Influences of Friedel’s Salt Produced by CaO-Activated Titanium-Extracted Tailing Slag on Chloride Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental and Program
2.3. Characterization of Solidified Samples
3. Results and Discussion
3.1. Chloride Binding Content and UCS of Solidified Samples
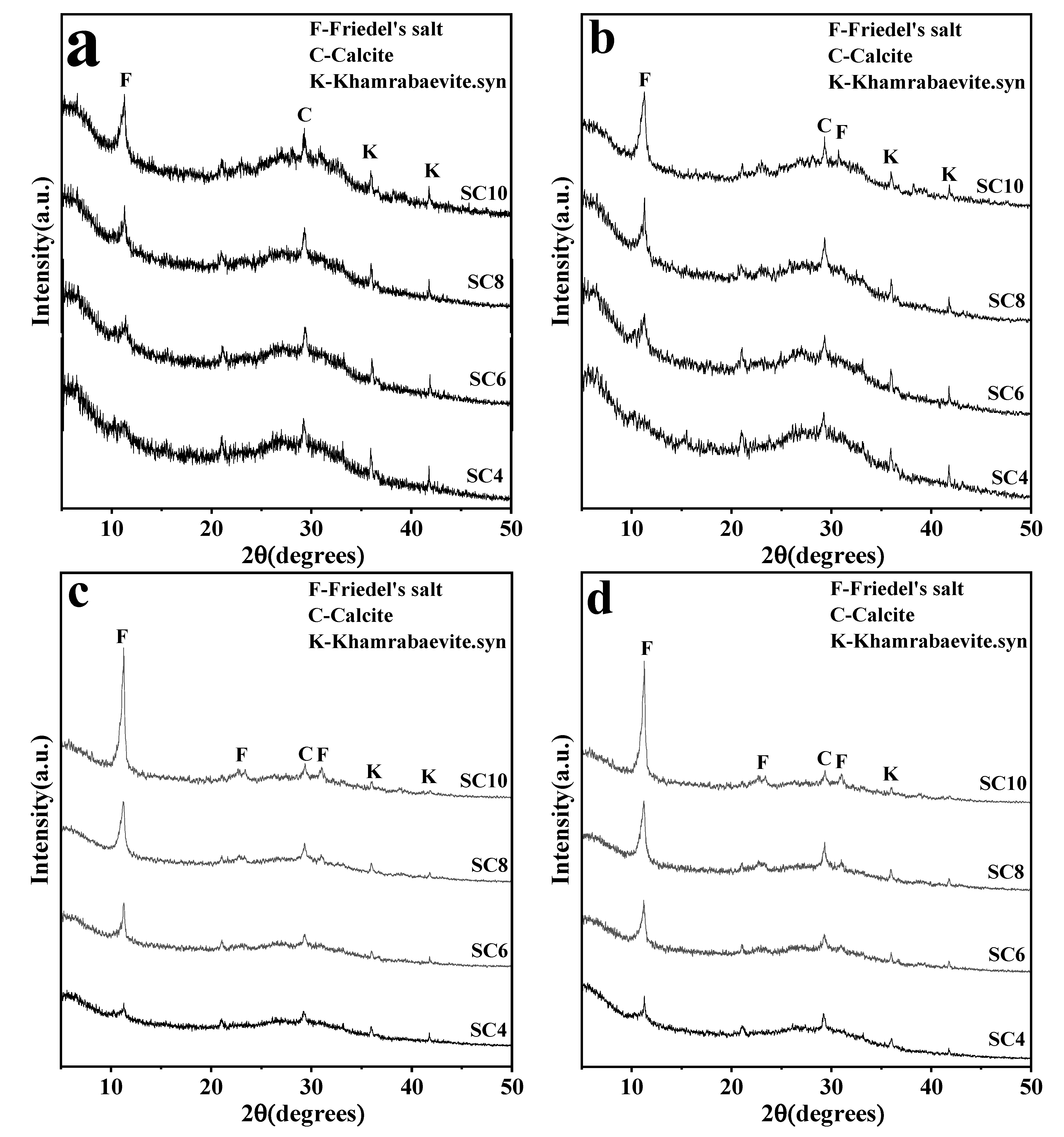
3.2. Phase Characteristics of Solidified Samples
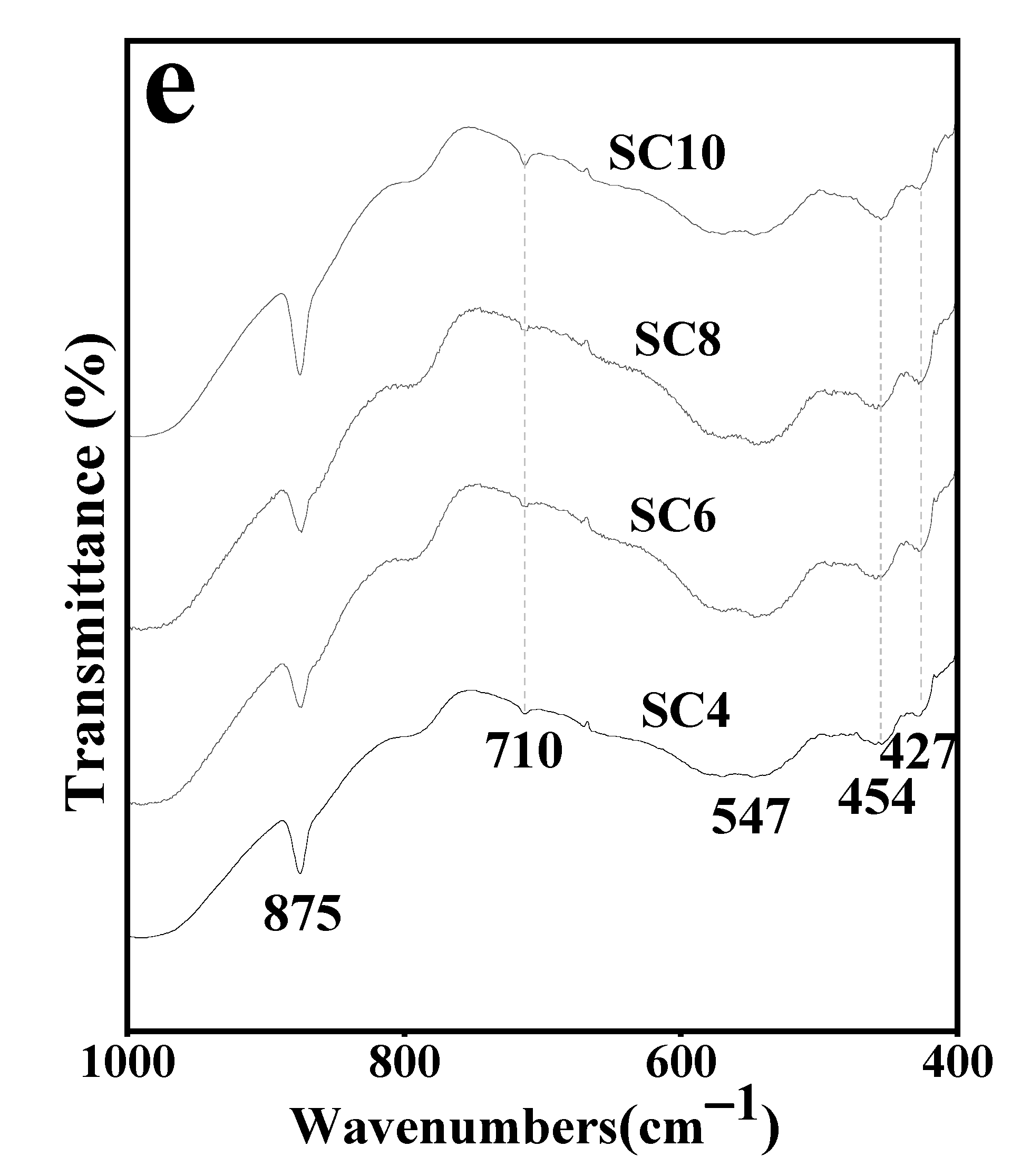
3.3. Infrared Spectral Characteristics of Hardened Body
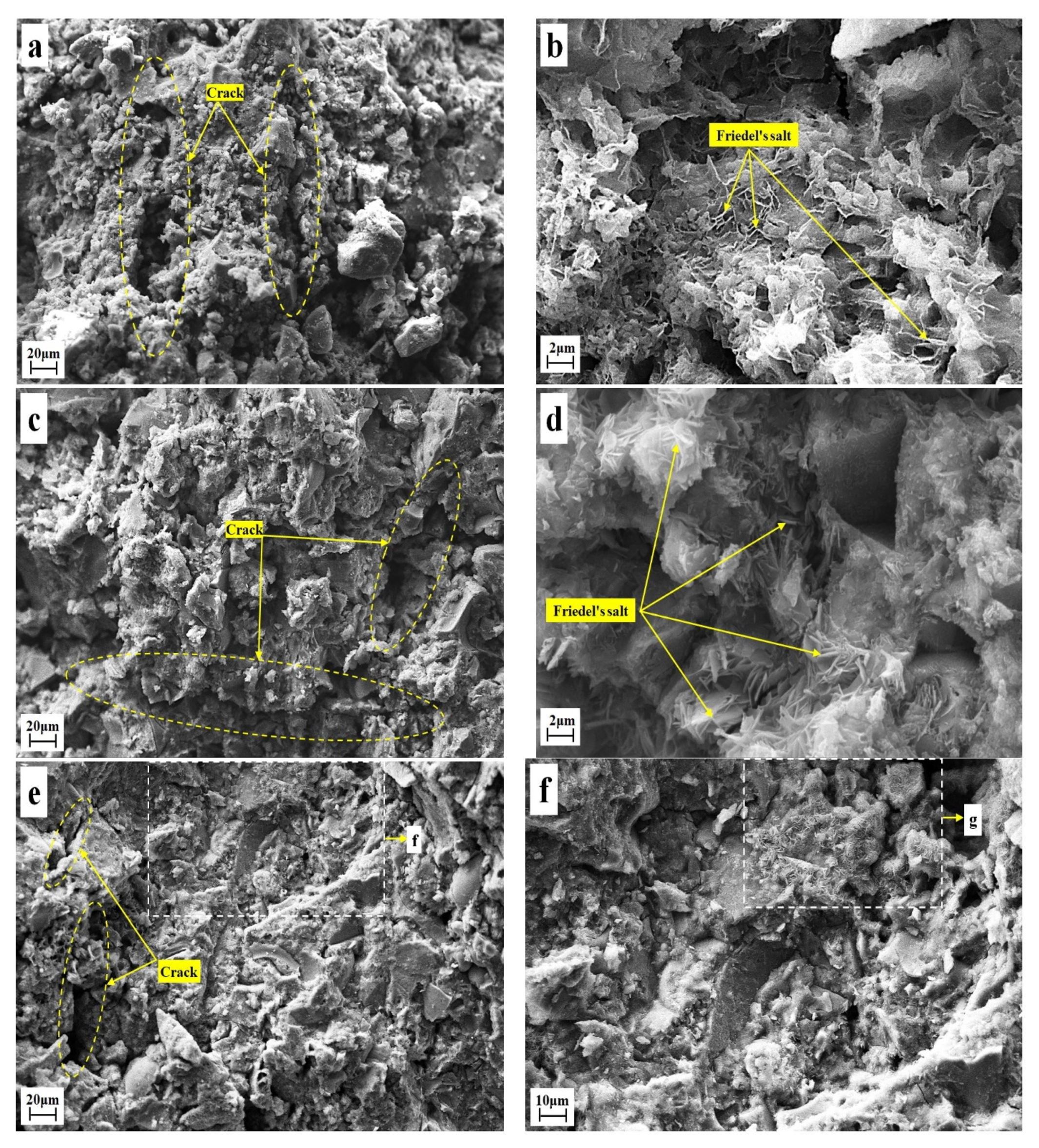
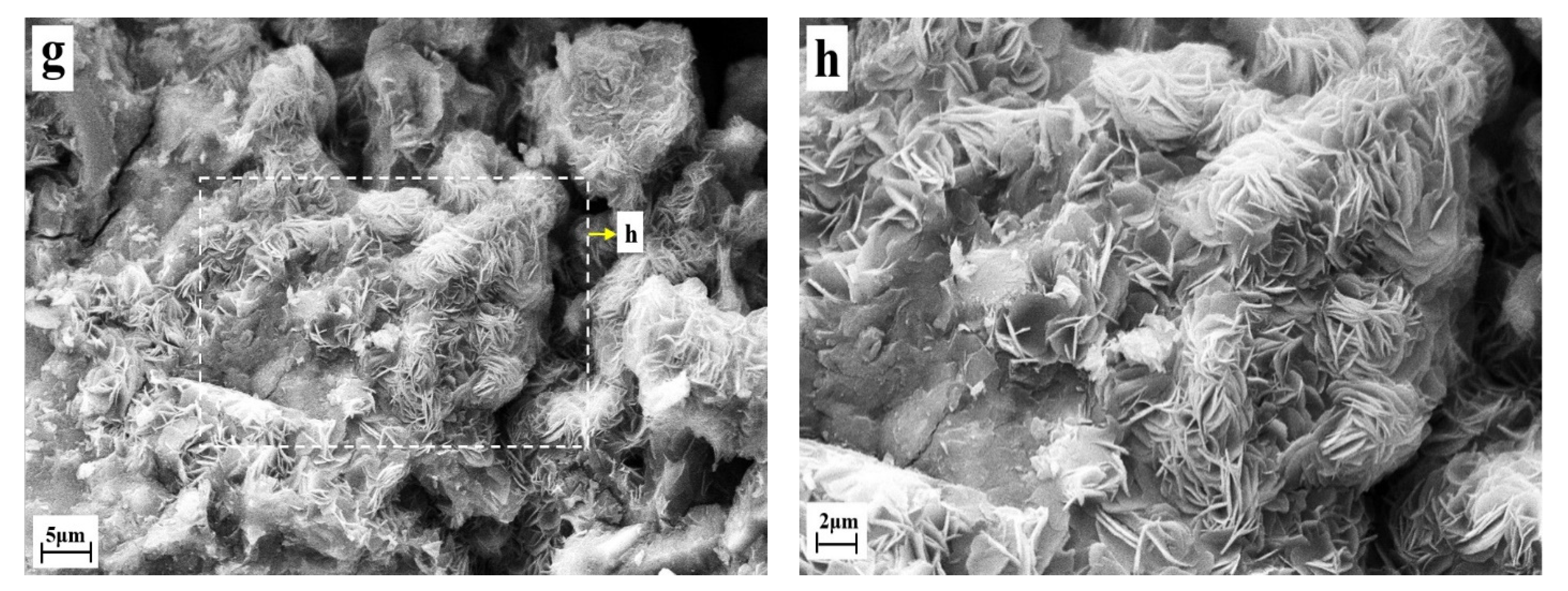
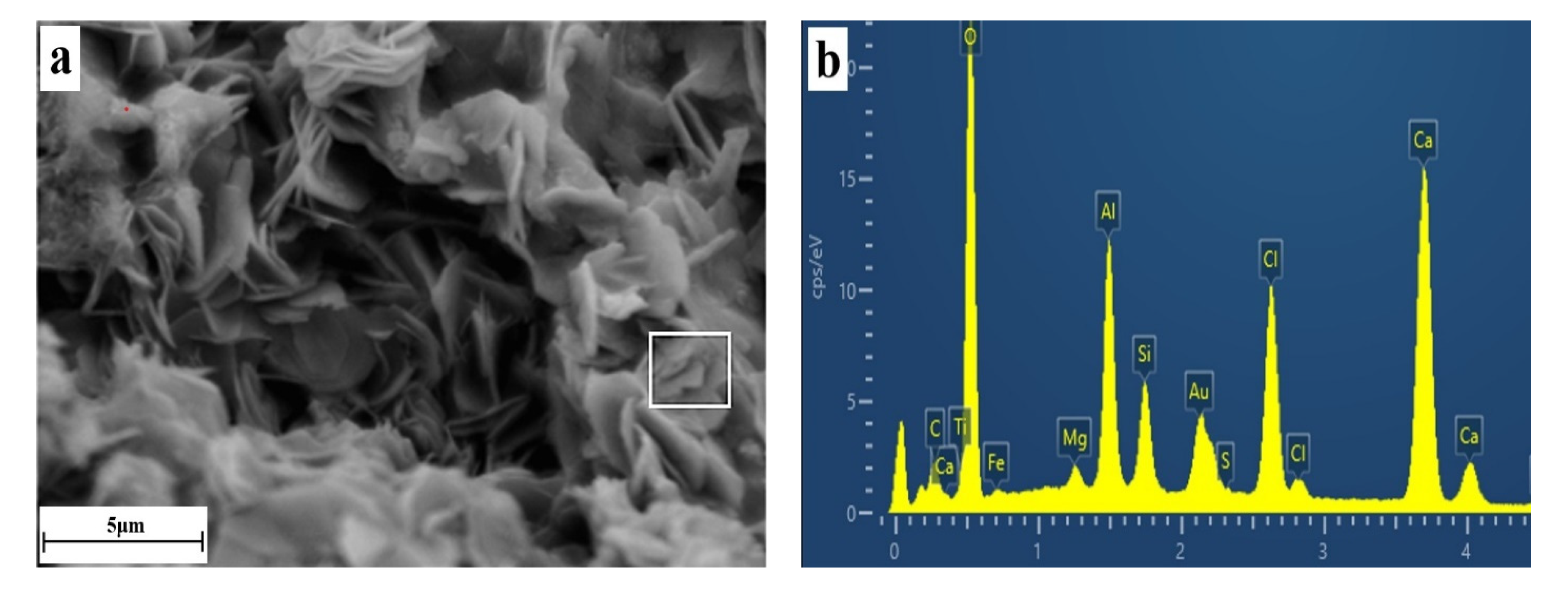
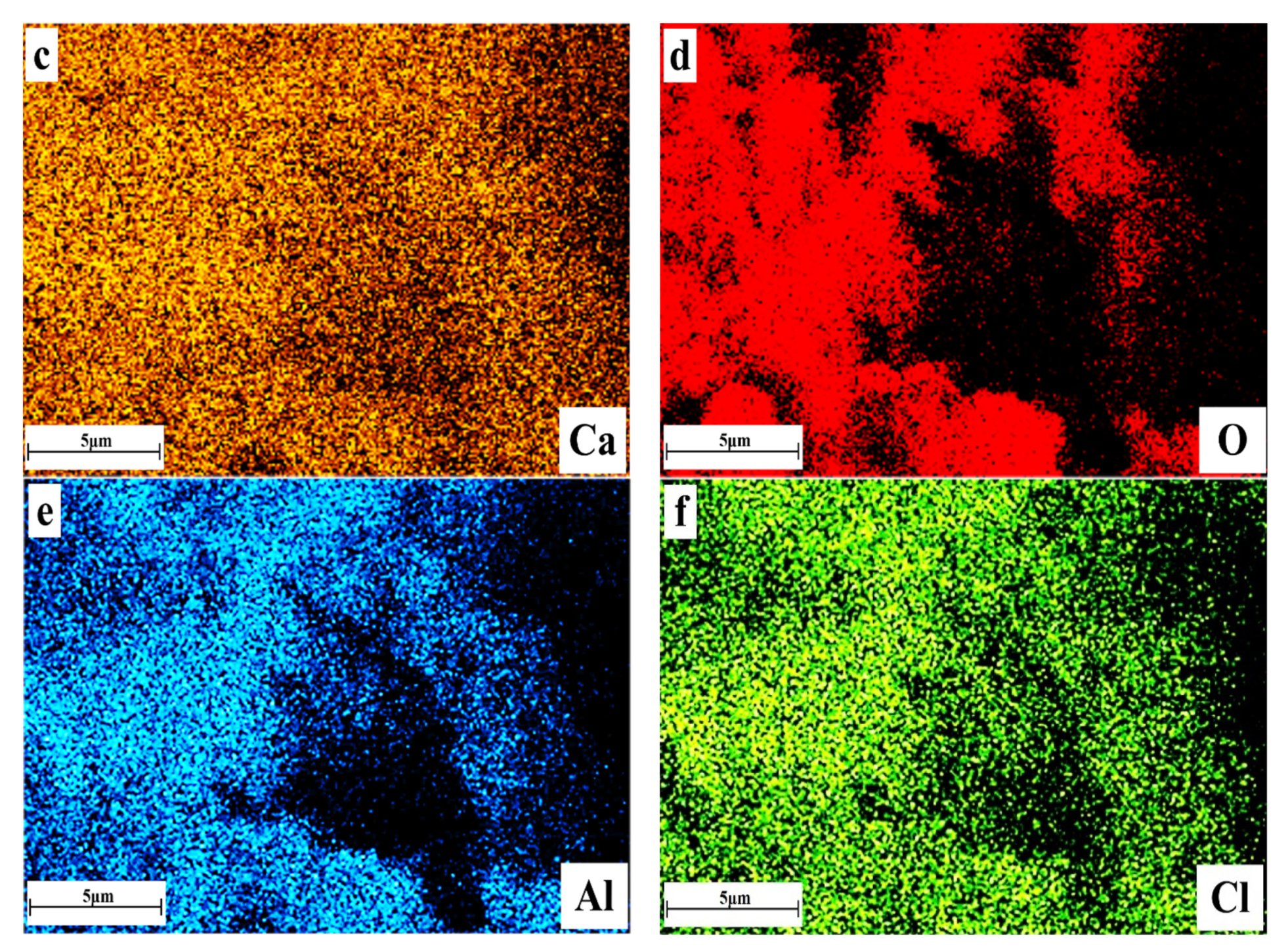
3.4. Microscopic Morphology of Solidified Samples
4. Conclusions
- (1)
- The solidified sample strength and chloride binding capacity of the CaO-activated TETS cementitious material were greatly affected by the CaO content and curing age. Increasing the CaO content and extending the curing age will not only help to improve the strength of the solidified sample, but also help to enhance the chloride binding capacity, especially within the curing age of 7 days. The growth rate of the strength and the chloride binding rate was the largest. The growth rate slowed down beyond 7 days. The higher the CaO content, the slower the reduction rate.
- (2)
- The main new phase produced in the solidified sample was FS and calcite. There were no C-S-H and portlandite diffraction peaks, which were commonly found in CaO-activated GGBS cementing material. The chloride binding in the solidified sample was mainly the chemical binding by FS. The diffraction peak intensity of FS was positively correlated with the content of CaO and the curing age. However, the diffraction peak of calcite was always weak, and its strength was less affected by the CaO content and the curing age.
- (3)
- FS is flaky and mainly existed in the pores of the solidified sample, and its production and development degree were affected by the content of CaO and the curing age. The higher the content of calcium oxide, the longer the curing age, the more FS production in the pores, and the more perfect the crystal development. FS can not only chemically bind chloride ions but can also optimize the pore structure of the solidified sample. It had two effects: chemically binding the chloride ions and improving the strength.
- (4)
- Since the strength of the solidified sample with 6 wt% CaO reached more than 5 MPa at 7 days and more than 11 MPa at 28 days, TETS can be used for pavement base, baking-free brick, and other engineering projects with low strength requirements and not sensitive to chloride ions. This study is helpful to realize the resource utilization of TETS, and can solve the problem that TETS only be stored at present. According to the law of strength and chloride binding capacity in this paper, it is necessary to increase the CaO content to more than 10 wt%. The addition of the activated alumina component may increase the production of FS and the chloride binding capacity. All the results presented here need to be further studied.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Chen, C.-X. Development Status of Global Titanium Resources Industry. Acta Geosci. Sin. 2021, 2, 245–250. [Google Scholar]
- Zhu, S.; Hu, J.; Zhang, C.; Li, S.; An, N. Study on optimizatio2n and mechanism of mechanical activation process of titanium-bearing blast furnace slag. J. Mater. Res. Technol. 2022, 19, 3130–3144. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Hu, Z.; Xie, X.; Yang, L. Properties and hydration behavior of Ti-extracted residues-red gypsum based cementitious materials. Constr. Build. Mater. 2019, 218, 610–617. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, G.; Yan, Y. Early hydration, mechanical strength and drying shrinkage of low-carbon alkali-activated Ti-extracted residues-fly ash cement and mortars. Constr. Build. Mater. 2021, 293, 123517. [Google Scholar] [CrossRef]
- Tao, M.; Lu, D.; Shi, Y.; Wu, C. Utilization and life cycle assessment of low activity solid waste as cementitious materials: A case study of titanium slag and granulated blast furnace slag. Sci. Total Environ. 2022, 849, 157797. [Google Scholar] [CrossRef]
- Qaidi, S.M.; Tayeh, B.A.; Isleem, H.F.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Sustainable utilization of red mud waste (bauxite residue) and slag for the production of geopolymer composites: A review. Case Stud. Constr. Mater. 2022, 16, e00994. [Google Scholar] [CrossRef]
- Qaidi, S.M.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Wang, Y.; Li, C.; Guo, Z.; Fan, S.; Zhang, H.; Feng, P. Chloride binding behavior of cement paste influenced by metakaolin dosage and chloride concentration. Cem. Concr. Compos. 2023, 135, 104821. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Hu, J.; Wang, Y.; Huang, H.; Ma, Y.; Zhang, Z.; Wang, H.; Yin, S.; Wei, J. Corrosion behavior of the reinforcement in chloride-contaminated alkali-activated fly ash pore solution. Compos. Part B 2021, 224, 109215. [Google Scholar] [CrossRef]
- Cai, W.; Xu, Z.; Zhang, Z.; Hu, J.; Huang, H.; Ma, Y.; Zhang, Z.; Wang, H.; Yin, S.; Wei, J. Chloride binding behavior of synthesized reaction products in alkali-activated slag. Compos. Part B 2022, 238, 109919. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Wilson, W.; Gonthier, J.N.; Georget, F.; Scrivener, K.L. Insights on chemical and physical chloride binding in blended cement pastes. Cem. Concr. Res. 2022, 156, 106747. [Google Scholar] [CrossRef]
- Ma, H.; Yi, C.; Zhu, H.; Fan, J.; Li, W.; Shi, Y.; Shi, Y.; Chen, H. Effect of auxiliary cementatious materials on the hardened cement paste chloride binding: An overview. Mater. Rev. 2018, 32, 469–474. [Google Scholar]
- Ming, X.; Liu, Q.; Wang, M.; Cai, Y.; Chen, B.; Li, Z. Improved chloride binding capacity and corrosion protection of cement-based materials by incorporating alumina nano particles. Cem. Concr. Compos. 2023, 136, 104898. [Google Scholar] [CrossRef]
- Yoon, S.; Moon, J.; Bae, S.; Duan, X.; Giannelis, E.P.; Monteiro, P.M. Chloride adsorption by calcined layered double hydroxides in hardened Portland cement paste. Mater. Chem. Phys. 2014, 145, 376–386. [Google Scholar] [CrossRef]
- Dousti, A.; Beaudoin, J.J.; Shekarchi, M. Chloride binding in hydrated MK, SF and natural zeolite-lime mixtures. Constr. Build. Mater. 2017, 154, 1035–1047. [Google Scholar] [CrossRef]
- Florea, M.; Brouwers, H. Chloride binding related to hydration products: Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, R.; Zhang, L.; Guan, X. Molecular insights into the adsorption of chloride ions in calcium silicate hydrate gels: The synergistic effect of calcium to silicon ratio and sulfate ion. Microporous Mesoporous Mater. 2022, 345, 112248. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P. Chloride in cement. Adv. Cem. Res. 2015, 27, 63–97. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.-y.; Mo, L.-w. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, Y.; Demopoulos, G.P. Desilication of sodium aluminate solution by Friedel’s salt (FS: 3CaO· A12O3· CaCl2· 10H2O). Hydrometallurgy 2009, 99, 225–230. [Google Scholar] [CrossRef]
- Sui, S.; Wu, M.; Yang, Z.; Wang, F.; Liu, Z.; Jiang, J. An investigation on the formation of Friedel’s salt in tricalcium silicate combined with metakaolin and limestone systems. Constr. Build. Mater. 2021, 284, 122855. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, C.; Sun, M.; Zhang, Y.; Duan, J. Review on Mechanism of Chloride Ion Binding and Its Influencing Factors in Cement-Based Materials. Bull. Chin. Ceram. Soc. 2022, 41, 2604–2617. [Google Scholar]
- Guo, Y.; Zhang, T.; Tian, W.; Wei, J.; Yu, Q. Physically and chemically bound chlorides in hydrated cement pastes: A comparison study of the effects of silica fume and metakaolin. J. Mater. Sci. 2019, 54, 2152–2169. [Google Scholar] [CrossRef]
- Das, J.K.; Pradhan, B. Experimental investigation on inhibiting compounds against steel corrosion in concrete admixed with different chloride salts. Mater. Struct. 2023, 56, 14. [Google Scholar] [CrossRef]
- Yang, T.; Fan, X.; Gao, X.; Gu, Q.; Xu, S.; Shui, Z. Modification on the chloride binding capacity of alkali activated slag by applying calcium and aluminium containing phases. Constr. Build. Mater. 2022, 358, 129427. [Google Scholar] [CrossRef]
- Ghadir, P.; Razeghi, H.R. Effects of sodium chloride on the mechanical strength of alkali activated volcanic ash and slag pastes under room and elevated temperatures. Constr. Build. Mater. 2022, 344, 128113. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Shen, P.; Lu, J.; Cai, Y.; Poon, C.S. Physicochemical investigation of Portland cement pastes prepared and cured with seawater. Mater. Struct. 2022, 55, 150. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Yu, Y. Chloride binding by calcined layered double hydroxides and alumina-rich cementitious materials in mortar mixed with seawater and sea sand. Constr. Build. Mater. 2021, 293, 123493. [Google Scholar] [CrossRef]
- Tang, S.; Peng, T.; Sun, H.; Ding, W.; Luo, L. Influencing Mechanism of Titanium-Extracted Tailing Slag on the Strength of CaO Steel Slag Hardened Paste. Materials 2023, 16, 937. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Cao, X.; Liu, Q.; Zang, H. Influence of the combination of calcium oxide and sodium carbonate on the hydration reactivity of alkali-activated slag binders. J. Clean. Prod. 2018, 171, 622–629. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O. Parameters affecting the properties and microstructure of quicklime (CaO)-Activated slag cement pastes. Cem. Concr. Compos. 2019, 103, 104–111. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Zhang, F.; Gao, Y.; Wu, Q. Chloride and heavy metal binding capacities of hydrotalcite-like phases formed in greener one-part sodium carbonate-activated slag cements. J. Clean. Prod. 2020, 253, 120047. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Song, Q. Chloride binding capacity and phase modification of alumina compound blended cement paste under chloride attack. Cem. Concr. Compos. 2020, 108, 103537. [Google Scholar] [CrossRef]
- Sun, C.; Sun, M.; Tao, T.; Qu, F.; Wang, G.; Zhang, P.; Li, Y.; Duan, J. Chloride binding capacity and its effect on the microstructure of mortar made with marine sand. Sustainability 2021, 13, 4169. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, J.; Cheng, Z. Chloride ion binding effect and corrosion resistance of geopolymer materials prepared with seawater and coral sand. Constr. Build. Mater. 2021, 309, 125126. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, Y.; Ma, J.; Li, Z. Removal of arsenic from water by Friedel’s salt (FS: 3CaO· Al2O3· CaCl2· 10H2O). J. Hazard. Mater. 2011, 195, 398–404. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al (OH) 6 (Cl, OH)· 2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Tian, Q.; Xu, Z.; Xu, R.; Zhang, L. Synthesis and application of Friedel’s salt in arsenic removal from caustic solution. Chem. Eng. J. 2017, 323, 304–311. [Google Scholar] [CrossRef]
- Dai, Y.; Qian, G.; Cao, Y.; Chi, Y.; Xu, Y.; Zhou, J.; Liu, Q.; Xu, Z.P.; Qiao, S. Effective removal and fixation of Cr (VI) from aqueous solution with Friedel’s salt. J. Hazard. Mater. 2009, 170, 1086–1092. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.J.; Basheer, P.M.; Bai, Y. Raman spectroscopic investigation of Friedel’s salt. Cem. Concr. Compos. 2018, 86, 306–314. [Google Scholar] [CrossRef]
- Yao, J.; Kong, Q.; Zhu, H.; Long, Y.; Shen, D. Adsorption characteristics of nitrite on Friedel’s salt under the landfill circumstance. Chem. Eng. J. 2014, 254, 479–485. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, Y.; Bai, H.; Qian, G.; Cao, Y.; Zhou, J.; Xu, Y.; Liu, Q.; Xu, Z.P.; Qiao, S. Effective removal of selenate from aqueous solutions by the Friedel phase. J. Hazard. Mater. 2010, 176, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, M.; Wang, W.; Hou, H. Identification of chromate binding mechanisms in Friedel’s salt. Constr. Build. Mater. 2013, 48, 942–947. [Google Scholar] [CrossRef]
- Ye, H.; Huang, L.; Chen, Z. Influence of activator composition on the chloride binding capacity of alkali-activated slag. Cem. Concr. Compos. 2019, 104, 103368. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yao, G.; Zhu, X.; Hu, S.; Qiu, J.; Chen, P.; Lyu, X. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Liu, J.; Feng, Z.; Kong, S. Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine. Materials 2020, 13, 4103. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jiang, J.; Wang, S.; Yang, T.; Qi, L.; Geng, J. The mechanical and interfacial properties of concrete prepared by recycled aggregates with chloride corrosion media. Constr. Build. Mater. 2021, 282, 122653. [Google Scholar] [CrossRef]
- Kim, T.; Kang, C.; Hong, S.; Seo, K.-Y. Investigating the effects of polyaluminum chloride on the properties of ordinary portland cement. Materials 2019, 12, 3290. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Z.-g.; Huang, X.; Bai, X.-h. Effect of Friedel’s salt on strength enhancement of stabilized chloride saline soil. J. Cent. South Univ. 2017, 24, 937–946. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Jin, R.; Liu, B. Formation of Friedel’s salt using steel slag and potash mine brine water. Constr. Build. Mater. 2019, 220, 119–127. [Google Scholar] [CrossRef]
- Kim, T. The effects of polyaluminum chloride on the mechanical and microstructural properties of alkali-activated slag cement paste. Cem. Concr. Compos. 2019, 96, 46–54. [Google Scholar] [CrossRef]



| Item | CaO | SiO2 | Al2O3 | TiO2 | MgO | Cl | Fe2O3 | SO3 | F | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 31.0 | 23.3 | 13.3 | 7.7 | 6.1 | 6.0 | 3.5 | 1.2 | 0.9 | 0.6 | 5.7 |
| Samples | CaO/wt% | TETS/wt% | Water/Solid/wt/wt |
|---|---|---|---|
| SC4 | 4.0 | 96.0 | 0.40 |
| SC6 | 6.0 | 94.0 | 0.40 |
| SC8 | 8.0 | 92.0 | 0.40 |
| SC10 | 10.0 | 90.0 | 0.40 |
| Samples | UCS (MPa) | Chloride Binding Amount (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 Day | 3 Days | 7 Days | 28 Days | 1 Day | 3 Days | 28 Days | 7 Days | |
| SC4 | 0 | 1.39 | 2.75 | 5.01 | 3.36 | 6.31 | 8.23 | 11.22 |
| SC6 | 0.46 | 2.59 | 5.34 | 11.49 | 7.23 | 11.31 | 15.65 | 20.57 |
| SC8 | 1.08 | 3.65 | 7.19 | 16.23 | 9.56 | 14.68 | 21.12 | 28.76 |
| SC10 | 2.12 | 5.23 | 9.61 | 20.74 | 16.32 | 22.89 | 30.07 | 38.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Peng, T.; Sun, H.; Ding, W.; Luo, L.; You, H.; Yao, X. Influences of Friedel’s Salt Produced by CaO-Activated Titanium-Extracted Tailing Slag on Chloride Binding. Materials 2023, 16, 2843. https://doi.org/10.3390/ma16072843
Tang S, Peng T, Sun H, Ding W, Luo L, You H, Yao X. Influences of Friedel’s Salt Produced by CaO-Activated Titanium-Extracted Tailing Slag on Chloride Binding. Materials. 2023; 16(7):2843. https://doi.org/10.3390/ma16072843
Chicago/Turabian StyleTang, Song, Tongjiang Peng, Hongjuan Sun, Wenjin Ding, Liming Luo, Hao You, and Xiaoman Yao. 2023. "Influences of Friedel’s Salt Produced by CaO-Activated Titanium-Extracted Tailing Slag on Chloride Binding" Materials 16, no. 7: 2843. https://doi.org/10.3390/ma16072843
APA StyleTang, S., Peng, T., Sun, H., Ding, W., Luo, L., You, H., & Yao, X. (2023). Influences of Friedel’s Salt Produced by CaO-Activated Titanium-Extracted Tailing Slag on Chloride Binding. Materials, 16(7), 2843. https://doi.org/10.3390/ma16072843








