1. Introduction
Silica-reinforced tire tread rubber compounds have been growing in use since their successful introduction to improve tire performance in order to achieve fuel-efficient and safe-driving tires [
1]. However, the improvement in the compatibility with and good dispersion of silica in a rubber matrix is challenging in compound processing. This is due to the different polarity causing incompatibility between silica and nonpolar rubber, i.e., natural rubber (NR), styrene-butadiene rubber (SBR), and butadiene rubber (BR), which are mostly used in tire treads. To enhance the compatibility and good dispersion of silica in these rubber matrices, bifunctional organosilanes are essential ingredients in silica-filled compounds. In addition, a mixing temperature of about 135–155 °C [
2,
3,
4,
5] is necessarily applied to ensure a complete reaction of the silanol groups of the silane and the hydroxyl groups on the silica surface, so-called “silanization”. The main governing factors of tire tread performance are determined by the intrinsic properties of the rubbery components, the elastomers, of the tire compounds. This is an important consideration when choosing a material for utilization in a tire tread application. Natural rubber is preferentially employed in truck and aircraft tire tread applications due to its excellent mechanical properties, high elasticity, and low heat build-up. Numerous studies of silica-reinforced NR-based tire tread compounds have been published. Kaewsakul et al. [
4] reported the optimum mixing conditions for silica-filled NR to achieve desirable mechanical properties and the rolling resistance of tire tread compounds. The appropriate mixing process was necessarily operated at high temperatures in the range of 135–150 °C and a silica–silane–rubber mixing interval of 10 min. The properties of vulcanizates were found to correlate with the processing temperature during the mixing of such silica-filled NR compounds. It was concluded that to achieve optimal final properties, the mixing of silica-filled NR compounds needed to be operated at the earlier mentioned high temperatures in order to reach sufficient silanization and proper silica–silane–rubber coupling [
3,
4,
5,
6,
7]. However, the mixing process at such high temperatures is prone to the thermal–oxidative degradation of NR [
8,
9].
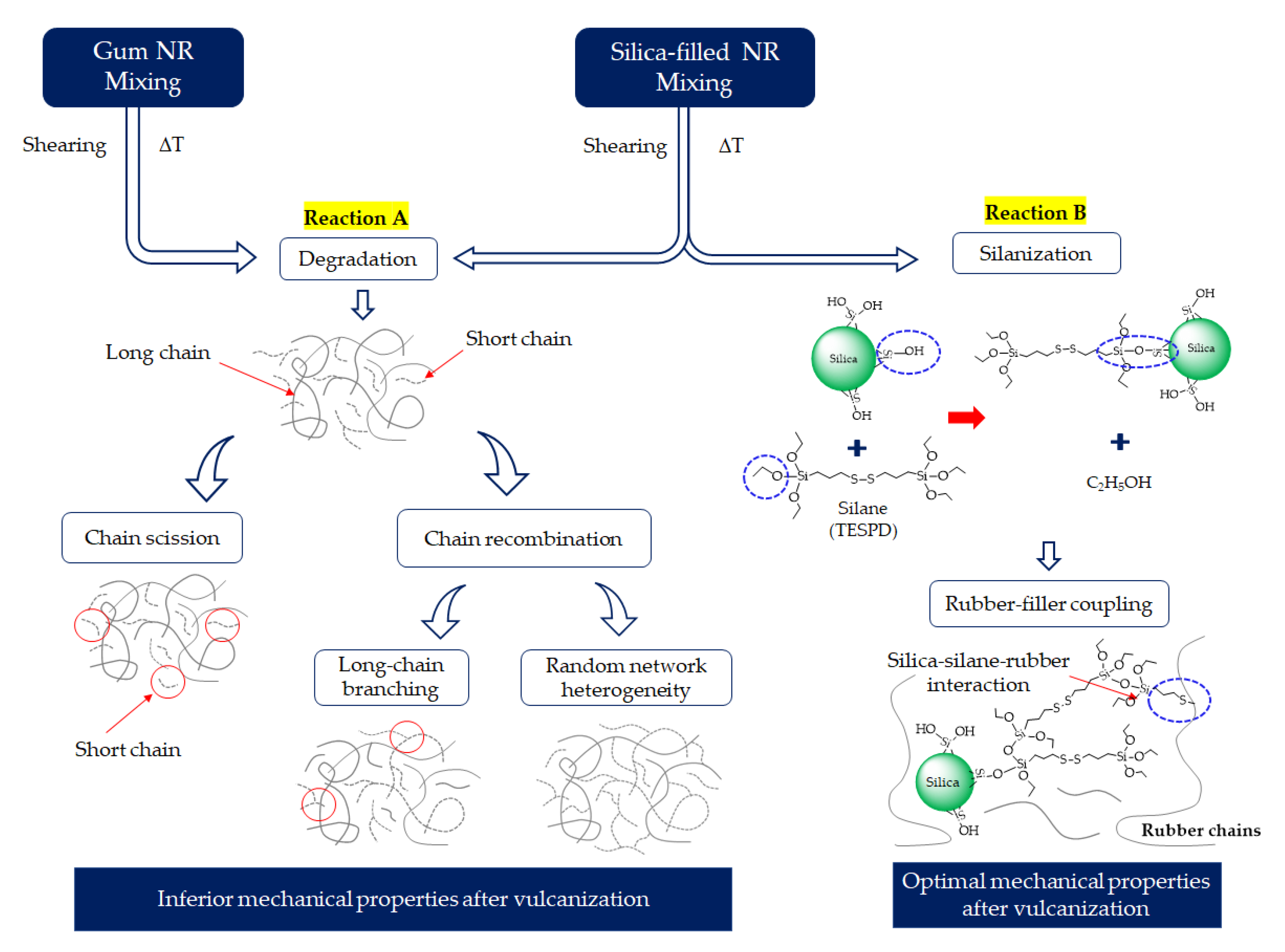
The degradation of NR is a free radical process that can be initiated by several modes, i.e., oxidative, thermal, and chemical. During mixing, the degradation of NR can occur due to mechanical effects: high shear forces during mixing generate heat and initiate rubber chain breakage. Consequently, unstable chain radicals are formed. These radicals are the initiators for further degradation and lead to chain scission, resulting in a decrease in the molecular weight and so deteriorated mechanical properties. All associated factors, chemicals, oxygen, temperature, and time, can contribute to the degradation of NR [
10]. Kumar et al. [
8] investigated the change in the molecular properties of NR films through thermo-oxidation. The extension of the exposure time of NR films at 100 °C led to a decrease in the molecular weight as a result of chain scission. A study on unfilled NR degradation by the evaluation of physical property changes reported that reduced tensile and tear strength were also due to a decreased NR molecular weight [
11]. Additionally, rubber degradation via decreased rubber molecular weight was observed with increasing mastication time and reaction time during the mechanical, chemical, and photooxidative degradation of epoxidized natural rubber (ENR) [
12]. The degradation of unfilled NR compounded at a high mixing temperature of 180 °C was studied. It was reported that an increase in the mixing time from 5 to 10 min at a certain mixing temperature led to the significant degradation of such unfilled NR compounds [
9].
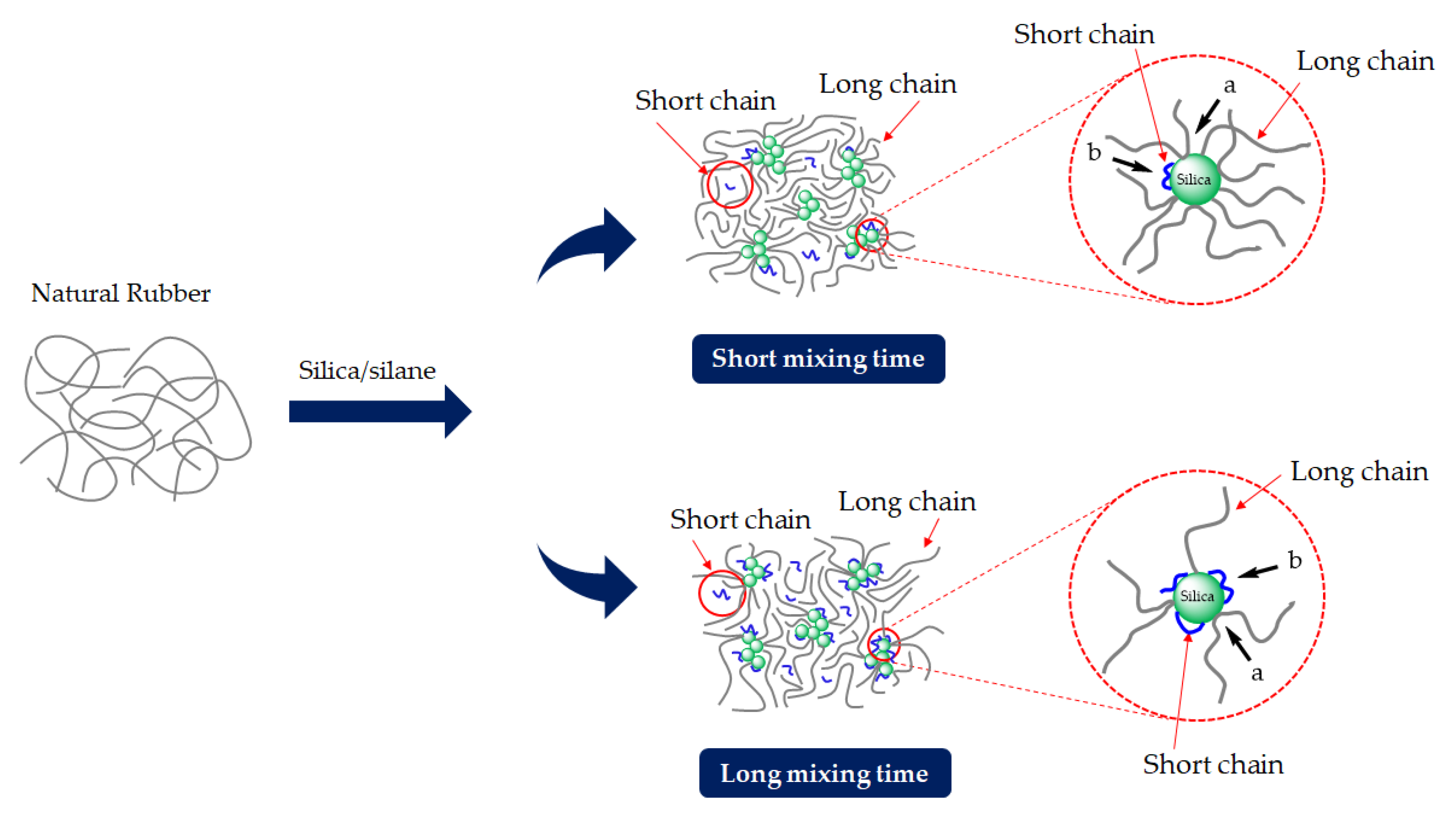
In our previous work, degradation phenomena during mixing were reported for silica-filled NR compounds with the main governing factor being temperature [
6]. The degradation during mixing was analyzed by changes in the dynamic mechanical response. At high discharge/dump temperatures above 150 °C, the Mooney stress relaxation rate and dynamic mechanical properties measured in a frequency sweep indicated a significant molecular chain modification that caused a heterogeneous network and the deterioration of the mechanical properties of the vulcanizates [
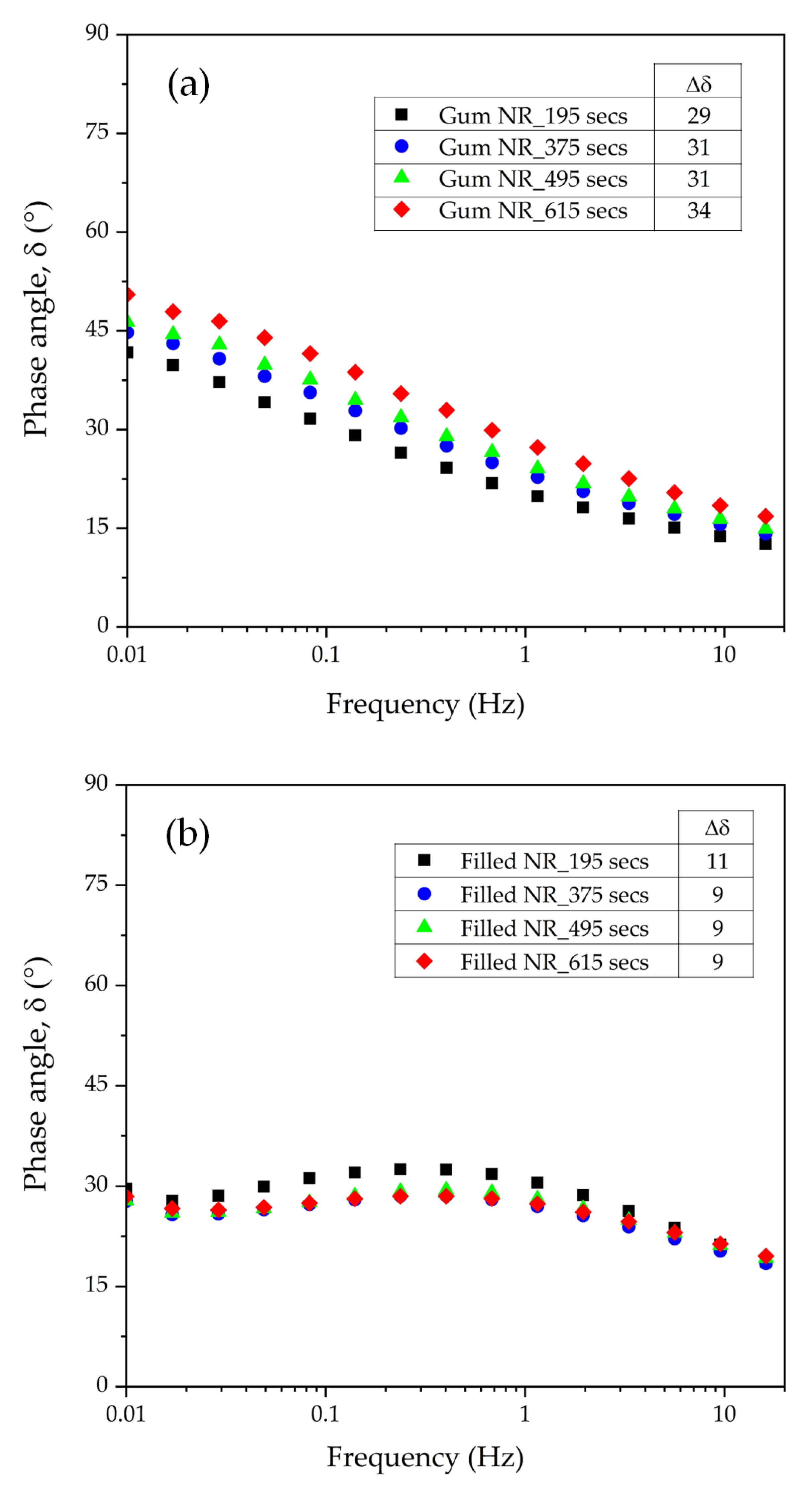
6]. In addition, other mixing conditions, i.e., shearing force and mixing time, may also contribute to rubber degradation. Especially, a long mixing time at the typical elevated temperature of silanization may lead to more radicals generated, thus influencing rubber degradation and the mechanical properties of rubber vulcanizates. Therefore, the present work aims to investigate the rubber degradation of silica-filled NR with different silanization times by using Gum (unfilled) NR compounds as references. Various techniques are applied to monitor rubber degradation, including structural changes, viscoelastic responses, and the creation of long-chain branching by using the technique of delta-delta (Δδ) as the different phase angle at a low (i.e., 0.01 Hz) and high frequency (i.e., 16 Hz). In addition, tire-related properties, i.e., the mechanical and dynamic characteristics of the silica-filled NR vulcanizates, are investigated.
2. Materials and Methods
2.1. Materials
Standard Malaysian Rubber 10 (SMR10), the NR grade mostly used in tire treads, was supplied by WEBER & SCHAER GmbH & Co. KG, Hamburg, Germany. Silica ULTRASIL 7005 with CTAB (Cetyl-Trimethyl-Ammonium Bromide) and BET (Brunauer–Emmett–Teller) specific surface areas of 171 and 190 m2/g, respectively, and the silane coupling agent bis-(3-TriEthoxySilylPropyl)-Disulfide (TESPD) were both obtained from Evonik, Essen, Germany. Treated Distillate Aromatic Extract oil (TDAE oil) (Vivatec 500) was obtained from Hansen & Rosenthal, Hamburg, Germany. DiPhenyl Guanidine (DPG), N-Cyclohexyl-2-Benzothiazyl Sulfenamide (CBS), 2,2,4-TriMethyl-1,2-dihydroQuinoline (TMQ), and N-(1,3-dimethylbutyl)-N′-Phenyl-p-PhenyleneDiamine (6PPD) were supplied by Lanxess Rhein Chemie GmbH, Cologne, Germany. Zinc oxide (ZnO), stearic acid, and sulfur were obtained from Merck KGaA, Darmstadt, Germany. All materials were used as received.
2.2. Compound Formulations
Silica-filled NR compounds were prepared by using chemicals and curatives according to typical truck tire tread formulations as given in
Table 1. An unfilled NR compound or so-called Gum NR was prepared as a reference.
2.3. Mixing
2.3.1. Silica-Filled NR Compounds
The rubber and chemicals were mixed in a lab-scale internal mixer with a mixing chamber of 50 cm
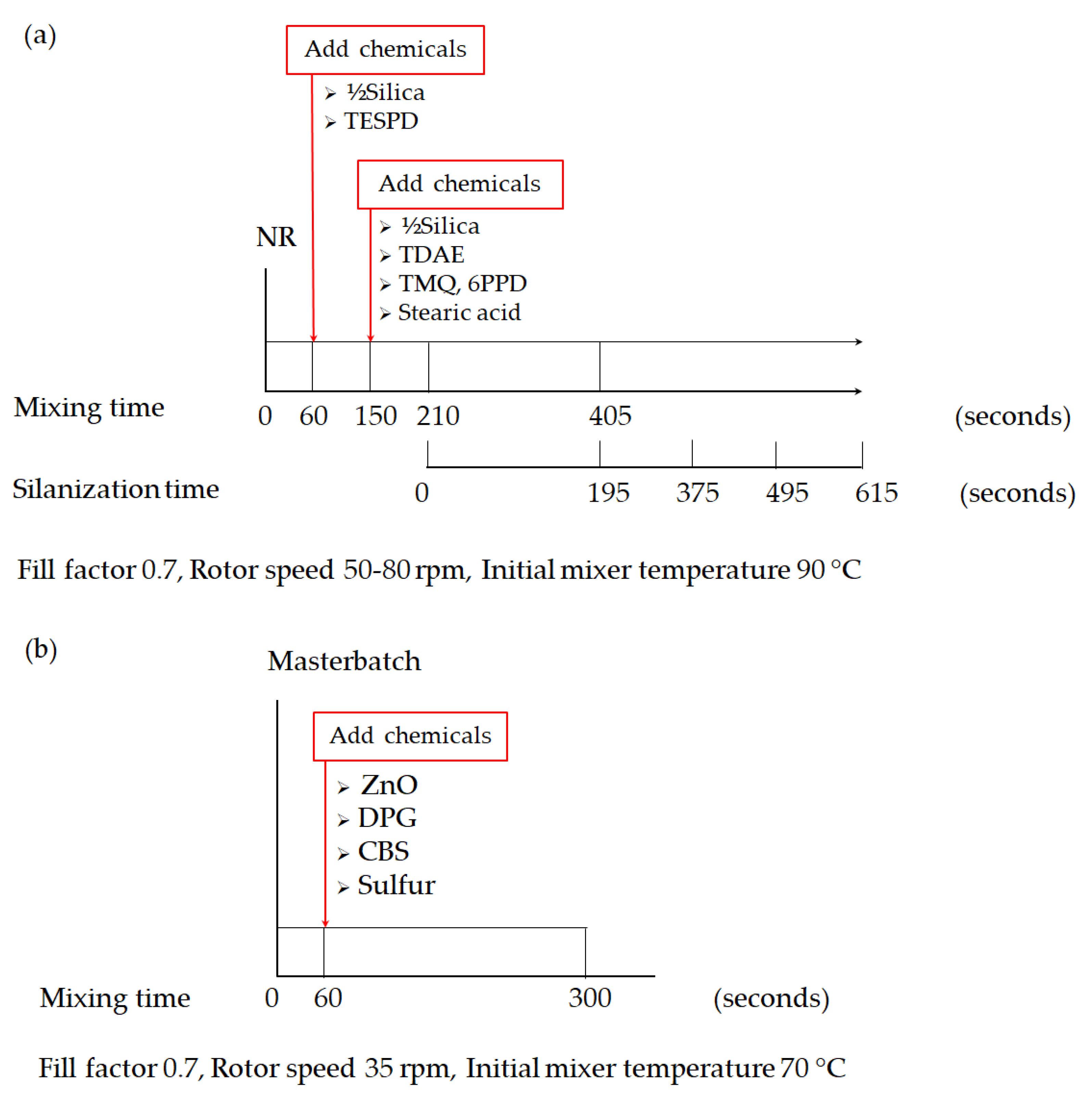
3 (Brabender GmbH & Co. KG, Duisburg, Germany) by using a fill factor of 0.7. The initial mixer temperature was set at 90 °C with varying rotor speeds from 50 to 80 rpm to achieve dump temperatures of 145–150 °C. The mixing was conducted in 2 steps according to the procedures shown in
Figure 1. In the first step, the nonproductive step, NR was masticated for 60 s, and then the first half of the silica and all of the silane were loaded. After 150 s of mixing, another half of the silica together with TDAE, stearic acid, TMQ, and 6PPD were loaded. The mixing continued for 210 s when the mixing temperature reached 140 °C and the silanization started. To ensure the completion of silanization, the mixing was continued for another 195 s to reach a total mixing time of 405 s. To examine the effect of longer mixing times at the typical elevated temperature of silanization in this work, the typical silanization time was extended to 375, 495, and 615 s. As shown in
Figure 1a, after reaching the total mixing time, the compound was then sheeted out on a two-roll mill to cool down in the final procedure of the nonproductive step. Subsequently, the productive mixing step was carried out at an initial mixer temperature of 70 °C to avoid premature vulcanization or scorching during the process in the same internal mixer. After the mastication of the masterbatch for 60 s, the curatives ZnO, DPG, CBS, and sulfur were added to the mixer. This productive mixing step was completed after 300 s as shown in
Figure 1b at an approximate dump temperature of 85 ± 5 °C. Finally, the compounds were sheeted out on a two-roll mill.
2.3.2. Gum Reference Compounds
To be compared with the silica-filled NR, an unfilled or Gum NR was prepared and used as a reference in this study. The Gum NR compounds were also prepared in 2 steps of mixing following the mixing procedures applied for silica-filled NR as shown in
Figure 1a, using the selected mixing conditions. Nevertheless, without the filler involved in the 1st mixing step, the dump temperature of the Gum compounds is lower than that of silica-filled NR. Therefore, the mixer temperature setting was adjusted to reach similar dump temperatures as found for the silica-filled NR compounds. The total mixing times of the Gum compounds were extended to be equal to the total mixing times after prolonged silanization times in the silica-filled compounds. Therefore, the term “prolonged mixing times” is used in the Gum compounds as well.
2.4. Characterization
2.4.1. Mooney Viscosity and Mooney Stress Relaxation
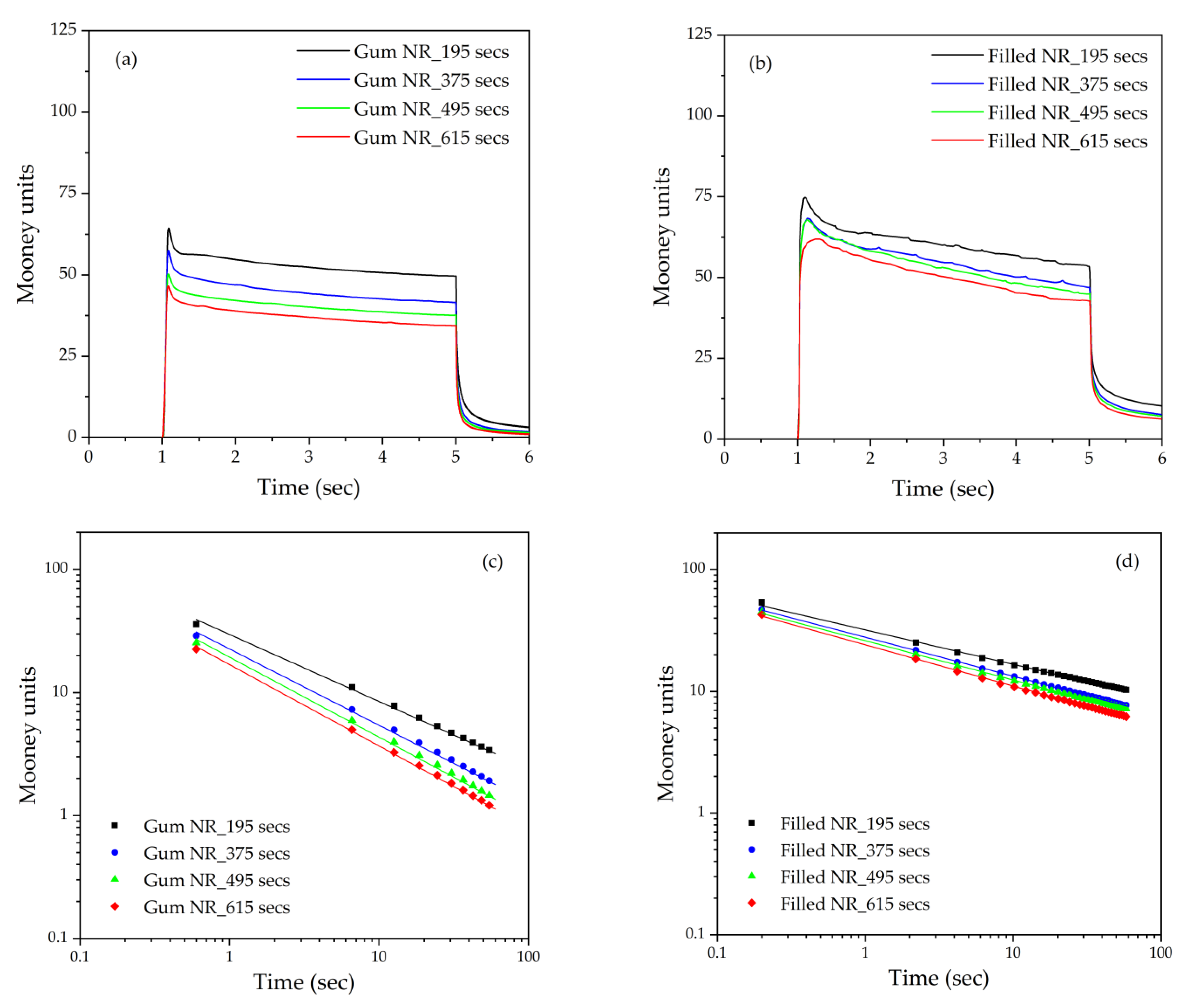
The nonproductive compounds were cooled down and left overnight at room temperature. The Mooney viscosity and Mooney stress relaxation were analyzed by using a Mooney viscometer (MV200VS, Alpha Technologies, Hudson, OH, USA) according to ASTM D1646 [
14]. The Mooney viscosity was tested at 4 min after preheating for 1 min and is reported as ML 1+4 (100 °C) (large rotor) for Gum and MS 1+4 (100 °C) (small rotor) for the filled NR. Note that due to the more common large rotor, some silica-filled compounds gave an initial value above the limit of the Mooney viscometer. Thus, the small rotor was used for all the filled compounds for consistency reasons. In the Mooney stress relaxation test, when the rotor of the Mooney viscometer stops after the completion of the viscosity test, the torque is detected for another 1 min. The stress relaxation of the compounds is described through a power-law model, as shown in Equation (1):
where
M = Mooney units (torque) after the completion of the viscosity test,
t = relaxation time (s),
k = a constant equal to the torque in Mooney units at 1 s after the disk is stopped,
a = exponent that determines the rate of stress relaxation.
Then, Equation (1) can be expressed in a log–log correlation, as given in Equation (2):
According to a log–log plot of Mooney units against time, the rate of stress relaxation is defined by the slope (
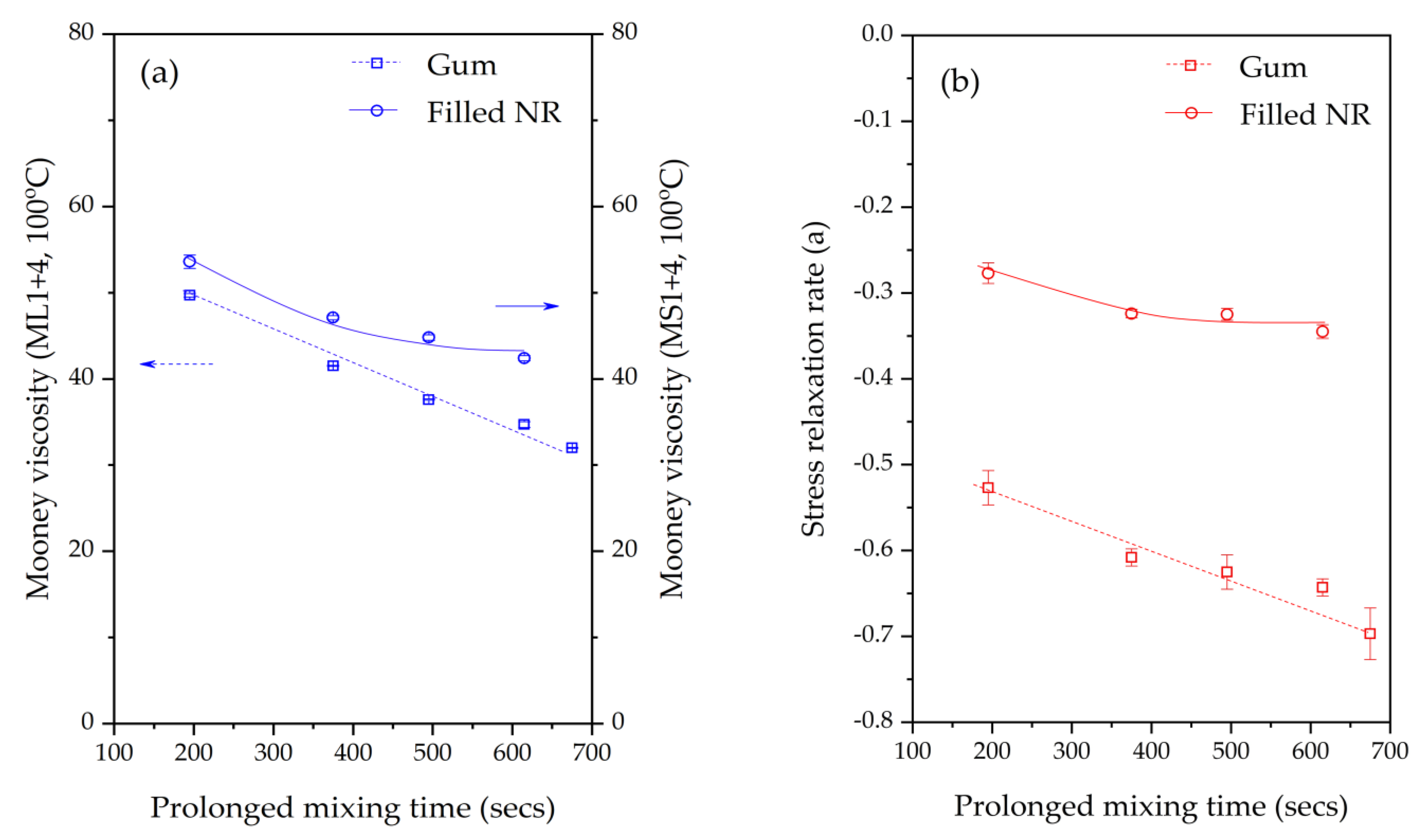
a). The Mooney stress relaxation is a combination of elastic and viscous responses. A higher value of the relaxation rate indicates an increase in the viscous/elastic ratio. Numerous studies of raw rubber and rubber compounds reported that the rate of stress relaxation correlates with the structural characteristics of the rubber, such as the molecular weight, molecular weight distribution, chain branching, and gel content [
6,
15].
2.4.2. Payne Effect
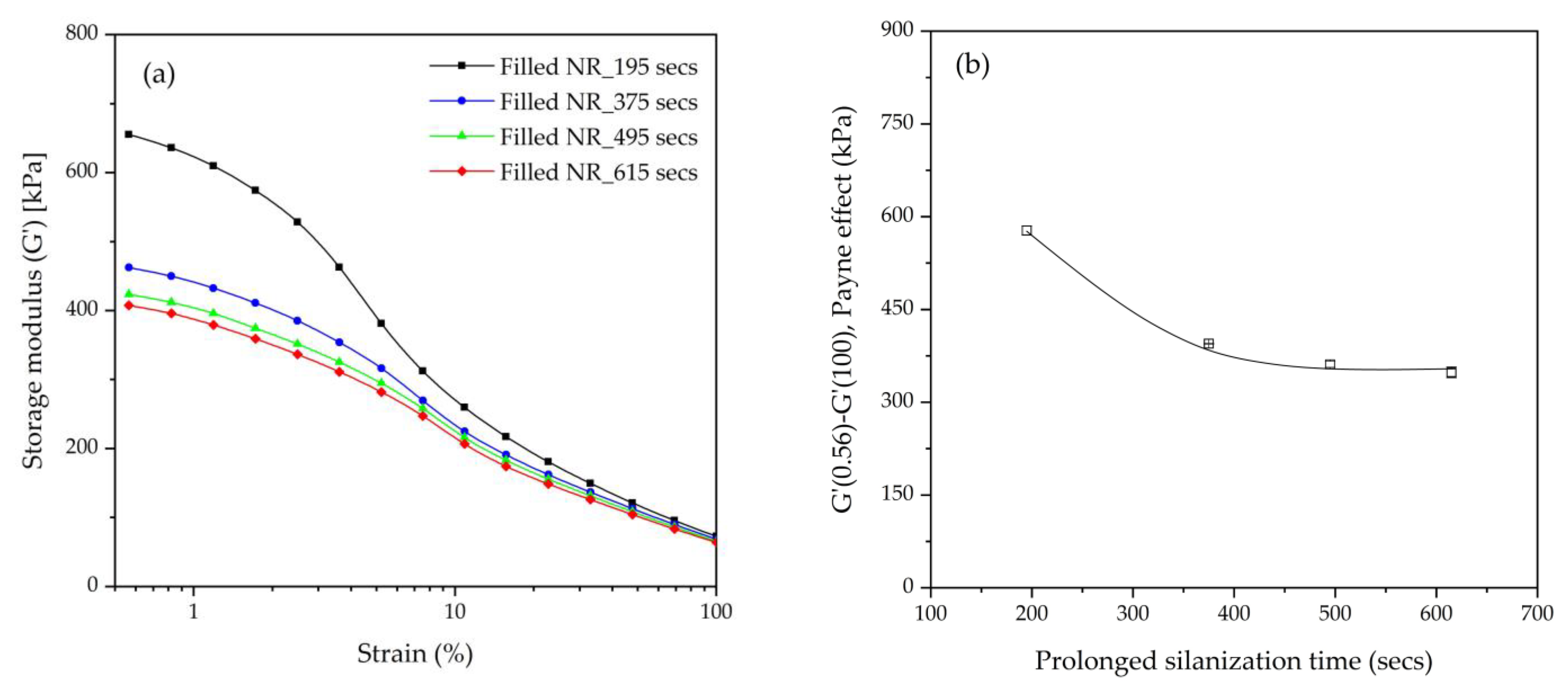
The storage shear moduli (G’) of the uncured silica-filled NR compounds obtained from the nonproductive mixing step were evaluated by using an RPA elite dynamic spectrometer (TA Instruments, New Castle, DE, USA) at a temperature of 100 °C, frequency of 0.5 Hz, and various strains in the range of 0.56–100%. The Payne effect, which indicates filler–filler interactions, was calculated by the differences in storage shear moduli at a low strain (0.5%) and high strain (100%) [
16].
2.4.3. Bound Rubber Content (%)
In total, 0.20 g by weight of the uncured nonproductive compounds were immersed in 20 mL of toluene for 3 days at room temperature in an air atmosphere. Thereafter, the sample was removed from the toluene, dried at 80 °C in a vacuum oven for 24 h, and weighed. The
bound rubber content (BRC) was calculated according to Equation (3) [
17]:
where
Wext = the weight of the sample after extraction,
Wf = the weight of silica in the original sample,
Wp = the weight of the polymer in the original sample.
2.4.4. Dynamic Properties as a Function of Frequency
The dynamic properties of the uncured nonproductive compounds were analyzed by using the RPA elite dynamic spectrometer as well. A frequency sweep mode was used at various frequencies in the range of 0.1 to 16 Hz with a strain amplitude of 5% and at a temperature of 100 °C. At this strain magnitude, the linear viscoelastic regime of the material was observed. The change in the phase angle (δ) with frequency was employed to characterize long-chain branch formation as described by Booij [
18].
2.4.5. Dynamic Properties as a Function of Temperature
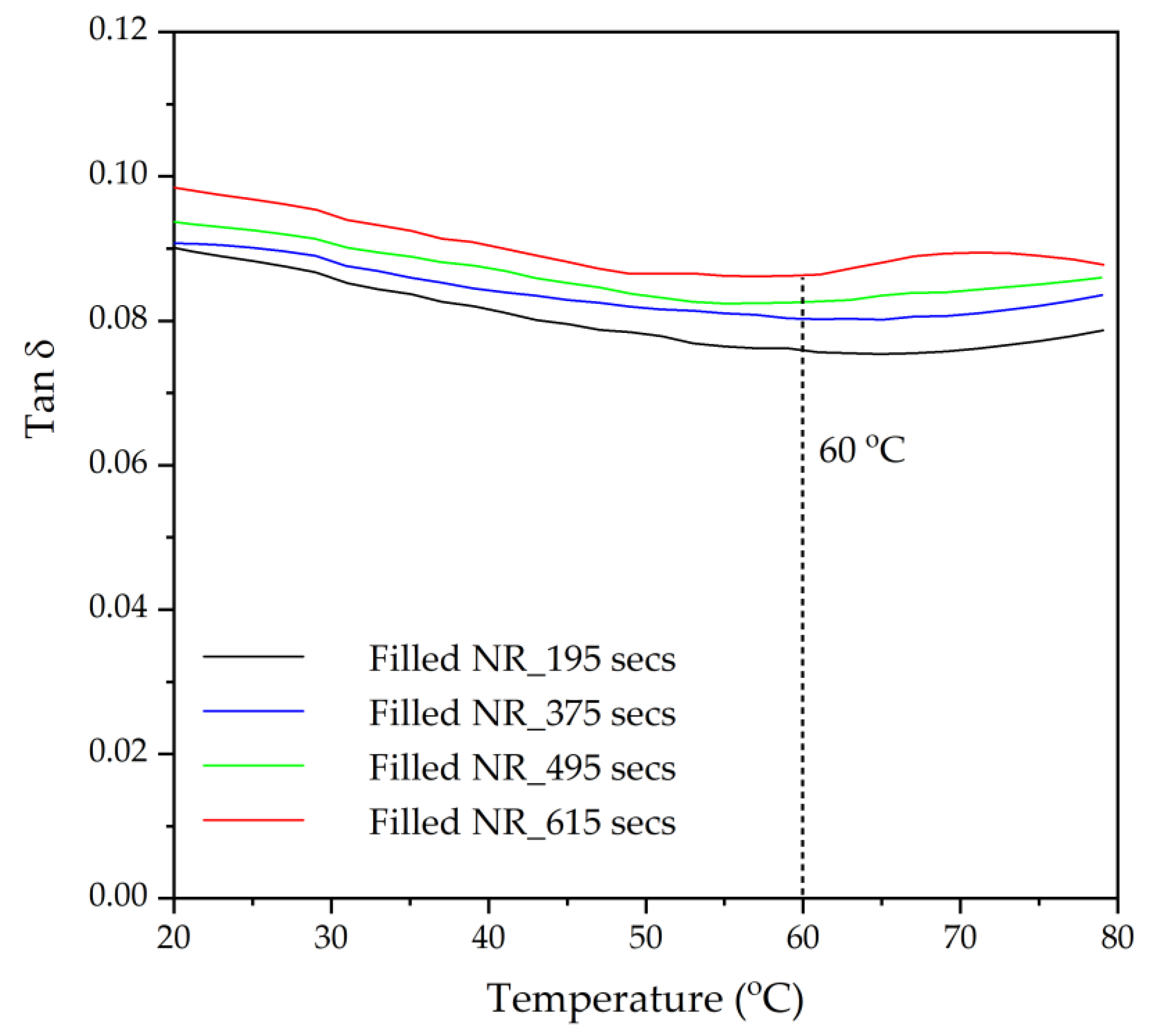
Dynamic mechanical properties of the rubber vulcanizates were characterized in tension mode by using a DMA Eplexor 2500 (NETZSCH-Gerätebau GmbH, Ahlden, Germany) with various temperatures in the range of 20 °C to 80 °C at a constant frequency of 10 Hz and a 0.1% strain. The rubber vulcanizate sample dimensions were 6 × 4 × 2 mm
3. The tangent of the phase angle (tanδ) at 60 °C was taken as indicative of the rolling resistance of the tire treads [
19].
2.4.6. Tensile Properties
The Gum NR and silica-filled NR compounds were vulcanized into 2 mm thick sheets by using a Wickert Laboratory Press (WLP1600, Wickert Maschinenbau GmbH, Landau in der Pfalz, Germany) at 150 °C and 100 bar by using their optimum cure times (t
c,95). The vulcanized sheets were die-cut to dumbbell-shaped specimens (type 2). Tensile tests were carried out with a Zwick tensile tester (model Z1.0/TH1S) at a crosshead speed of 500 mm/min according to ASTM D412 [
20]. The tensile modulus at a 300% strain as well as stress and elongation at break were analyzed.
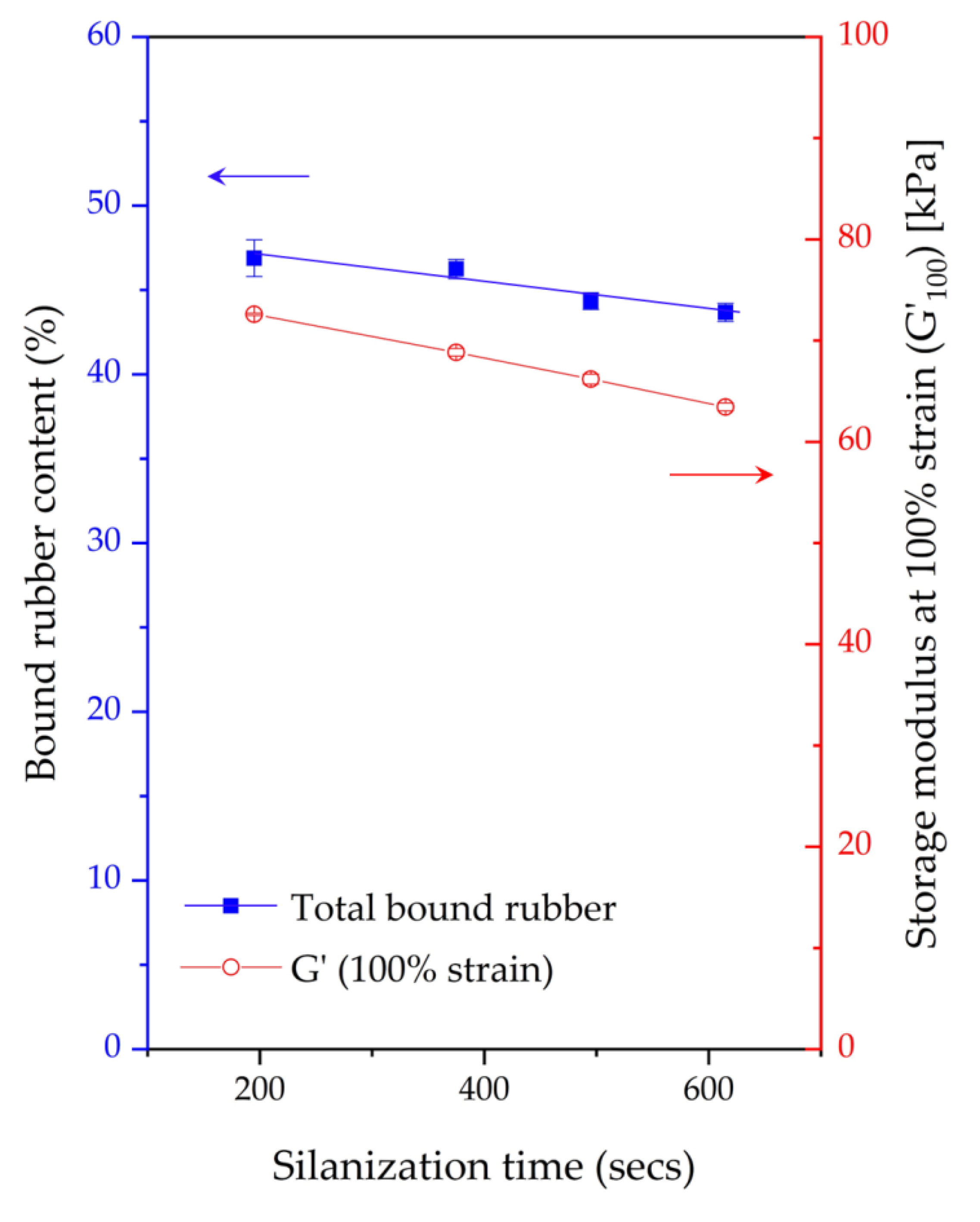
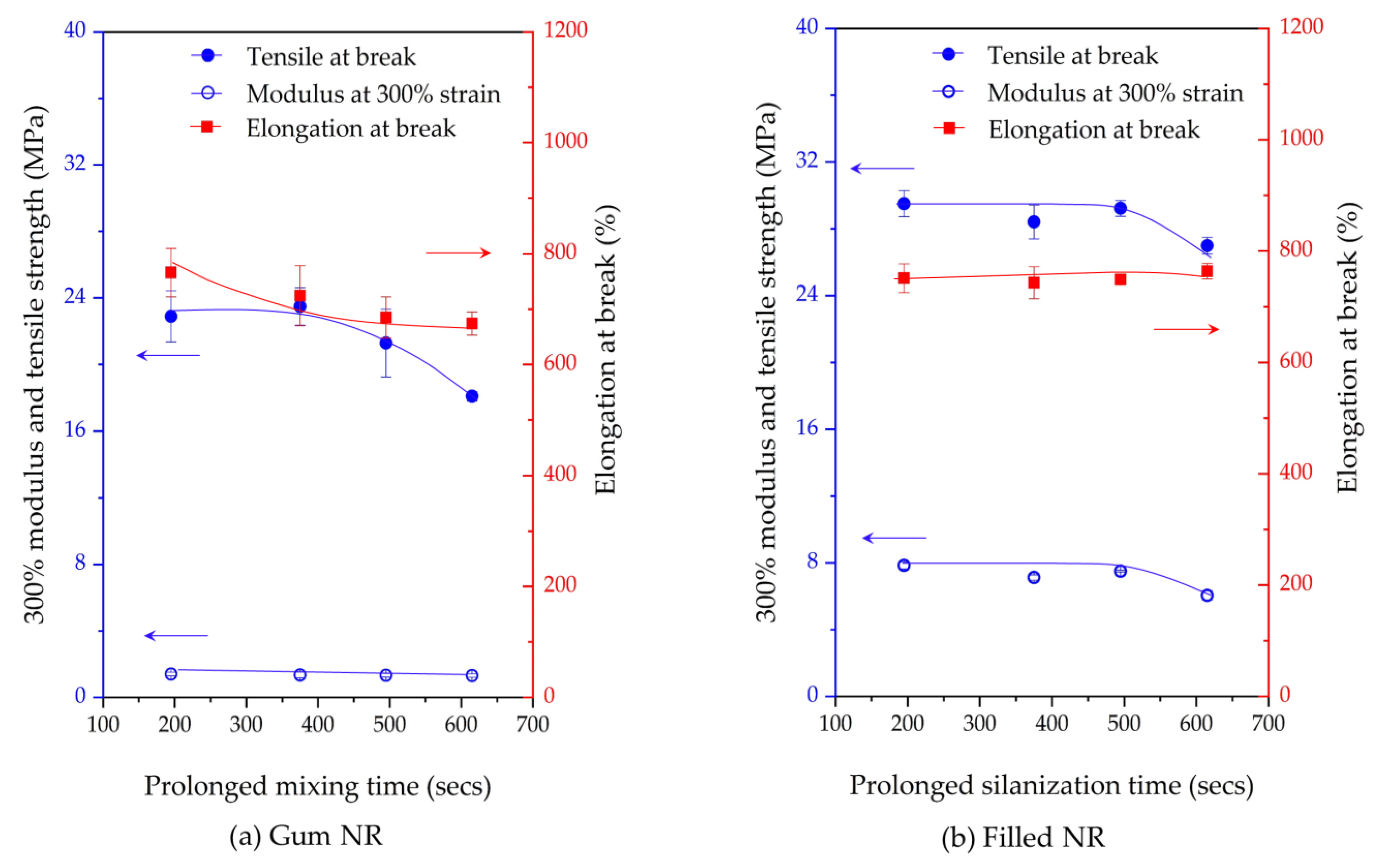
4. Conclusions
The influence of prolonged silanization on the degradation of Gum and silica-filled NR due to molecular chain modification was analyzed by the changes in dynamic responses. This study elucidates the proper silanization time needed for the mixing of silica-filled NR to minimize rubber degradation. The results illustrate an appropriate silanization time in the range of 375–495 s to provide sufficient silanization as well as a permissible degree of rubber degradation. For Gum NR, long durations of mixing during nonproductive compounding cause changes in the properties of the vulcanized rubber due to chain scission. The degradation or chain scission results in low molecular weight fractions as evidenced by a reduced Mooney viscosity and an increased Mooney stress relaxation rate for long mixing times. This is accompanied by a rise in Δδ, which indicates higher chain mobility. A different trend is observed for silica-filled NR, whose properties reveal a balance in degradation and silanization/coupling reactions on viscous/elastic responses at extended silanization times over 375 s. Drastic changes in the Mooney viscosity, Mooney stress relaxation, Payne effect, and Δδ demonstrate a combination of degradation and silanization at the beginning of the silanization period. But thereafter, balanced reactions between degradation and premature crosslinking or branch formation due to coupling reactions are noticed as indicated by the leveling off of those properties. The bound rubber content and storage modulus at 100% strain still showed an increase in short chains with continually prolonged silanization times. Therefore, the mechanical properties of the filled NR vulcanizates slightly decrease with an increasing silanization time up to 495 s but are more pronounced with a further increase over 495 s. The strongly reduced tensile strength and modulus at 300% strain are therefore most likely due to abundant short chains and the resulting network heterogeneity. Moreover, the higher degree of chain scission with a longer duration of silanization negatively influences the dynamic properties at 60 °C, indicative of the rolling resistance of a tire tread made from silica-reinforced NR.