Strength–Toughness of a Low-Alloy 0.25C Steel Treated by Q&P Processing
Abstract
1. Introduction
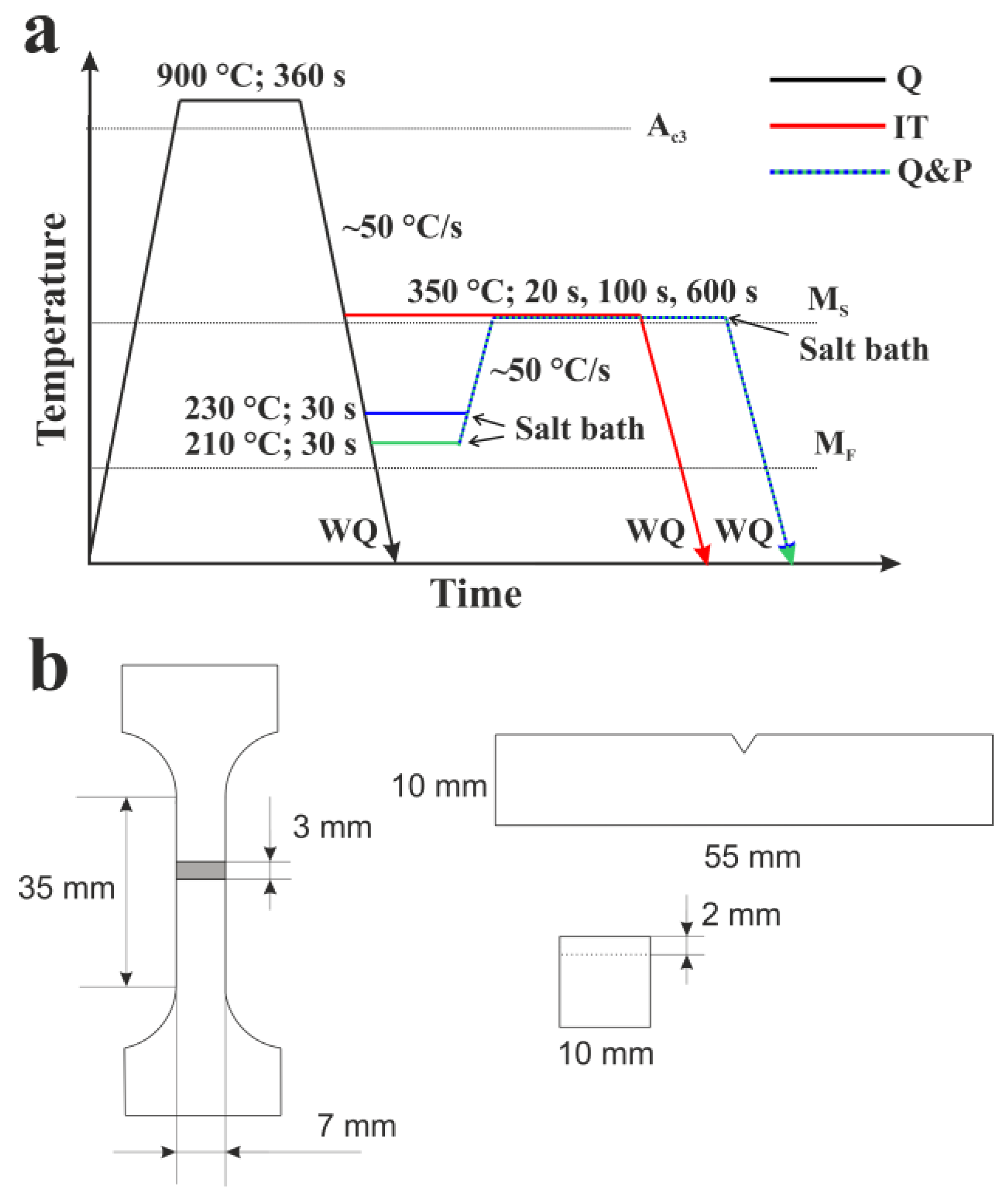
2. Materials and Methods
3. Results
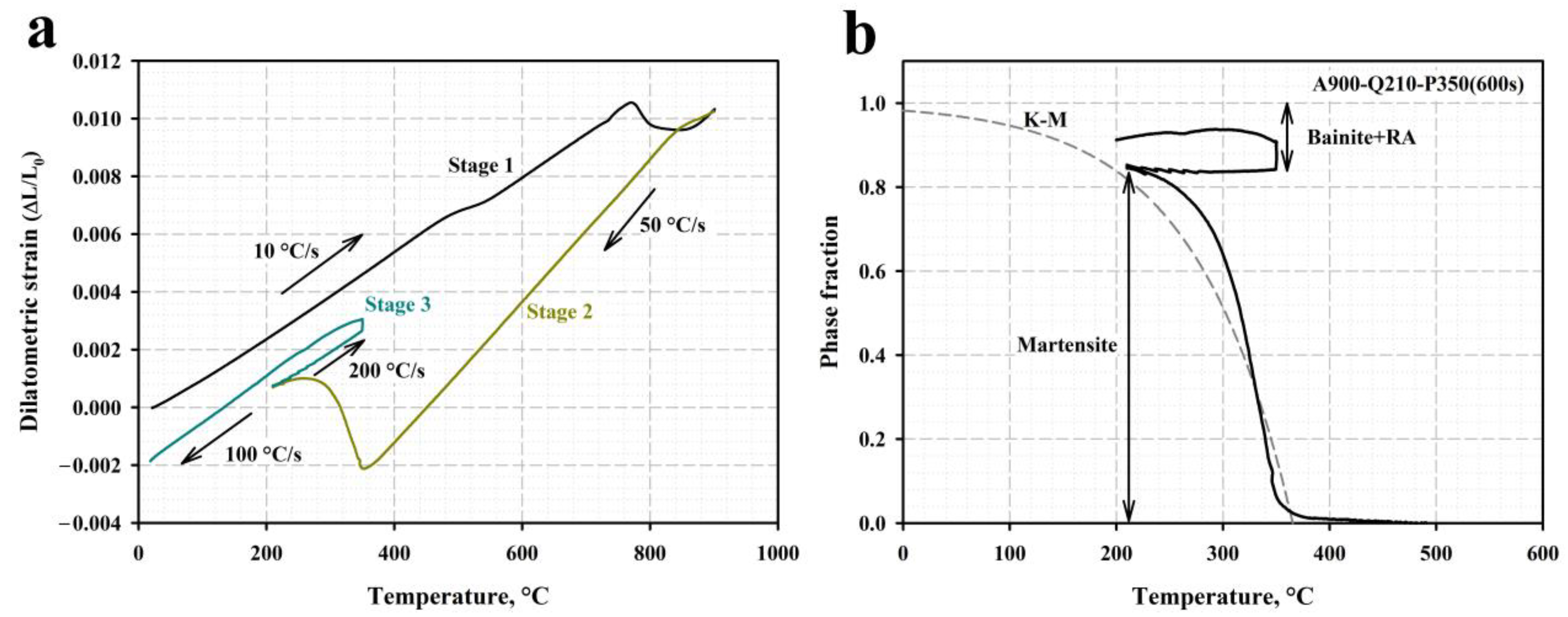
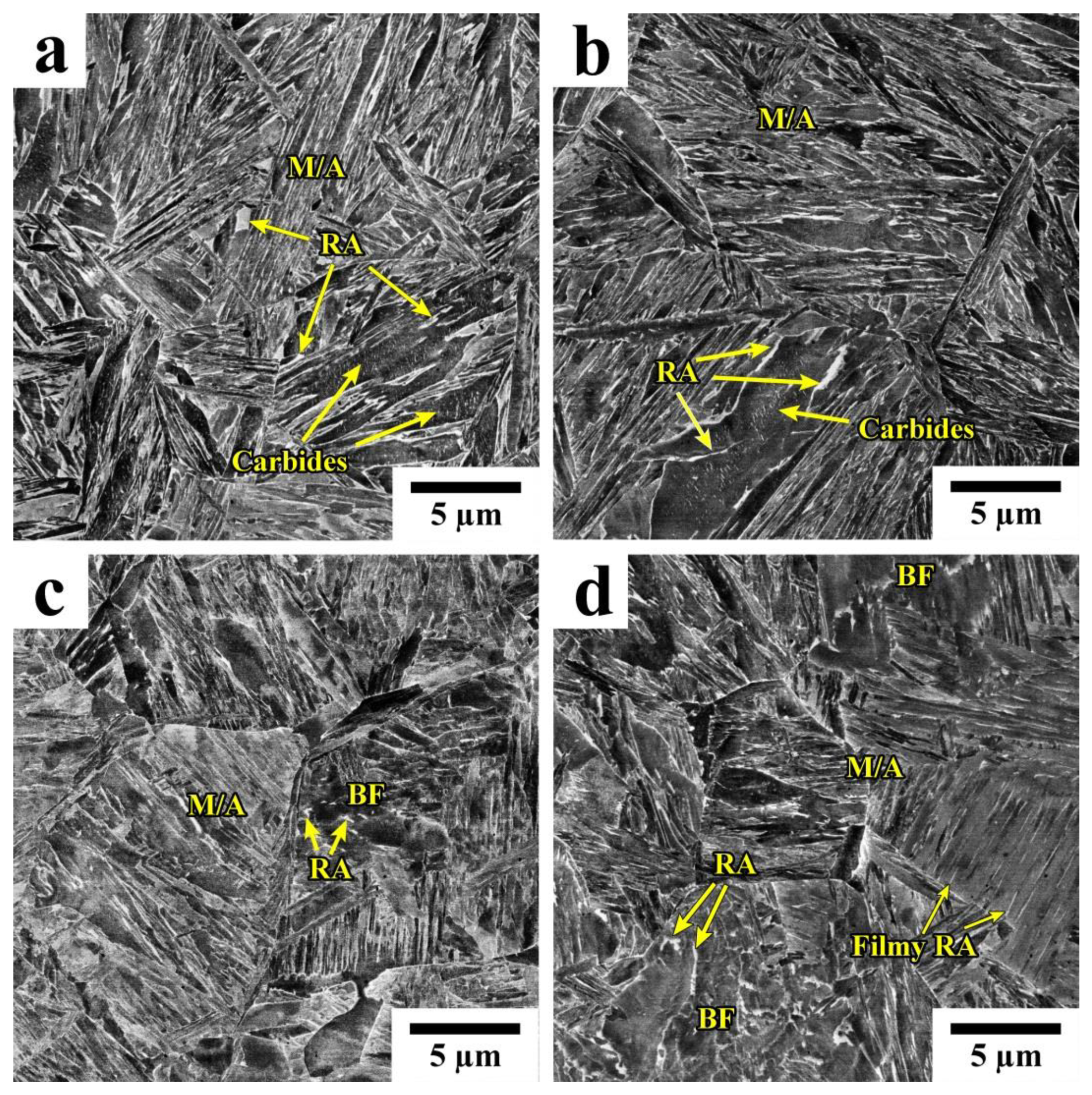
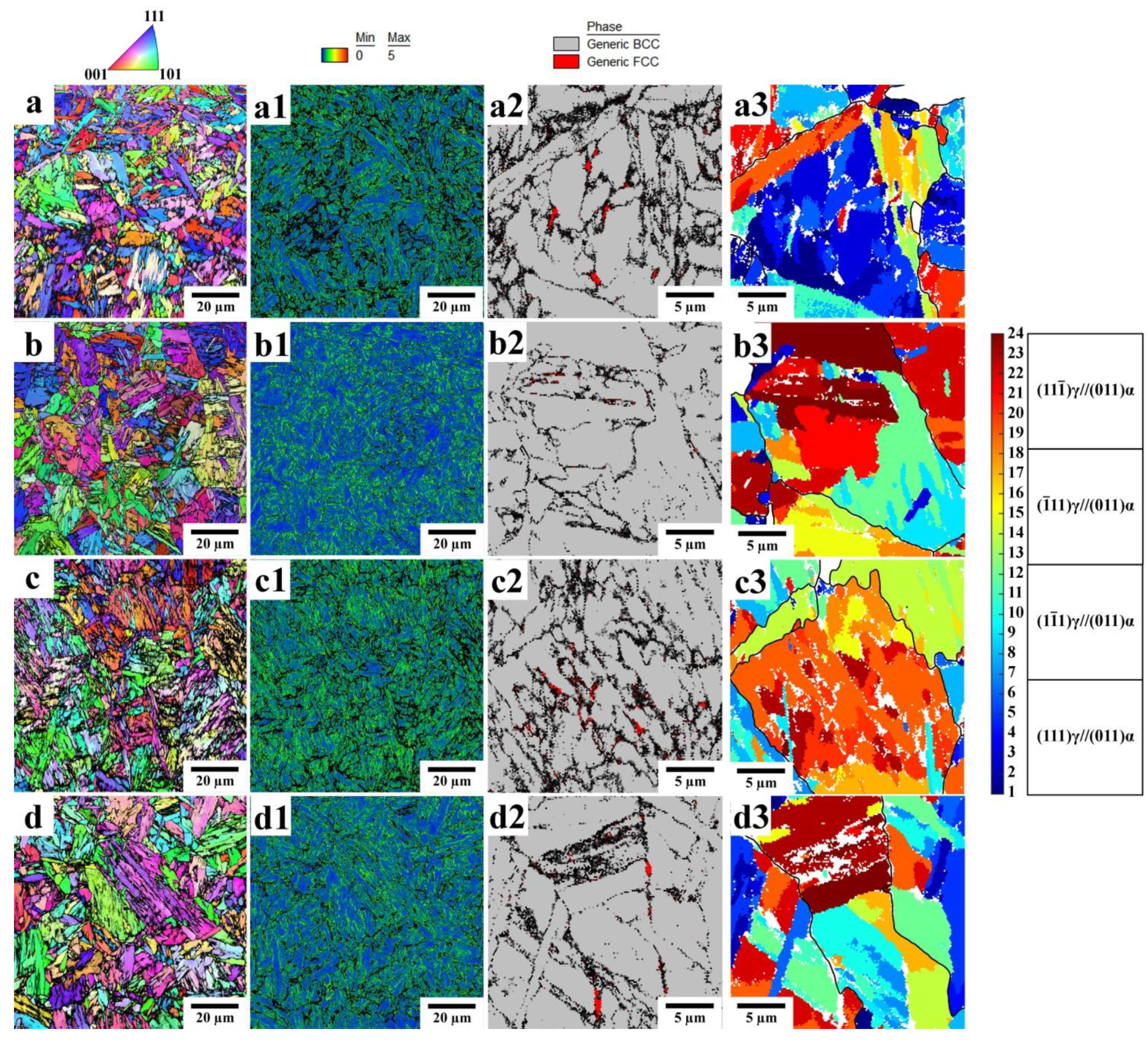
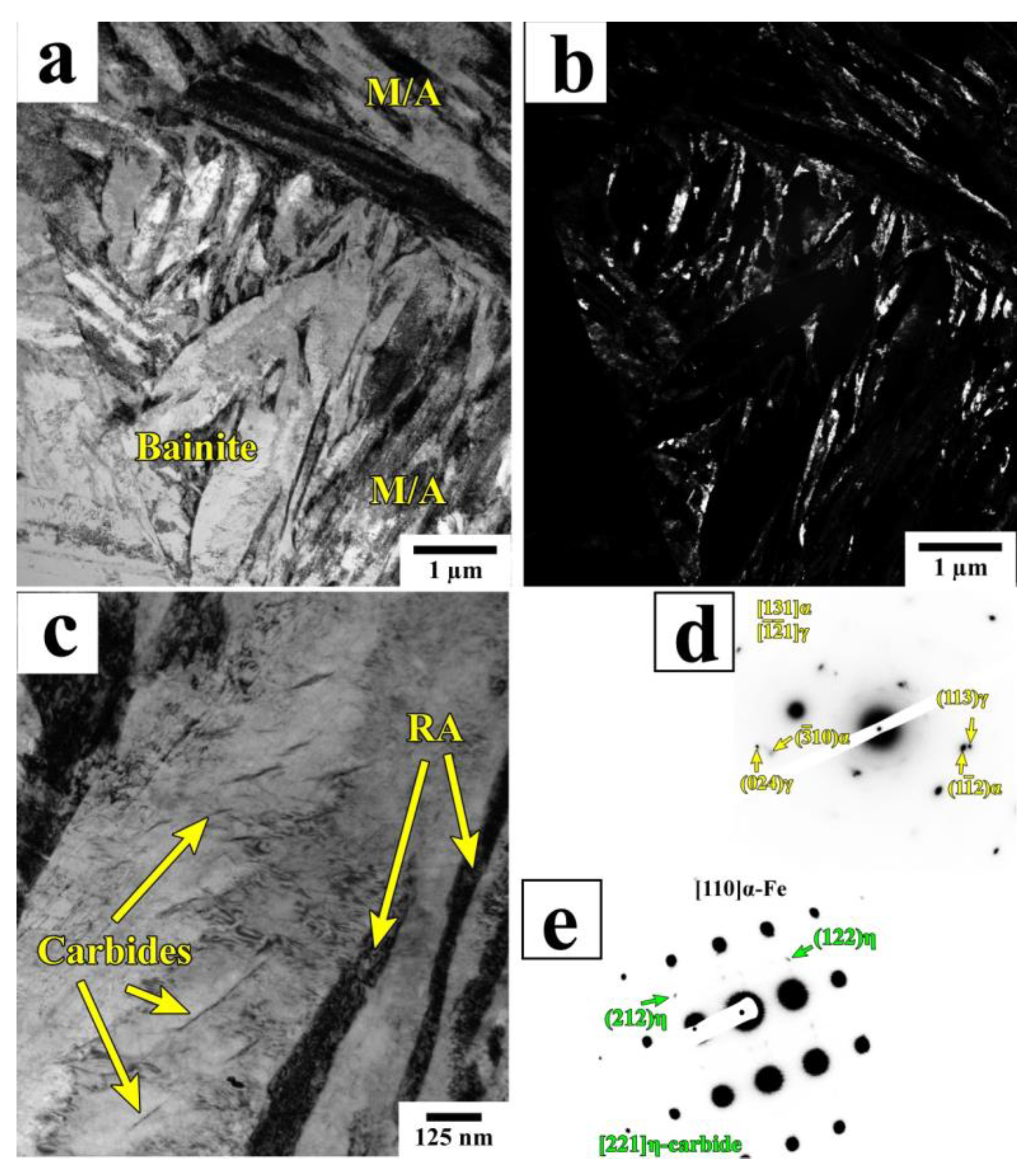
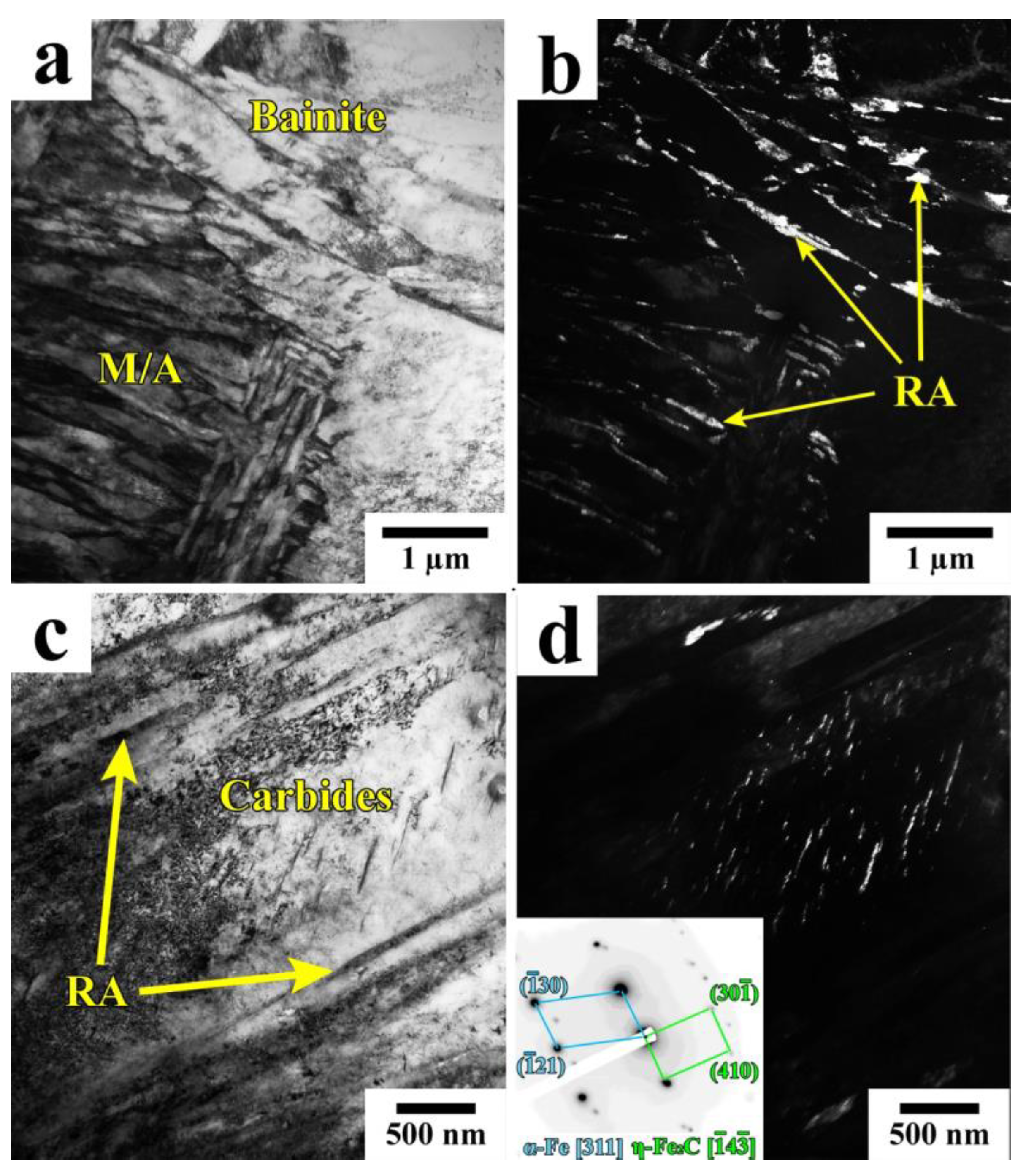
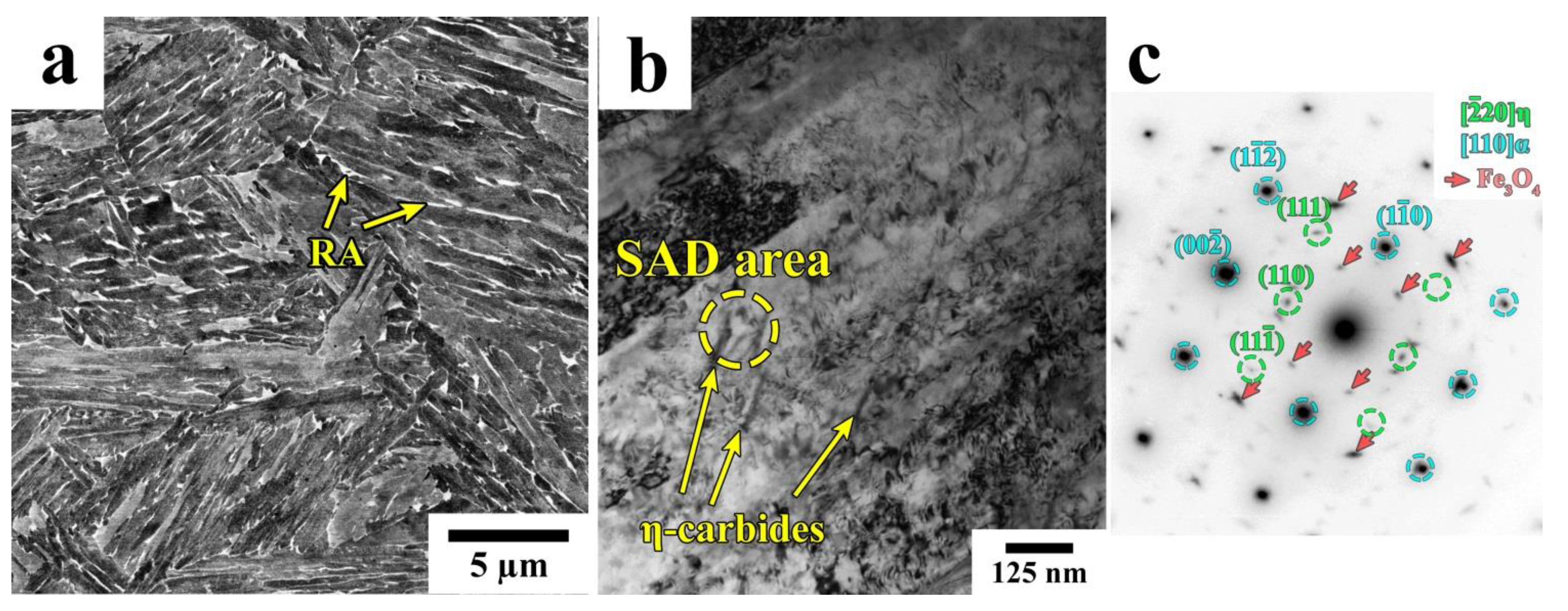
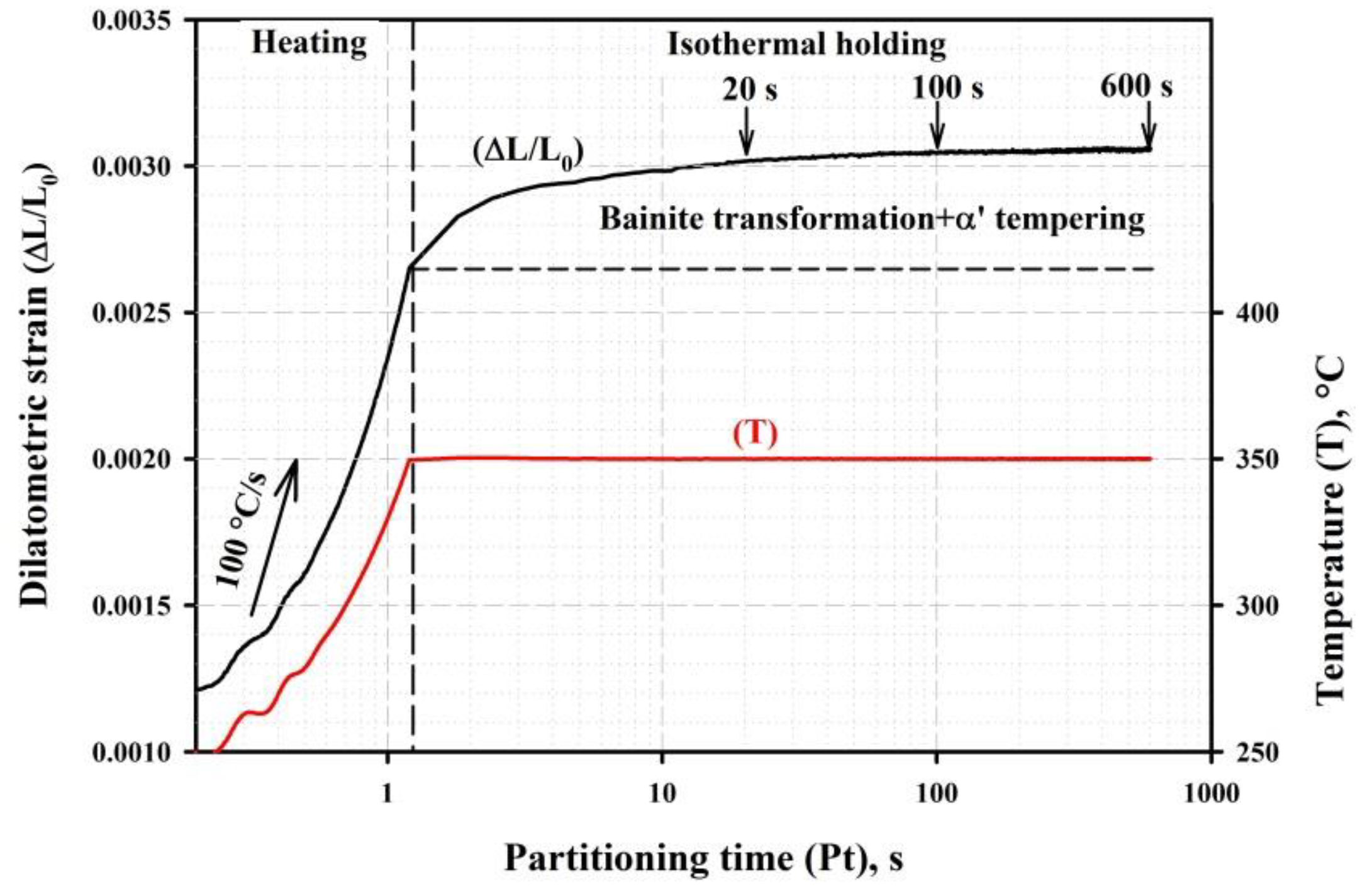
3.1. Phase Analysis and Microstructure
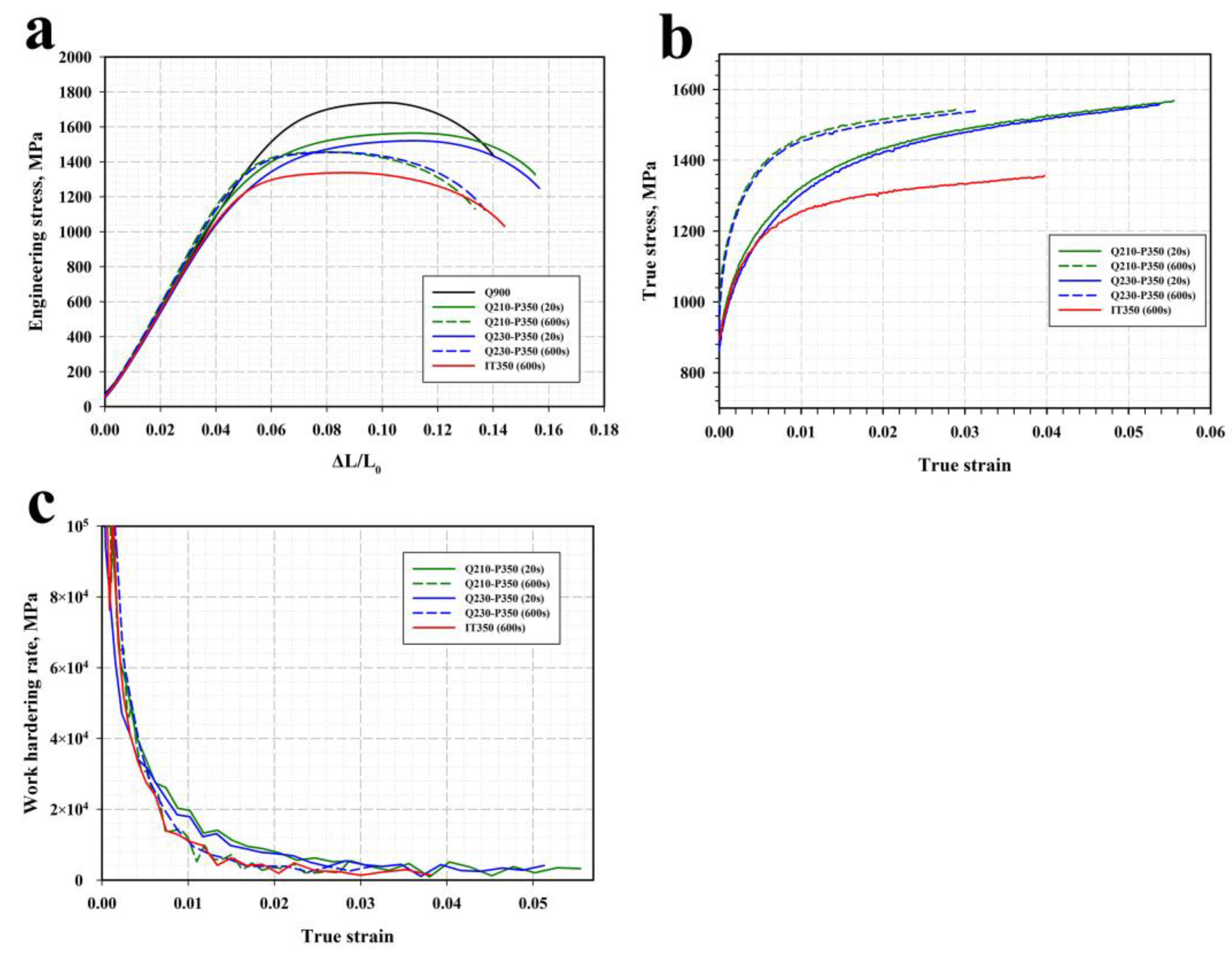
3.2. Mechanical Properties
4. Discussion
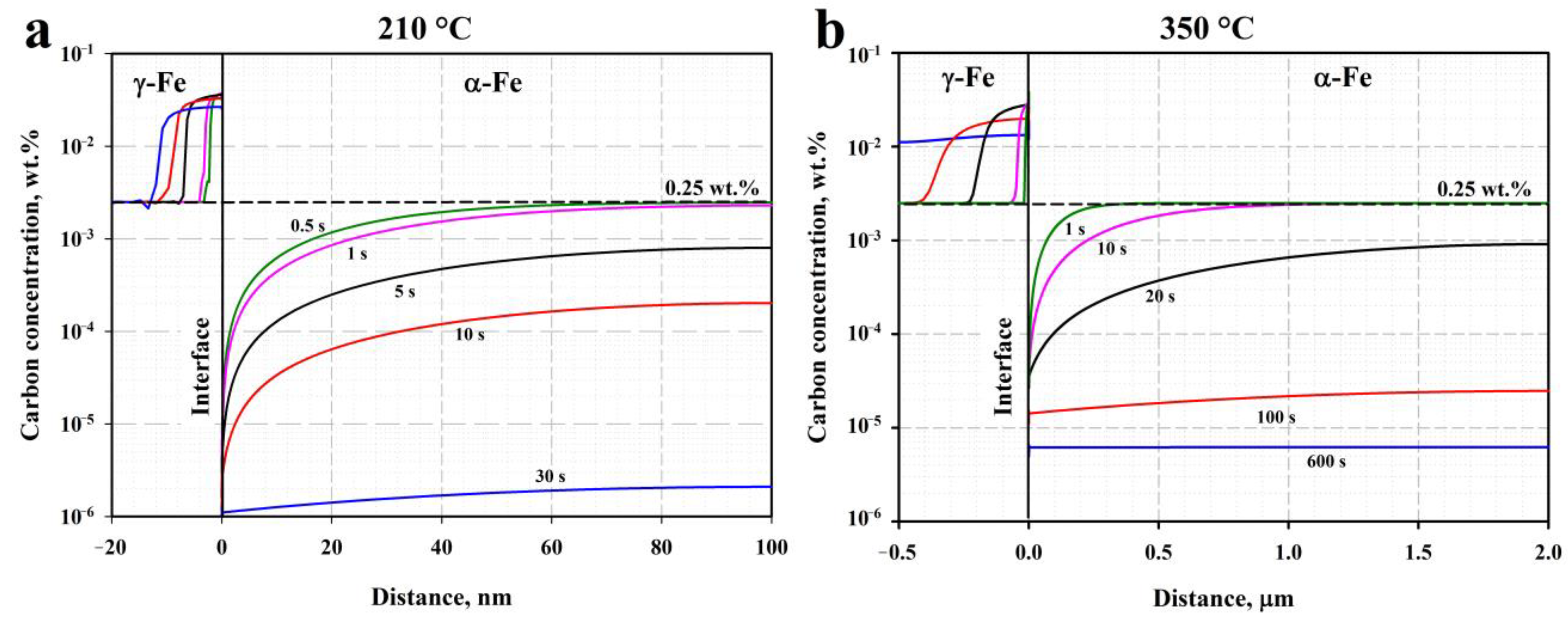
4.1. Microstructural Evolution during Q&P
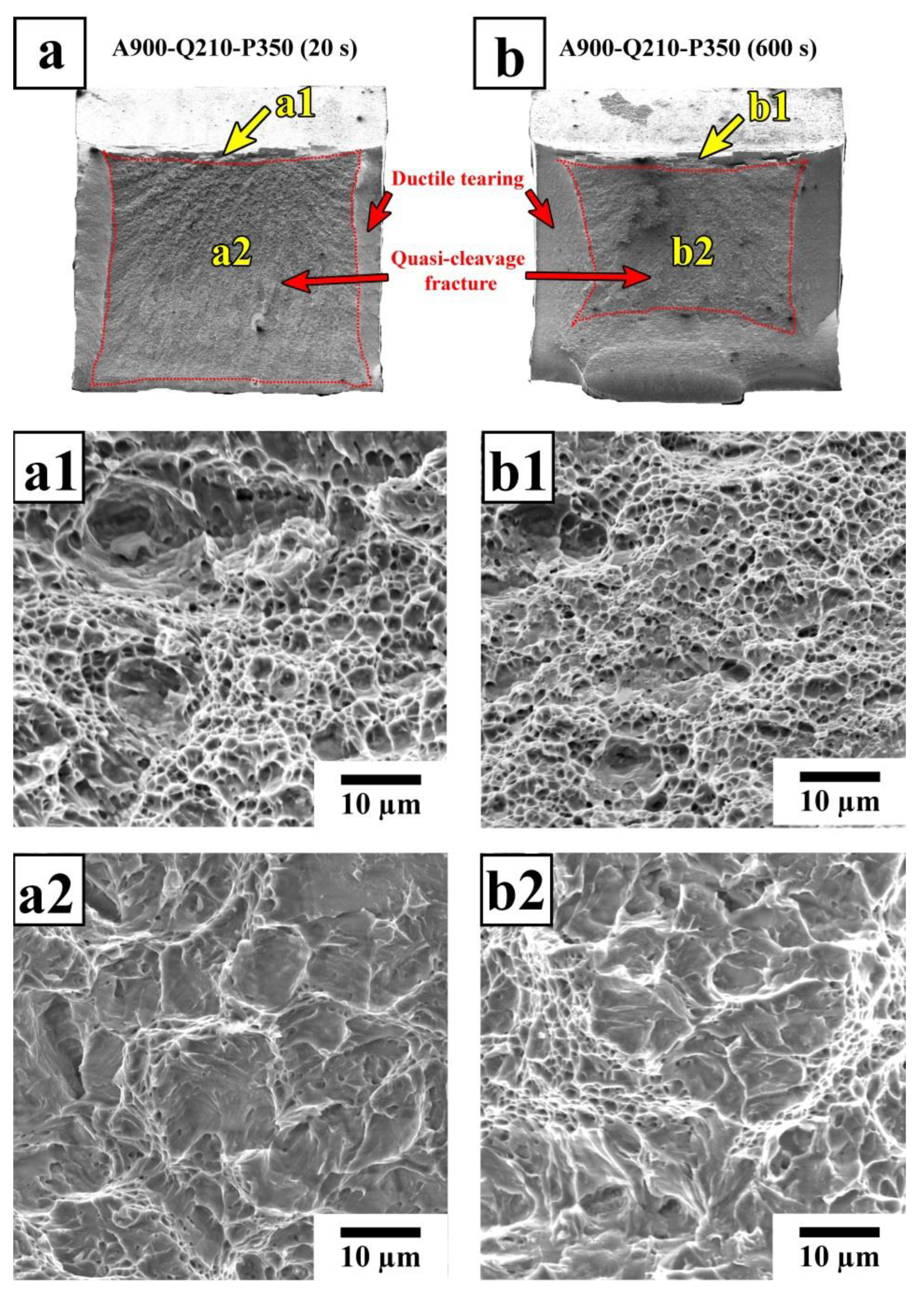
4.2. Relationship between Microstructure and Mechanical Properties
5. Conclusions
- Quenching to 210–230 °C with subsequent partitioning at 350 °C allows for the stabilizing of 6–11% of RA, and the brittle secondary martensite is eliminated during the final cooling. Stabilized RA with a film-like morphology is located on the lath and block boundaries of the tempered martensite, while relatively wide RA islands with irregular shapes are the product of the bainite transformation of large austenite regions during the partitioning stage.
- The bainitic transformation in the Q&P samples is nearly completed within 600 s at 350 °C. A decrease in the volume fraction and the mean width of RA as the Pt increases from 20 s to 600 s is mainly caused by the decomposition of coarse austenite islands.
- The concurrent increase in the yield strength and decrease in ductility with the increasing Pt is attributed to the decomposition of less strengthened RA and the precipitation of η-carbide particles in martensite.
- The large austenite islands have a detrimental effect on the fracture resistance of the Q&P steel. Martensite tempering and the stabilization of RA during partitioning is responsible for an excellent combination of a high-impact energy of ~100 J and a yield strength of 1200 MPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon Partitioning to Austenite from Martensite or Bainite during the Quench and Partition (Q&P) Process: A Critical Assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of Carbon Partitioning, Carbide Precipitation and Bainite Formation during the Q&P Process in a Low C Steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wang, C.; Martín, D.S.; Xu, W. Modeling Retained Austenite in Q&P Steels Accounting for the Bainitic Transformation and Correction of Its Mismatch on Optimal Conditions. Acta Mater. 2020, 188, 528–538. [Google Scholar] [CrossRef]
- Li, H.Y.; Lu, X.W.; Wu, X.C.; Min, Y.A.; Jin, X.J. Bainitic Transformation during the Two-Step Quenching and Partitioning Process in a Medium Carbon Steel Containing Silicon. Mater. Sci. Eng. A 2010, 527, 6255–6259. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.B. Evolution of Microstructure during the “Quenching and Partitioning (Q&P)” Treatment. Materialia 2021, 18, 101135. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of Carbon from Supersaturated Plates of Ferrite, with Application to Steel Processing and Fundamentals of the Bainite Transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon Partitioning into Austenite after Martensite Transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.G.; Sietsma, J. Microstructural Development during the Quenching and Partitioning Process in a Newly Designed Low-Carbon Steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- Wang, C.Y.; Shi, J.; Cao, W.Q.; Dong, H. Characterization of Microstructure Obtained by Quenching and Partitioning Process in Low Alloy Martensitic Steel. Mater. Sci. Eng. A 2010, 527, 3442–3449. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhang, T.; Li, J.; Hou, X.; Sun, W. Effects of Quenching Temperature on Bainite Transformation, Retained Austenite and Mechanical Properties of Hot-Galvanized Q&P Steel. Mater. Sci. Eng. A 2021, 822, 141643. [Google Scholar] [CrossRef]
- Tan, X.; Lu, W.; Rao, X. Effect of Ultra-Fast Heating on Microstructure and Mechanical Properties of Cold-Rolled Low-Carbon Low-Alloy Q&P Steels with Different Austenitizing Temperature. Mater. Charact. 2022, 191, 112086. [Google Scholar] [CrossRef]
- Hosoya, Y.; Matsumura, Y.; Tomota, Y.; Onuki, Y.; Harjo, S. Mechanism of Improved Ductility of 1500 MPa-Class Ultra-High Strength Cold-Rolled Steel Sheet Produced by Rolling and Partitioning Method. ISIJ Int. 2020, 60, 2097–2106. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Ge, Z.; Li, J.; Wang, Y. Effect of Heat Treatment on Microstructure and Mechanical Properties of New Cold-Rolled Automotive Steels. Metals 2020, 10, 1414. [Google Scholar] [CrossRef]
- Soleimani, M.; Kalhor, A.; Mirzadeh, H. Transformation-Induced Plasticity (TRIP) in Advanced Steels: A Review. Mater. Sci. Eng. A 2020, 795, 140023. [Google Scholar] [CrossRef]
- Dong, X.X.; Shen, Y.F.; Jia, N.; Zhu, Y.T. Improving Mechanical Properties and Retained-Austenite Stability of a Medium Carbon Q&P Steel by Adjusting Phase Ratio. Mater. Sci. Eng. A 2022, 833, 142580. [Google Scholar] [CrossRef]
- Mishnev, R.; Borisova, Y.; Gaidar, S.; Kniaziuk, T.; Vagina, O.; Kaibyshev, R. Q&P Response of a Medium Carbon Low Alloy Steel. Metals 2023, 13, 689. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Huang, X.; Huang, W. Effect of Bainitic Isothermal Transformation plus Q&P Process on the Microstructure and Mechanical Properties of 0.2C Bainitic Steel. Mater. Sci. Eng. A 2016, 678, 339–346. [Google Scholar] [CrossRef]
- Godbole, K.; Das, C.R.; Albert, S.K.; Panigrahi, B.B. Grain Boundary Engineering to Overcome Temper Embrittlement in Martensitic Steel. Mater. Lett. 2020, 264, 127321. [Google Scholar] [CrossRef]
- Blankart, C.; Wesselmecking, S.; Krupp, U. Article Influence of Quenching and Partitioning Parameters on Phase Transformations and Mechanical Properties of Medium Manganese Steel for Press-Hardening Application. Metals 2021, 11, 1879. [Google Scholar] [CrossRef]
- Bagliani, E.P.; Santofimia, M.J.; Zhao, L.; Sietsma, J.; Anelli, E. Microstructure, Tensile and Toughness Properties after Quenching and Partitioning Treatments of a Medium-Carbon Steel. Mater. Sci. Eng. A 2013, 559, 486–495. [Google Scholar] [CrossRef]
- De Diego-Calderón, I.; Sabirov, I.; Molina-Aldareguia, J.M.; Föjer, C.; Thiessen, R.; Petrov, R.H. Microstructural Design in Quenched and Partitioned (Q&P) Steels to Improve Their Fracture Properties. Mater. Sci. Eng. A 2016, 657, 136–146. [Google Scholar] [CrossRef]
- Lan, H.F.; Du, L.X.; Misra, R.D.K. Effect of Microstructural Constituents on Strength-Toughness Combination in a Low Carbon Bainitic Steel. Mater. Sci. Eng. A 2014, 611, 194–200. [Google Scholar] [CrossRef]
- Speich, G.R.; Dabkowski, D.S.; Porter, L.F. Strength and Toughness of Fe-10ni Alloys Containing C, Cr, Mo, and Co. Metall. Trans. 1973, 4, 303–315. [Google Scholar] [CrossRef]
- Linke, B.M.; Gerber, T.; Hatscher, A.; Salvatori, I.; Aranguren, I.; Arribas, M. Impact of Si on Microstructure and Mechanical Properties of 22MnB5 Hot Stamping Steel Treated by Quenching & Partitioning (Q&P). Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 54–65. [Google Scholar] [CrossRef]
- Xiong, Z.; Timokhina, I.; Pereloma, E. Clustering, Nano-Scale Precipitation and Strengthening of Steels. Prog. Mater. Sci. 2021, 118, 100764. [Google Scholar] [CrossRef]
- Miettunen, I.; Ghosh, S.; Somani, M.C.; Pallaspuro, S.; Kömi, J. Competitive Mechanisms Occurring during Quenching and Partitioning of Three Silicon Variants of 0.4 Wt.% Carbon Steels. J. Mater. Res. Technol. 2021, 11, 1045–1060. [Google Scholar] [CrossRef]
- Tkachev, E.; Borisov, S.; Belyakov, A.; Kniaziuk, T.; Vagina, O.; Gaidar, S.; Kaibyshev, R. Effect of Quenching and Tempering on Structure and Mechanical Properties of a Low-Alloy 0.25C Steel. Mater. Sci. Eng. A 2023, 868, 144757. [Google Scholar] [CrossRef]
- Kaar, S.; Krizan, D.; Schneider, R.; Sommitsch, C. On Competing Reactions and Austenite Stabilization: Advanced Model for Exact Microstructural Prediction in Q&P Steels with Elevated Mn-Content. Materialia 2022, 26, 101584. [Google Scholar] [CrossRef]
- Speer, J.G.; Streicher, A.M.; Matlock, D.K.; Rizzo, F.; Krauss, G. Quenching and Partitioning: A Fundamentally New Process to Create High Strength Trip Sheet Microstructures. In Proceedings of the Materials Science and Technology 2003 Meeting, Chicago, IL, USA, 9–12 November 2003; pp. 505–522. [Google Scholar]
- Koistinen, D.P.; Marburger, R.E. A General Equation Prescribing the Extent of the Austenite-Martensite Transformation in Pure Iron-Carbon Alloys and Plain Carbon Steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Kung, C.Y.; Rayment, J.J. An Examination of the Validity of Existing Empirical Formulae for the Calculation of Ms Temperature. Metall. Trans. A 1982, 13, 328–331. [Google Scholar] [CrossRef]
- Jatczak, C.F. Retained Austenite and Its Measurement by X-Ray Diffraction. In Proceedings of the SAE Technical Papers; SAE: Warrendale, PA, USA, 1980. [Google Scholar]
- Scott, C.P.; Drillet, J. A Study of the Carbon Distribution in Retained Austenite. Scr. Mater. 2007, 56, 489–492. [Google Scholar] [CrossRef]
- Ungár, T. Dislocation Densities, Arrangements and Character from X-Ray Diffraction Experiments. Mater. Sci. Eng. A 2001, 309–310, 14–22. [Google Scholar] [CrossRef]
- Macchi, J.; Gaudez, S.; Geandier, G.; Teixeira, J.; Denis, S.; Bonnet, F.; Allain, S.Y.P. Dislocation Densities in a Low-Carbon Steel during Martensite Transformation Determined by in Situ High Energy X-ray Diffraction. Mater. Sci. Eng. A 2021, 800, 140249. [Google Scholar] [CrossRef]
- Ungár, T.; Dragomir, I.; Révész, Á.; Borbély, A. The Contrast Factors of Dislocations in Cubic Crystals: The Dislocation Model of Strain Anisotropy in Practice. J. Appl. Crystallogr. 1999, 32, 992–1002. [Google Scholar] [CrossRef]
- Niessen, F.; Nyyssönen, T.; Gazder, A.A.; Hielscher, R. Parent Grain Reconstruction from Partially or Fully Transformed Microstructures in MTEX. J. Appl. Crystallogr. 2022, 55, 180–194. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation Gradients and Geometrically Necessary Dislocations in Ultrafine Grained Dual-Phase Steels Studied by 2D and 3D EBSD. Mater. Sci. Eng. A 2010, 527, 2738–2746. [Google Scholar] [CrossRef]
- Ruiz-Jimenez, V.; Kuntz, M.; Sourmail, T.; Caballero, F.G.; Jimenez, J.A.; Garcia-Mateo, C. Retained Austenite Destabilization during Tempering of Low-Temperature Bainite. Appl. Sci. 2020, 10, 8901. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G. Bainitic steels: Tempering. In Encyclopedia of Iron, Steel, and Their Alloys; Taylor & Francis: Abingdon, UK, 2016; pp. 1–14. ISBN 1-4665-1104-4. [Google Scholar]
- Santajuana, M.A.; Eres-Castellanos, A.; Ruiz-Jimenez, V.; Allain, S.; Geandier, G.; Caballero, F.G.; Garcia-Mateo, C. Quantitative Assessment of the Time to End Bainitic Transformation. Metals 2019, 9, 925. [Google Scholar] [CrossRef]
- Im, Y.R.; Kim, E.Y.; Song, T.; Lee, J.S.; Suh, D.W. Tensile Properties and Stretch-Flangeability of Trip Steels Produced by Quenching and Partitioning (Q&P) Process with Different Fractions of Constituent Phases. ISIJ Int. 2021, 61, 572–581. [Google Scholar] [CrossRef]
- Lu, W.; Herbig, M.; Liebscher, C.H.; Morsdorf, L.; Marceau, R.K.W.; Dehm, G.; Raabe, D. Formation of Eta Carbide in Ferrous Martensite by Room Temperature Aging. Acta Mater. 2018, 158, 297–312. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wan, X.; Liu, G.; Yang, Z.; Chen, H. Flash Annealing Yields a Strong and Ductile Medium Mn Steel with Heterogeneous Microstructure. Scr. Mater. 2021, 198, 113819. [Google Scholar] [CrossRef]
- Ebner, S.; Schnitzer, R.; Suppan, C.; Stark, A.; Liu, H.; Hofer, C. Characterization of Carbides in Q&P Steels Using a Combination of High-Resolution Methods. Mater. Charact. 2020, 163, 110242. [Google Scholar] [CrossRef]
- Hidalgo, J.; Findley, K.O.; Santofimia, M.J. Thermal and Mechanical Stability of Retained Austenite Surrounded by Martensite with Different Degrees of Tempering. Mater. Sci. Eng. A 2017, 690, 337–347. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Danoix, F.; Soler, M.; Goune, M. Carbon Heterogeneities in Austenite during Quenching & Partitioning (Q&P) Process Revealed by in Situ High Energy X-Ray Diffraction (HEXRD) Experiments. Scr. Mater. 2020, 181, 108–114. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, H.; Gui, X.; Luo, P.; Tan, Z.; Bai, B. Enhanced Ductility and Toughness in an Ultrahigh-Strength Mn-Si-Cr-C Steel: The Great Potential of Ultrafine Filmy Retained Austenite. Acta Mater. 2014, 76, 425–433. [Google Scholar] [CrossRef]
- Wu, R.; Li, W.; Zhou, S.; Zhong, Y.; Wang, L.; Jin, X. Effect of Retained Austenite on the Fracture Toughness of Quenching and Partitioning (Q&P)-Treated Sheet Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 1892–1902. [Google Scholar]
- Zhou, Q.; Qian, L.; Tan, J.; Meng, J.; Zhang, F. Inconsistent Effects of Mechanical Stability of Retained Austenite on Ductility and Toughness of Transformation-Induced Plasticity Steels. Mater. Sci. Eng. A 2013, 578, 370–376. [Google Scholar] [CrossRef]
- Du, H.; Gong, Y.; Liang, T.; Li, Y.; Xu, Y.; Lu, X.; Zeng, Q.; Jin, X. Enhancement of Impact Toughness Via Tailoring Deformation Compatibility of Constituent Phases in Duplex Q&P Steel with Excellent Strength and Ductility. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 2097–2117. [Google Scholar] [CrossRef]




| Treatment | State | Phase Volume Fractions, % | Carbon in RA (XRD), wt.% | ||||
|---|---|---|---|---|---|---|---|
| MINITIAL (Dilatometry) | RAINITIAL (1 − MINITIAL) | RAFINAL (XRD) | RAFINAL (Magnetic Saturation) | Bainitic Ferrite (RAINITIAL − RAXRD) | |||
| As quenched | A900-Qwater | ~100 | -//- | -//- | 1.4 ± 0.3 | -//- | -//- |
| Q&P | A900-Q210-P350 (20 s) | 85 | 15 | 10 | 13.3 ± 0.2 | 5 | 1.04 |
| A900-Q210-P350 (600 s) | 85 | 15 | 7 | 5.3 ± 0.4 | 8 | 0.96 | |
| A900-Q230-P350 (20 s) | 83 | 17 | 11 | 12.3 ± 0.4 | 6 | 1.06 | |
| A900-Q230-P350 (600 s) | 83 | 17 | 6 | 5.2 ± 0.2 | 11 | 1.05 | |
| IT | A900-IT350 (600 s) | 5 | 95 | 7 | 7.7 ± 0.2 | 88 | 1.07 |
| Treatment | State | dPAG (OM), µm | dP (EBSD), µm | db (EBSD), µm | Length/Width of η-Carbide Particles, nm | KAM Average, (Scan Step is 0.15 µm), ° | ρKAM, m−2 × 1015 | ρXRD, m−2 × 1015 |
|---|---|---|---|---|---|---|---|---|
| As quenched | A900-Qwater | 16.2 ± 0.8 | 4.6 ± 0.4 | 0.89 ± 0.02 | - | 0.69 | 0.65 | 3.2 ± 0.5 |
| Q&P | A900-Q210-P350 (20 s) | 6.0 ± 0.8 | 0.79 ± 0.03 | 93/13 | 0.81 | 0.75 | 4.0 ± 0.7 | |
| A900-Q210-P350 (600 s) | 5.5 ± 0.6 | 0.80 ± 0.04 | 131/13 | 0.56 | 0.53 | 1.6 ± 0.3 | ||
| A900-Q230-P350 (20 s) | 7.0 ± 1.1 | 0.95 ± 0.06 | 87/9 | 0.81 | 0.75 | 3.4 ± 0.7 | ||
| A900-Q230-P350 (600 s) | 6.3 ± 1.1 | 0.93 ± 0.06 | 121/13 | 0.62 | 0.59 | 2.4 ± 0.4 | ||
| IT | A900-IT350 (600 s) | 6.7 ± 0.8 | 0.87 ± 0.04 | 126/17 | 0.54 | 0.51 | 1.9 ± 0.3 |
| Treatment | State | Hardness, HRC | Yield Strength (YS), MPa | Ultimate Tensile Strength (UTS), MPa | UTS/YS | Total Elongation (TE), % | Uniform Elongation, % | Charpy Toughness at 20 °C, J |
|---|---|---|---|---|---|---|---|---|
| As quenched | A900-Qwater | 51.3 ± 0.6 | 1360 | 1740 | 1.28 | 8.8 | 3.6 | 49 |
| Q&P | A900-Q210-P350 (20 s) | 47.6 ± 1.0 | 1150 | 1560 | 1.36 | 11.0 | 5.6 | 38 |
| A900-Q210-P350 (100 s) | 45.5 ± 0.9 | 1220 | 1440 | 1.18 | 10.2 | 3.9 | 93 | |
| A900-Q210-P350 (600 s) | 45.6 ± 0.4 | 1230 | 1450 | 1.18 | 9.5 | 2.9 | 93 | |
| A900-Q230-P350 (20 s) | 46.5 ± 1.1 | 1090 | 1520 | 1.39 | 11.2 | 5.5 | 44 | |
| A900-Q230-P350 (100 s) | 46.1 ± 0.2 | 1210 | 1460 | 1.21 | 10.5 | 4.0 | 64 | |
| A900-Q230-P350 (600 s) | 46.4 ± 0.4 | 1230 | 1450 | 1.18 | 9.9 | 3.3 | 101 | |
| IT | A900-IT350 (600 s) | 43.8 ± 1.1 | 1070 | 1340 | 1.25 | 11.1 | 3.9 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachev, E.; Borisov, S.; Borisova, Y.; Kniaziuk, T.; Gaidar, S.; Kaibyshev, R. Strength–Toughness of a Low-Alloy 0.25C Steel Treated by Q&P Processing. Materials 2023, 16, 3851. https://doi.org/10.3390/ma16103851
Tkachev E, Borisov S, Borisova Y, Kniaziuk T, Gaidar S, Kaibyshev R. Strength–Toughness of a Low-Alloy 0.25C Steel Treated by Q&P Processing. Materials. 2023; 16(10):3851. https://doi.org/10.3390/ma16103851
Chicago/Turabian StyleTkachev, Evgeniy, Sergey Borisov, Yuliya Borisova, Tatiana Kniaziuk, Sergey Gaidar, and Rustam Kaibyshev. 2023. "Strength–Toughness of a Low-Alloy 0.25C Steel Treated by Q&P Processing" Materials 16, no. 10: 3851. https://doi.org/10.3390/ma16103851
APA StyleTkachev, E., Borisov, S., Borisova, Y., Kniaziuk, T., Gaidar, S., & Kaibyshev, R. (2023). Strength–Toughness of a Low-Alloy 0.25C Steel Treated by Q&P Processing. Materials, 16(10), 3851. https://doi.org/10.3390/ma16103851







