral nutrient acquisition, decreased photosynthesis and root activity | [112] | |
| 30–40 | 0.02–2 g/L | Zea mays | Negative effect on seed germination and seedling growth | [113] | |
| 10 | 250, 500, 750 mg/kg | Medicago sativa | Reduced root biomass up to 80% | [114] | |
| 64, 80 | 20–200 mg/L | Vigna angularis | Disrupted plant physiology of plant, enhanced oxidative stress and reduced photosynthetic pigment | [115] | |
| Ag | 10 | 10–50 mg/kg | Lysopersicon esculentum | Induced reactive oxidative stress that reduced photosynthesis, CO2 assimilation and fruit yield | [116] |
| 10 | 0.001–10,000 mg/L | Allium cepa | Root growth was inhibited | [117] | |
| 10 | 25, 50, 75 and 100 μM | Allium cepa | Strongly reduced the root growth, induced mitotic index, induced ROS formation, caused oxidative DNA damage in higher concentrations | [118] | |
| 5–10 | 300–900 ppm | Lupinus termis | Decreased germination percentage, reduced the root and shoot elongation, root and shoot fresh weights, total chlorophyll, total protein content and sugar content | [119] | |
| CuO | 40–80 | 500, 1000, 2000 mg/L | Zea mays, Oryza sativa | 95% and 97% inhibition in root length of maize and rice at 2000 mgL−1 | [120] |
| 25–55 | 50–500 mg/L | Brassica rapa | Synthesis of photosynthetic pigments and sugar was decreased | [121] | |
| 43 | 50–1000 mg/kg | Oryza sativa | Low water uptake by root and aerial parts; grain production was considerably reduced | [122] | |
| TiO2 | 20 ± 5 | 50, 200 mg/L | Oryza sativa | Reduced grain yield and biomass | [123] |
| 25 | 250–1000 mg/L | Cannabls sativa, Brassica oleracea var. capitata, Avena sativa, Zea mays, Lactuca sativa, Allium cepa | Inhibition of root growth for oat, corn, cabbage, lettuce and reduction of soybean and cucumber germination | [124] | |
| 5–15 | 0.02–2 g/L | Zea mays | Inhibition of germination, root and shoot growth | [113] | |
| 20 | 100–500 mg/L | Oryza sativa | Reduced biomass and altered antioxidant defense | [125] | |
| Al2O3 | <50 | 10–1000 mg/L | Sinapis alba | At all concentrations, seed germination was affected negatively | [126] |
| 13 | 5, 25, 59 mg/L | Triticum aseivum | H2O2 content was increased, reduced production of photosynthetic pigment and anthocyanin | [127] | |
| Au | 2–21 | 5, 8, 10 mg/L | Hordeum vulgare | Leaves became yellow and necrotic, the roots colored dark brown and decreased fresh plant biomass, leaf and root lengths | [128] |
| 3.5, 18 | 48 ppm | Nicotiana xanthi | Caused biotoxicity and observed leaf necrotic lesions | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. https://doi.org/10.3390/ma16083097
Wang X, Xie H, Wang P, Yin H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials. 2023; 16(8):3097. https://doi.org/10.3390/ma16083097
Chicago/Turabian StyleWang, Xueran, Hongguo Xie, Pei Wang, and Heng Yin. 2023. "Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root" Materials 16, no. 8: 3097. https://doi.org/10.3390/ma16083097
APA StyleWang, X., Xie, H., Wang, P., & Yin, H. (2023). Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials, 16(8), 3097. https://doi.org/10.3390/ma16083097




