The Effect of Casting Technique and Severe Straining on the Microstructure, Electrical Conductivity, Mechanical Properties and Thermal Stability of the Al–1.7 wt.% Fe Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure of Al–1.7Fe Alloys in the Initial State and After Cold Deformation
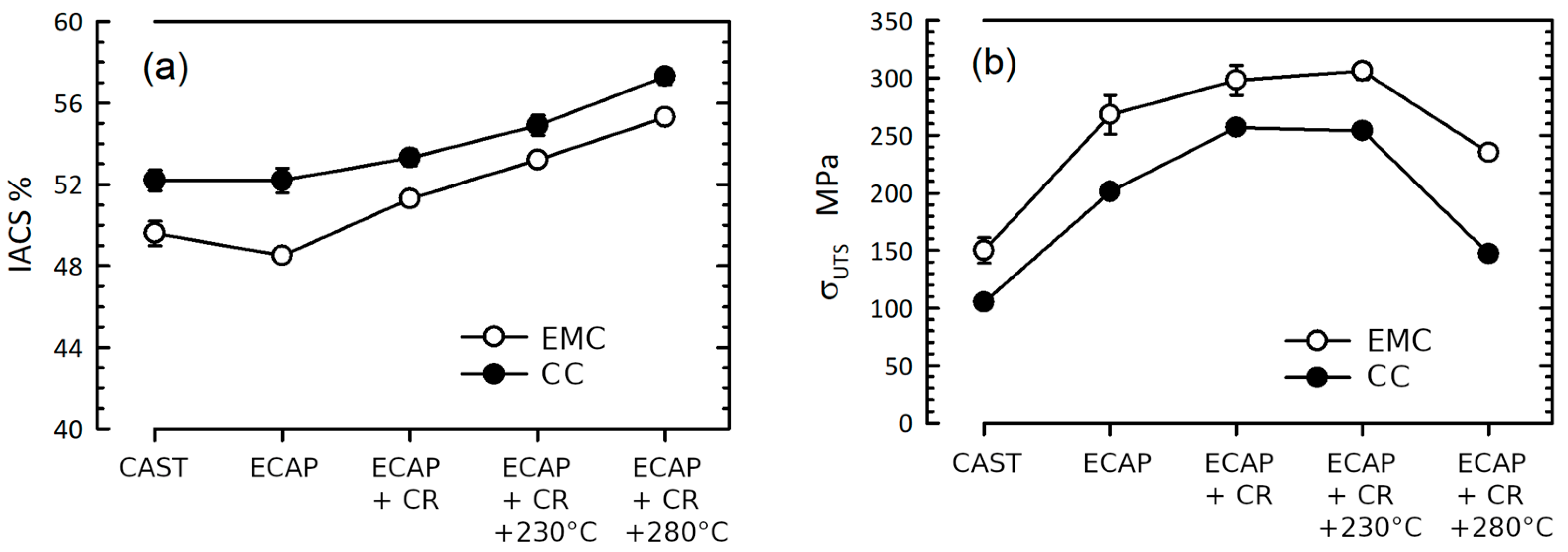
3.2. Mechanical Properties and Electrical Conductivity
3.3. Thermal Stability of UFG Al–1.7Fe Alloys
4. Discussion
4.1. Microstructural Basis for Electricity-Strength Relationship Evolutionary Behavior
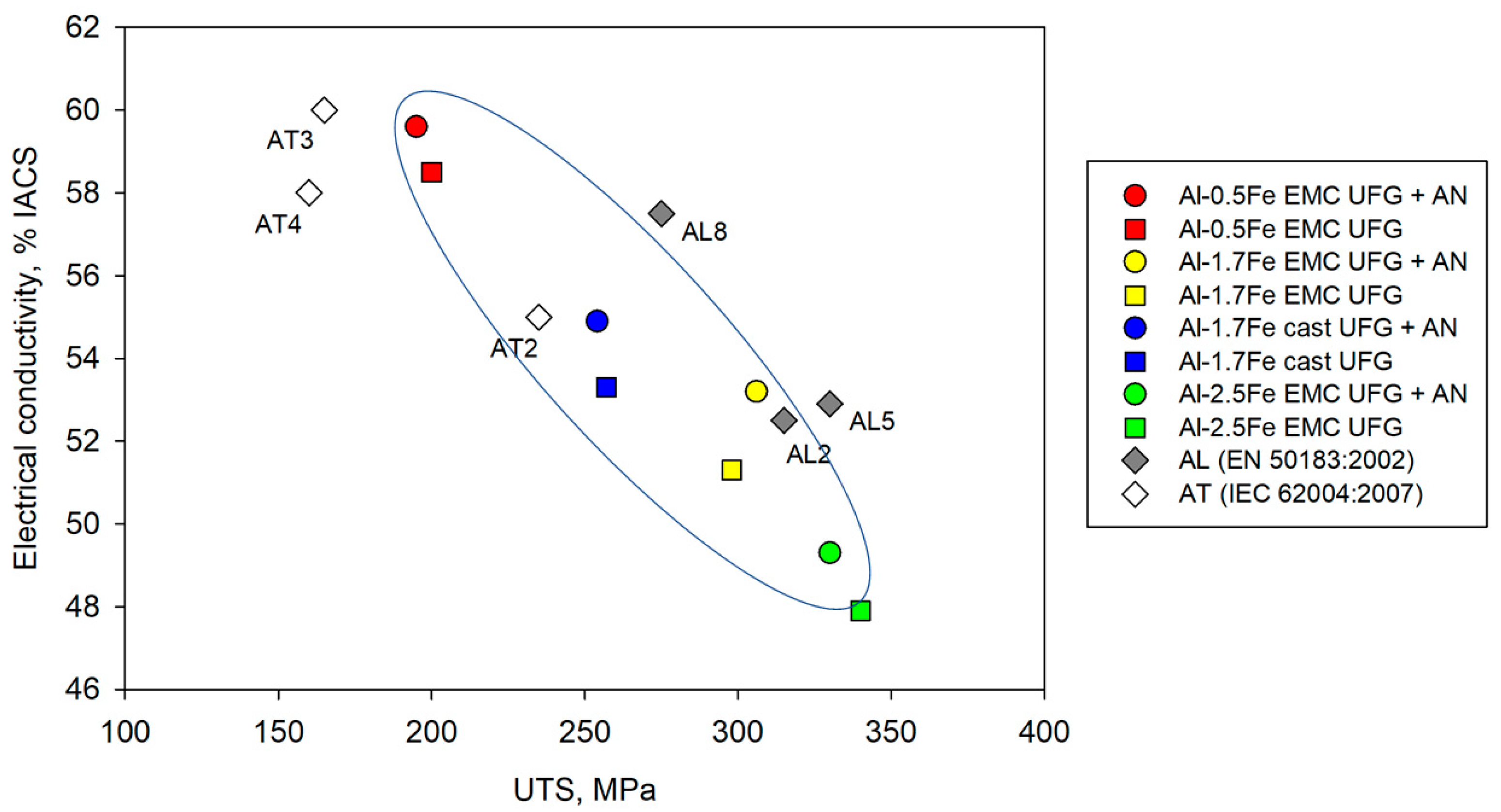
4.2. Combination of Strength and Electrical Conductivity
5. Conclusions
- It is shown that the casting method affects the structure and properties of aluminum alloys. The EMC method allows, firstly, to obtain smaller particles of the intermetallic phase (to change the quantitative phase composition), and secondly, to change the type of crystallizing intermetallic compounds (the qualitative phase composition).
- The formation of the Al2Fe phase in this system was confirmed, which was previously shown for Al–0.5Fe and Al–2.5Fe alloys. This phase is metastable, and, as the analysis of the contributions of microstructural mechanisms to strength and electrical conductivity showed, its presence, together with the changed morphology of intermetallic particles, leads to an increase in the strength of the alloy without loss of electrical conductivity during ECAP and subsequent CR.
- ECAP and subsequent CR lead to the expected increase in the strength of the alloys under study, and the increase is more pronounced in the EMC alloy due to the initially finer fraction of intermetallic particles and their crystal structure. The more unexpected change was the non-decrease and even increase in electrical conductivity in these alloys, which allows us to talk about overcoming the concept of finding a balance between strength and electrical conductivity in aluminum alloys.
- Alloys have shown high thermal stability—up to 230 °C for 1 hour, which is equivalent to operating at 150 °C for a theoretically unlimited time. The data obtained make it possible to recommend these alloys as thermally stable conductor materials in addition to commercial Al–Zr and Al–Mg–Si systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polmear, I.; St John, D.; Nie, J.-F.; Qian, M. Light Alloys: Metallurgy of the Light Metals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Kiessling, F.; Nefzger, P.; Nolasco, J.F.; Kaintzyk, U. Overhead Power Lines: Planning, Design, Construction; Springer: Berlin/Heidelberg, Germany, 2003; 759p. [Google Scholar] [CrossRef]
- Jawalkar, C.S.; Kant, S. A Review on Use of Aluminium Alloys in Aircraft Components. i-Manag. J. Mater. Sci. 2015, 3, 33–38. [Google Scholar] [CrossRef]
- Gueydan, A.; Domengès, B.; Hug, E. Study of the Intermetallic Growth in Copper-Clad Aluminum Wires after Thermal Aging. Intermetallics 2014, 50, 34–42. [Google Scholar] [CrossRef]
- Hug, E.; Bellido, N. Brittleness Study of Intermetallic (Cu, Al) Layers in Copper-Clad Aluminium Thin Wires. Mater. Sci. Eng. A 2011, 528, 7103–7106. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent Developments in Advanced Aircraft Aluminium Alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Cordier, G.; Dorsam, G.; Kniep, R. New Intermediate Phases in the Ternary Systems Rare-Earth-Transition Element-Aluminium. J. Magn. Magn. Mater. 1988, 76, 653–654. [Google Scholar] [CrossRef]
- Matveeva, I.; Dovzhenko, N.; Sidelnikov, S.; Trifonenkov, L.; Baranov, V.; Lopatina, E. Development and Research of New Aluminium Alloys with Transition and Rare-Earth Metals and Equipment for Production of Wire for Electrotechnical Applications by Methods of Combined Processing. In Light Metals; Sadler, B.A., Ed.; Springer: Cham, Switzerland, 2013; pp. 443–447. [Google Scholar] [CrossRef]
- Kvackaj, T.; Bidulska, J.; Fujda, M.; Kocisko, R.; Pokorny, I.; Milkovic, O. Nanostructure Formation and Properties in Some Al Alloys after SPD and Heat Treatment. Mater. Sci. Forum 2010, 633–634, 273–302. [Google Scholar] [CrossRef]
- Wawer, K.; Lewandowska, M.; Kurzydlowski, K.J. Improvement of Mechanical Properties of 7475 Aluminium Alloy by the Combination of SPD Processing and Annealing. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 690, pp. 311–314. [Google Scholar]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Bikmukhametov, I.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Effect of the Eutectic Al-(Ce,La) Phase Morphology on Microstructure, Mechanical Properties, Electrical Conductivity and Heat Resistance of Al-4.5(Ce,La) Alloy after SPD and Subsequent Annealing. J. Alloys Compd. 2019, 796, 321–330. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of Equal-Channel Angular Pressing as a Processing Tool for Grain Refinement. Progr. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Ciemiorek, M.; Bartkowski, P.; Chromiński, W.; Olejnik, L.; Lewandowska, M.; Candidate, P. Forming Ability of Ultrafine-Grained Aluminium Plates Processed by Incremental ECAP. Adv. Eng. Mater. 2019, 21, 1900473. [Google Scholar] [CrossRef]
- Vysotskii, I.; Malopheyev, S.; Mironov, S.; Kaibyshev, R. Deformation Behavior of Friction-Stir Welded Al-Mg-Mn Alloy with Ultrafine-Grained Structure. Mater. Charact. 2022, 185, 111758. [Google Scholar] [CrossRef]
- Sauvage, X.; Wilde, G.; Divinski, S.V.; Horita, Z.; Valiev, R.Z. Grain Boundaries in Ultrafine Grained Materials Processed by Severe Plastic Deformation and Related Phenomena. Mater. Sci. Eng. A 2012, 540, 1–12. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; In, H.; Arita, M.; Horita, Z. High-pressure torsion for fabrication of high-strength and high-electrical conductivity Al micro-wires. J. Mater. Sci. 2014, 49, 6550–6557. [Google Scholar] [CrossRef]
- Ivanisenko, Y.; Kulagin, R.; Fedorov, V.; Mazilkin, A.; Scherer, T.; Baretzky, B.; Hahn, H. High Pressure Torsion Extrusion as a New Severe Plastic Deformation Process. Mater. Sci. Eng. A 2016, 664, 247–256. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Polyakov, A.V.; Semenova, I.P.; Raab, G.I.; Churakova, A.A.; Gimaltdinova, E.I.; Sabirov, I.; Segurado, J.; Sitdikov, V.D.; Alexandrov, I.V.; et al. Evolution of Microstructure, Macrotexture and Mechanical Properties of Commercially Pure Ti during ECAP-Conform Processing and Drawing. Mater. Sci. Eng. A 2013, 562, 128–136. [Google Scholar] [CrossRef]
- Xu, C.; Schroeder, S.; Berbon, P.B.; Langdon, T.G. Principles of ECAP-Conform as a Continuous Process for Achieving Grain Refinement: Application to an Aluminum Alloy. Acta Mater. 2010, 58, 1379–1386. [Google Scholar] [CrossRef]
- Raab, G.J.; Valiev, R.Z.; Lowe, T.C.; Zhu, Y.T. Continuous Processing of Ultrafine Grained Al by ECAP-Conform. Mater. Sci. Eng. A 2004, 382, 30–34. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, Z.; Cui, X.; Wu, Y.; Liu, X. Optimizing Microstructures of Dilute Al-Fe-Si Alloys Designed with Enhanced Electrical Conductivity and Tensile Strength. J. Alloys Compd. 2015, 650, 768–776. [Google Scholar] [CrossRef]
- Cieslar, M.; Fressengeas, C.; Karimi, A.; Martin, J.L. Portevin-Le Chatelier Effect in Biaxially Strained Al-Fe-Si Foils. Scripta Mater. 2003, 48, 1105–1110. [Google Scholar] [CrossRef]
- Korotkova, N.O.; Belov, N.A.; Timofeev, V.N.; Motkov, M.M.; Cherkasov, S.O. Influence of Heat Treatment on the Structure and Properties of an Al–7% REM Conductive Aluminum Alloy Casted in an Electromagnetic Crystallizer. Phys. Met. Metall. 2020, 121, 173–179. [Google Scholar] [CrossRef]
- Belov, N.; Akopyan, T.; Korotkova, N.; Murashkin, M.; Timofeev, V.; Fortuna, A. Structure and Properties of ca and Zr Containing Heat Resistant Wire Aluminum Alloy Manufactured by Electromagnetic Casting. Metals 2021, 11, 236. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Zhukova, O.O.; Kazykhanov, V.U.; Shaikhulova, A.F.; Enikeev, N.A.; Timofeev, V.N.; Murashkin, M.Y. On the Effect of ECAP and Subsequent Cold Rolling on the Microstructure and Properties of Electromagnetically Cast Al–Fe Alloys. Int. J. Lightweight Mater. Manuf. 2022, 5, 484–495. [Google Scholar] [CrossRef]
- Belov, N.A.; Alabin, A.N.; Eskin, D.G.; Istomin-Kastrovskii, V.V. Optimization of Hardening of Al-Zr-Sc Cast Alloys. J. Mater. Sci. 2006, 41, 5890–5899. [Google Scholar] [CrossRef]
- Murashkin, M.; Medvedev, A.; Kazykhanov, V.; Krokhin, A.; Raab, G.; Enikeev, N.; Valiev, R.Z. Enhanced Mechanical Properties and Electrical Conductivity in Ultrafine-Grained Al 6101 Alloy Processed via ECAP-Conform. Metals 2015, 5, 2148–2164. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Jin, Z.; Valiev, R.Z.; Zhu, Y.T. Microstructures and Mechanical Properties of Ultrafine Grained 7075 Al Alloy Processed by ECAP and Their Evolutions during Annealing. Acta Mater. 2004, 52, 4589–4599. [Google Scholar] [CrossRef]
- IEC 62004:2007; Thermal-Resistant Aluminium Alloy Wire for Overhead Line Conductor. IEC: Geneva, Switzerland, 2007.
- Medvedev, A.; Murashkin, M.; Enikeev, N.; Medvedev, E.; Sauvage, X. Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys. Metals 2021, 11, 815. [Google Scholar] [CrossRef]
- Saller, B.D.; Hu, T.; Ma, K.; Kurmanaeva, L.; Lavernia, E.J.; Schoenung, J.M. A Comparative Analysis of Solubility, Segregation, and Phase Formation in Atomized and Cryomilled Al–Fe Alloy Powders. J. Mater. Sci. 2015, 50, 4683–4697. [Google Scholar] [CrossRef]
- Topping, T.D.; Ahn, B.; Li, Y.; Nutt, S.R.; Lavernia, E.J. Influence of Process Parameters on the Mechanical Behavior of an Ultrafine-Grained Al Alloy. Metall. Mater. Trans. A 2012, 43, 505–519. [Google Scholar] [CrossRef]
- Asgharzadeh, H.; Simchi, A.; Kim, H.S. Microstructure and Mechanical Properties of Oxide-Dispersion Strengthened Al6063 Alloy with Ultra-Fine Grain Structure. Metall. Mater. Trans. A 2011, 42, 816–824. [Google Scholar] [CrossRef]
- Kamikawa, N.; Huang, X.; Tsuji, N.; Hansen, N. Strengthening Mechanisms in Nanostructured High-Purity Aluminium Deformed to High Strain and Annealed. Acta Mater. 2009, 57, 4198–4208. [Google Scholar] [CrossRef]
- Murashkin, M.Y.; Sabirov, I.; Medvedev, A.E.; Enikeev, N.A.; Lefebvre, W.; Valiev, R.Z.; Sauvage, X. Mechanical and Electrical Properties of an Ultrafine Grained Al-8.5 wt. % RE (RE = 5.4 wt.% Ce, 3.1 wt.% La) Alloy Processed by Severe Plastic Deformation. Mater. Des. 2016, 90, 433–442. [Google Scholar] [CrossRef]
- Sfikas, A.K.; Gonzalez, S.; Lekatou, A.G.; Kamnis, S.; Karantzalis, A.E. A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties. Metals 2022, 12, 1092. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworths and Co., Ltd.: London, UK, 1976; 971p. [Google Scholar]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Optimization of Strength-Electrical Conductivity Properties in Al–2Fe Alloy by Severe Plastic Deformation and Heat Treatment. Adv. Eng. Mater. 2018, 20, 1700867. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Lapovok, R.; Brodova, I.G.; Thomson, P.F. Ultrafine-Grained Al-5 Wt.% Fe Alloy Processed by ECAP with Backpressure. Mater. Sci. Eng. A 2003, 357, 159–167. [Google Scholar] [CrossRef]
- EN 50183:2002; Overhead Power Line Conductors—Bare Conductors of Aluminium Alloy with Magnesium and Silicon Content. European Committee for Standardization (CEN): Bruxelles, Belgium, 2002.
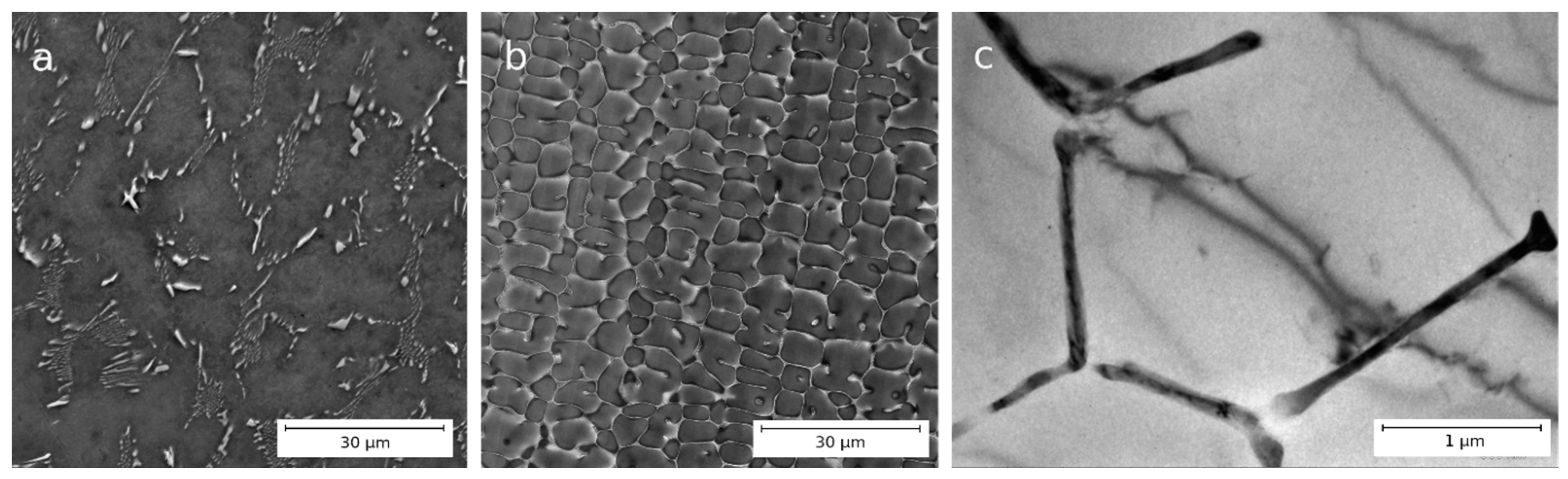

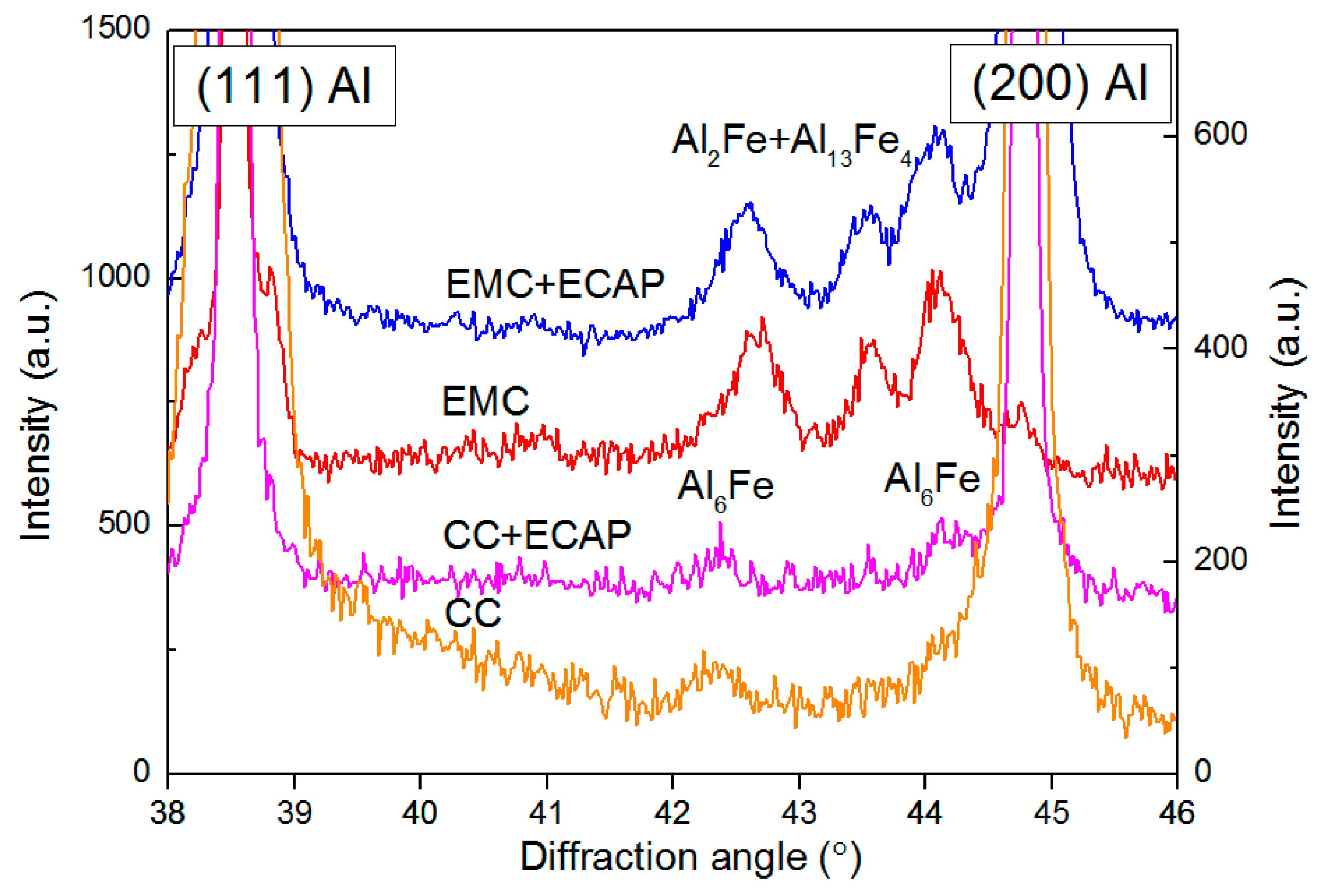
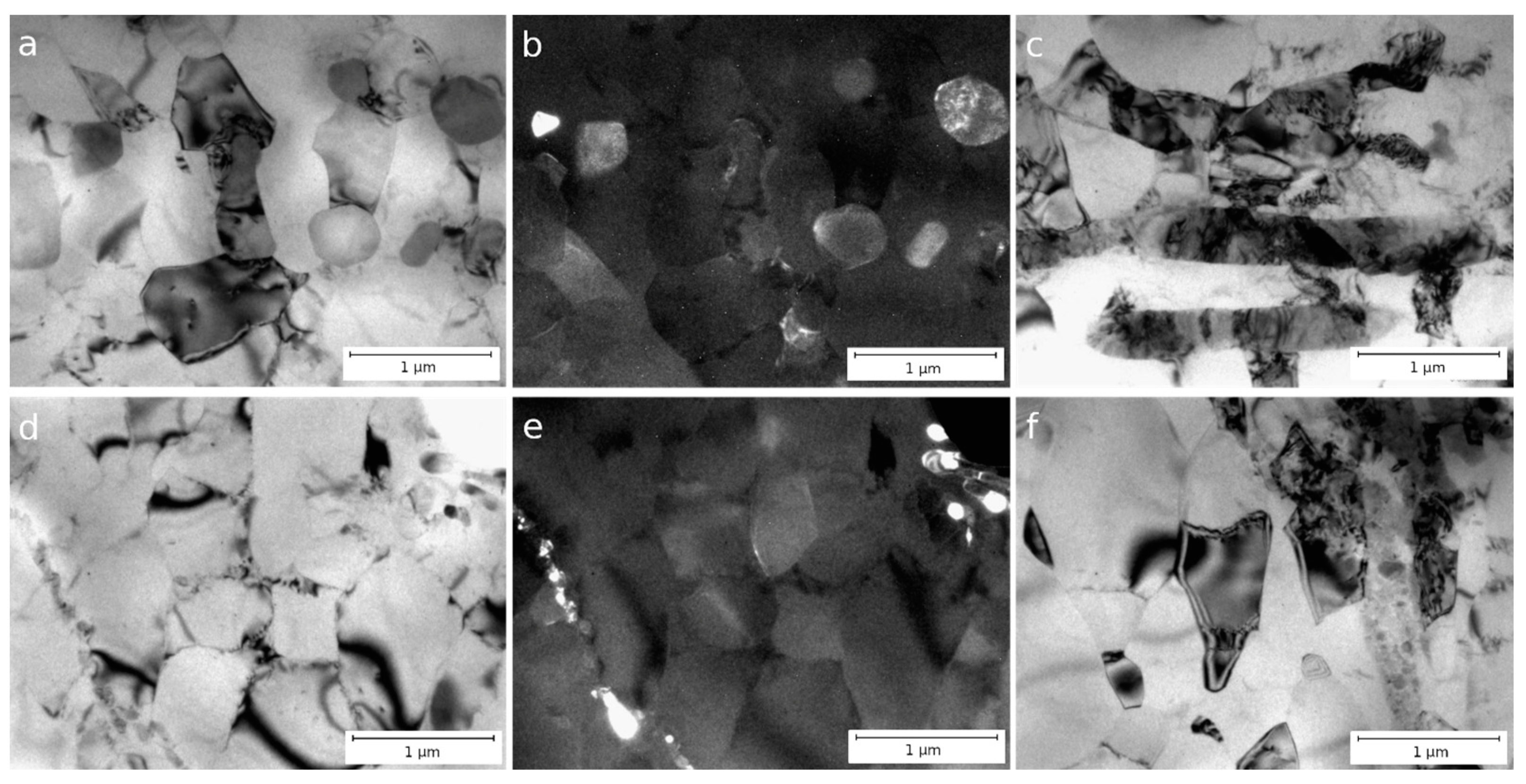
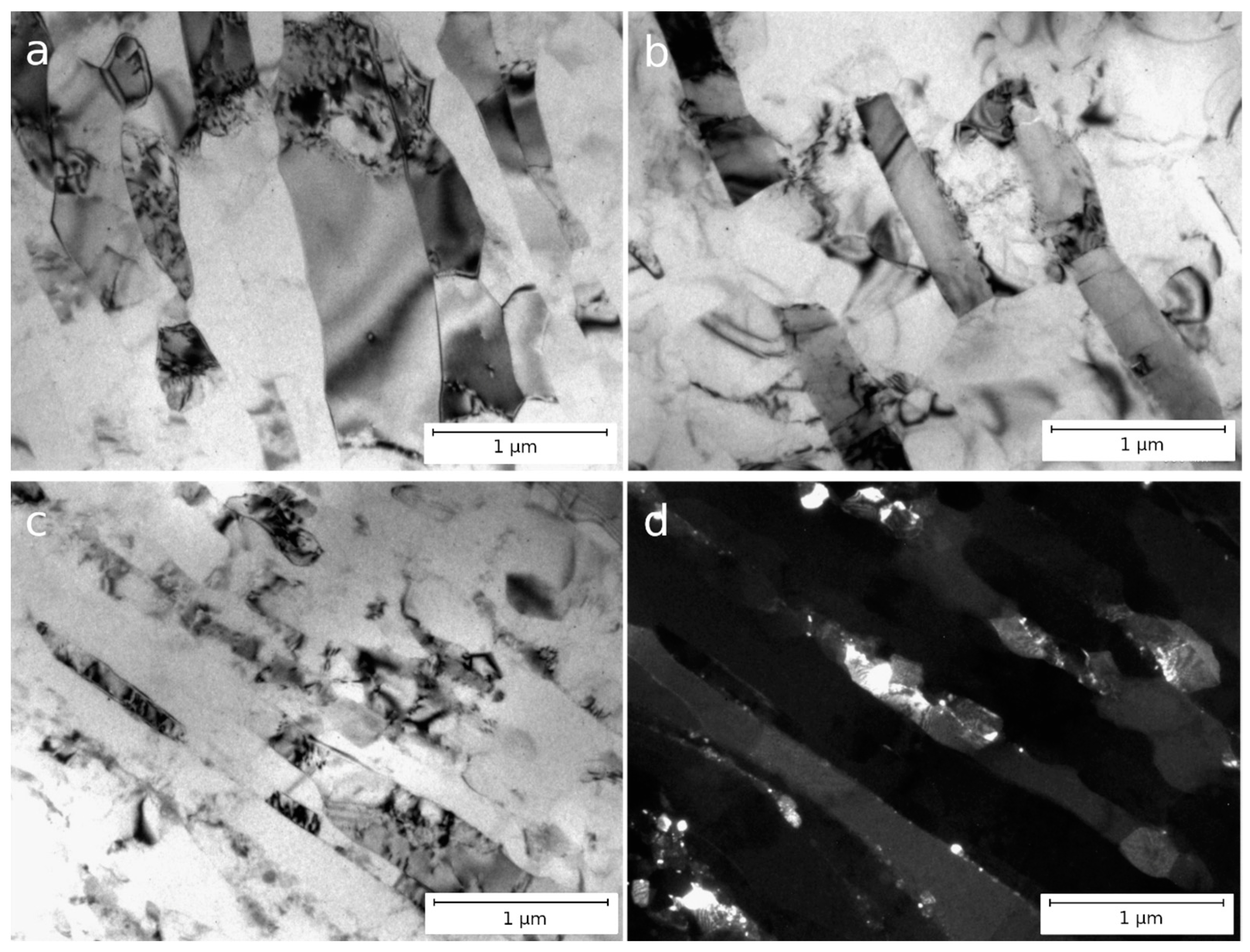

| Casting Method | Al | Fe | Si | Σ (Zn, Ti, Ni, V, Cu) |
|---|---|---|---|---|
| Conventional casting (CC) | Rem. | 1.71 | 0.12 | <0.04 |
| Electromagnetic casting (EMC) | Rem. | 1.65 | 0.03 | <0.04 |
| State | DXRD, nm | <ε2>1/2, % | a, Å | ρdis × 1013, m−2 | |
|---|---|---|---|---|---|
| CC | Cast | - | - | 4.0493 | - |
| ECAP | 330 ± 9 | 0.0035 ± 0.0006 | 4.0500 | 1.3 | |
| EMC | Cast | - | - | 4.0499 | - |
| ECAP | 200 ± 5 | 0.0043 ± 0.0010 | 4.0498 | 2.6 | |
| Initial (Dendritic Cell Size) | ECAP | ECAP + CR (Grain Width) | ECAP + CR + AN (Grain Width) | |||||
|---|---|---|---|---|---|---|---|---|
| Casting type | CC | EMC | CC | EMC | CC | EMC | CC | EMC |
| Grain size, nm | 13 ± 5 µm | 4.6 ± 1.5 µm | 694 ± 159 | 620 ± 20 | 256 ± 100 | 290 ± 20 | 685 ± 252 | 575 ± 25 |
| Particle size, nm | 370 ± 130 | 90 ± 20 | 334 ± 65 | 94 ± 24 | 264 ± 40 | 70 ± 50 | 77 ± 18 | 50 ± 21 |
| State | Alloy | ω, MS/m | IACS, % | σ0.2, MPa | σUTS, MPa | δ, % |
|---|---|---|---|---|---|---|
| Cast | CC | 30.36 ± 0.26 | 52.2 ± 0.5 | 40 ± 7 | 105 ± 4 | 18.3 ± 0.5 |
| EMC | 28.77 ± 0.21 | 49.6 ± 0.6 | 60 ± 6 | 150 ± 11 | 28.8 ± 2.1 | |
| ECAP | CC | 30.27 ± 0.34 | 52.2 ± 0.6 | 179 ± 8 | 201 ± 4 | 9.6 ± 0.2 |
| EMC | 28.10 ± 0.40 | 48.5 ± 0.2 | 251 ± 8 | 268 ± 17 | 11.7 ± 1.6 | |
| ECAP + CR | CC | 30.96 ± 0.19 | 53.3 ± 0.4 | 220 ± 4 | 257 ± 1 | 15.9 ± 1.3 |
| EMC | 29.77 ± 0.19 | 51.3 ± 0.1 | 276 ± 11 | 298 ± 13 | 17.1 ± 1.6 |
| State | Alloy | ω, MS/m | IACS, % | σ0.2, MPa | σUTS, MPa | δ, % |
|---|---|---|---|---|---|---|
| ECAP + CR + 230 °C, 1 h | C | 31.88 ± 0.29 | 54.9 ± 0.6 | 190 ± 3 | 254 ± 1 | 9.3 ± 0.2 |
| EMC | 30.86 ± 0.20 | 53.2 ± 0.1 | 284 ± 8 | 306 ± 7 | 14.4 ± 1.4 | |
| ECAP + CR + 280 °C, 1 h | C | 33.24 ± 0.21 | 57.3 ± 0.4 | 126 ± 3 | 147 ± 1 | 31.5 ± 2.2 |
| EMC | 32.10 ± 0.14 | 55.3 ± 0.1 | 228 ± 2 | 235 ± 3 | 17.9 ± 0.6 |
| Yield Strength, MPa | σ0 | σGB | σdisl | σdisp | σ0.2calc | σ0.2exp | Δexp-calc |
|---|---|---|---|---|---|---|---|
| CC ECAP | 10 | 48 | 9 | n\a | 67 | 179 | 112 |
| EMC ECAP | 10 | 51 | 12 | n\a | 73 | 251 | 178 |
| Electrical Resistivity, mΩ × m | ρAlpure | ρGB | ρdisl | ρSS | ρcalc | ρexp | Δexp-calc |
| CC ECAP | 0.02655 | 0.002248 | 0.0000004 | 0 | 0.02880 | 0.03304 | 0.00424 |
| EMC ECAP | 0.02655 | 0.002516 | 0.0000007 | 0 | 0.02907 | 0.03559 | 0.00652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedev, A.; Zhukova, O.; Enikeev, N.; Kazykhanov, V.; Timofeev, V.; Murashkin, M. The Effect of Casting Technique and Severe Straining on the Microstructure, Electrical Conductivity, Mechanical Properties and Thermal Stability of the Al–1.7 wt.% Fe Alloy. Materials 2023, 16, 3067. https://doi.org/10.3390/ma16083067
Medvedev A, Zhukova O, Enikeev N, Kazykhanov V, Timofeev V, Murashkin M. The Effect of Casting Technique and Severe Straining on the Microstructure, Electrical Conductivity, Mechanical Properties and Thermal Stability of the Al–1.7 wt.% Fe Alloy. Materials. 2023; 16(8):3067. https://doi.org/10.3390/ma16083067
Chicago/Turabian StyleMedvedev, Andrey, Olga Zhukova, Nariman Enikeev, Vil Kazykhanov, Victor Timofeev, and Maxim Murashkin. 2023. "The Effect of Casting Technique and Severe Straining on the Microstructure, Electrical Conductivity, Mechanical Properties and Thermal Stability of the Al–1.7 wt.% Fe Alloy" Materials 16, no. 8: 3067. https://doi.org/10.3390/ma16083067
APA StyleMedvedev, A., Zhukova, O., Enikeev, N., Kazykhanov, V., Timofeev, V., & Murashkin, M. (2023). The Effect of Casting Technique and Severe Straining on the Microstructure, Electrical Conductivity, Mechanical Properties and Thermal Stability of the Al–1.7 wt.% Fe Alloy. Materials, 16(8), 3067. https://doi.org/10.3390/ma16083067







