Killing Two Birds with One Stone: Upgrading Organic Compounds via Electrooxidation in Electricity-Input Mode and Electricity-Output Mode
Abstract
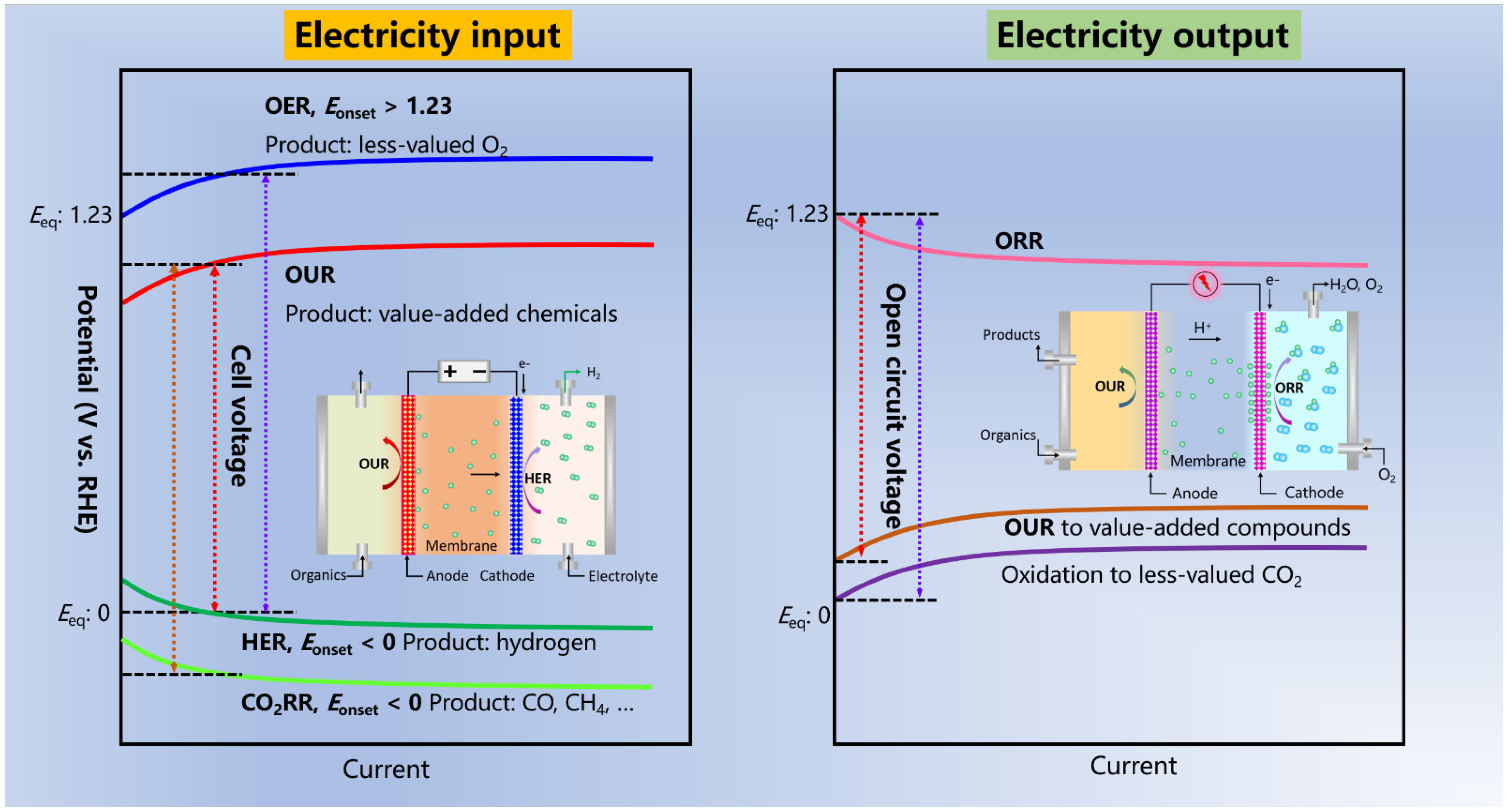
1. Introduction
2. Anodic OUR of Organic Compounds in Parallel with HER (OUR||HER)
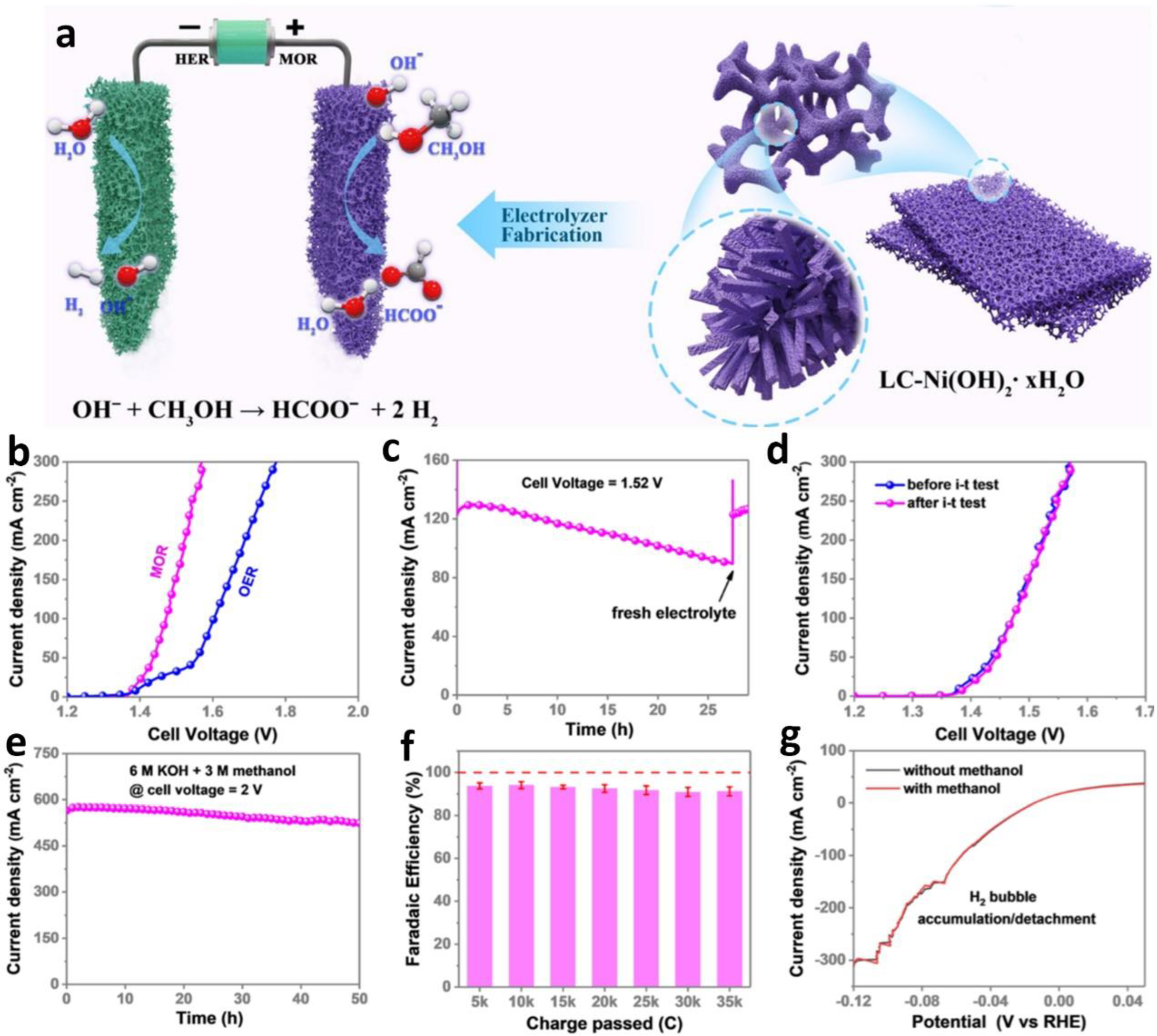
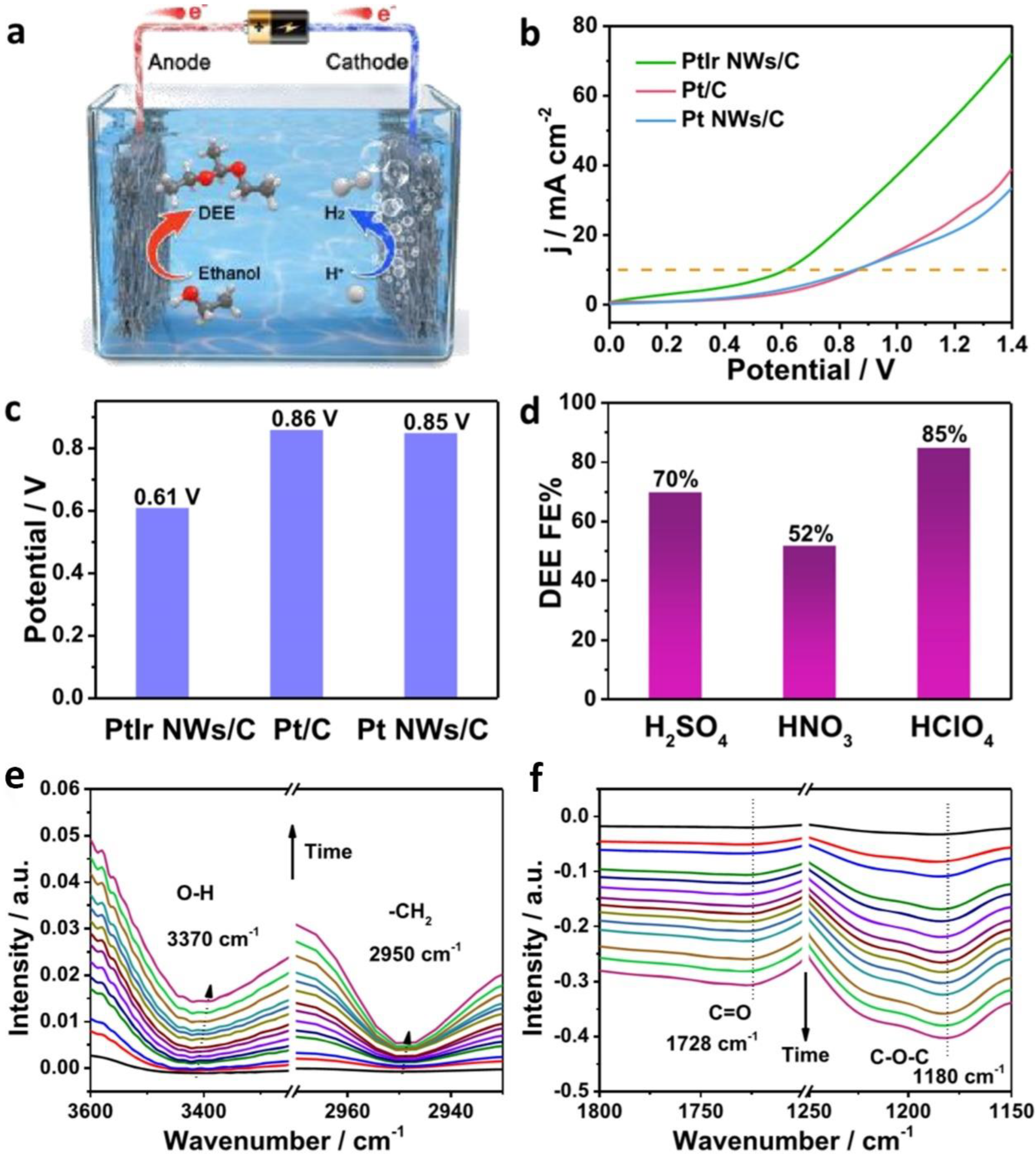
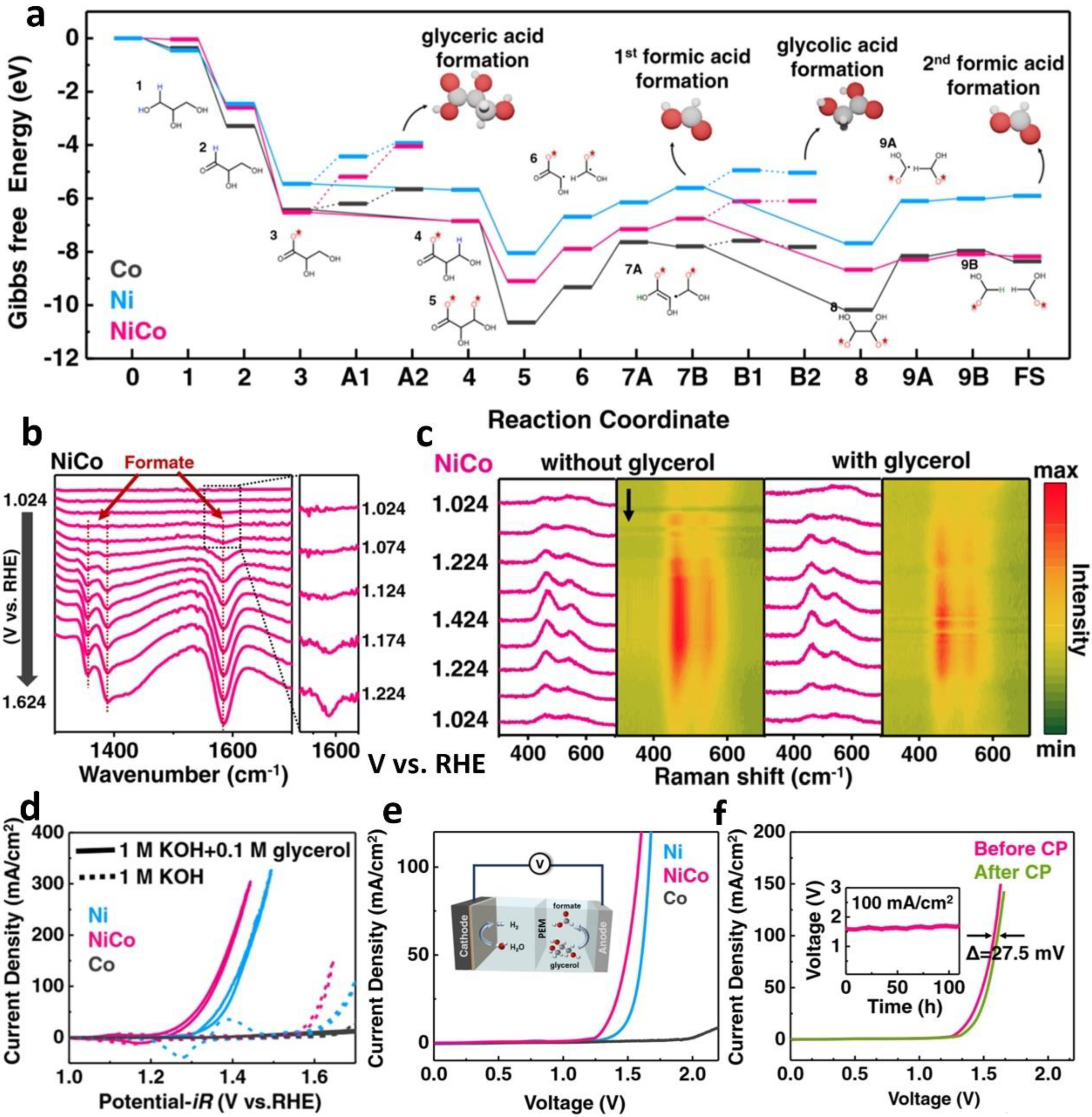
2.1. Anodic OUR of Alcohol Integrating with Cathodic HER
2.2. Anodic OUR of Aldehyde Integrating with Cathodic HER
2.3. Anodic OUR of Carboxylates Integrating with Cathodic HER
2.4. Anodic OUR of Nitrogen-Contained Molecules Integrating with Cathodic HER
2.5. Anodic OUR of Other Organic Molecules Integrating with Cathodic HER
| Working Mode | Reaction Type | Anode Catalyst | Substrate | Electrolyte | Product | 3-Electrode System | 2-Electrode System | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EOER at j10 (VRHE) | EOUR at j10 (VRHE) | VOER-HER at j10 | VOUR-HER at j10 | |||||||
| OUR||HER on electricity-input mode | Alcohol oxidation | Vp-Ni2P-Pt/CC | methanol | 2 M in 1 M KOH | Formate/H2 | 0.72 at J50 | 1.651 at J50 | - | ca. 0.7 | [38] |
| CeO2/RuO2 | methanol | 2.5 M in 0.5 M H2SO4 | Formic acid/H2 | 1.495 | 1.195 | 1.568 | 1.308 | [39] | ||
| Co(OH)2@HOS | methanol | 3 M in 1 M KOH | formate/H2 | 1.571 | 1.385 | 1.631 | 1.497 | [41] | ||
| PtIr NWs | ethanol | 4 M in 0.5 M HClO4 | DEE/H2 | - | 0.45 | - | 0.61 | [26] | ||
| Co(OH)2@Ni(OH)2 | ethanol | 1.0 M in 1.0 M KOH | acetate/H2 | - | 1.3 | - | 1.39 | [33] | ||
| Gold | glycerol | 0.1 M in 0.1 M NaOH | glyceric acid/H2 | - | 1.0 | - | - | [47] | ||
| NiCo hydroxide | glycerol | 0.1 M 1 M KOH | formate/H2 | - | 1.39 at J100 | - | 1.33 | [50] | ||
| PdAg/NF | ethylene glycol | 1 M in 0.5 M KOH | glycolic acid/H2 | 1.55 | 0.57 | - | 1.02 at j20 | [57] | ||
| CoNC | glucose | 0.1 M in 1.0 M KOH | gluconic acid, glucaric acid/H2 | 1.7 at J100 | 1.5 at J100 | 1.78 V at J100 | 0.9 V at J100 | [69] | ||
| Ni(OH)2 | benzyl alcohol | benzoic acid/H2 | ~1.33 at J100 | [58] | ||||||
| aldehyde oxidation | NixB | HMF | 10 mM in 1 M KOH | FDCA/H2 | 1.62 at J100 | 1.45 at J100 | - | - | [68] | |
| carboxylate oxidation | NiCl2•dme, Ligand L4 | carboxylic acids | NaI (0.2 M), DMF | decarboxylative products | - | - | - | 4 mA, 4 F per mol | [77] | |
| Pt-foil | valeric acid | 0.5 M | n-octane | - | - | - | - | [82] | ||
| amine oxidation | NiSe | Benzyl-amine | 1 mM in 1.0 M KOH | benzyl nitrile/H2 | 1.48 | 1.34 | 1.70 at J20 | 1.49 at J20 | [83] | |
| t-Ni/Co MOF | Benzyl-amine | 0.02 M in 1.0 M KOH | benzonitrile/H2 | - | - | ~1.75 | ~1.5 | [14] | ||
| S-Ni(OH)2 | Propyl-amine | 0.1 M in 1.0 M KOH | propionitrile/H2 | - | 1.327 at J100 | - | - | [84] | ||
| sulfides oxidation | CoFe-LDH | sulfides | 0.25 M in MeCN/H2O | sulfoxides/H2 | 1.90 at J5 | 1.39 at J5 | - | - | [87] | |
| Ni(ii)–bipyridine | phenyl sulfide | H2O (30 Equiv.), n-Bu4NBF4, MeCN | phenyl sulfoxides/H2 | [89] | ||||||
| nitroalkanes | NiSe | nitrotoluene | 0.4 mM in 1.0 M KOH | E-nitroethene | - | - | 1.69 | 1.36 | [12] | |
| alkane oxidation | Pt | ethylene | 1 M KCl | ethylene oxide/H2 | FE 70% | product specificities 97% | [95] | |||
3. Anodic OUR of Organic Compounds in Parallel with CO2RR (OUR||CO2RR)
4. OUR-Based Fuel Cells or Other Devices
| Working Mode | Reaction Type | Anode Catalyst | Substrate | Electrolyte | Product | 3-Electrode System | 2-Electrode System | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EOER at j10 (VRHE) | EOUR at j10 (VRHE) | VOER-HER at j10 | VOUR-HER at j10 | |||||||
| OUR||CO2RR on electricity-input mode | CuONS/CF | methanol | 1 M KOH | Formic acid | - | 1.47 at J100 | - | 0.93 | [99] | |
| CuSn | methanol | 1 M in 1 M KOH | formate | 1.76 at J200 | 1.49 at J200 | VOER-CO2RR: 3.84 at J100 | VMOR-CO2RR: 3.23 at J100 | [100] | ||
| PdOx/ZIF-8 | HMF | 20 mM in 0.5 M [Bmim]BF4, 1.0 M CH3CN | FDCA | FEco, 97% | FEorganic acid, 84.3% | - | - | [101] | ||
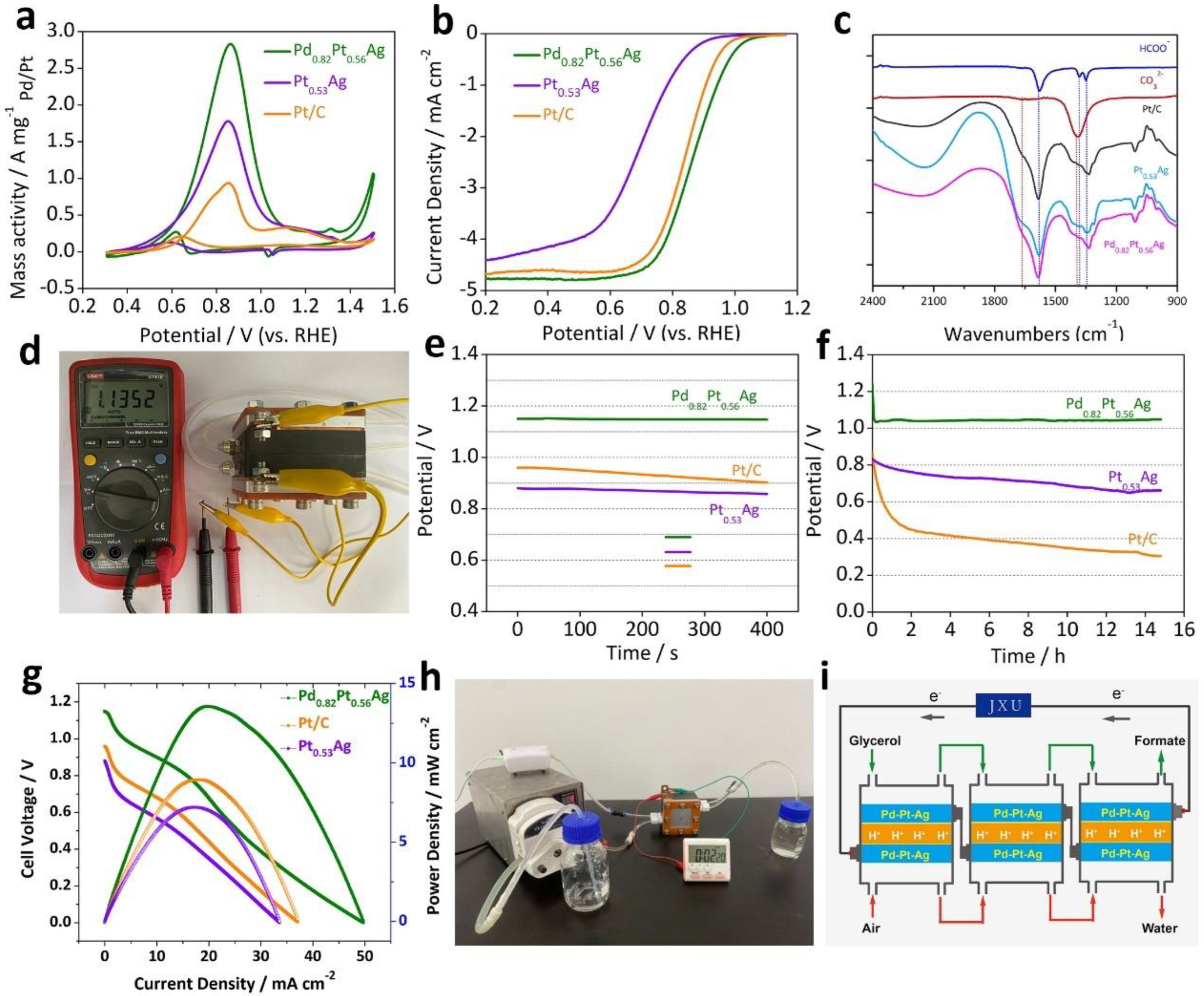
| OUR-based battery on electricity output mode | fuel cells | Pd0.82Pt0.56Ag | glycerol | 1 M in 0.5 M NaOH | formate | - | - | - | Vocp: 1.13 V | [32] |
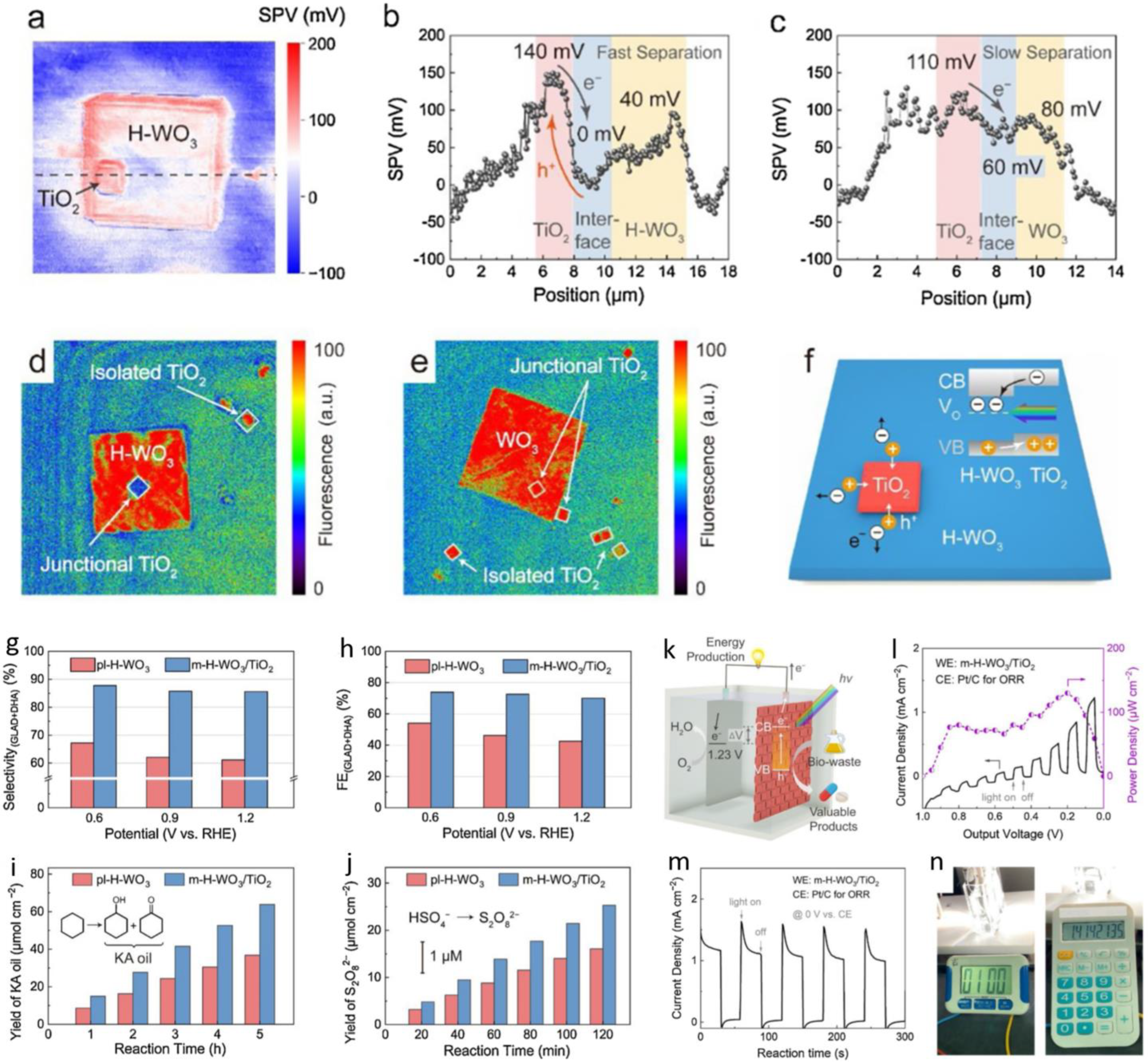
| WO3/TiO2 | glycerol | 0.5 M H2SO4 | GLA, DHA | - | - | - | Vocp: 0.9 V | [106] | ||
| Bi-Pt | glycerol | 0.1 M in 0.1 M KOH | glycolate and formate | - | - | - | Vocp: 1.0 V | [107] | ||
| Zinc–air battery | Co(OH)2@Ni(OH)2 | ethanol | 1.0 M | acetate | - | - | - | - | [33] | |
5. Conclusions and Future Perspectives
- Future work should focus on theory calculation-guided smart design and the precise synthesis of advanced catalysts. The prerequisite to realize the architecture is the advanced catalysts, especially for the anode oxidation reaction and CO2RR. The ideal catalysts must possess high intrinsic catalytic activity, large electrochemical surface areas, maximum utilization of catalytic sites, significant robustness, etc. Therefore, smart design of advanced catalysts is desirable. To screen the best catalysts, machine learning has been demonstrated to be effective. Besides the assistance of theory calculations, advanced synthesis and characterization methods are also crucial to obtain the target catalyst materials. Considering that noble metals are limited to scarce reserves in the Earth’s crust and their resulting high costs, earth-abundant metals and or carbon-based catalysts should be preferentially focused and developed;
- Future work should work towards the optimization of reaction conditions. Besides the catalyst materials, reaction conditions (e.g., the solvent, additives and temperature) have a great influence on the thermodynamics and kinetics of substrate adsorption and conversion, the target product desorption, on processes such as mass transfer and the microenvironment, and thus on the final catalytic efficiency. Therefore, the optimization of reaction conditions is quite necessary. If conditions render them necessary, theoretical simulations and in situ and/or operando technologies can be helpful;
- In situ and/or operando technology assisted the characterization and identification on the molecular/electronic level of the active sites, reaction pathways, important intermediates and the final structure-property relationship. In order to explore the advanced catalysts and improve the final catalytic efficiency, the study of the catalytic mechanism is indispensable. In situ and/or operando technologies (e.g., High Performance Liquid Chromatography (HPLC), Differential Electrochemical Mass Spectrometry (DEMS), Fourier-transform infrared spectroscopy (FTIR), Raman Spectra, X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS)) are powerful tools able to reveal the catalytic mechanism, which conversely guides the optimization of catalysts and reaction conditions;
- Electrolysis system optimization plays a key role in this research. The state-of-the-art systems also determine efficiency, cost, operability and the safety of these three strategies in both the laboratory and the industry. The design and optimization of systems include but are not limited to the basic cell units (e.g., electrode materials, current collector and diaphragm), feeding units, separation and purification units, controlling system, etc.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| OUR | Electrochemically oxidative upgrading reaction |
| OER | oxygen evolution reaction |
| HER | hydrogen evolution reaction |
| CO2RR | CO2 electroreduction reaction |
| PEM | proton exchange membrane |
| ROS | reactive oxygen species |
| MOR | methanol electrooxidation reaction |
| EOR | ethanol electrooxidation reaction |
| DEE | 1,1-diethoxyethane |
| GOR | Glycerol electrochemical oxidation |
| HMF | 5-hydroxymethylfurfural |
| FDCA | 2, 5-furandicarboxylic acid |
| SOR | electrooxidation of organic sulfides |
| AEOR | electrooxidation of alkene |
| LDH | Layered Double Hydroxide |
| GLAD | glyceraldehyde |
| DHA | 1, 3-dihydroxyacetone |
| KA oil | cyclohexanol and cyclohexanone |
| ORR | oxygen reduction reaction |
| j10 | 10 mA cm−2 |
References
- Deng, C.; Toe, C.Y.; Li, X.; Tan, J.; Yang, H.; Hu, Q.; He, C. Earth-Abundant Metal-Based Electrocatalysts Promoted Anodic Reaction in Hybrid Water Electrolysis for Efficient Hydrogen Production: Recent Progress and Perspectives. Adv. Energy Mater. 2022, 12, 2201047. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. Progress in Hydrogen Production Coupled with Electrochemical Oxidation of Small Molecules. Angew. Chem. Int. Ed. 2022, 61, e202213328. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Feng, X. Upgrading Organic Compounds through the Coupling of Electrooxidation with Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202209014. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Hung, S.-F.; Cao, A.; Wang, Z.; Karmodak, N.; Huang, J.E.; Yan, Y.; Sedighian Rasouli, A.; Ozden, A.; Wu, F.-Y. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 2022, 5, 1081–1088. [Google Scholar] [CrossRef]
- Du, H.-L.; Chatti, M.; Hodgetts, R.Y.; Cherepanov, P.V.; Nguyen, C.K.; Matuszek, K.; MacFarlane, D.R.; Simonov, A.N. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 2022, 609, 722–727. [Google Scholar] [CrossRef]
- Ali, T.; Wang, H.; Iqbal, W.; Bashir, T.; Shah, R.; Hu, Y. Electro-Synthesis of Organic Compounds with Heterogeneous Catalysis. Adv. Sci. 2022, 10, 2205077. [Google Scholar] [CrossRef] [PubMed]
- Schröder, V.; Emonts, B.; Janßen, H.; Schulze, H.P. Explosion Limits of Hydrogen/oxygen Mixtures at Initial Pressures up to 200 bar. Chem. Eng. Technol. 2004, 27, 847–851. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Kreuer, K.D.; Maier, J.; Müller, K. Chemical Degradation of Nafion Membranes under Mimic Fuel Cell Conditions as Investigated by Solid-State NMR Spectroscopy. J. Phys. Chem. C 2010, 114, 14635–14645. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Q.; Cai, J.; Ni, C.; Wang, Q.; Wang, Q.; Yang, J.; Geng, R.; Fang, Z. Synthesis of 3-Thiocyanobenzothiophene via Difunctionalization of Active Alkyne Promoted by Electrochemical-Oxidation. Chem. Eur. J. 2022, e202203306. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, Y.-N.; Sun, L.; Wang, Z.; Wang, W.; Jiang, L.; Tao, X.; Li, L.; Li, X.-H.; Zhao, G. Strain-induced in situ formation of NiOOH species on CoCo bond for selective electrooxidation of 5-hydroxymethylfurfural and efficient hydrogen production. Appl. Catal. B Environ. 2022, 305, 121072. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Z.; Liu, T.; Tao, L.; Tian, J.; Gu, K.; Wei, X.; Zhou, P.; Gan, L.; Du, S.; et al. Transforming Electrocatalytic Biomass Upgrading and Hydrogen Production from Electricity Input to Electricity Output. Angew. Chem. Int. Ed. 2021, 134, e202115636. [Google Scholar]
- Chong, X.; Liu, C.; Wang, C.; Yang, R.; Zhang, B. Integrating Hydrogen Production and Transfer Hydrogenation with Selenite Promoted Electrooxidation of α-Nitrotoluenes to E-Nitroethenes. Angew. Chem. Int. Ed. 2021, 133, 22181–22187. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Qin, B.; Gan, J.; Peng, X. Energy-efficient monosaccharides electrooxidation coupled with green hydrogen production by bifunctional Co9S8/Ni3S2 electrode. Chem. Eng. J. 2022, 446, 136950. [Google Scholar] [CrossRef]
- Xiang, M.; Xu, Z.; Wu, Q.; Wang, Y.; Yan, Z. Selective electrooxidation of primary amines over a Ni/Co metal-organic framework derived electrode enabling effective hydrogen production in the membrane-free electrolyzer. J. Power Sources 2022, 535, 231461. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Qi, Y.; Li, W.; Wang, C. Efficient Electrooxidation of 5-Hydroxymethylfurfural Using Co-Doped Ni3S2 Catalyst: Promising for H2 Production under Industrial-Level Current Density. Adv. Sci. 2022, 9, 2200957. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Zhang, C.; Ma, H.; Guo, Z.; Zhong, C.; Xu, M.; Wang, X.; Wang, Y.; Ma, H. Green Electrosynthesis of 5, 5′-Azotetrazolate Energetic Materials Plus Energy-Efficient Hydrogen Production Using Ruthenium Single-Atom Catalysts. Adv. Mater. 2022, 34, 2203900. [Google Scholar] [CrossRef]
- Liu, X.; Qin, H.; Wang, G.; Li, Q.; Huang, Q.; Wen, Z.; Mao, S. Co-doped Ni–Mo oxides: Highly efficient and robust electrocatalysts for urea electrooxidation assisted hydrogen production. J. Mater. Chem. A 2022, 10, 16825–16833. [Google Scholar] [CrossRef]
- Ding, X.; Pei, L.; Huang, Y.; Chen, D.; Xie, Z. Hollow NiCoP Nanoprisms Derived from Prussian Blue Analogues as Bifunctional Electrocatalysts for Urea-Assisted Hydrogen Production in Alkaline Media. Small 2022, 18, 2205547. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.Z. Stable and Highly Efficient Hydrogen Evolution from Seawater Enabled by an Unsaturated Nickel Surface Nitride. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef]
- Zhai, X.; Yu, Q.; Chi, J.; Wang, X.; Li, B.; Yang, B.; Li, Z.; Lai, J.; Wang, L. Accelerated dehydrogenation kinetics through Ru, Fe dual-doped Ni2P as bifunctional electrocatalyst for hydrazine-assisted self-powered hydrogen generation. Nano Energy 2023, 105, 108008. [Google Scholar] [CrossRef]
- Yousefi Rizi, H.A.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energy 2022, 15, 8246. [Google Scholar] [CrossRef]
- Du, J.; Xiang, D.; Zhou, K.; Wang, L.; Yu, J.; Xia, H.; Zhao, L.; Liu, H.; Zhou, W. Electrochemical hydrogen production coupled with oxygen evolution, organic synthesis, and waste reforming. Nano Energy 2022, 104, 107875. [Google Scholar] [CrossRef]
- Deng, X.; Xu, G.Y.; Zhang, Y.J.; Wang, L.; Zhang, J.; Li, J.F.; Fu, X.Z.; Luo, J.L. Understanding the Roles of Electrogenerated Co3+ and Co4+ in Selectivity-Tuned 5-Hydroxymethylfurfural Oxidation. Angew. Chem. Int. Ed. 2021, 133, 20698–20705. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, B.; Li, Y.; Zou, Y.; Chen, W.; Zhou, W.; Song, M.; Wu, Y.; Lu, Y.; Liu, J. Pt-modulated Redox Property and HMF Adsorption Kinetics of Ni (OH)2 for Biomass Upgrading. Angew. Chem. Int. Ed. 2021, 60, 22908–22914. [Google Scholar]
- Gu, K.; Wang, D.; Xie, C.; Wang, T.; Huang, G.; Liu, Y.; Zou, Y.; Tao, L.; Wang, S. Defect-Rich High-Entropy Oxide Nanosheets for Efficient 5-Hydroxymethylfurfural Electrooxidation. Angew. Chem. Int. Ed. 2021, 133, 20415–20420. [Google Scholar] [CrossRef]
- Yin, K.; Chao, Y.; Lv, F.; Tao, L.; Zhang, W.; Lu, S.; Li, M.; Zhang, Q.; Gu, L.; Li, H.; et al. One Nanometer PtIr Nanowires as High-Efficiency Bifunctional Catalysts for Electrosynthesis of Ethanol into High Value-Added Multicarbon Compound Coupled with Hydrogen Production. J. Am. Chem. Soc. 2021, 143, 10822–10827. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, D.; Ogihara, H.; Kurokawa, H. Upgrading of Ethanol to 1,1-Diethoxyethane by Proton-Exchange Membrane Electrolysis. ChemSusChem 2021, 14, 4431–4438. [Google Scholar] [CrossRef]
- Bellini, M.; Pagliaro, M.V.; Marchionni, A.; Filippi, J.; Miller, H.A.; Bevilacqua, M.; Lavacchi, A.; Oberhauser, W.; Mahmoudian, J.; Innocenti, M. Hydrogen and Chemicals from Alcohols through Electrochemical Reforming by Pd-CeO2/C Electrocatalyst. Inorg. Chim. Acta 2021, 518, 120245. [Google Scholar] [CrossRef]
- Verma, S.; Lu, S.; Kenis, P.J. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 2019, 4, 466–474. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Turk, S.; Latsuzbaia, R.; Bhardwaj, R.; Anastasopol, A.; Sastre-Calabuig, F.; Garcia, A.C.; Giling, E.; Goetheer, E. Electroreduction of CO2 to CO paired with 1, 2-propanediol oxidation to lactic acid. Toward an economically feasible system. Ind. Eng. Chem. Res. 2019, 58, 6195–6202. [Google Scholar] [CrossRef]
- Houache, M.S.; Safari, R.; Nwabara, U.O.; Rafaïdeen, T.; Botton, G.A.; Kenis, P.J.; Baranton, S.; Coutanceau, C.; Baranova, E.A. Selective Electrooxidation of Glycerol to Formic Acid over Carbon Supported Ni1–x M x (M = Bi, Pd, and Au) Nanocatalysts and Coelectrolysis of CO2. ACS Appl. Energy Mater. 2020, 3, 8725–8738. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Huang, L.; Zhang, X.; Gu, L.; Cao, Y.; Li, W.; Hu, J.; Cao, X. Co-generation of electricity and formate in glycerol fuel cells with a bifunctional PdPtAg alloy nanowire electrocatalyst. Green Chem. 2022, 24, 9721–9733. [Google Scholar] [CrossRef]
- Li, Z.; Ning, S.; Xu, J.; Zhu, J.; Yuan, Z.; Wu, Y.; Chen, J.; Xie, F.; Jin, Y.; Wang, N.; et al. In situ electrochemical activation of Co(OH)2@Ni(OH)2 heterostructures for efficient ethanol electrooxidation reforming and innovative zinc–ethanol–air batteries. Energy Environ. Sci. 2022, 15, 5300–5312. [Google Scholar]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Wang, H.; Jusys, Z.; Behm, R.J. Adsorption and electrooxidation of ethylene glycol and its C2 oxidation products on a carbon-supported Pt catalyst: A quantitative DEMS study. Electrochim. Acta 2009, 54, 6484–6498. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Recent achievements in direct ethylene glycol fuel cells (DEGFC). Appl. Catal. B Environ. 2010, 97, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liu, T.; Ma, X.; Feng, Y.; Xu, B.; Cai, W.; Li, Y.; Su, D.; Shao, Q. Rhombohedral Pd–Sb Nanoplates with Pd-Terminated Surface: An Efficient Bifunctional Fuel-Cell Catalyst. Adv. Mater. 2022, 34, 2202333. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Yang, Y.; Fu, G.; Si, F.; Chen, J.; Ahmad, M.; Zhang, Z.; Ye, C.; Zhang, J. Ni2P with phosphorus vacancy supported Pt clusters for efficiently electrocatalytic co-production of hydrogen and value-added chemicals from methanol-water at low potential. Chem. Eng. J. 2023, 452, 139057. [Google Scholar] [CrossRef]
- Li, M.; Zhang, D.; Yi, Y.; Xue, B.; Liu, B. Boosting Anodic Methanol Upgrading over RuO2 through Integration with CeO2 for Energy-saving H2 Generation in Acidic Environment. Electrochim. Acta 2022, 423, 140566. [Google Scholar] [CrossRef]
- Chen, J.; Ahmad, M.; Zhang, Y.; Ye, H.; Wang, L.; Zhang, J.; Fu, X.-Z.; Luo, J.-L. Versatile Mo modulation effects enable efficient electrocatalytic aqueous methanol electro-reforming over surface-engineered NiCoMo alloy. Chem. Eng. J. 2023, 454, 140056. [Google Scholar] [CrossRef]
- Xiang, K.; Wu, D.; Deng, X.; Li, M.; Chen, S.; Hao, P.; Guo, X.; Luo, J.L.; Fu, X.Z. Boosting H2 generation coupled with selective oxidation of methanol into value-added chemical over cobalt hydroxide@hydroxysulfide nanosheets electrocatalysts. Adv. Funct. Mater. 2020, 30, 1909610. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Wang, M.; Ren, T.; Ren, K.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Methanol electroreforming coupled to green hydrogen production over bifunctional NiIr-based metal-organic framework nanosheet arrays. Appl. Catal. B Environ. 2022, 300, 120753. [Google Scholar] [CrossRef]
- Fu, G.; Kang, X.; Zhang, Y.; Yang, X.; Wang, L.; Fu, X.-Z.; Zhang, J.; Luo, J.-L.; Liu, J. Coordination effect-promoted durable Ni (OH)2 for energy-saving hydrogen evolution from water/methanol co-electrocatalysis. Nano-Micro Lett. 2022, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, J.; Wu, M.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. PdAg@Pd core-shell nanotubes: Superior catalytic performance towards electrochemical oxidation of formic acid and methanol. J. Power Sources 2018, 398, 201–208. [Google Scholar] [CrossRef]
- Huang, L.; Zou, J.; Ye, J.Y.; Zhou, Z.Y.; Lin, Z.; Kang, X.; Jain, P.K.; Chen, S. Synergy between plasmonic and electrocatalytic activation of methanol oxidation on palladium–silver alloy nanotubes. Angew. Chem. Int. Ed. 2019, 131, 8886–8890. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, A.; Dorado, F.; Sanchez, P.; De La Osa, A.R. Boosting hydrogen and chemicals production through ethanol electro-reforming on Pt-transition metal anodes. J. Energy Chem. 2022, 70, 394–406. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, L.; Pan, X.; Zhou, Z.; Zheng, Y.; Yang, X.; Zhao, G. Carbon paper supported gold nanoflowers for tunable glycerol electrooxidation boosting efficient hydrogen evolution. Carbon 2023, 203, 88–96. [Google Scholar] [CrossRef]
- Miao, Y.; Li, Z.; Song, Y.; Fan, K.; Guo, J.; Li, R.; Shao, M. Surface active oxygen engineering of photoanodes to boost photoelectrochemical water and alcohol oxidation coupled with hydrogen production. Appl. Catal. B Environ. 2023, 323, 122147. [Google Scholar] [CrossRef]
- Li, S.; Xie, W.; Song, Y.; Li, Y.; Song, Y.; Li, J.; Shao, M. Integrated CoPt electrocatalyst combined with upgrading anodic reaction to boost hydrogen evolution reaction. Chem. Eng. J. 2022, 437, 135473. [Google Scholar] [CrossRef]
- He, Z.; Hwang, J.; Gong, Z.; Zhou, M.; Zhang, N.; Kang, X.; Han, J.W.; Chen, Y. Promoting biomass electrooxidation via modulating proton and oxygen anion deintercalation in hydroxide. Nat. Commun. 2022, 13, 3777. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, X.; Zou, S.; Cai, W. Recent Advances in Glycerol Electrooxidation on Pt and Pd: From Reaction Mechanisms to Catalytic Materials. J. Electrochem. 2021, 27, 233–256. [Google Scholar]
- Qiao, B.; Yang, T.; Shi, S.; Jia, N.; Chen, Y.; Chen, X.; An, Z.; Chen, P. Highly Active Hollow RhCu Nanoboxes toward Ethylene Glycol Electrooxidation. Small 2021, 17, 2006534. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, S.; Meng, X.; Zeng, J.; Yin, S.; Li, X.; Chen, Y. Ultrathin Rh nanosheets as a highly efficient bifunctional electrocatalyst for isopropanol-assisted overall water splitting. Nanoscale 2019, 11, 9319–9326. [Google Scholar] [CrossRef]
- Mahmoudian, J.; Bellini, M.; Pagliaro, M.V.; Oberhauser, W.; Innocenti, M.; Vizza, F.; Miller, H.A. Electrochemical coproduction of acrylate and hydrogen from 1, 3-propandiol. ACS Sustain. Chem. Eng. 2017, 5, 6090–6098. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Z.; Teng, X.; Niu, Y.; Gong, S.; Chen, W.; Meyer, T.J.; Chen, Z. Paired formate and H2 productions via efficient bifunctional Ni-Mo nitride nanowire electrocatalysts. J. Energy Chem. 2022, 72, 432–441. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Hu, M.-K.; Wei, W.; Zhou, S.-H.; Wu, X.-T.; Zhu, Q.-L. Ordered macroporous superstructure of bifunctional cobalt phosphide with heteroatomic modification for paired hydrogen production and polyethylene terephthalate plastic recycling. Appl. Catal. B Environ. 2022, 316, 121667. [Google Scholar] [CrossRef]
- Si, D.; Xiong, B.; Chen, L.; Shi, J. Highly selective and efficient electrocatalytic synthesis of glycolic acid in coupling with hydrogen evolution. Chem. Catal. 2021, 1, 941–955. [Google Scholar] [CrossRef]
- Ming, L.; Wu, X.-Y.; Wang, S.-S.; Wu, W.; Lu, C.-Z. Facile growth of transition metal hydroxide nanosheets on porous nickel foam for efficient electrooxidation of benzyl alcohol. Green Chem. 2021, 23, 7825–7830. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Y.; Xu, S.M.; Zhou, H.; Xu, M.; Ma, L.; Shao, M.; Kong, X.; Wang, B.; Zheng, L.; et al. Alcohols electrooxidation coupled with H2 production at high current densities promoted by a cooperative catalyst. Nat. Commun. 2022, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kuang, P.; Wageh, S.; Al-Ghamdi, A.A.; Tang, H.; Yu, J. Potential-dependent reconstruction of Ni-based cuboid arrays for highly efficient hydrogen evolution coupled with electro-oxidation of organic compound. Chem. Eng. J. 2023, 453, 139797. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Han, X.; Huang, H.; Wei, Q.; Guo, W.; Wang, Z.; Qiu, J. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm−2. Energy Environ. Sci. 2020, 13, 4990–4999. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Grabowski, G.; Lewkowski, J.; Skowroński, R. The Electrochemical Oxidation of 5-hydroxymethylfurfural with the Nickel Oxide/hydroxide Electrode. Electrochim. Acta 1991, 36, 1995. [Google Scholar] [CrossRef]
- Cha, H.G.; Choi, K.-S. Combined Biomass Valorization and Hydrogen Production in a Photoelectrochemical Cell. Nat. Chem. 2015, 7, 328–333. [Google Scholar] [CrossRef]
- Taitt, B.J.; Nam, D.-H.; Choi, K.-S. A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic Acid. ACS Catal. 2018, 9, 660–670. [Google Scholar] [CrossRef]
- Zhang, N.; Zou, Y.; Tao, L.; Chen, W.; Zhou, L.; Liu, Z.; Zhou, B.; Huang, G.; Lin, H.; Wang, S. Electrochemical Oxidation of 5-Hydroxymethylfurfural on Nickel Nitride/Carbon Nanosheets: Reaction Pathway Determined by In Situ Sum Frequency Generation Vibrational Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 15895–15903. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Boonstra, R.; Terrero Rodriguez, I.M.; Sun, Y. Integrating Electrocatalytic 5-hydroxymethylfurfural Oxidation and Hydrogen Production via Co–P-derived Electrocatalysts. ACS Energy Lett. 2016, 1, 386–390. [Google Scholar] [CrossRef]
- Barwe, S.; Weidner, J.; Cychy, S.; Morales, D.M.; Dieckhofer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic Oxidation of 5-(Hydroxymethyl)furfural Using High-Surface-Area Nickel Boride. Angew. Chem. Int. Ed. 2018, 57, 11460–11464. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, F.; Chen, L.; Li, Y.; Shen, K. Superior bifunctional cobalt/nitrogen-codoped carbon nanosheet arrays on copper foam enable stable energy-saving hydrogen production accompanied with glucose upgrading. Green Chem. 2022, 24, 6544–6555. [Google Scholar] [CrossRef]
- Zhao, L.; Kuang, X.; Sun, X.; Zhang, Y.; Wei, Q. Synchronously achieving highly efficient hydrogen evolution and high-yield synthesis of glucaric acid by MOF nanorod arrays. J. Electrochem. Soc. 2019, 166, H534. [Google Scholar] [CrossRef]
- Hao, S.; Yang, L.; Liu, D.; Kong, R.M.; Du, G.; Asiri, A.M.; Yang, Y.; Sun, X. Integrating natural biomass electro-oxidation and hydrogen evolution: Using porous Fe-doped CoP nanosheets array as a bifunctional catalyst. Chem. Commun. 2017, 53, 5710–5713. [Google Scholar] [CrossRef] [PubMed]
- Svadkovskaya, G.; Voitkevich, S. Electrolytic condensation of carboxylic acids. Russ. Chem. Rev. 1960, 29, 161. [Google Scholar] [CrossRef]
- Schäfer, H.J. Anodic and Cathodic CC-Bond Formation. Angew. Chem. Int. Ed. 1981, 20, 911–934. [Google Scholar] [CrossRef]
- Chen, N.; Ye, Z.; Zhang, F. Recent progress on electrochemical synthesis involving carboxylic acids. Org. Biomol. Chem. 2021, 19, 5501–5520. [Google Scholar] [CrossRef]
- Häring, A.P.; Pollok, D.; Strücker, B.R.; Kilian, V.; Schneider, J.; Waldvogel, S.R. Beyond Kolbe and Hofer–Moest: Electrochemical Synthesis of Carboxylic Anhydrides from Carboxylic Acids. ChemistryOpen 2022, 11, e202200059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, M.; Zhang, X.; He, M.-K.; Yang, C.; Guo, L.; Xia, W. Electrochemical synthesis of functionalized gem-difluoroalkenes with diverse alkyl sources via a defluorinative alkylation process. Org. Chem. Front. 2022, 9, 95–101. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Hioki, Y.; Oderinde, M.S.; Qiao, J.X.; Rodriguez, K.X.; Zhang, H.-J.; Kawamata, Y.; Baran, P.S. Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling. Nature 2022, 606, 313–318. [Google Scholar] [CrossRef]
- Becking, L.; Schäfer, H.J. Synthesis of a prostaglandine precursor by mixed kolbe electrolysis of 3-(cyclopent-2-enyloxy)propionate. Tetrahedron Lett. 1988, 29, 2801–2802. [Google Scholar] [CrossRef]
- Schierle, K.; Hopke, J.; Niedt, M.L.; Boland, W.; Steckhan, E. Homologues of dihydro-12-oxo-phytodienoic acid and jasmonic acid by mixed kolbe electrolysis. Tetrahedron Lett. 1996, 37, 8715–8718. [Google Scholar] [CrossRef]
- Harenbrock, M.; Matzeit, A.; Schäfer, H.J. Anodic heterocoupling (mixed Kolbe electrolysis) of carbohydrate carboxylic acids with alkanoic acids to C-glycosides. Liebigs Ann. 1996, 1996, 55–62. [Google Scholar] [CrossRef]
- Quertenmont, M.; Goodall, I.; Lam, K.; Markó, I.; Riant, O. Kolbe Anodic Decarboxylation as a Green Way to Access 2-Pyrrolidinones. Org. Lett. 2020, 22, 1771–1775. [Google Scholar] [CrossRef]
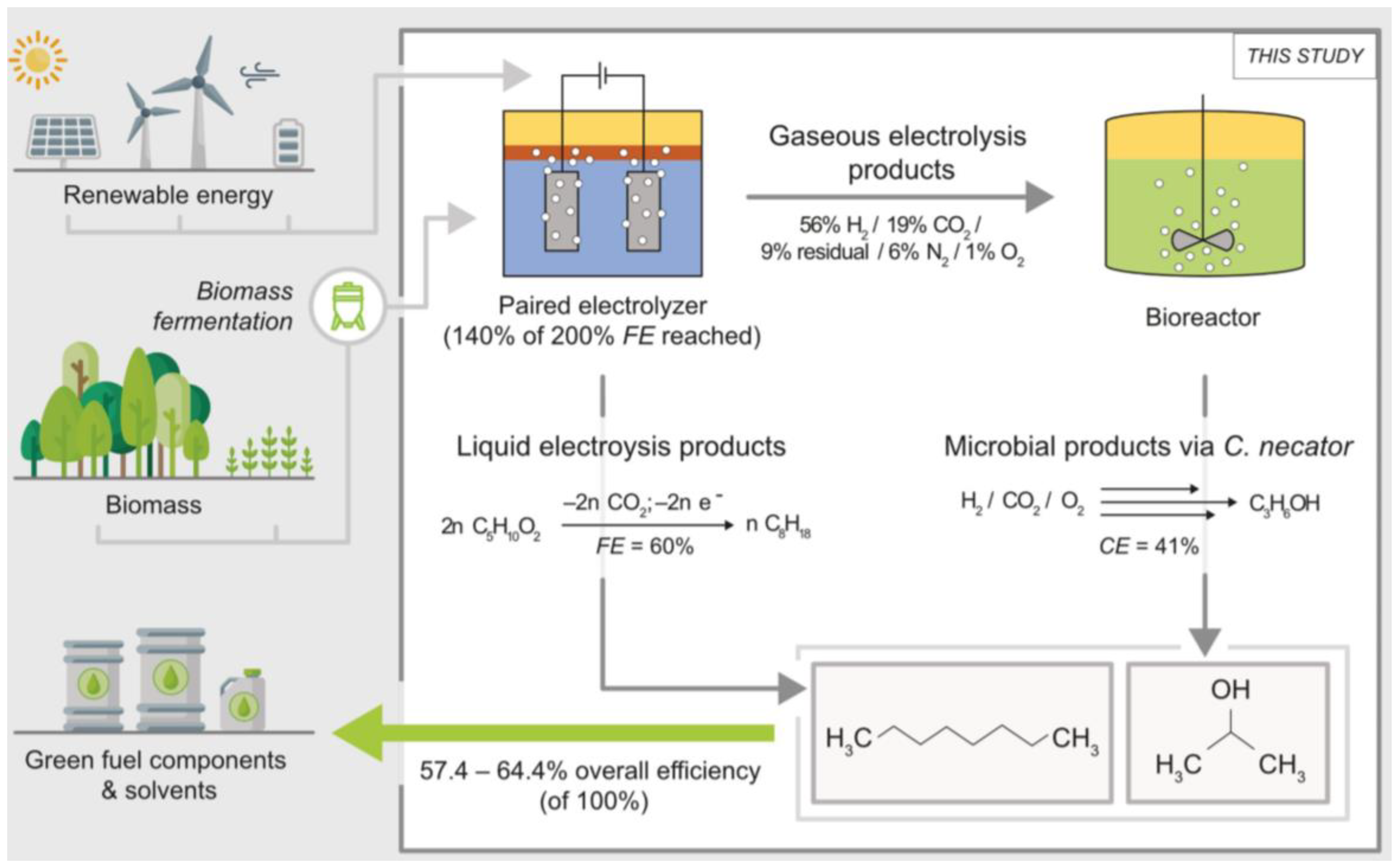
- Teetz, N.; Holtmann, D.; Harnisch, F.; Stöckl, M. Upgrading Kolbe Electrolysis—Highly Efficient Production of Green Fuels and Solvents by Coupling Biosynthesis and Electrosynthesis. Angew. Chem. Int. Ed. 2022, 134, e202210596. [Google Scholar] [CrossRef]
- Huang, Y.; Chong, X.; Liu, C.; Liang, Y.; Zhang, B. Boosting Hydrogen Production by Anodic Oxidation of Primary Amines over a NiSe Nanorod Electrode. Angew Chem. Int. Ed. 2018, 57, 13163–13166. [Google Scholar] [CrossRef] [PubMed]
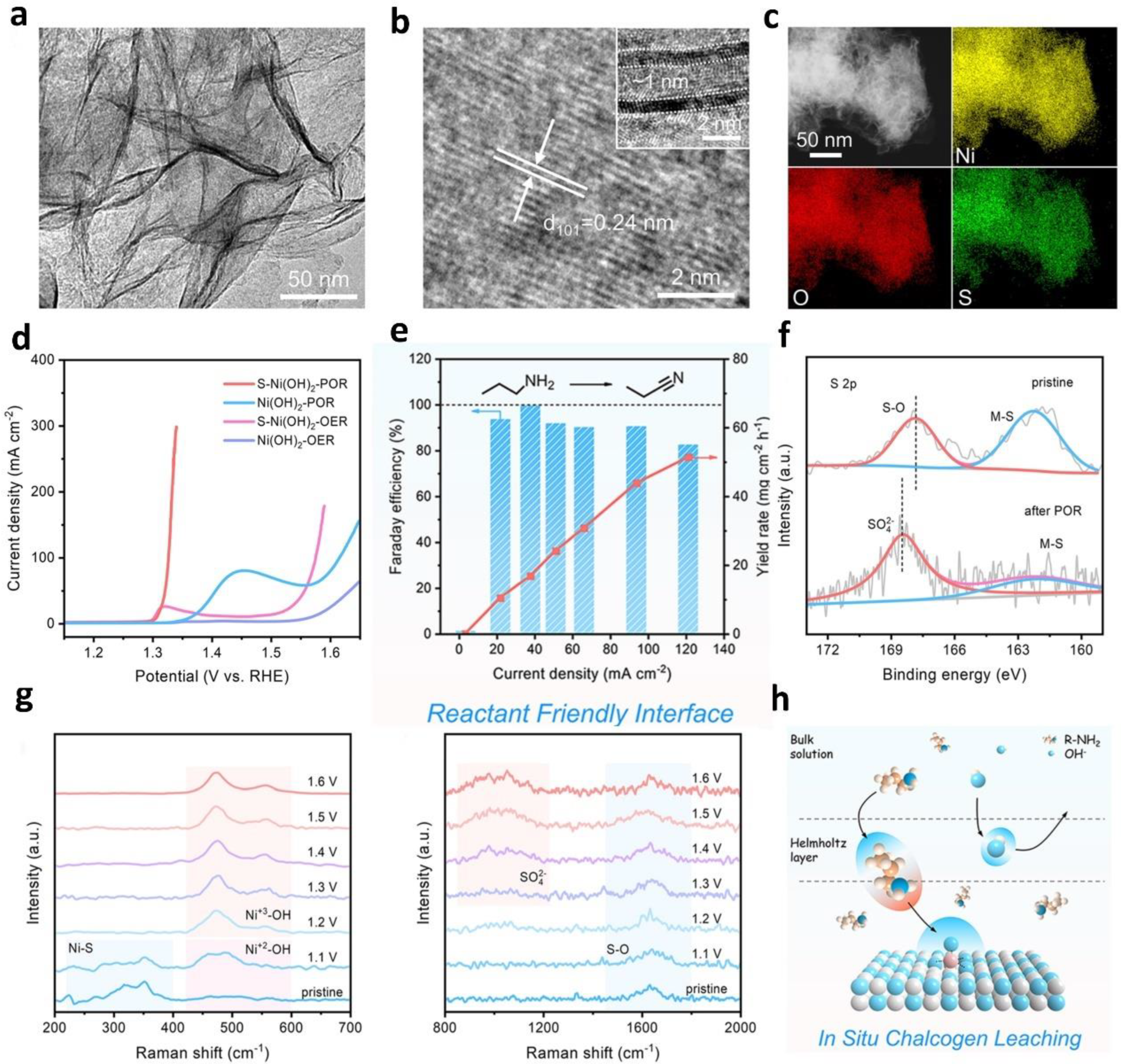
- Wen, Q.; Lin, Y.; Yang, Y.; Gao, R.; Ouyang, N.; Ding, D.; Liu, Y.; Zhai, T. In Situ Chalcogen Leaching Manipulates Reactant Interface toward Efficient Amine Electrooxidation. ACS Nano 2022, 16, 9572–9582. [Google Scholar] [CrossRef]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Fu, Z.-H.; Tian, H.-D.; Ni, S.-F.; Wright, J.S.; Li, M.; Wen, L.-R.; Zhang, L.-B. Scalable selective electrochemical oxidation of sulfides to sulfoxides. Green Chem. 2022, 24, 4772–4777. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, H.; Xu, M.; Hao, P.; Kong, X.; Duan, H. Integrating hydrogen production with anodic selective oxidation of sulfides over a CoFe layered double hydroxide electrode. Chem. Sci. 2021, 12, 938–945. [Google Scholar] [CrossRef]
- Laudadio, G.; Straathof, N.J.; Lanting, M.D.; Knoops, B.; Hessel, V.; Noël, T. An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor. Green Chem. 2017, 19, 4061–4066. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, S.-H.; Jin, R.; Qiu, X.; Wei, J.; Tan, H.; Jiang, X.; Shi, X.; Song, S.; Jiao, N. Electrochemically induced nickel catalysis for oxygenation reactions with water. Nat. Catal. 2021, 4, 116–123. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, Q.; Zhang, T.-T.; Li, Y.; Li, J.-H. Electrochemical oxygenation of sulfides with molecular oxygen or water: Switchable preparation of sulfoxides and sulfones. Org. Biomol. Chem. 2021, 19, 10314–10318. [Google Scholar] [CrossRef]
- Paim, L.L.; Stradiotto, N.R. Electrooxidation of sulfide by cobalt pentacyanonitrosylferrate film on glassy carbon electrode by cyclic voltammetry. Electrochim. Acta 2010, 55, 4144–4147. [Google Scholar] [CrossRef]
- Sharma, A.S.; Sharma, V.S.; Kaur, H.; Varma, R.S. Supported heterogeneous nanocatalysts in sustainable, selective and eco-friendly epoxidation of olefins. Green Chem. 2020, 22, 5902–5936. [Google Scholar] [CrossRef]
- Moyer, B.A.; Thomas, M.S.; Meyer, T.J. Chemically catalyzed net electrochemical oxidation of alcohols, aldehydes, and unsaturated hydrocarbons using the system (trpy)(bpy)Ru(OH2)2+/(trpy)(bpy)RuO2+. J. Am. Chem. Soc. 1980, 102, 2310–2312. [Google Scholar] [CrossRef]
- Lum, Y.; Huang, J.E.; Wang, Z.; Luo, M.; Nam, D.H.; Leow, W.R.; Chen, B.; Wicks, J.; Li, Y.C.; Wang, Y.; et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glyco. Nat. Catal. 2020, 3, 14–22. [Google Scholar] [CrossRef]
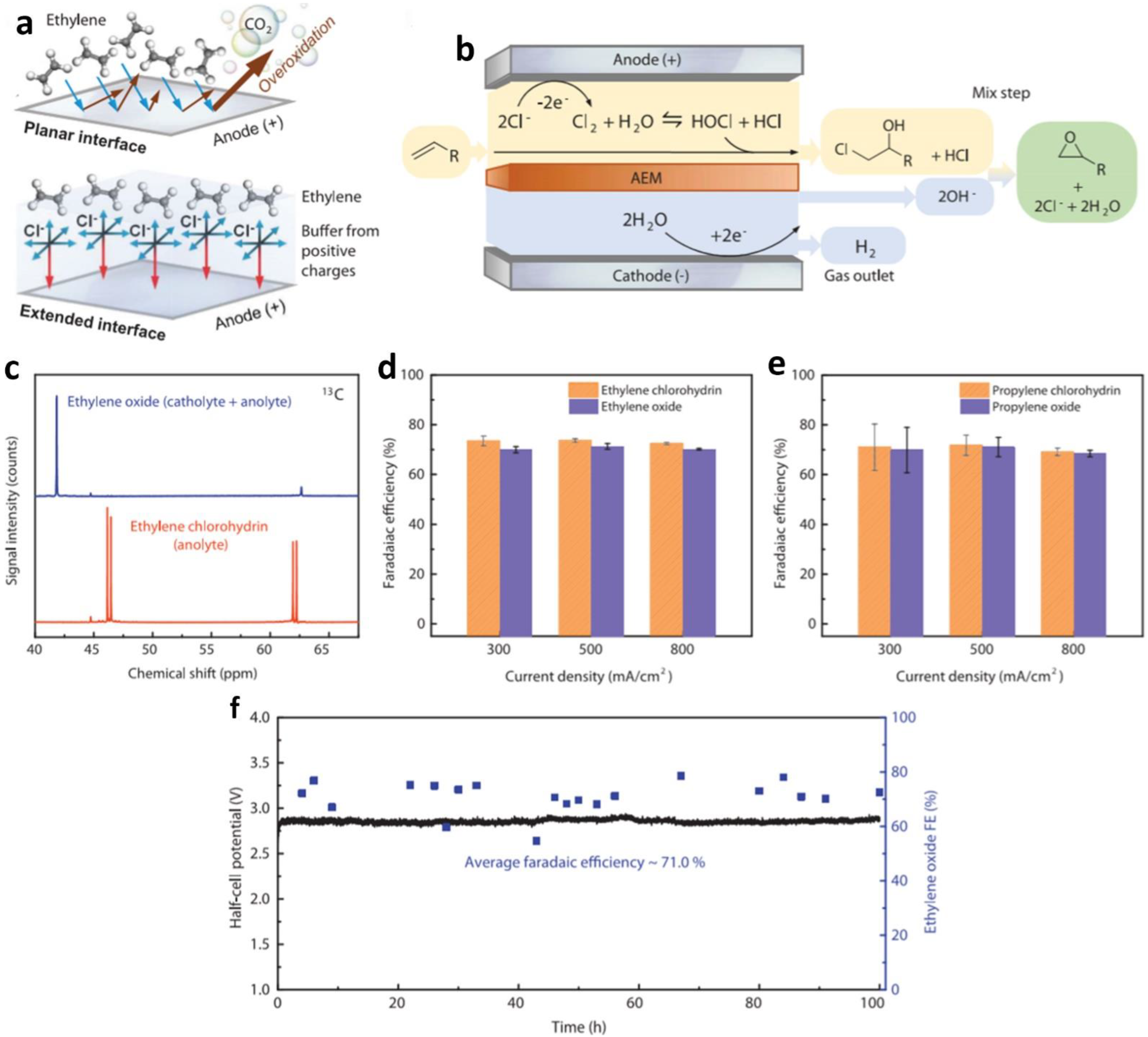
- Leow, W.R.; Lum, Y.; Ozden, A.; Wang, Y.; Nam, D.H.; Chen, B.; Wicks, J.; Zhuang, T.-T.; Li, F.; Sinton, D.; et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 2020, 368, 1228–1233. [Google Scholar] [CrossRef]
- Li, Y.; Ozden, A.; Leow, W.R.; Ou, P.; Huang, J.E.; Wang, Y.; Bertens, K.; Xu, Y.; Liu, Y.; Roy, C.; et al. Redox-mediated electrosynthesis of ethylene oxide from CO2 and water. Nat. Catal. 2022, 5, 185–192. [Google Scholar] [CrossRef]
- Vass, Á.; Kormányos, A.; Kószó, Z.; Endrődi, B.; Janáky, C. Anode Catalysts in CO2 Electrolysis: Challenges and Untapped Opportunities. ACS Catal. 2022, 12, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Bajada, M.A.; Roy, S.; Warnan, J.; Abdiaziz, K.; Wagner, A.; Roessler, M.M.; Reisner, E. A Precious-Metal-Free Hybrid Electrolyzer for Alcohol Oxidation Coupled to CO2-to-Syngas Conversion. Angew. Chem. Int. Ed. 2020, 59, 15633–15641. [Google Scholar] [CrossRef]
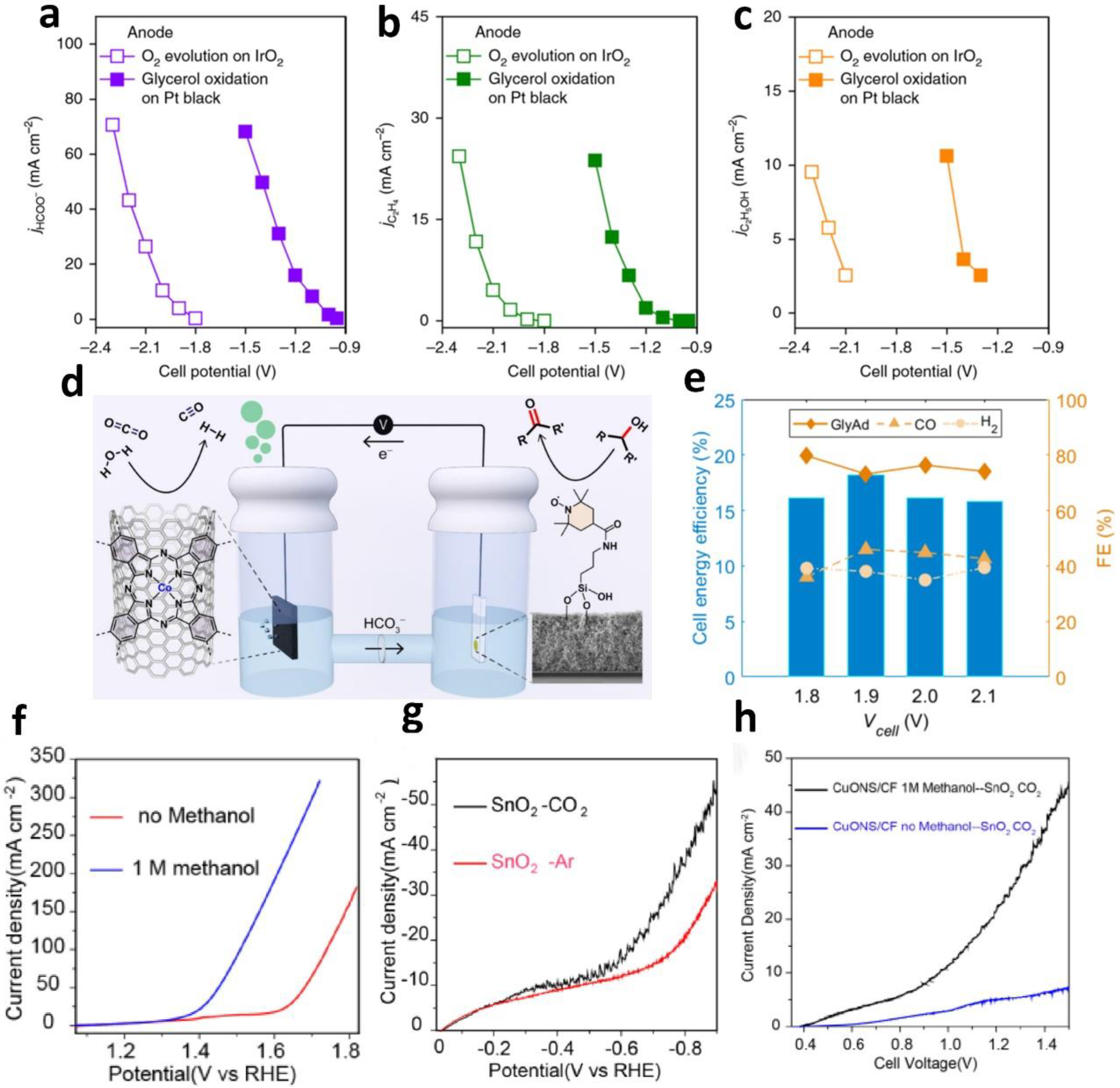
- Wei, X.; Li, Y.; Chen, L.; Shi, J. Formic acid electro-synthesis by concurrent cathodic CO2 reduction and anodic CH3OH oxidation. Angew. Chem. Int. Ed. 2021, 60, 3148–3155. [Google Scholar] [CrossRef]
- Li, Y.; Huo, C.-Z.; Wang, H.-J.; Ye, Z.-X.; Luo, P.-P.; Cao, X.-X.; Lu, T.-B. Coupling CO2 reduction with CH3OH oxidation for efficient electrosynthesis of formate on hierarchical bifunctional CuSn alloy. Nano Energy 2022, 98, 107277. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, Q.; Guo, W.; Li, P.; Jia, S.; Liu, J.; Ma, J.; Zhang, J.; Liu, Z.; Han, B. Engineering, Simultaneous CO2 Reduction and 5-Hydroxymethylfurfural Oxidation to Value-Added Products by Electrocatalysis. ACS Sustain. Chem. Eng. 2022, 10, 8043–8050. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Park, J.E.; Kim, S.; Kim, O.-H.; Hwang, W.; Her, M.; Kang, S.Y.; Park, S.; Kwon, O.J.; Park, H.S. Differences in the electrochemical performance of Pt-based catalysts used for polymer electrolyte membrane fuel cells in liquid half-and full-cells. Chem. Rev. 2021, 121, 15075–15140. [Google Scholar] [CrossRef]
- He, Y.; Wu, G. PGM-free oxygen-reduction catalyst development for proton-exchange membrane fuel cells: Challenges, solutions, and promises. Acc. Mater Res. 2022, 3, 224–236. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Zachman, M.J.; Zeng, Y.; Yu, H.; Li, B.; Wang, M.; Braaten, J.; Liu, J.; Meyer, H.M. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 2022, 7, 652–663. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Yu, R.; Xia, L.; Yang, J.; Lang, Z.; Zhu, J.; Huang, J.; Wang, J.; Wang, Y. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat. Catal. 2022, 5, 300–310. [Google Scholar] [CrossRef]
- Gu, Z.; An, X.; Liu, R.; Xiong, L.; Tang, J.; Hu, C.; Liu, H.; Qu, J. Interface-modulated nanojunction and microfluidic platform for photoelectrocatalytic chemicals upgrading. Appl. Catal. B Environ. 2021, 282, 119541. [Google Scholar] [CrossRef]
- de Souza, M.B.; Guima, K.-E.; Fernández, P.S.; Martins, C.A. Glycerol Is Converted into Energy and Carbonyl Compounds in a 3D-Printed Microfluidic Fuel Cell: In Situ and In Operando Bi-Modified Pt Anodes. ACS Appl. Mater. Interfaces 2022, 14, 25457–25465. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Chen, K.; Wang, J.; Huang, L.; Dang, C.; Gu, L.; Cao, X. Killing Two Birds with One Stone: Upgrading Organic Compounds via Electrooxidation in Electricity-Input Mode and Electricity-Output Mode. Materials 2023, 16, 2500. https://doi.org/10.3390/ma16062500
Ma J, Chen K, Wang J, Huang L, Dang C, Gu L, Cao X. Killing Two Birds with One Stone: Upgrading Organic Compounds via Electrooxidation in Electricity-Input Mode and Electricity-Output Mode. Materials. 2023; 16(6):2500. https://doi.org/10.3390/ma16062500
Chicago/Turabian StyleMa, Jiamin, Keyu Chen, Jigang Wang, Lin Huang, Chenyang Dang, Li Gu, and Xuebo Cao. 2023. "Killing Two Birds with One Stone: Upgrading Organic Compounds via Electrooxidation in Electricity-Input Mode and Electricity-Output Mode" Materials 16, no. 6: 2500. https://doi.org/10.3390/ma16062500
APA StyleMa, J., Chen, K., Wang, J., Huang, L., Dang, C., Gu, L., & Cao, X. (2023). Killing Two Birds with One Stone: Upgrading Organic Compounds via Electrooxidation in Electricity-Input Mode and Electricity-Output Mode. Materials, 16(6), 2500. https://doi.org/10.3390/ma16062500






