Heterostructure of NiFe@NiCr-LDH for Active and Durable Oxygen Evolution Reactions in Alkaline Media
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
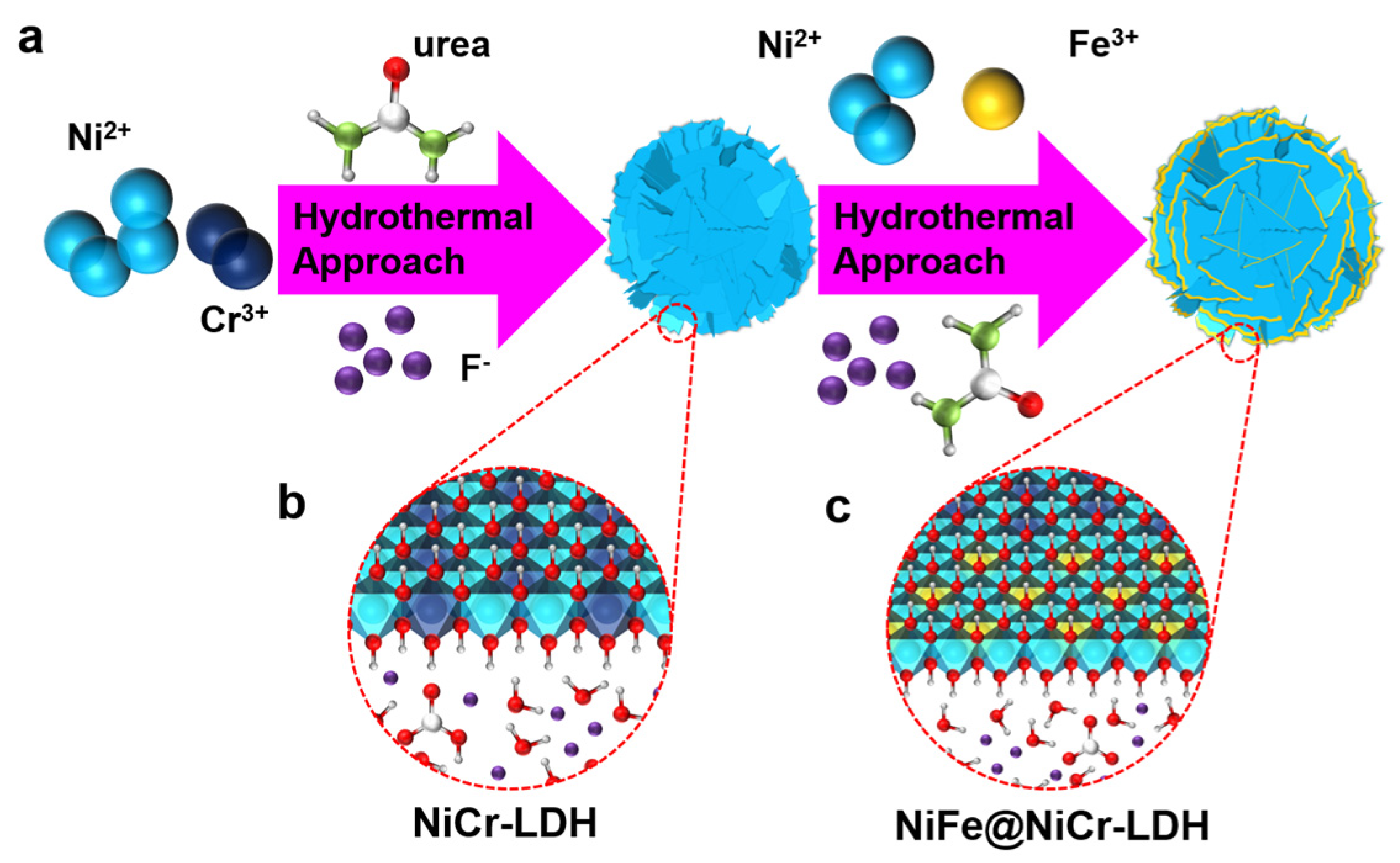
2.2. Synthesis of NiCr-LDH
2.3. Synthesis of NiFe@NiCr-LDH
2.4. Characterizations
2.5. Electrochemical Tests
3. Results and Discussion
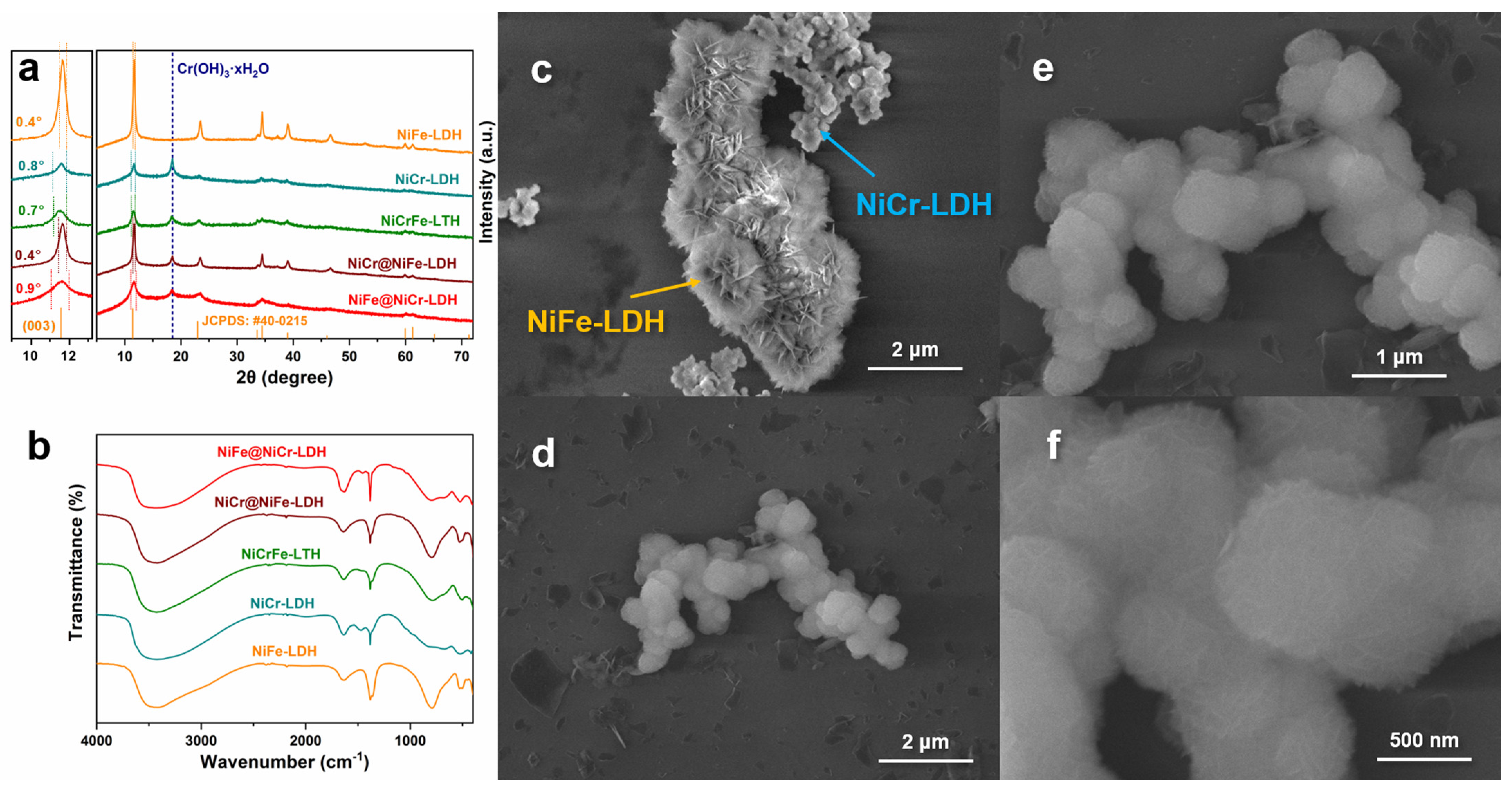
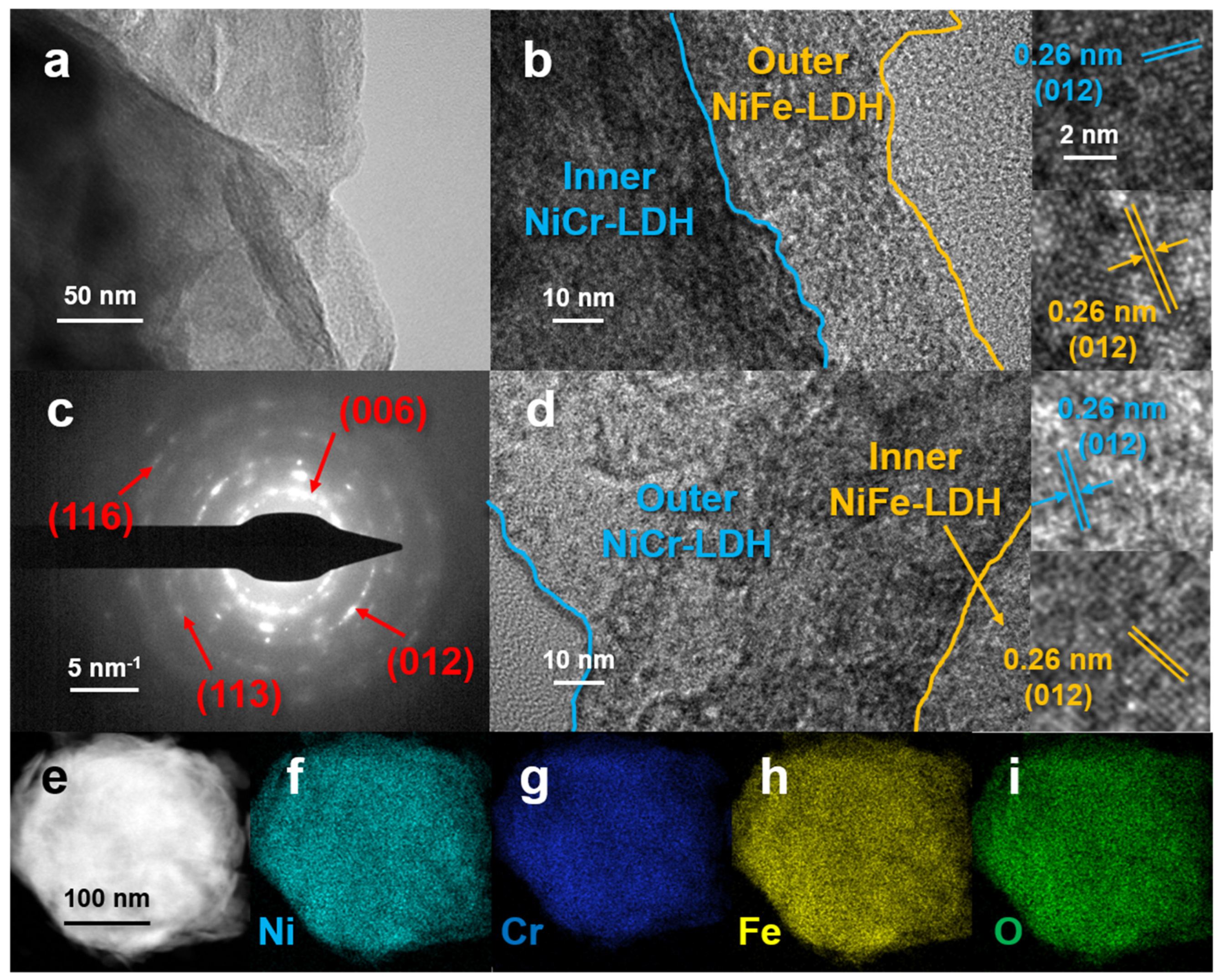
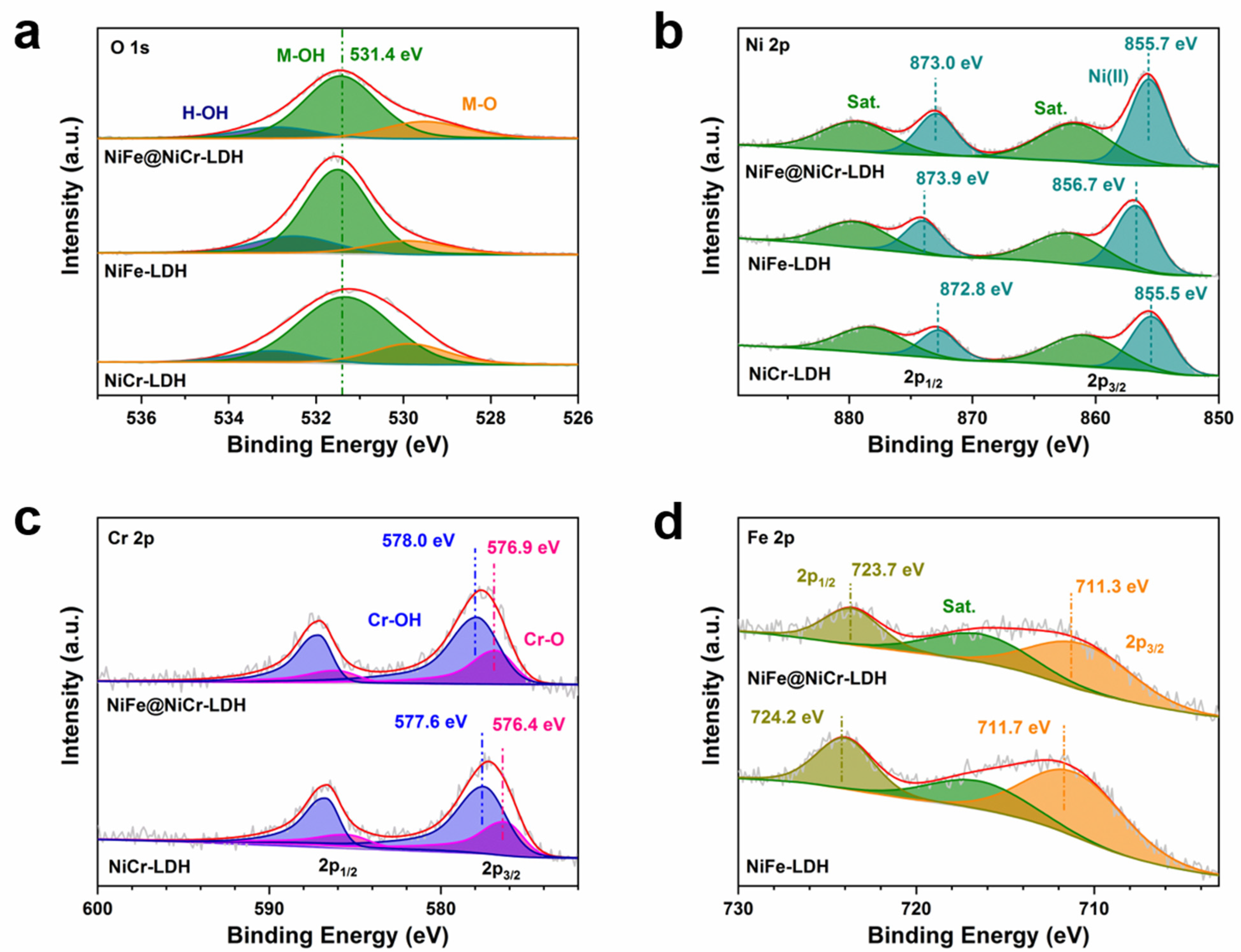
3.1. Characterization of the Samples
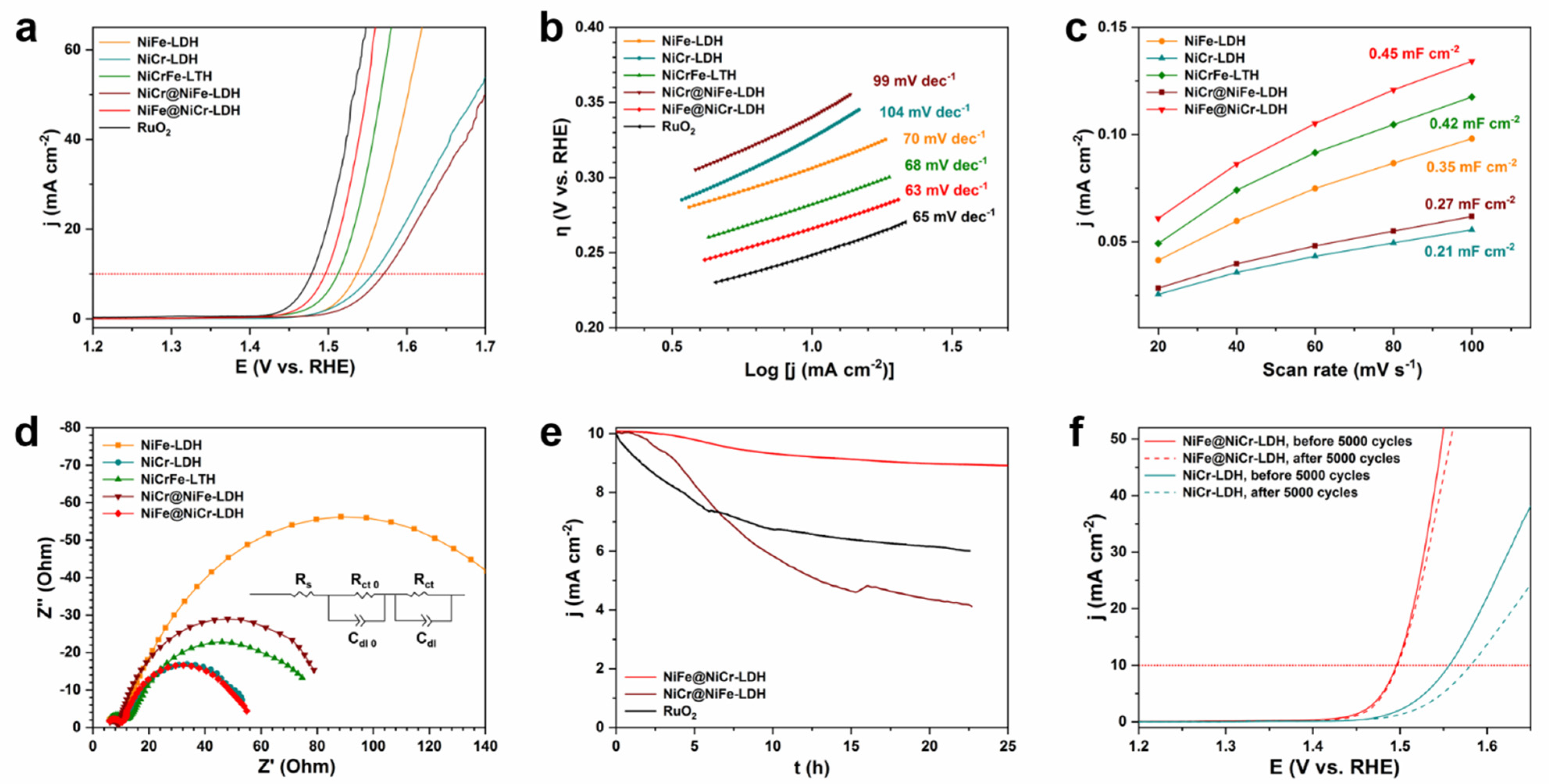
3.2. Electrochemical Performance toward OER
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Hu, T.; Dai, L.; Li, C.M. Metal-free photo- and electro-catalysts for hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 23674–23698. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Wang, Q.; Schweidler, S.; Botros, M.; Fu, T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-entropy energy materials: Challenges and new opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Li, J.; Chen, Q.; Du, Y.; Rao, P.; Li, R.; Jia, C.; Kang, Z.; Deng, P.; et al. Engineering Ruthenium-Based Electrocatalysts for Effective Hydrogen Evolution Reaction. Nano-Micro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and Interface Effects in Water-Splitting Electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Lai, J.; Wang, L.; Feng, S. Strong High Entropy Alloy-Support Interaction Enables Efficient Electrocatalytic Water Splitting at High Current Density. Chin. J. Struct. Chem. 2022, 41, 2208003–2208011. [Google Scholar]
- Wang, F.-G.; Liu, X.; Lv, Q.-X.; Liu, B.; Chai, Y.-M.; Dong, B. Transition Metal Boride-Based Materials for Electrocatalytic Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2209008–2209044. [Google Scholar]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.; Li, C.; Li, Y. Fe-Based Electrocatalysts for Oxygen Evolution Reaction: Progress and Perspectives. ACS Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Rao, R.R.; Kolb, M.J.; Halck, N.B.; Pedersen, A.F.; Mehta, A.; You, H.; Stoerzinger, K.A.; Feng, Z.; Hansen, H.A.; Zhou, H.; et al. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 2017, 10, 2626–2637. [Google Scholar] [CrossRef]
- Binninger, T.; Doublet, M.L. The Ir-OOOO-Ir transition state and the mechanism of the oxygen evolution reaction on IrO2(110). Energy Environ. Sci. 2022, 15, 2519–2528. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Chen, H.; Gao, R.; Shi, L.; Yang, L.; Zou, X. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 2021, 42, 1054–1077. [Google Scholar] [CrossRef]
- Shan, J.; Guo, C.; Zhu, Y.; Chen, S.; Song, L.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Charge-Redistribution-Enhanced Nanocrystalline Ru@IrOx Electrocatalysts for Oxygen Evolution in Acidic Media. Chem 2019, 5, 445–459. [Google Scholar] [CrossRef]
- Wang, C.; Jin, L.; Shang, H.; Xu, H.; Shiraishi, Y.; Du, Y. Advances in engineering RuO2 electrocatalysts towards oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent Advances and Prospective in Ruthenium-Based Materials for Electrochemical Water Splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Sun, H.; Tung, C.W.; Qiu, Y.; Zhang, W.; Wang, Q.; Li, Z.; Tang, J.; Chen, H.C.; Wang, C.; Chen, H.M. Atomic Metal-Support Interaction Enables Reconstruction-Free Dual-Site Electrocatalyst. J. Am. Chem. Soc. 2022, 144, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Gao, X.; Chu, W.; Gao, G.; Wang, L.-W. Recent advances in single-atom electrocatalysts supported on two-dimensional materials for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 9979–9999. [Google Scholar] [CrossRef]
- Wei, M.; Li, J.; Chu, W.; Wang, N. Phase control of 2D binary hydroxides nanosheets via controlling-release strategy for enhanced oxygen evolution reaction and supercapacitor performances. J. Energy Chem. 2019, 38, 26–33. [Google Scholar] [CrossRef]
- Fu, C.-L.; Wang, Y.; Huang, J.-H. Hybrid of Quaternary Layered Double Hydroxides and Carbon Nanotubes for Oxygen Evolution Reaction. Chin. J. Struct. Chem. 2020, 39, 1807–1816. [Google Scholar]
- Luo, M.; Cai, Z.; Wang, C.; Bi, Y.; Qian, L.; Hao, Y.; Li, L.; Kuang, Y.; Li, Y.; Lei, X.; et al. Phosphorus oxoanion-intercalated layered double hydroxides for high-performance oxygen evolution. Nano Res. 2017, 10, 1732–1739. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Singh, T.I.; Kim, N.H.; Lee, J.H. Remarkable Bifunctional Oxygen and Hydrogen Evolution Electrocatalytic Activities with Trace-Level Fe Doping in Ni- and Co-Layered Double Hydroxides for Overall Water-Splitting. ACS Appl. Mater. Interfaces 2018, 10, 42453–42468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning Surface Electronic Configuration of NiFe LDHs Nanosheets by Introducing Cation Vacancies (Fe or Ni) as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Cai, Z.; Bi, Y.; Tian, W.; Luo, M.; Zhang, Q.; Zhang, Q.; Xie, Q.; Wang, J.; Li, Y.; et al. Effects of redox-active interlayer anions on the oxygen evolution reactivity of NiFe-layered double hydroxide nanosheets. Nano Res. 2018, 11, 1358–1368. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Li, X.; Zai, J.; Liu, Y.; He, X.; Xiang, S.; Ma, Z.; Qian, X. Atomically thin layered NiFe double hydroxides assembled 3D microspheres with promoted electrochemical performances. J. Power Sources 2016, 325, 675–681. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wang, C.; Liang, J.; Liu, X.; Zhou, K.; Sasaki, T. A Superlattice of Alternately Stacked Ni-Fe Hydroxide Nanosheets and Graphene for Efficient Splitting of Water. ACS Nano 2015, 9, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-L.; Xu, H.; Li, G.-R. NiCoFe Layered Triple Hydroxides with Porous Structures as High-Performance Electrocatalysts for Overall Water Splitting. ACS Energy Lett. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Liu, S.; Wan, R.; Lin, Z.; Liu, Z.; Liu, Y.; Tian, Y.; Qin, D.-D.; Tang, Z. Probing the Co role in promoting the OER and Zn–air battery performance of NiFe-LDH: A combined experimental and theoretical study. J. Mater. Chem. A 2022, 10, 5244–5254. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Xi, L.; Yu, Y.; Chen, N.; Sun, S.; Wang, W.; Lange, K.M.; Zhang, B. Single-Atom Au/NiFe Layered Double Hydroxide Electrocatalyst: Probing the Origin of Activity for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef]
- Li, P.; Wang, M.; Duan, X.; Zheng, L.; Cheng, X.; Zhang, Y.; Kuang, Y.; Li, Y.; Ma, Q.; Feng, Z.; et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 2019, 10, 1711. [Google Scholar] [CrossRef]
- Xu, S.-J.; Zhou, Y.-N.; Shen, G.-P.; Dong, B. Ni(OH)2 Derived from NiS2 Induced by Reflux Playing Three Roles for Hydrogen/Oxygen Evolution Reaction. Chin. J. Struct. Chem. 2022, 41, 2208052–2208057. [Google Scholar]
- Wu, Y.; Song, M.; Huang, Y.-C.; Dong, C.-L.; Li, Y.; Lu, Y.; Zhou, B.; Wang, D.; Jia, J.; Wang, S.; et al. Promoting surface reconstruction of NiFe layered double hydroxides via intercalating [Cr(C2O4)3]3− for enhanced oxygen evolution. J. Energy Chem. 2022, 74, 140–148. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, Z.; Cheng, F.; Li, W.; Cui, P.; Xu, G.; Xu, S.; Wang, P.; Sheng, G.; Yan, Y.; et al. Unlocking the potential of graphene for water oxidation using an orbital hybridization strategy. Energy Environ. Sci. 2018, 11, 407–416. [Google Scholar] [CrossRef]
- Babu, S.P.; Falch, A. Recent Developments on Cr-Based Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Media. ChemCatChem 2022, 14, e202200364. [Google Scholar] [CrossRef]
- Liu, T.; Diao, P. Nickel foam supported Cr-doped NiCo2O4/FeOOH nanoneedle arrays as a high-performance bifunctional electrocatalyst for overall water splitting. Nano Res. 2020, 13, 3299–3309. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, M.; Yang, H.; Lin, L.; Jiang, H.; Ge, S.; Li, H.; Sun, G.; Ma, S.; Yang, X. 3D Porous Amorphous gamma-CrOOH on Ni Foam as Bifunctional Electrocatalyst for Overall Water Splitting. Inorg. Chem. 2019, 58, 4014–4018. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, X.; Xu, J.; Si, R.; Sheng, J.; Luo, J.; Zhang, S.; Dong, W.; Li, G.; Wang, W.; et al. Ruthenium-Doped Cobalt-Chromium Layered Double Hydroxides for Enhancing Oxygen Evolution through Regulating Charge Transfer. Small 2020, 16, 1905328. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yuan, X.; Wang, X.; Liu, X.; Dong, W.; Wang, R.; Duan, Y.; Huang, F. Rational design of cobalt–chromium layered double hydroxide as a highly efficient electrocatalyst for water oxidation. J. Mater. Chem. A 2016, 4, 11292–11298. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Hu, Y.; Guan, M.; Xu, L.; Li, H.; Bao, J.; Li, H. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.; Shearer, M.J.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Ye, W.; Fang, X.; Chen, X.; Yan, D. A three-dimensional nickel-chromium layered double hydroxide micro/nanosheet array as an efficient and stable bifunctional electrocatalyst for overall water splitting. Nanoscale 2018, 10, 19484–19491. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, F.; Chen, W.; Ma, Y.; Wu, Y.; Gu, X.; Qin, Y.; Tao, P.; Song, C.; Shang, W.; et al. In Situ Vertical Growth of Fe–Ni Layered Double-Hydroxide Arrays on Fe–Ni Alloy Foil: Interfacial Layer Enhanced Electrocatalyst with Small Overpotential for Oxygen Evolution Reaction. ACS Energy Lett. 2018, 3, 2357–2365. [Google Scholar] [CrossRef]
- Chen, S.; Yu, C.; Cao, Z.; Huang, X.; Wang, S.; Zhong, H. Trimetallic NiFeCr-LDH/MoS2 composites as novel electrocatalyst for OER. Int. J. Hydrog. Energy 2021, 46, 7037–7046. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Hocking, R.K.; Zhao, C. NiFeCr Hydroxide Holey Nanosheet as Advanced Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 41239–41245. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, T.; Zhang, S.; Li, D.; Tang, P.; Alonso-Vante, N.; Feng, Y. Template-free synthesis of three-dimensional NiFe-LDH hollow microsphere with enhanced OER performance in alkaline media. J. Energy Chem. 2019, 33, 130–137. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, X.; Gao, D.; He, H.; Ou, Y.; Zhou, M.; Lai, Q.; Wei, X.; Xiao, P.; Zhang, Y. Trimetallic CoFeCr hydroxide electrocatalysts synthesized at a low temperature for accelerating water oxidationviatuning the electronic structure of active sites. Sustain. Energ. Fuels 2020, 4, 3647–3653. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Chen, X.; Zhao, C. High valence chromium regulated cobalt-iron-hydroxide for enhanced water oxidation. J. Power Sources 2018, 402, 381–387. [Google Scholar] [CrossRef]
- Thenuwara, A.C.; Attanayake, N.H.; Yu, J.; Perdew, J.P.; Elzinga, E.J.; Yan, Q.; Strongin, D.R. Cobalt Intercalated Layered NiFe Double Hydroxides for the Oxygen Evolution Reaction. J. Phys. Chem. B 2018, 122, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Lou, Z.X.; Wu, X.; Liu, Y.; Zhao, J.Y.; Sun, K.Z.; Li, W.X.; Chen, J.; Yuan, H.Y.; Zhu, M.; et al. Operando High-Valence Cr-Modified NiFe Hydroxides for Water Oxidation. Small 2022, 18, 2200303. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chem. Int. Ed. 2018, 130, 178–182. [Google Scholar] [CrossRef]
- Yan, L.; Du, Z.; Lai, X.; Lan, J.; Liu, X.; Liao, J.; Feng, Y.; Li, H. Synergistically modulating the electronic structure of Cr-doped FeNi LDH nanoarrays by O-vacancy and coupling of MXene for enhanced oxygen evolution reaction. Int. J. Hydrog. Energy 2023, 48, 1892–1903. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tang, Y.; Guo, C.; Liu, Y.; Tang, Z. Heterostructure of NiFe@NiCr-LDH for Active and Durable Oxygen Evolution Reactions in Alkaline Media. Materials 2023, 16, 2968. https://doi.org/10.3390/ma16082968
Liu S, Tang Y, Guo C, Liu Y, Tang Z. Heterostructure of NiFe@NiCr-LDH for Active and Durable Oxygen Evolution Reactions in Alkaline Media. Materials. 2023; 16(8):2968. https://doi.org/10.3390/ma16082968
Chicago/Turabian StyleLiu, Sanchuan, Yujun Tang, Chengyu Guo, Yonggang Liu, and Zhenghua Tang. 2023. "Heterostructure of NiFe@NiCr-LDH for Active and Durable Oxygen Evolution Reactions in Alkaline Media" Materials 16, no. 8: 2968. https://doi.org/10.3390/ma16082968
APA StyleLiu, S., Tang, Y., Guo, C., Liu, Y., & Tang, Z. (2023). Heterostructure of NiFe@NiCr-LDH for Active and Durable Oxygen Evolution Reactions in Alkaline Media. Materials, 16(8), 2968. https://doi.org/10.3390/ma16082968






