Influence of Illite and Its Amine Modifications on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Filler Modification
2.3. Preparation of One-Side Self-Adhesive Tape
2.4. Infrared Spectroscopy FTIR
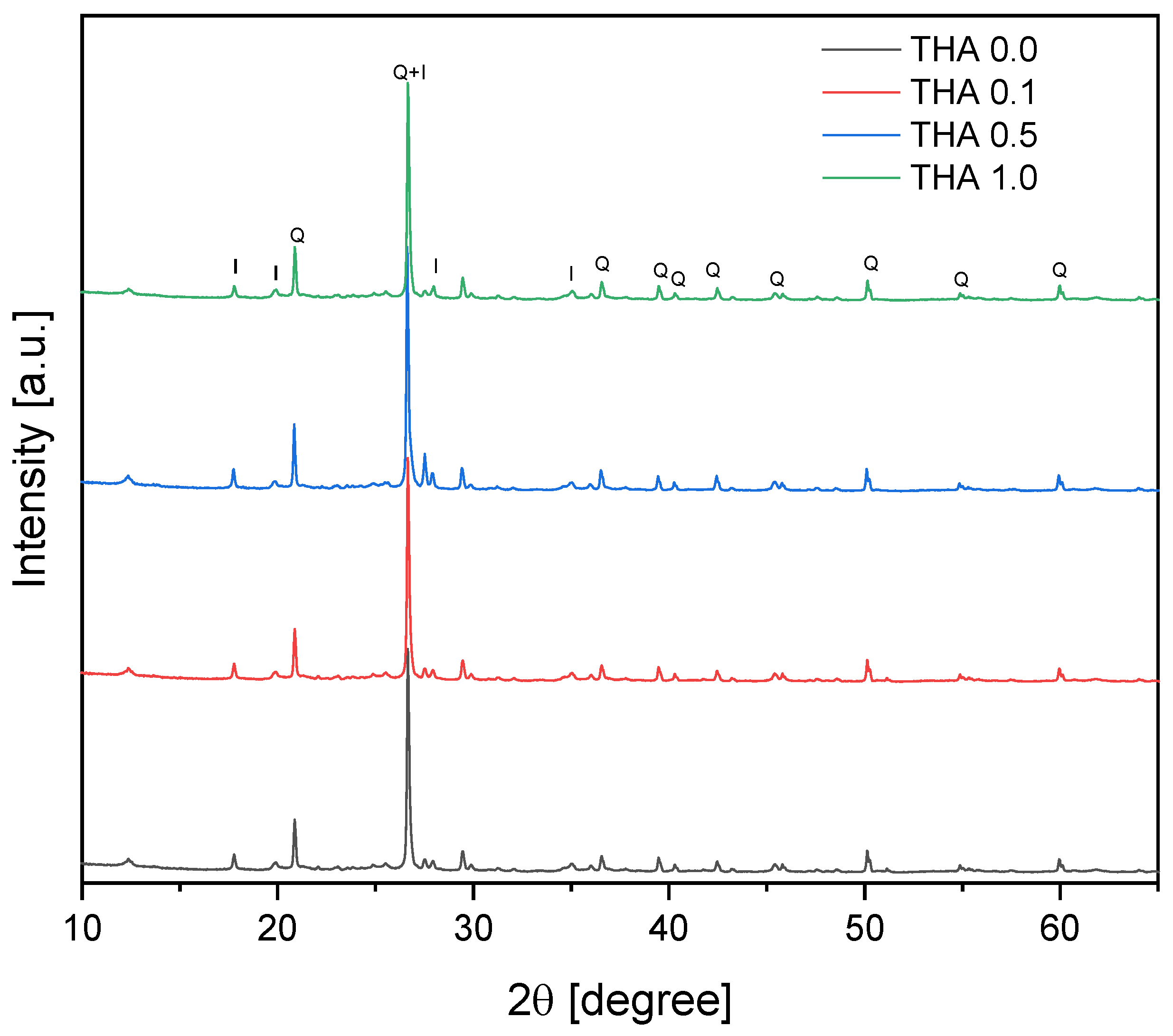
2.5. X-ray Diffraction (XRD)
2.6. Pot-Life
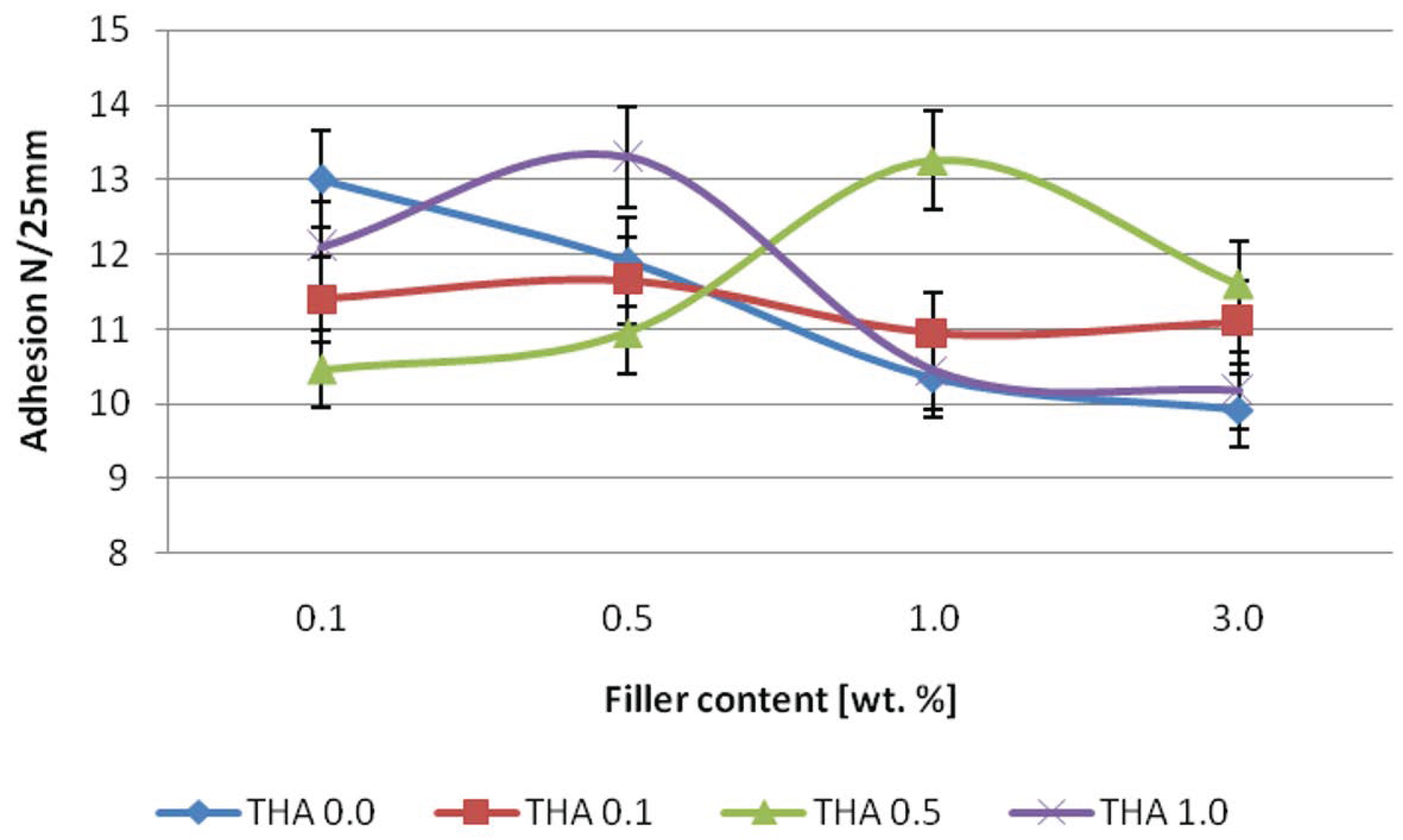
2.7. Adhesion
2.8. Cohesion
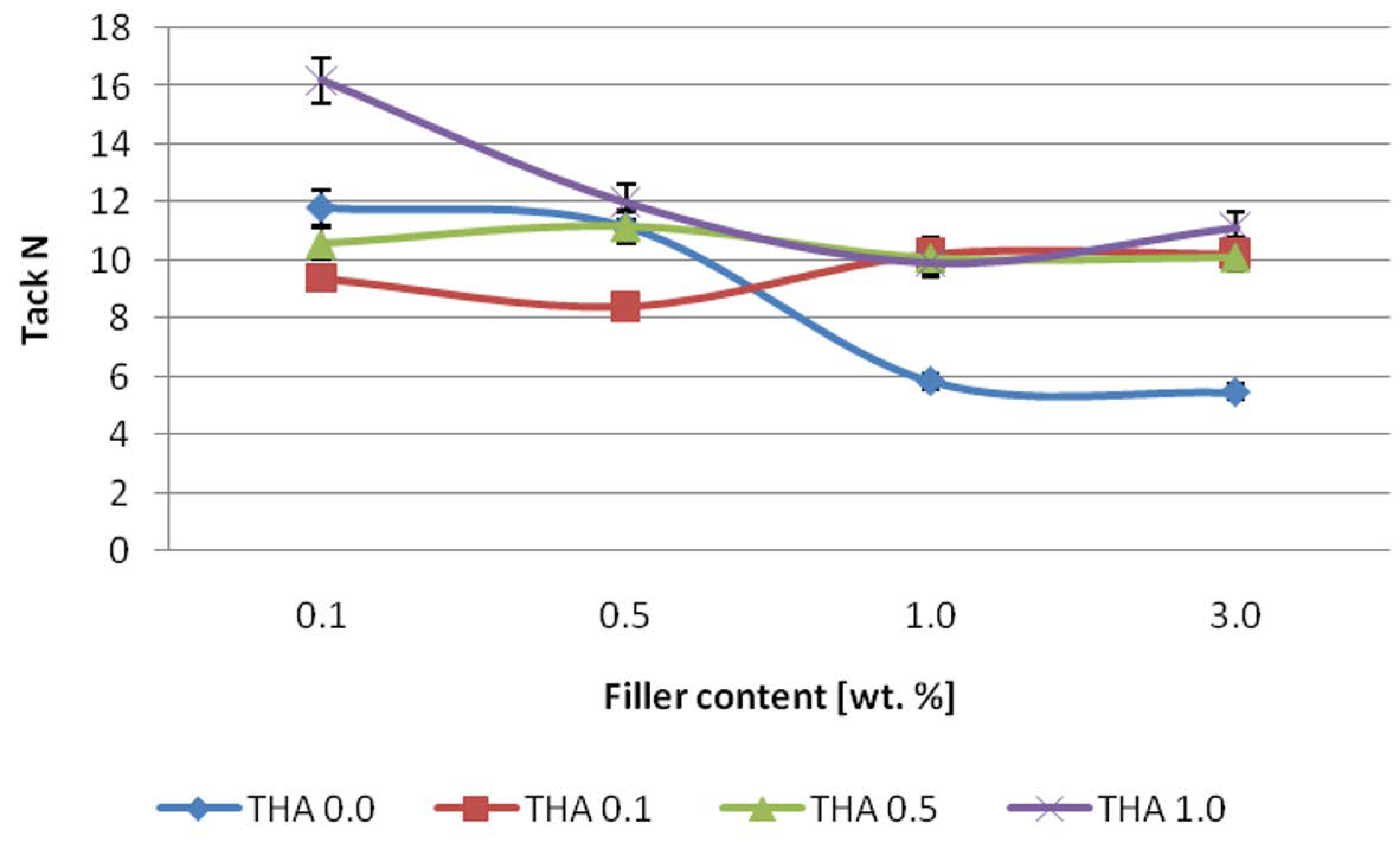
2.9. Tack
2.10. Thermal Resistance
2.11. Shrinkage
2.12. Thermogravimetric Analysis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Licbarski, A.; Bartkowiak, M.; Czech, Z. Influence of Selected Crosslinking Agents and Selected Unsaturated Copolymerizable Photoinitiators Referring to the Shrinkage Resistance of Solvent-Based Acrylic Pressure-Sensitive Adhesives. Polymers 2022, 14, 5190. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.J.; Bayne, S.C.; Baier, R.; Tomsia, A.P.; Marshall, G.W. A review of adhesion science. Dent. Mater. 2010, 26, e11–e16. [Google Scholar] [CrossRef]
- Kim, H.-J.; Czech, Z.; Bartkowiak, M.; Shim, G.-S.; Kabatc, J.; Licbarski, A. Study of UV-initiated polymerization and UV crosslinking of acrylic monomers mixture for the production of solvent-free pressure-sensitive adhesive films. Polym. Test. 2022, 105, 107424. [Google Scholar] [CrossRef]
- Shim, G.-S.; Kim, H.-J.; Bartkowiak, M. Curing behavior and impact of crosslinking agent variation in stepwise UV/UV cured acrylic pressure-sensitive adhesives. J. Mater. Res. Technol. 2021, 15, 1622–1629. [Google Scholar] [CrossRef]
- Czech, Z.; Butwin, A. New Developments in the Area of Solvent-Borne Acrylic Pressure-Sensitive Adhesives. J. Adhes. Sci. Technol. 2009, 23, 1689–1707. [Google Scholar] [CrossRef]
- Mapari, S.; Mestry, S.; Mhasje, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Czech, Z.; Milker, R. Development trends in pressure-sensitive adhesives systems. Mater. Sci. 2005, 23, 1015–1022. [Google Scholar]
- Mozelewska, K.; Czech, Z.; Bartkowiak, M.; Nowak, M.; Bednarczyk, P.; Niezgoda, P.; Kabatc, J.; Skotnicka, A. Preparation and characterization of acrylic pressure-sensitive adhesives crosslinked with UV radiation-influence of monomer composition on adhesive proporties. Materials 2021, 15, 246. [Google Scholar] [CrossRef]
- Chew, J.Y.M.; Tonneijk, S.J.; Paterson, W.R.; Wilson, D.I. Solvent-based cleaning of emulsion polymerization reactors. Chem. Eng. J. 2006, 117, 61–69. [Google Scholar] [CrossRef]
- Xu, C.; Liang, W.; Wu, X.; Jiao, E.; Lu, M.; Wu, K.; Hao, X.; Shi, J. Effect of novel silicone/vanillin monomer on the thermal stability and adhesion properties of UV-curable polyurethane pressure sensitive adhesive and its application in functional glass. Prog. Org. Coat. 2022, 171, 107019. [Google Scholar] [CrossRef]
- Pradeep, S.V.; Kandasubramanian, B.; Sidharth, S. A review on recent trends in bio-based pressure sensitive adhesives. J. Adhes. 2023, 2023, 2176761. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, Q.; Wang, S.; Yan, S. Effect of 3-Mercaptopropyltriethoxysilane Modified Illite on the Reinforcement of SBR. Materials 2022, 15, 3459. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Sung, H.; Hwang, Y.; Kyun Noh, S.; Lee, D. Modification of montmorillonite with oligomeric amine derivatives for polymer nanocomposite preparation. Appl. Clay Sci. 2007, 38, 1–8. [Google Scholar] [CrossRef]
- Song, T.; Ren, Z.; Li, H.; Sun, X.; Xue, M.; Yan, S. Modification of illite with calcium pimelate and its influence on the crystallization and mechanical property of isotactic polypropylene. Compos. Part A Appl. Sci. Manuf. 2019, 123, 200–207. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers Based on Phosphoric Acid and Illito-Kaolinitic Clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef]
- Alkan, M.; Tekin, G.; Namli, H. FTIR and zeta potential measurements of sepiolite treated with some organosilanes. Microporous Mesoporous Mater. 2005, 84, 75–83. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Kadhum, A.; Nassir, M.; Al-Amiery, A. Impact of Sulfuric Acid Treatment of Halloysite on Physico-Chemic Property Modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K. Influence of Nanoclay on the Thermo-Mechanical Properties of Silicone Pressure-Sensitive Adhesives. Materials 2022, 15, 7460. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K.; Piątek-Hnat, M.; Czech, Z.; Bartkowiak, M. Silicone pressure-sensitive adhesives with increased thermal resistance. J. Therm. Anal. Calorim. 2022, 147, 7719–7727. [Google Scholar] [CrossRef]
- Antosik, A.K.; Musik, M.; Mozelewska, K.; Miądlicki, P. Influence of cleaned granite dust on the properties of silicone pressure-sensitive adhesives. J. Anal. Pharm. Res. 2022, 11, 27–30. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K. Influence of storage time on the useful properties of silicone pressure-sensitive adhesives. Polimery/Polymers 2022, 67, 317–323. [Google Scholar] [CrossRef]
- Joo, H.-S.; Do, H.-S.; Park, Y.-J.; Kim, H.-J. Adhesion performance of UV-cured semi-IPN structure acrylic pressure sensitive adhesives. J. Adhes. Sci. Technol. 2006, 20, 1573–1594. [Google Scholar] [CrossRef]
- Antosik, A.K.; Makuch, E.; Gziut, K. Influence of modified attapulgite on silicone pressure-sensitive adhesives properties. J. Polym. Res. 2022, 29, 135. [Google Scholar] [CrossRef]
- Kowalski, A.; Czech, Z.; Byczyński, Ł. How does the surface free energy influence the tack of acrylic pressure-sensitive adhesives (PSAs)? J. Coat. Technol. Res. 2013, 10, 879–885. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K.; Czech, Z.; Piątek-Hnat, M. Influence of Montmorillonite on the Properties of Silicone Pressure-Sensitive Adhesives: Preparation of a Double-Sided Tape Based on the Best Composition. Silicon 2020, 12, 1887–1893. [Google Scholar] [CrossRef]
- Antosik, A.K.; Weisbrodt, M.; Mozelewska, K.; Czech, Z.; Piątek-Hnat, M. Impact of environmental conditions on silicone pressure-sensitive adhesives. Polym. Bull. 2020, 77, 6625–6639. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Matusik, J. The Effect of Surfactant-Modified Montmorillonite on the Cross-Linking Efficiency of Polysiloxanes. Materials 2021, 14, 2623. [Google Scholar] [CrossRef] [PubMed]


| Filler | Treatment | Symbols |
|---|---|---|
| Illite | Natural, unmodified | THA 0.0 |
| 0.1 M solution of N,N,4-trimethylaniline | THA 0.1 | |
| 0.5 M solution of N,N,4-trimethylaniline | THA 0.5 | |
| 1.0 M solution of N,N,4-trimethylaniline | THA 1.0 |
| Wavenumber (cm−1) | Corresponding Species |
|---|---|
| 3432, 3625, 3697 | Hydroxyl groups |
| 1647 | H-O-H of water |
| 1455 | Hydroxyl groups |
| 1033 | Si-O |
| 911 | Al-OH |
| 789, 777 | Doublet of quartz |
| 693 | Si-O-Si of quartz |
| 532, 753 | Al-OSi |
| 468 | Si-O |
| Resin Acronym | Tack [N] | Adhesion [N/25 mm] | Cohesion [h] | SAFT [°C] | Viscosity [Pa·s] | |
|---|---|---|---|---|---|---|
| 20 °C | 70 °C | |||||
| Q2-7358 | 6.9 | 10.2 | >72 | >72 | 147 | 16.7 |
| Filler | Viscosity [Pa·s] | ||||
|---|---|---|---|---|---|
| 1 Day | 2 Days | 3 Days | 5 Days | 7 Days | |
| THA 0.0 | 21.0 | 22.8 | 25.1 | 28.7 | 31.5 |
| THA 0.1 | 39.2 | 41.0 | 49.2 | 58.0 | 69.0 |
| THA 0.5 | 35.2 | 36.9 | 44.8 | 53.0 | 71.0 |
| THA 1.0 | 33.9 | 38.5 | 46.1 | 54.5 | 75.0 |
| Filler Content [wt.%] | THA 0.0 | THA 0.1 | THA 0.5 | THA 1.0 |
|---|---|---|---|---|
| Cohesion at 20 °C [h] | ||||
| 0.1 | >72 | >72 | >72 | >72 |
| 0.5 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 |
| 3.0 | >72 | >72 | >72 | >72 |
| Cohesion at 70 °C [h] | ||||
| 0.1 | >72 | >72 | >72 | >72 |
| 0.5 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 |
| 3.0 | 32 | >72 | >72 | >72 |
| SAFT [°C] | ||||
| 0.1 | >225 | >225 | >225 | >225 |
| 0.5 | >225 | 215 | >225 | 206 |
| 1.0 | >225 | 213 | 219 | 165 |
| 3.0 | >160 | 156 | 180 | 160 |
| Shrinkage [%] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Filler Content [wt.%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| Pure | ||||||||||||
| 0.0 | 0.41 | 0.42 | 0.64 | 0.90 | 0.96 | 1.02 | 1.14 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 |
| THA 0.0 | ||||||||||||
| 0.1 | 0.23 | 0.32 | 0.35 | 0.39 | 0.42 | 0.47 | 0.50 | 0.52 | 0.55 | 0.57 | 0.60 | 0.61 |
| 0.5 | 0.10 | 0.12 | 0.17 | 0.22 | 0.28 | 0.30 | 0.33 | 0.36 | 0.38 | 0.40 | 0.40 | 0.40 |
| 1.0 | 0.08 | 0.13 | 0.16 | 0.19 | 0.20 | 0.23 | 0.25 | 0.26 | 0.28 | 0.29 | 0.29 | 0.29 |
| 3.0 | 0.04 | 0.06 | 0.08 | 0.11 | 0.13 | 0.14 | 0.18 | 0.21 | 0.23 | 0.24 | 0.25 | 0.20 |
| THA 0.1 | ||||||||||||
| 0.1 | 0.24 | 0.30 | 0.33 | 0.36 | 0.41 | 0.46 | 0.49 | 0.53 | 0.55 | 0.58 | 0.59 | 0.60 |
| 0.5 | 0.14 | 0.17 | 0.21 | 0.26 | 0.31 | 0.34 | 0.36 | 0.39 | 0.40 | 0.40 | 0.40 | 0.45 |
| 1.0 | 0.09 | 0.10 | 0.14 | 0.16 | 0.18 | 0.20 | 0.23 | 0.25 | 0.27 | 0.30 | 0.30 | 0.30 |
| 3.0 | 0.07 | 0.08 | 0.10 | 0.12 | 0.13 | 0.14 | 0.16 | 0.18 | 0.20 | 0.21 | 0.21 | 0.21 |
| THA 0.5 | ||||||||||||
| 0.1 | 0.10 | 0.20 | 0.28 | 0.32 | 0.35 | 0.38 | 0.43 | 0.47 | 0.49 | 0.52 | 0.56 | 0.58 |
| 0.5 | 0.15 | 0.18 | 0.20 | 0.26 | 0.30 | 0.33 | 0.35 | 0.38 | 0.40 | 0.43 | 0.48 | 0.48 |
| 1.0 | 0.10 | 0.13 | 0.16 | 0.20 | 0.25 | 0.31 | 0.33 | 0.35 | 0.37 | 0.40 | 0.40 | 0.40 |
| 3.0 | 0.08 | 0.12 | 0.14 | 0.16 | 0.18 | 0.20 | 0.22 | 0.25 | 0.27 | 0.27 | 0.27 | 0.25 |
| THA 1.0 | ||||||||||||
| 0.1 | 0.25 | 0.30 | 0.35 | 0.37 | 0.39 | 0.43 | 0.46 | 0.48 | 0.51 | 0.54 | 0.55 | 0.55 |
| 0.5 | 0.21 | 0.25 | 0.28 | 0.31 | 0.35 | 0.38 | 0.40 | 0.43 | 0.46 | 0.50 | 0.51 | 0.51 |
| 1.0 | 0.17 | 0.20 | 0.23 | 0.25 | 0.28 | 0.30 | 0.33 | 0.35 | 0.37 | 0.40 | 0.40 | 0.40 |
| 3.0 | 0.08 | 0.11 | 0.14 | 0.16 | 0.18 | 0.20 | 0.23 | 0.24 | 0.25 | 0.25 | 0.25 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosik, A.K.; Mozelewska, K.; Musik, M.; Miądlicki, P.; Wilpiszewska, K. Influence of Illite and Its Amine Modifications on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Materials 2023, 16, 2879. https://doi.org/10.3390/ma16072879
Antosik AK, Mozelewska K, Musik M, Miądlicki P, Wilpiszewska K. Influence of Illite and Its Amine Modifications on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Materials. 2023; 16(7):2879. https://doi.org/10.3390/ma16072879
Chicago/Turabian StyleAntosik, Adrian Krzysztof, Karolina Mozelewska, Marlena Musik, Piotr Miądlicki, and Katarzyna Wilpiszewska. 2023. "Influence of Illite and Its Amine Modifications on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives" Materials 16, no. 7: 2879. https://doi.org/10.3390/ma16072879
APA StyleAntosik, A. K., Mozelewska, K., Musik, M., Miądlicki, P., & Wilpiszewska, K. (2023). Influence of Illite and Its Amine Modifications on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Materials, 16(7), 2879. https://doi.org/10.3390/ma16072879







