Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis
Abstract
1. Introduction
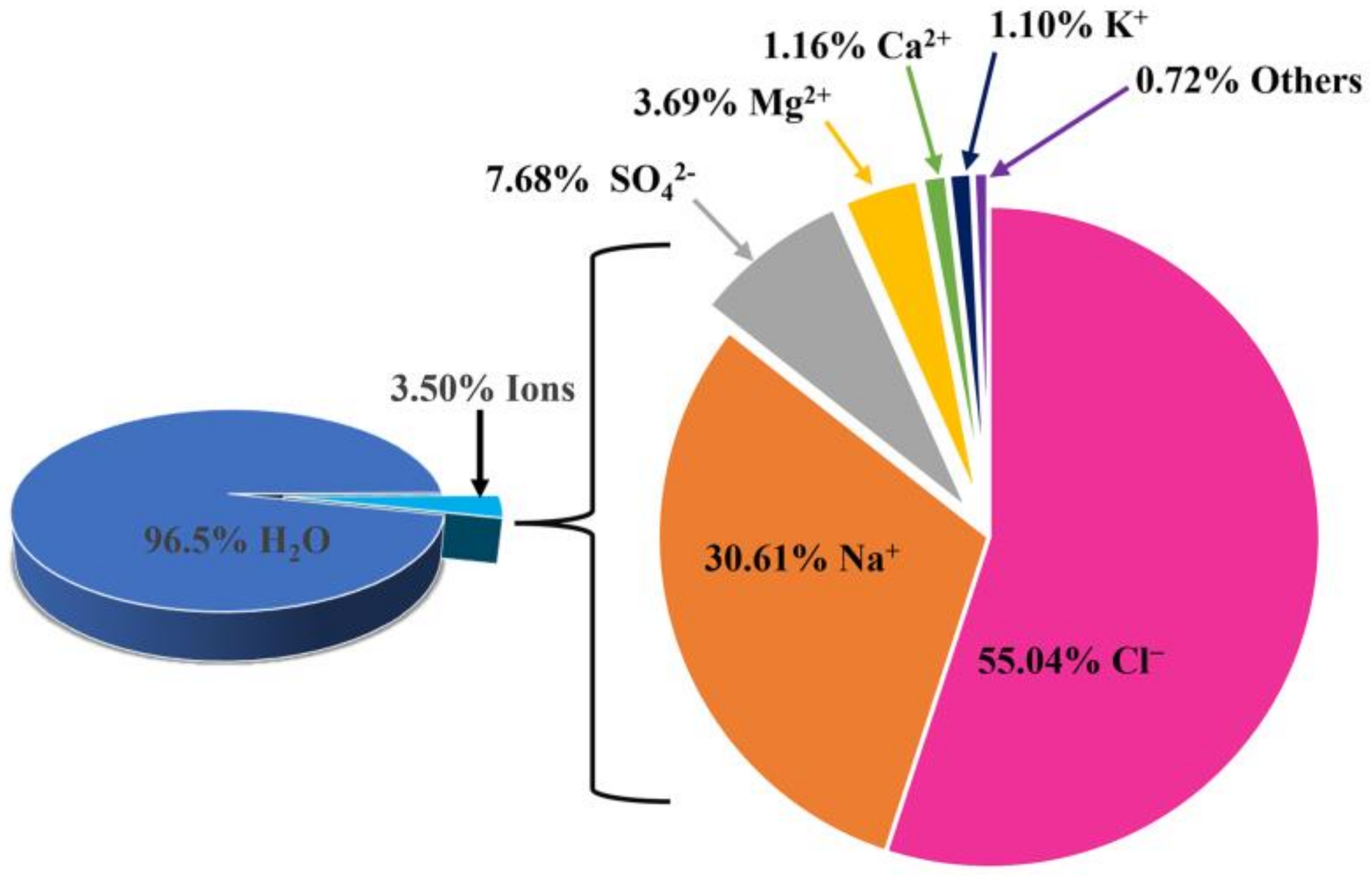
2. Fundamentals of Seawater Electrolysis
2.1. Reaction Mechanism
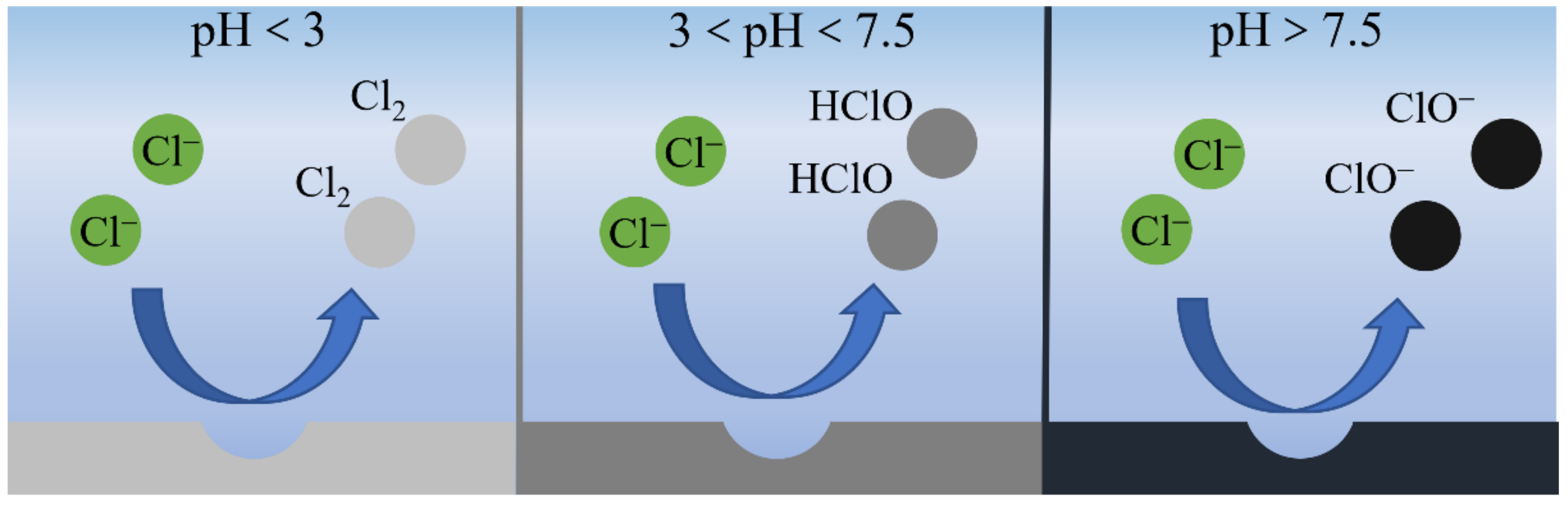
2.2. Chloride Oxidation
2.3. Corrosion and Poisoning of the Electrode
2.4. Evaluation Metrics for Electrocatalytic Performance
2.4.1. Overpotential
2.4.2. Tafel Slope
2.4.3. Electrochemical Surface Area
2.4.4. Long-Term Stability
2.5. Key Aspects for Designing a Corrosion-Resistant Electrode
3. Design Strategies for a Corrosion-Resistant Electrode
3.1. Design Strategies for a Corrosion-Resistant Anode
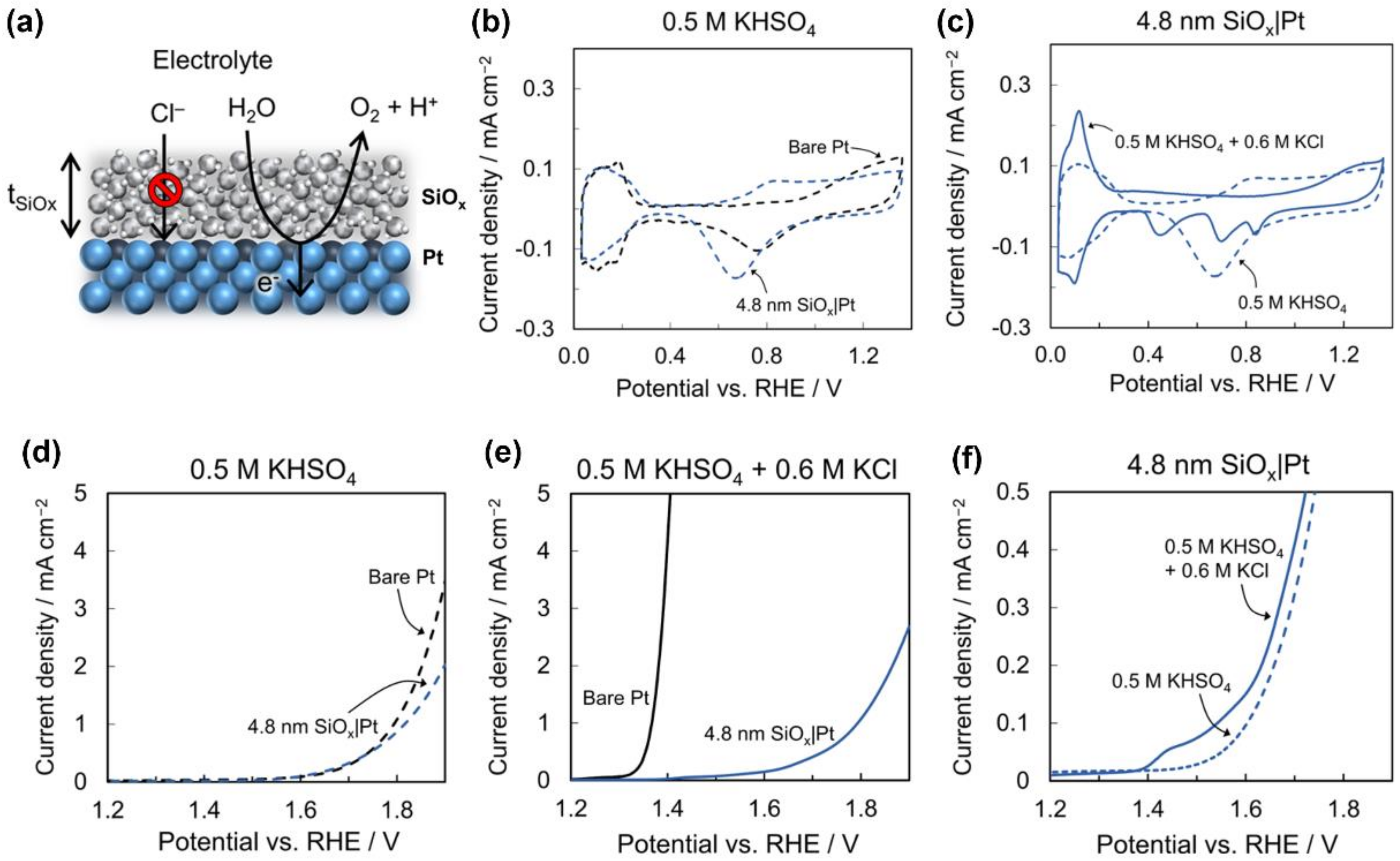
3.1.1. Permselective Strategy
3.1.2. Electrostatic Repulsion Strategy
3.2. Design Strategies for a Corrosion-Resistant Cathode
Blocking Strategy
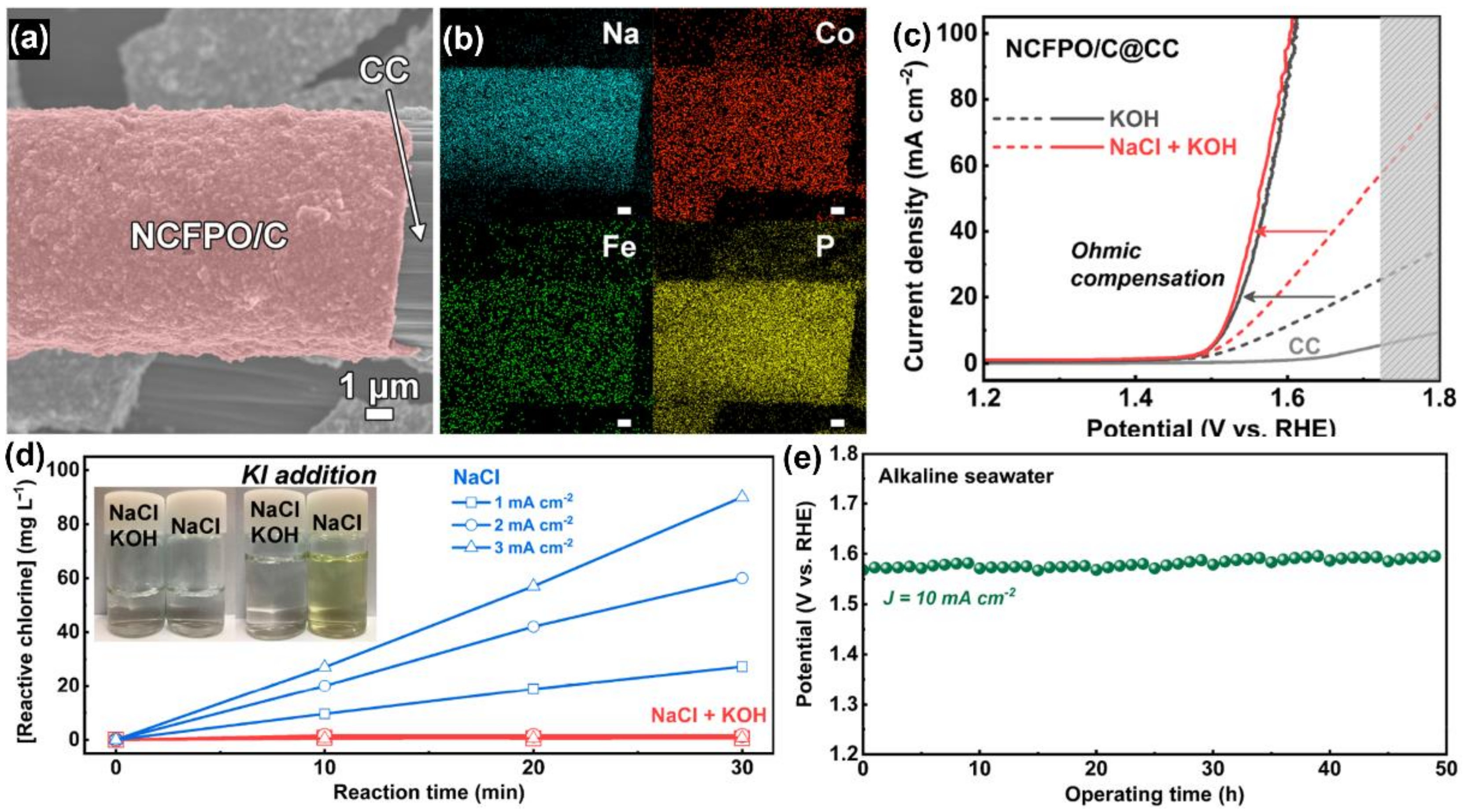
3.3. pH Buffer Strategy
4. Conclusions and Outlook
4.1. Efficient Screening Electrocatalysts
4.2. Understand the Reaction Mechanism In-Depth
4.3. Establish a Standard Criteria System
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PEM | Proton exchange membrane |
| HER | hydrogen evolution reaction |
| OER | oxygen evolution reaction |
| CER | chlorine evolution reaction |
| LSV | linear sweep voltammetry |
| CV | cyclic voltammetry |
| ECSA | electrochemical surface area |
| FESEM | field emission scanning electron microscopy |
| HRTEM | high resolution transmission electron microscope |
| SEM | scanning electron microscope |
| EDS | energy dispersive spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| DCA | dicyandiamide |
| TEM | transmission electron microscope |
References
- Ma, X.; Chang, C.; Zhang, Y.; Niu, P.; Liu, X.; Wang, S.; Li, L. Synthesis of Co-based Prussian Blue Analogues/Dual-Doped Hollow Carbon Microsphere Hybrids as High-Performance Bifunctional Electrocatalysts for Oxygen Evolution and Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 8318–8326. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef]
- Van Drunen, J.; Pilapil, B.K.; Makonnen, Y.; Beauchemin, D.; Gates, B.D.; Jerkiewicz, G. Electrochemically Active Nickel Foams as Support Materials for Nanoscopic Platinum Electrocatalysts. ACS Appl. Mater. Interfaces 2014, 6, 12046–12061. [Google Scholar] [CrossRef]
- Lee, B.; Wang, L.; Wang, Z.; Cooper, N.J.; Elimelech, M. Directing the research agenda on water and energy technologies with process and economic analysis. Energy Environ. Sci. 2023, 16, 714–722. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef]
- Xue, Q.; Bai, X.; Zhao, Y.; Li, Y.; Wang, T.; Sun, H.; Li, F.; Chen, P.; Jin, P.; Yin, S.; et al. Au core-PtAu alloy shell nanowires for formic acid electrolysis. J. Energy Chem. 2022, 65, 94–102. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2020, 4, 295–400. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Lv, X.; Zhang, Y.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Hegner, F.S.; Garcés-Pineda, F.A.; González-Cobos, J.; Rodríguez-García, B.; Torréns, M.; Palomares, E.J.; López, N.; Galán-Mascarós, J.R. Understanding the Catalytic Selectivity of Cobalt Hexacyanoferrate toward Oxygen Evolution in Seawater Electrolysis. ACS Catal. 2021, 11, 13140–13148. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, K.; He, J.; Li, F.; Huang, H.; Chen, P.; Chen, Y. Nitrogen-doped graphene aerogel-supported ruthenium nanocrystals for pH-universal hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 1535–1543. [Google Scholar] [CrossRef]
- Dingenen, F.; Verbruggen, S. Tapping hydrogen fuel from the ocean: A review on photocatalytic, photoelectrochemical and electrolytic splitting of seawater. Renew. Sustain. Energy Rev. 2021, 142, 110866. [Google Scholar] [CrossRef]
- Lokesh, S.; Srivastava, R. Advanced Two-Dimensional Materials for Green Hydrogen Generation: Strategies toward Corrosion Resistance Seawater Electrolysis─Review and Future Perspectives. Energy Fuels 2022, 36, 13417–13450. [Google Scholar] [CrossRef]
- Lu, J.; Li, C.; Wang, H.; Ji, S.; Wang, X.; Wang, R. How to get to best oxygen evolution behavior from the electrolysis practice of the seawater. Int. J. Hydrogen Energy 2021, 46, 12936–12943. [Google Scholar] [CrossRef]
- Maril, M.; Delplancke, J.; Cisternas, N.; Tobosque, P.; Maril, Y.; Carrasco, C. Critical aspects in the development of anodes for use in seawater electrolysis. Int. J. Hydrogen Energy 2022, 47, 3532–3549. [Google Scholar] [CrossRef]
- Huynh, M.; Ozel, T.; Liu, C.; Lau, E.; Nocera, D. Design of template-stabilized active and earth-abundant oxygen evolution catalysts in acid. Chem. Sci. 2017, 8, 4779–4794. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, C.; Huang, Z.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, Z.; Deng, B.; Ye, C.; Zhang, L.; Wang, Y.; Li, T.; Liu, Q.; Cui, G.; Asiri, A.M.; et al. Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt. Int. J. Hydrogen Energy 2022, 47, 3580–3586. [Google Scholar] [CrossRef]
- Meng, C.; Cao, Y.; Luo, Y.; Zhang, F.; Kong, Q.; Alshehri, A.A.; Alzahrani, K.A.; Li, T.; Liu, Q.; Sun, X. A Ni-MOF nanosheet array for efficient oxygen evolution electrocatalysis in alkaline media. Inorg. Chem. Front. 2021, 8, 3007–3011. [Google Scholar] [CrossRef]
- Qiu, Y.; Feng, Z.; Ji, X.; Liu, J. Surface self-reconstruction of nickel foam triggered by hydrothermal corrosion for boosted water oxidation. Int. J. Hydrogen Energy 2021, 46, 1501–1508. [Google Scholar] [CrossRef]
- Guang, H.; Zhu, S.; Liang, Y.; Wu, S.; Li, Z.; Luo, S.; Cui, Z.; Inoue, A. Highly efficient nanoporous CoBP electrocatalyst for hydrogen evolution reaction. Rare Met. 2021, 40, 1031–1039. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G. One-Dimensional Earth-Abundant Nanomaterials for Water-Splitting Electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, L.; Zhou, H.; Li, D.; Tang, D.; Yu, F. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Mater. Today Phys. 2022, 26, 100727. [Google Scholar] [CrossRef]
- Lee, K.; Balamurugan, M.; Park, S.; Ha, H.; Jin, K.; Seo, H.; Nam, K. Importance of Entropic Contribution to Electrochemical Water Oxidation Catalysis. ACS Energy Lett. 2019, 4, 1918–1929. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, D.; Zou, R. Metal-organic frameworks and their derivatives as bifunctional electrocatalysts. Coord. Chem. Rev. 2018, 376, 430–448. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, S.; Feng, J.; Tong, Y.; Zhou, F.; Li, Q.; Song, L.; Chen, S.; Wu, K.; Su, P.; et al. Confined Fe–Cu Clusters as Sub-Nanometer Reactors for Efficiently Regulating the Electrochemical Nitrogen Reduction Reaction. Adv. Mater. 2020, 32, 2004382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Z.; Li, Z.; Han, M.; Cheng, Y.; Zheng, Z. Standing NiFe LDH nanosheets on stainless steel fibers felt: A synergistic impact on the oxygen evolution reaction (OER) for the water splitting. Catal. Commun. 2022, 164, 106425. [Google Scholar] [CrossRef]
- Jiao, S.; Fu, X.; Wang, S.; Zhao, Y. Perfecting electrocatalysts via imperfections: Towards the large-scale deployment of water electrolysis technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Bodhankar, P.; Sarawade, P.; Singh, G.; Vinu, A.; Dhawale, D. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Pengju, Y.; Zhao, J.; Qiu, J. Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Z.; Li, J.; Li, Z.; Meng, X. Recent advances in electrocatalytic seawater splitting. Recent advances in electrocatalytic seawater splitting. Rare Met. 2023, 42, 751–768. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Kato, Z.; Bhattarai, J.; Kumagai, N.; Izumiya, K.; Hashimoto, K. Durability enhancement and degradation of oxygen evolution anodes in seawater electrolysis for hydrogen production. Appl. Surf. Sci. 2011, 257, 8230–8236. [Google Scholar] [CrossRef]
- Kato, Z.; Sato, M.; Sasaki, Y.; Izumiya, K.; Kumagai, N.; Hashimoto, K. Electrochemical characterization of degradation of oxygen evolution anode for seawater electrolysis. Electrochim. Acta 2014, 116, 152–157. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.; Zhao, C.; Zhang, Q. Seawater electrolyte-based metal–air batteries: From strategies to applications. Energy Environ. Sci. 2020, 13, 3253–3268. [Google Scholar] [CrossRef]
- Wang, H.; Weng, C.; Ren, J.; Yuan, Z. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426. [Google Scholar] [CrossRef]
- Yu, X.; Xu, S.; Wang, Z.; Wang, S.; Zhang, J.; Liu, Q.; Luo, Y.; Du, Y.; Sun, X.; Wu, Q. Self-supported Ni3S2@Ni2P/MoS2 heterostructures on nickel foam for an outstanding oxygen evolution reaction and efficient overall water splitting. Dalton Trans. 2021, 50, 15094–15102. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrogen Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Karlsson, R.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef]
- Consonni, V.; Trasatti, S.; Pollak, F.; O’Grady, W.E. Mechanism of chlorine evolution on oxide anodes study of pH effects. J. Electroanal. Chem. Interfacial Electrochem. 1987, 228, 393–406. [Google Scholar] [CrossRef]
- Smith, R.; Prévot, M.; Fagan, R.; Zhang, Z.; Sedach, P.; Siu, M.; Trudel, S.; Berlinguette, C. Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Getaye Sendeku, M.; Zhang, X.; Xu, L.; Wang, Z.; Wang, S.; Duan, X.; Liu, H.; Liu, W.; et al. Phosphate-decorated Ni3Fe-LDHs@CoPx nanoarray for near-neutral seawater splitting. Chem. Eng. J. 2023, 460, 141413. [Google Scholar]
- Liu, X.; Gong, M.; Xiao, D.; Deng, S.; Liang, J.; Zhao, T.; Lu, Y.; Shen, T.; Zhang, J.; Wang, D. Turning Waste into Treasure: Regulating the Oxygen Corrosion on Fe Foam for Efficient Electrocatalysis. Small 2020, 16, 2000663. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liang, Z.; Zhao, J.; Zhu, B.; Cai, K.; Zou, R.; Xu, Q. Hierarchical Cobalt Phosphide Hollow Nanocages toward Electrocatalytic Ammonia Synthesis under Ambient Pressure and Room Temperature. Small Methods 2018, 2, 1800204. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Z.; Li, J.; Li, Z.; Meng, X. Recent advances in transition metal selenides-based electrocatalysts: Rational design and applications in water splitting. J. Alloys Compd. 2022, 918, 165719. [Google Scholar] [CrossRef]
- Oh, B.S.; Oh, S.G.; Hwang, Y.Y.; Yu, H.; Kang, J.; Kim, I.S. Formation of hazardous inorganic by-products during electrolysis of seawater as a disinfection process for desalination. Sci. Total Environ. 2010, 408, 5958–5965. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Balaji, R.; Kannan, B.S.; Lakshmi, J.; Senthil, N.; Vasudevan, S.; Sozhan, G.; Shukla, A.K.; Ravichandran, S. An alternative approach to selective sea water oxidation for hydrogen production. Electrochem. Commun. 2009, 11, 1700–1702. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef]
- Abdel-Aal, H.K.; Hussein, I.A. Parametric study for saline water electrolysis: Part III—Precipitate formation and recovery of magnesium salts. Int. J. Hydrogen Energy 1993, 18, 553–556. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G. Electrochemical analysis of seawater electrolysis with molybdenum-oxo catalysts. Int. J. Hydrogen Energy 2013, 38, 2589–2595. [Google Scholar] [CrossRef]
- Khatun, S.; Hirani, H.; Roy, P. Seawater electrocatalysis: Activity and selectivity. J. Mater. Chem. A 2021, 9, 74–86. [Google Scholar] [CrossRef]
- Kuhn, A.T.; Chan, C.Y. pH changes at near-electrode surfaces. J. Appl. Electrochem. 1983, 13, 189–207. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, X.; Han, H.; Liu, Z.; Liu, J.; Ji, X. Advantageous metal-atom-escape towards super-hydrophilic interfaces assembly for efficient overall water splitting. J. Power Sources 2021, 499, 229941. [Google Scholar] [CrossRef]
- Vos, J.G.; Liu, Z.; Speck, F.D.; Perini, N.; Fu, W.; Cherevko, S.; Koper, M.T.M. Selectivity Trends Between Oxygen Evolution and Chlorine Evolution on Iridium-Based Double Perovskites in Acidic Media. ACS Catal. 2019, 9, 8561–8574. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, W.; Wang, W.; Wang, Z.; Li, J.; Xu, J.; Yu, J. Research on performance optimization and mechanism of electrochemical water softening applied by pulse power supply. Water Sci. Technol. 2021, 84, 2432–2445. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Fourmond, V.; Jacques, P.; Fontecave, M.; Artero, V. H2 Evolution and Molecular Electrocatalysts: Determination of Overpotentials and Effect of Homoconjugation. Inorg. Chem. 2010, 49, 10338–10347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, X.; Xu, P.; Liu, D.; Cui, L.; Zhao, H.; Du, Y. Synthesis of pomegranate-like Mo2C@C nanospheres for efficient microwave absorption. Chem. Eng. J. 2019, 372, 312–320. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]
- Conway, B.E.; Tilak, B.V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, Y.; Zhao, Z.; Meng, X.; Li, Z. Modification strategies to improve electrocatalytic activity in seawater splitting: A review. J. Mater. Sci. 2022, 57, 19243–19259. [Google Scholar] [CrossRef]
- An, C.; Kang, W.; Deng, Q.; Hu, N. Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range. Rare Met. 2022, 41, 378–384. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Liu, S.; Chen, X.; Ma, T.; Wang, G.; Lu, Z.; Sun, J. Enhanced interface interaction in Cu2S@Ni core-shell nanorod arrays as hydrogen evolution reaction electrode for alkaline seawater electrolysis. J. Power Sources 2021, 506, 230235. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Lv, H.; Wang, M.; Cui, X.; Liu, G.; Jiang, L. Triggering the Intrinsic Catalytic Activity of Ni-Doped Molybdenum Oxides via Phase Engineering for Hydrogen Evolution and Application in Mg/Seawater Batteries. ACS Sustain. Chem. Eng. 2021, 9, 13106–13113. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, C.; Feng, X.; Tan, H.; Yan, L.; Liu, Y.; Kang, Z.; Wang, E.; Li, Y. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Bu, X.; Liang, X.; Bu, Y.; Quan, Q.; Meng, Y.; Lai, Z.; Wang, W.; Liu, C.; Lu, J.; Lawrence Wu, C.; et al. NiMo@C3N5 heterostructures with multiple electronic transmission channels for highly efficient hydrogen evolution from alkaline electrolytes and seawater. Chem. Eng. J. 2022, 438, 135379. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhu, Q.; McElhenny, B.; Zhang, F.; Wu, C.; Xing, X.; Bao, J.; Chen, S.; Ren, Z. Boron-modified cobalt iron layered double hydroxides for high efficiency seawater oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Haq, T.U.; Haik, Y. S doped Cu2O-CuO nanoneedles array: Free standing oxygen evolution electrode with high efficiency and corrosion resistance for seawater splitting. Catal. Today 2022, 400–401, 14–25. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Song, H.J.; Yoon, H.; Ju, B.; Lee, D.; Kim, D. Electrocatalytic Selective Oxygen Evolution of Carbon-Coated Na2Co1–xFexP2O7 Nanoparticles for Alkaline Seawater Electrolysis. ACS Catal. 2020, 10, 702–709. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cao, Y.; Liu, H.; Wang, A.; Zhang, C.; Zheng, X. In situ growth of NiSe@Co0.85Se heterointerface structure with electronic modulation on nickel foam for overall water splitting. Rare Met. 2021, 40, 1373–1382. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, W.; Lai, W.; Wang, M.; Li, Q.; Wang, Z.; Yuan, J.; Deng, Y.; Bao, J.; Ji, H. NiFe Layered Double Hydroxide/FeOOH Heterostructure Nanosheets as an Efficient and Durable Bifunctional Electrocatalyst for Overall Seawater Splitting. Inorg. Chem. 2021, 60, 17371–17378. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Wang, J.; Wei, H.; Zhang, S.; Zhu, S.; Li, Z.; Wu, S.; Jiang, H.; Liang, Y. Sandwich structured Ni3S2-MoS2-Ni3S2@Ni foam electrode as a stable bifunctional electrocatalyst for highly sustained overall seawater splitting. Electrochim. Acta 2021, 390, 138833. [Google Scholar] [CrossRef]
- Wang, H.; Ren, J.; Wang, L.; Sun, M.; Yang, H.; Lv, X.; Yuan, Z. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 66–73. [Google Scholar] [CrossRef]
- Ke, S.; Chen, R.; Chen, G.; Ma, X. Mini Review on Electrocatalyst Design for Seawater Splitting: Recent Progress and Perspectives. Energy Fuels 2021, 35, 12948–12956. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Ren, G.; Meng, X. Vacancy-engineered bismuth-based semiconductor with enhanced photocatalytic activity: A review. Mater. Sci. Semicond. Process. 2022, 137, 106230. [Google Scholar] [CrossRef]
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru Incorporated Amorphous Cobalt-Based Oxides for High-Current-Density Overall Water Splitting in Alkaline and Seawater Media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef]
- Singh, B.; Singh, A.; Yadav, A.; Indra, A. Modulating electronic structure of metal-organic framework derived catalysts for electrochemical water oxidation. Coord. Chem. Rev. 2021, 447, 214144. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, B.; Zheng, Y.; Jin, H.; Jiao, Y.; Qiao, S. Charge State Manipulation of Cobalt Selenide Catalyst for Overall Seawater Electrolysis. Adv. Energy Mater. 2018, 8, 1801926. [Google Scholar] [CrossRef]
- Zhou, G.; Guo, Z.; Shan, Y.; Wu, S.; Zhang, J.; Yan, K.; Liu, L.; Chu, P.K.; Wu, X. High-efficiency hydrogen evolution from seawater using hetero-structured T/Td phase ReS2 nanosheets with cationic vacancies. Nano Energy 2019, 55, 42–48. [Google Scholar] [CrossRef]
- Gao, G.; Yang, S.; Wang, S.; Li, L. Construction of 3D porous MXene supercapacitor electrode through a dual-step freezing strategy. Scr. Mater. 2022, 213, 114605. [Google Scholar] [CrossRef]
- Sun, J.; Meng, X. Modulating the Electronic Properties of MoS2 Nanosheets for Electrochemical Hydrogen Production: A Review. ACS Appl. Nano Mater. 2021, 4, 11413–11427. [Google Scholar] [CrossRef]
- Haq, T.U.; Pasha, M.; Tong, Y.; Mansour, S.A.; Haik, Y. Au nanocluster coupling with Gd-Co2B nanoflakes embedded in reduced TiO2 nanosheets: Seawater electrolysis at low cell voltage with high selectivity and corrosion resistance. Appl. Catal. B Environ. 2022, 301, 120836. [Google Scholar] [CrossRef]
- Bhardwaj, A.A.; Vos, J.G.; Beatty, M.E.S.; Baxter, A.F.; Koper, M.T.M.; Yip, N.Y.; Esposito, D.V. Ultrathin Silicon Oxide Overlayers Enable Selective Oxygen Evolution from Acidic and Unbuffered pH-Neutral Seawater. ACS Catal. 2021, 11, 1316–1330. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Liu, F.; Liu, J.; Xu, W.; Hu, H.; Chen, X.; Wang, W.; Cheng, F. Anionic formulation of electrolyte additive towards stable electrocatalytic oxygen evolution in seawater splitting. J. Energy Chem. 2022, 72, 361–369. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Qiu, H.; Su, C.; Shao, Z. High Selectivity Electrocatalysts for Oxygen Evolution Reaction and Anti-Chlorine Corrosion Strategies in Seawater Splitting. Catalysts 2022, 12, 261. [Google Scholar] [CrossRef]
- Kirk, D.W.; Ledas, A.E. Precipitate formation during sea water electrolysis. Int. J. Hydrogen Energy 1982, 7, 925–932. [Google Scholar] [CrossRef]
- Han, J.; Jwa, E.; Lee, H.; Kim, E.; Nam, J.; Hwang, K.S.; Jeong, N.; Choi, J.; Kim, H.; Jeung, Y.; et al. Direct seawater electrolysis via synergistic acidification by inorganic precipitation and proton flux from bipolar membrane. Chem. Eng. J. 2022, 429, 132383. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]




| Catalysts | Electrolyte | η@j (mV@mA cm2) | Tafel Slope (mV dec−1) | ECSA (mF cm−2) | Stability (h) | Refs. |
|---|---|---|---|---|---|---|
| HER | ||||||
| Co80B5P15 | 1 M KOH | 42@10 | 39.8 | 8.26 | 20 | [26] |
| Mn–NiO–Ni/Ni-F | Seawater | 200@35 | 121.0 | / | 50 | [46] |
| Pt-Te-MoS2 | 1 M KOH | 52@10 | 62.3 | / | 8 | [74] |
| Cu2S@Ni | 1 M NaOH + 0.5 M NaCl | 200@530 | 95.1 | / | 150 | [75] |
| Ni-MoO3 | Seawater | 412@10 | 133.0 | 13.24 | 24 | [76] |
| CoMoP@C | Seawater | 450@10 | 49.7 | 37.60 | 10 | [77] |
| NiMo@C3N5 | Seawater | 486@10 | 68.3 | 14.60 | 10 | [78] |
| OER | ||||||
| SSFF@NiFe LDH | 1 M KOH | 198@10 | 31.6 | 3.63 | 10 | [34] |
| B-Co2Fe LDH | 1 M KOH + Seawater | 205@10 | 39.2 | / | 100 | [79] |
| S-Cu2O-CuO NDLs | 1 M KOH + Seawater | 450@1000 | 45.0 | / | 100 | [80] |
| CoPx@FeOOH | 1 M KOH + Seawater | 337@500 | 37.6 | / | 80 | [81] |
| Na2CoP2O7/C | 1 M KOH + 0.5 M NaCl | 480@100 | 47.0 | 53 | 100 | [82] |
| NiFe/NiSx/Ni | 1 M KOH + 0.5 M NaCl | 510@400 | / | / | 1000 | [83] |
| Bifunction | ||||||
| Ni3S2@Ni2P/MoS2 | 1 M KOH | 175@10 | 46.0 | 4.50 | 40 | [44] |
| FeNi OH-100 | 1 M KOH | 236@50 | 48.9 | 66.86 | 25 | [62] |
| NiSe@Co0.85Se/NF | 1 M KOH | 258@10 | 50.0 | 5.61 | 20 | [84] |
| NiFe-LDH/FeOOH | 1 M NaOH + 0.5 M NaCl | 274@100 | 69.8 | / | 105 | [85] |
| Ni3S2-MoS2-Ni3S2@Ni | 1 M KOH + 0.5 M NaCl | 188@100 | 48.0 | 15.20 | 100 | [86] |
| Ni2P/NiS2 | 1 M KOH + Seawater | 391@500 | 23.0 | 75.10 | 48 | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, X.; Yu, J.; Zhou, W. Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis. Materials 2023, 16, 2709. https://doi.org/10.3390/ma16072709
Zhao L, Li X, Yu J, Zhou W. Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis. Materials. 2023; 16(7):2709. https://doi.org/10.3390/ma16072709
Chicago/Turabian StyleZhao, Li, Xiao Li, Jiayuan Yu, and Weijia Zhou. 2023. "Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis" Materials 16, no. 7: 2709. https://doi.org/10.3390/ma16072709
APA StyleZhao, L., Li, X., Yu, J., & Zhou, W. (2023). Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis. Materials, 16(7), 2709. https://doi.org/10.3390/ma16072709







