Microstructure and Mechanical Properties of Spark Plasma Sintered CoCrFeNiNbX High-Entropy Alloys with Si Addition
Abstract
1. Introduction
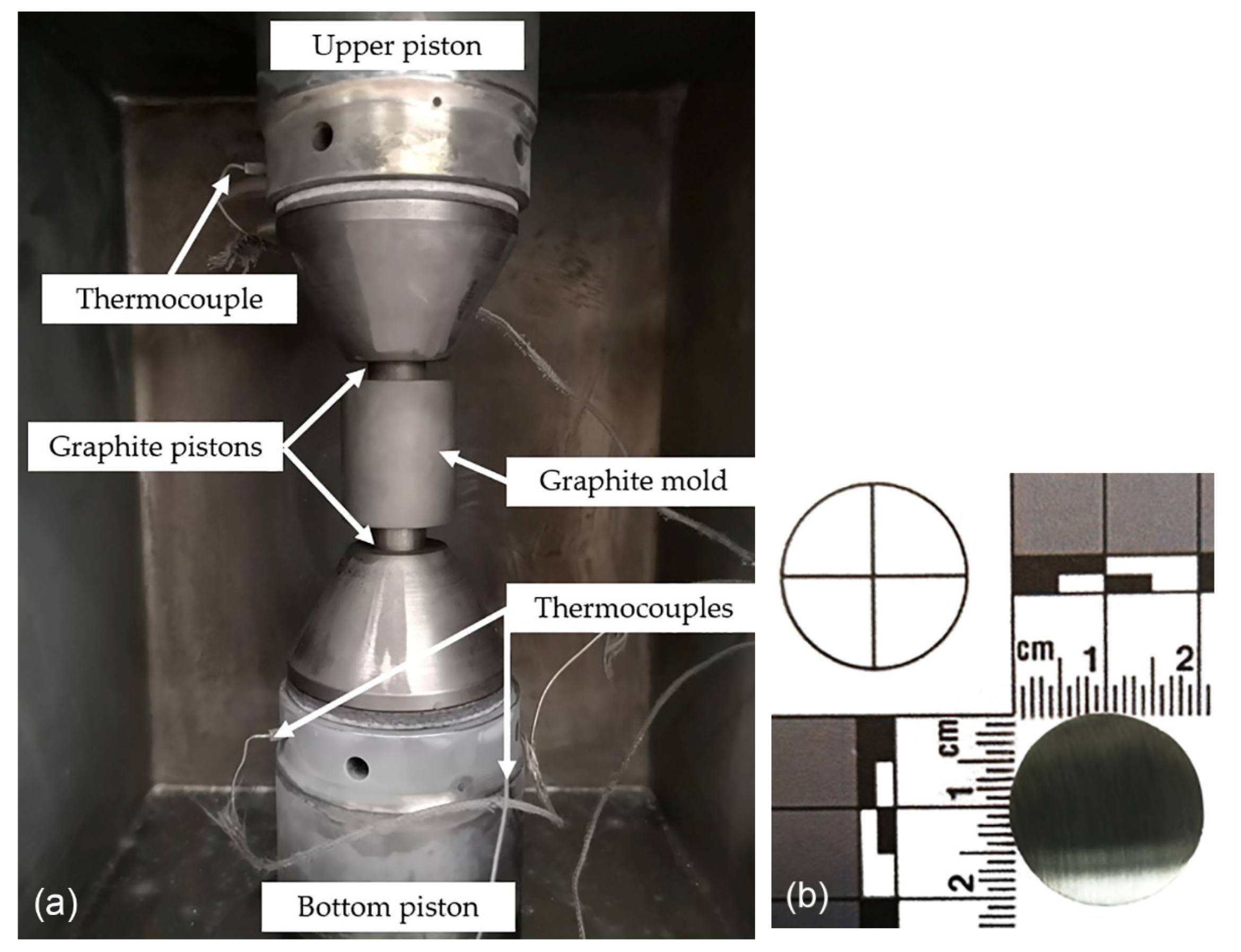
2. Materials and Methods
3. Results and Discussion
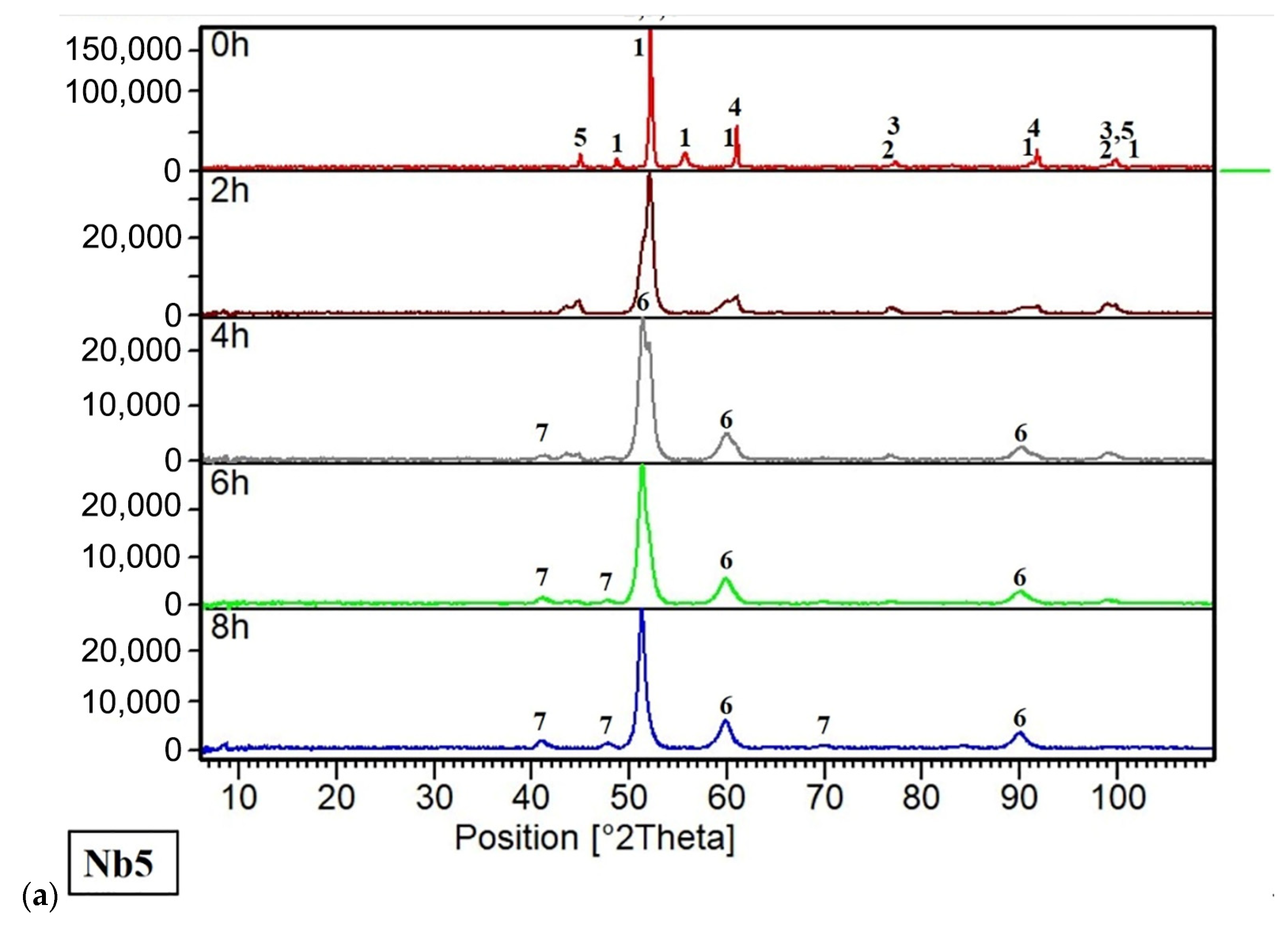
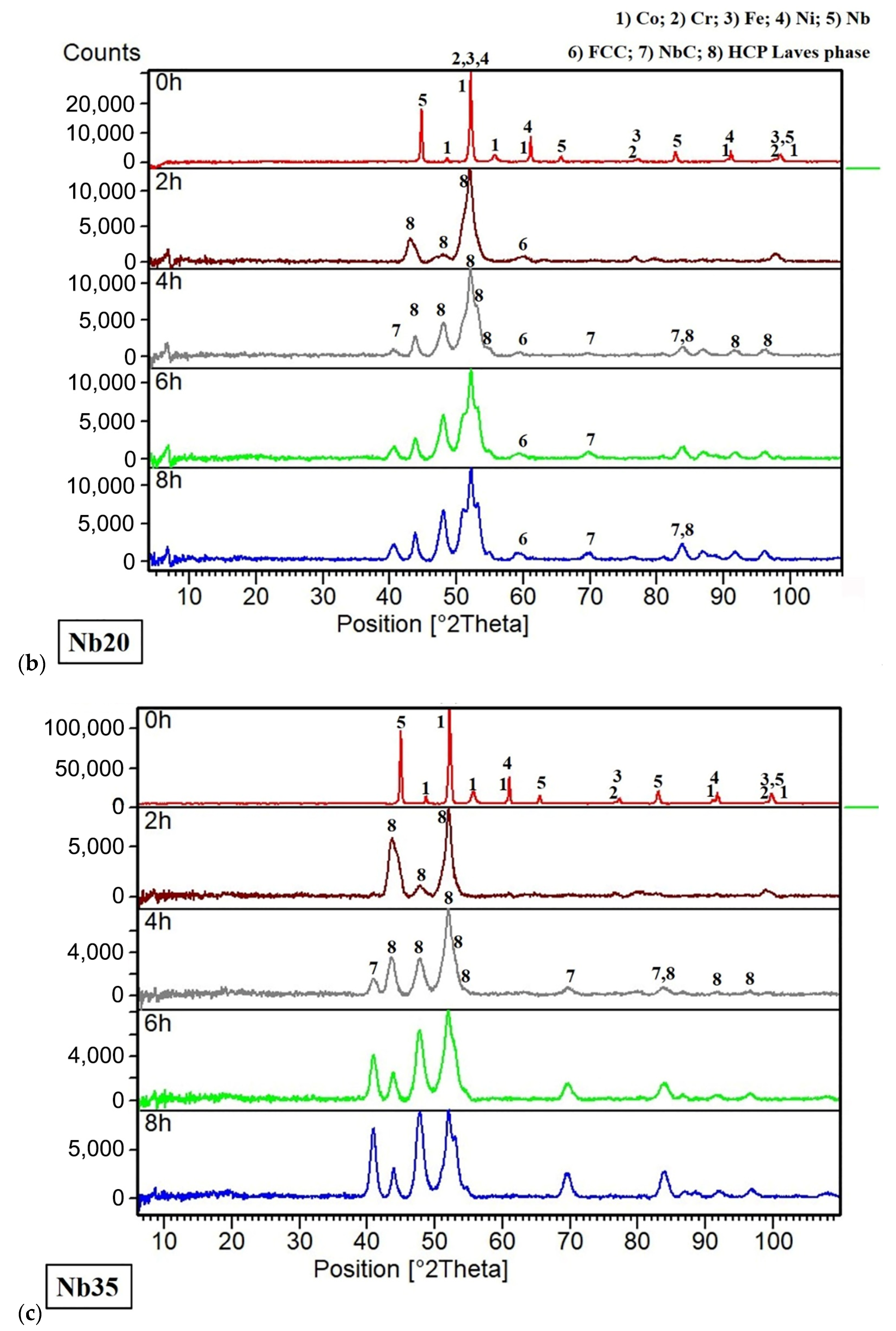
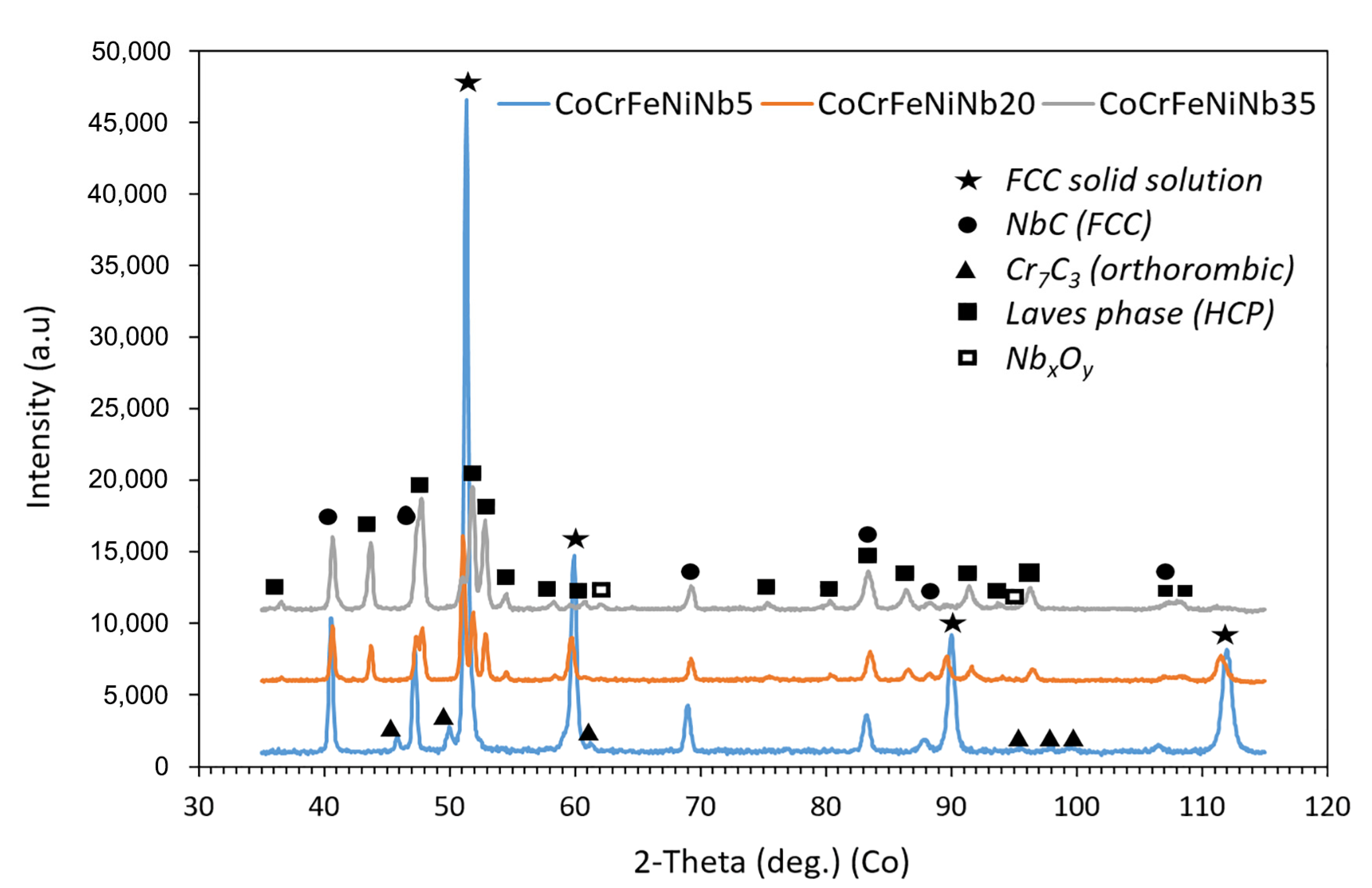
3.1. Chemical and Phase Composition
3.1.1. Powders and Compacts
3.1.2. Annealed Compacts
3.2. Mechanical Properties
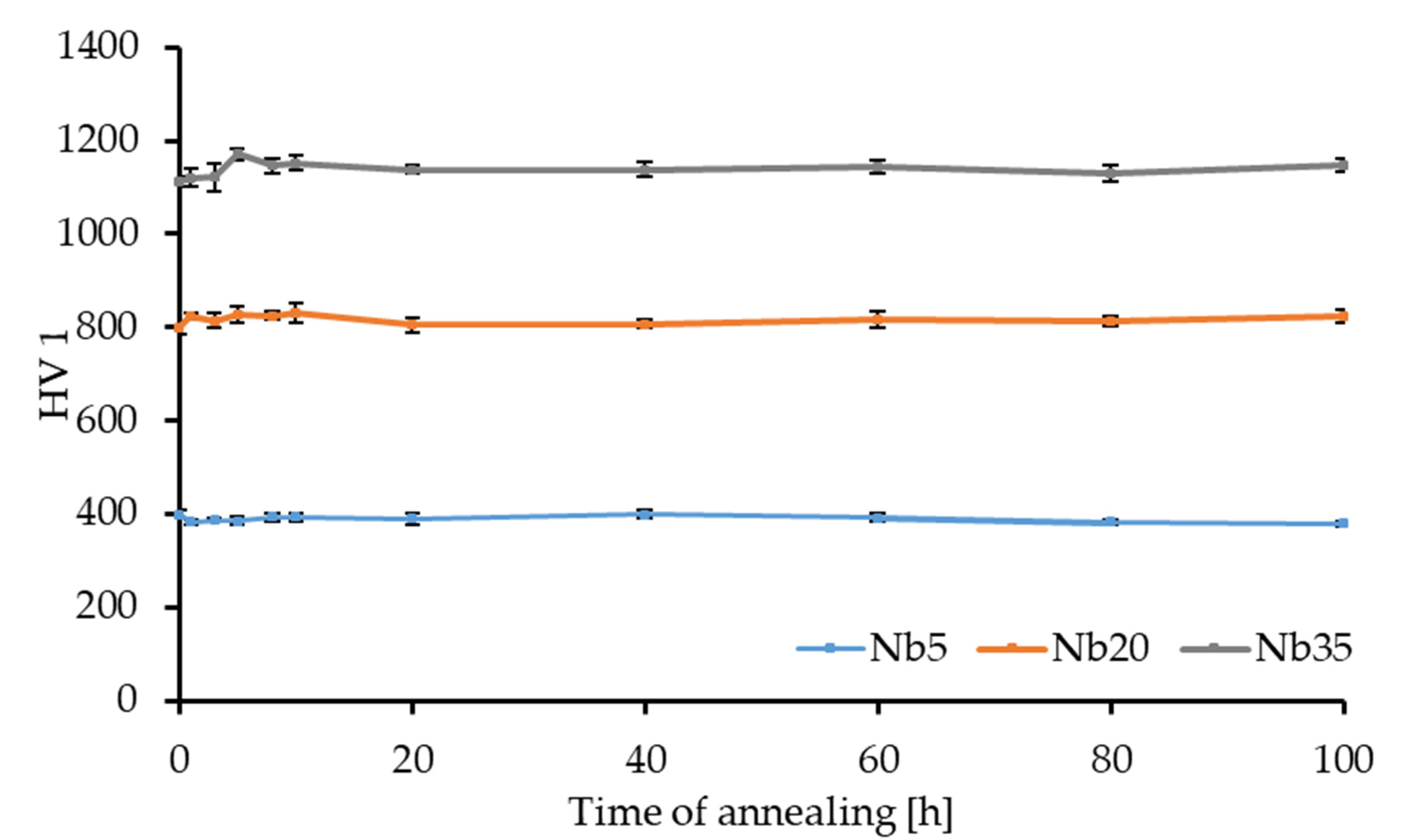
3.2.1. Hardness and Instrumented Microhardness Measurements
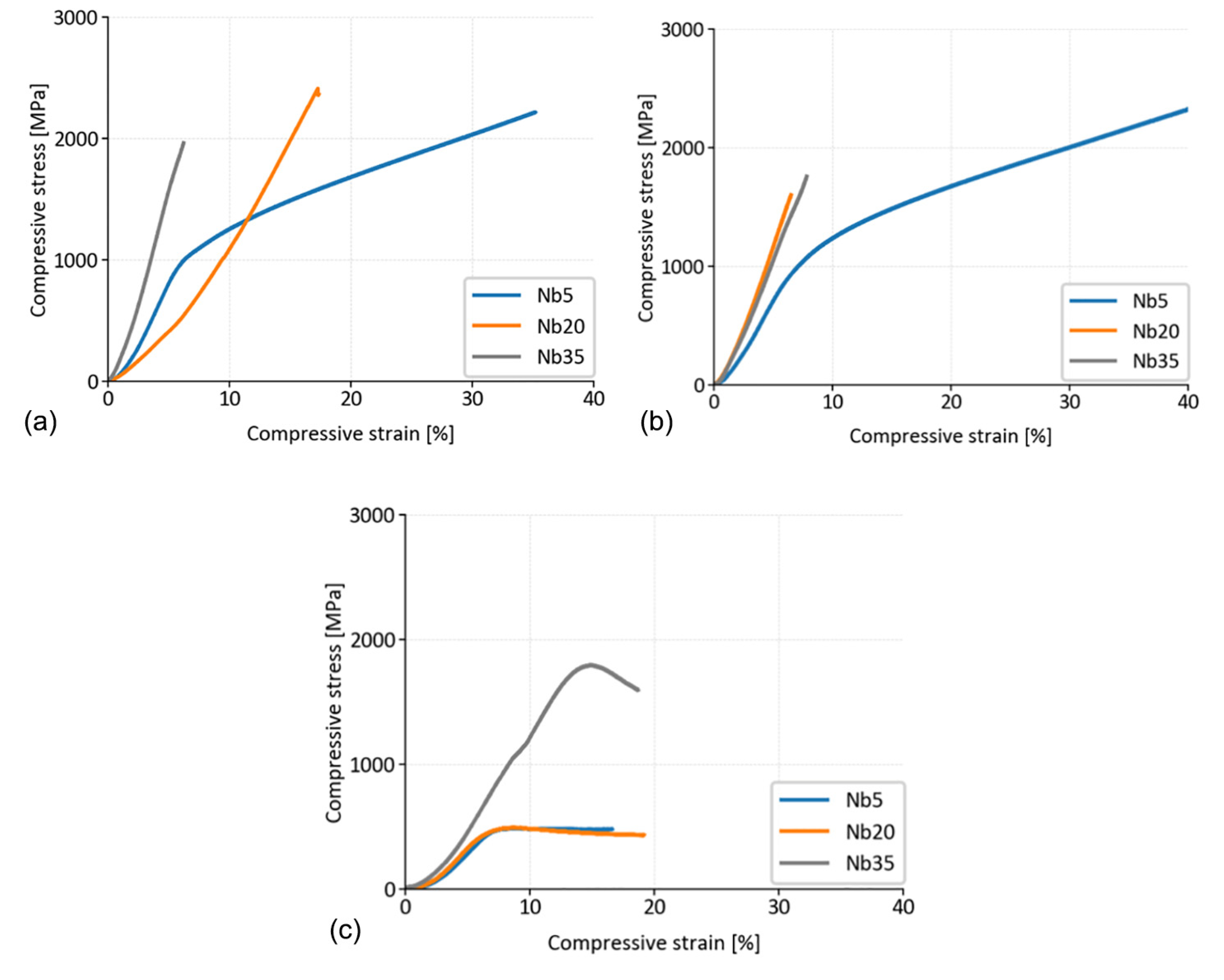
3.2.2. Compressive Deformation Tests
Laboratory Temperature Testing
Testing at 800 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, F.; Wang, Z.; Wang, J.; Wu, Q.; Chen, D.; Han, B.; Li, J.; Wang, J.; Kai, J.J. Abnormal γ″—ε phase transformation in the CoCrFeNiNb0.25 high entropy alloy. Scr. Mater. 2018, 146, 281–285. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Cheng, P.; Wang, Q.; Li, J.; Dang, Y.; Wang, J.; Liu, C.T. Designing eutectic high entropy alloys of CoCrFeNiNbX. J. Alloys Compd. 2016, 656, 284–289. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNbxNi High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Shang, X.; Leng, C.; Li, J.; Wang, J. Stability of lamellar structures in CoCrFeNiNbx eutectic high entropy alloys at elevated temperatures. Mater. Des. 2016, 104, 259–264. [Google Scholar] [CrossRef]
- Tsai, M.H.; Fan, A.-C.; Wang, H.-A. Effect of atomic size difference on the type of major intermetallic phase in arc-melted CoCrFeNiX high-entropy alloys. J. Alloys Compd. 2017, 695, 1479–1487. [Google Scholar] [CrossRef]
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the tribological properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Gao, X.; Lu, Y.; Cao, Z.; Gao, M.C.; Hawk, J.A.; Li, T. A new strategy to design eutectic high-entropy alloys using simple mixture method. Mater. Des. 2018, 142, 101–105. [Google Scholar] [CrossRef]
- Fan, A.-C.; Li, J.-H.; Tsai, M.-H. On the phase constituents of three CoCrFeNiX (X = V, Nb, Ta) high-entropy alloys after prolonged annealing. J. Alloys Compd. 2020, 823, 153524. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Zhao, L.; Wang, L.; Zhao, S.; Zhang, Y.; Cui, B. Synergistic effect of Nb and Mo alloying on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2022, 829, 142153. [Google Scholar] [CrossRef]
- Chanda, B.; Jana, P.P.; Das, J. A tool to predict the evolution of phase and Young’s modulus in high entropy alloys using an artificial neural network. Comput. Mater. Sci. 2021, 197, 110619. [Google Scholar] [CrossRef]
- An, X.; Chu, C.; Zhao, H.; Shen, B.; Zhou, L.; Chu, P.K. CoNiFeNb0.45 eutectic multi-principal element alloy with excellent mechanical properties and corrosion resistance. Mater. Sci. Eng. A 2020, 777, 139026. [Google Scholar] [CrossRef]
- Chanda, B.; Das, J. Evolution of microstructure homogeneity and mechanical properties in nano-/ultrafine eutectic CoCrFeNiNbx (0.45 ≤ x ≤ 0.65) high entropy alloy ingots and cast rods. J. Alloys Compd. 2022, 901, 163610. [Google Scholar] [CrossRef]
- Zhou, K.; Li, J.; Wang, L.; Yang, H.; Wang, Z.; Wang, J. Direct laser deposited bulk CoCrFeNiNbx high entropy alloys. Intermetallics 2019, 114, 106592. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Jayalakshmi, S.; Singh, R.A.; Deev, V.B.; Prusov, E.S. Factors determining solid solution phase formation and stability in CoCrFeNiX0.4 (X = Al, Nb, Ta) high entropy alloys fabricated by powder plasma arc additive manufacturing. J. Alloys Compd. 2021, 857, 157625. [Google Scholar] [CrossRef]
- Matěj, Z.; Kužel, R. MStruct—Software/Library for MicroStructure Analysis by Powder Diffraction; X-ray Group, School of Physics, Charles University: Prague, Czech Republic; Lund University: Lund, Sweden; Available online: http://www.xray.cz/mstruct (accessed on 17 October 2022).
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- ISO 14577; Metallic Materials—Instrumented Indentation Test for Hardness and Material Parameters. 2002. Available online: https://www.iso.org/standard/56626.html (accessed on 17 March 2023).
- Sergi, A.; Khan, R.H.U.; Georgilas, K.; Meisnar, M.; Makaya, A.; Attallah, M.M. Powder HIP of pure Nb and C-103 alloy: The influence of powder characteristics on mechanical properties. Int. J. Refract. Met. Hard Mater. 2022, 104, 105803. [Google Scholar] [CrossRef]
- Čech, J.; Haušild, P.; Karlík, M.; Bouček, V.; Nová, K.; Průša, F.; Novák, P.; Kopeček, J. Effect of initial powders on properties of FeAlSi intermetallics. Materials 2019, 12, 2846. [Google Scholar] [CrossRef]
- Průša, F.; Cabibbo, M.; Šenková, A.; Kučera, V.; Veselka, Z.; Školáková, A.; Vojtěch, D.; Cibulková, J.; Čapek, J. High-strength ultrafine-grained CoCrFeNiNb high-entropy alloy prepared by mechanical alloying: Proper-ties and strengthening mechanism. J. Alloys Compd. 2020, 835, 155308. [Google Scholar] [CrossRef]
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Niu, S.; Wu, Q.; Li, J.; Wang, J.; Liu, C.T.; Dang, Y. Strengthening the CoCrFeNiNb0.25 high entropy alloy by FCC precipitate. J. Alloys Compd. 2016, 667, 53–57. [Google Scholar] [CrossRef]
- Cao, X.; Wu, C.; Liu, Y.; Peng, H.; Su, X. Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys. Metals 2022, 12, 756. [Google Scholar] [CrossRef]
- Freund, M.; Andre, D.; Zehnder, C.; Rempel, H.; Gerber, D.; Zubair, M.; Sandlöbes-Haut, S.; Gibson, J.S.K.-L.; Korte-Kerzel, S. Plastic deformation of the CaMg2 C14-Laves phase from 50–250 °C. Materialia 2021, 20, 101237. [Google Scholar] [CrossRef]
- Lin, D.; Xi, X.; Li, X.; Hu, J.; Xu, L.; Han, Y.; Zhang, Y.; Zhao, L. High-temperature mechanical properties of FeCoCrNi high-entropy alloys fabricated via selective laser melting. Mater. Sci. Eng. A 2022, 832, 142354. [Google Scholar] [CrossRef]


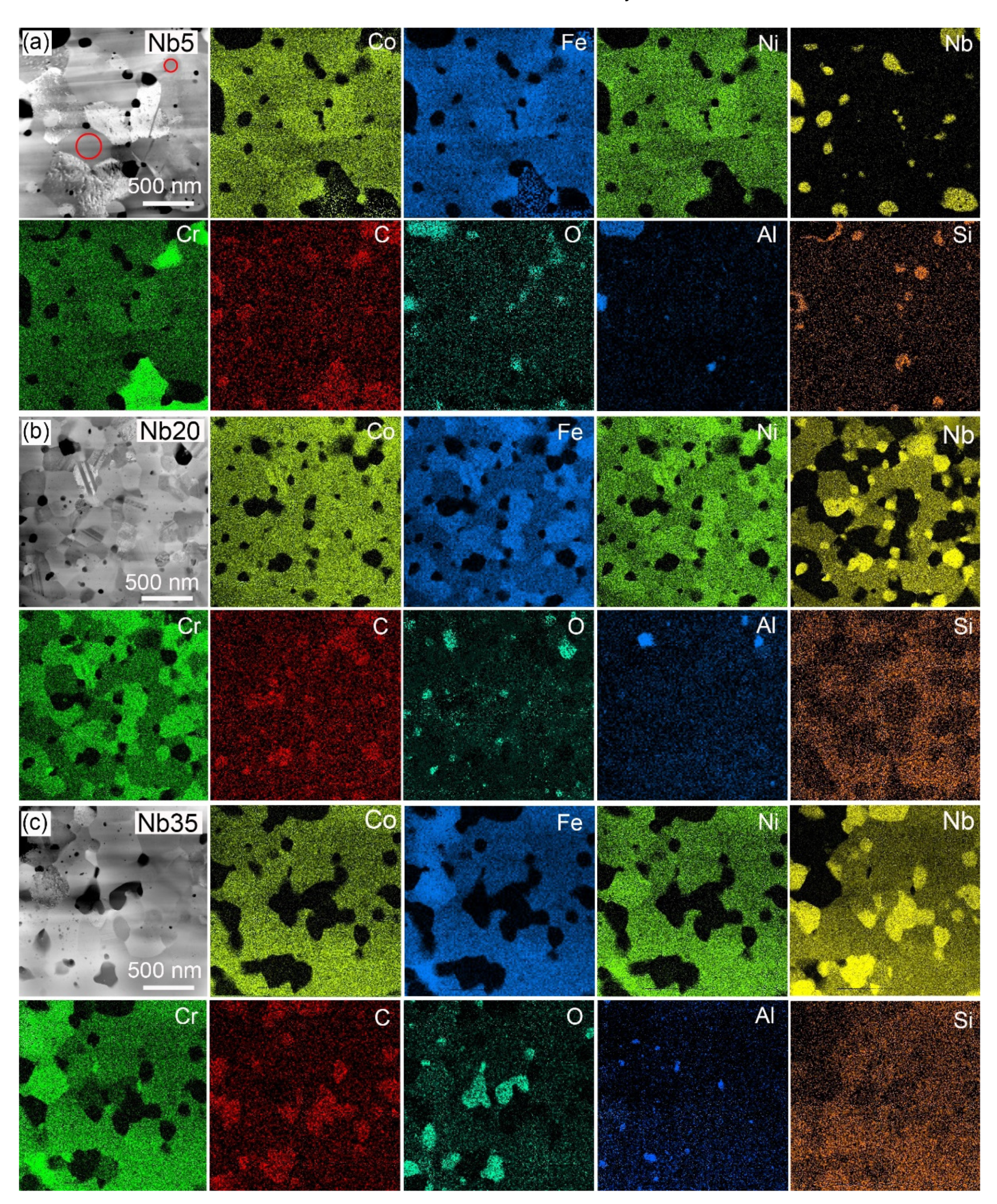
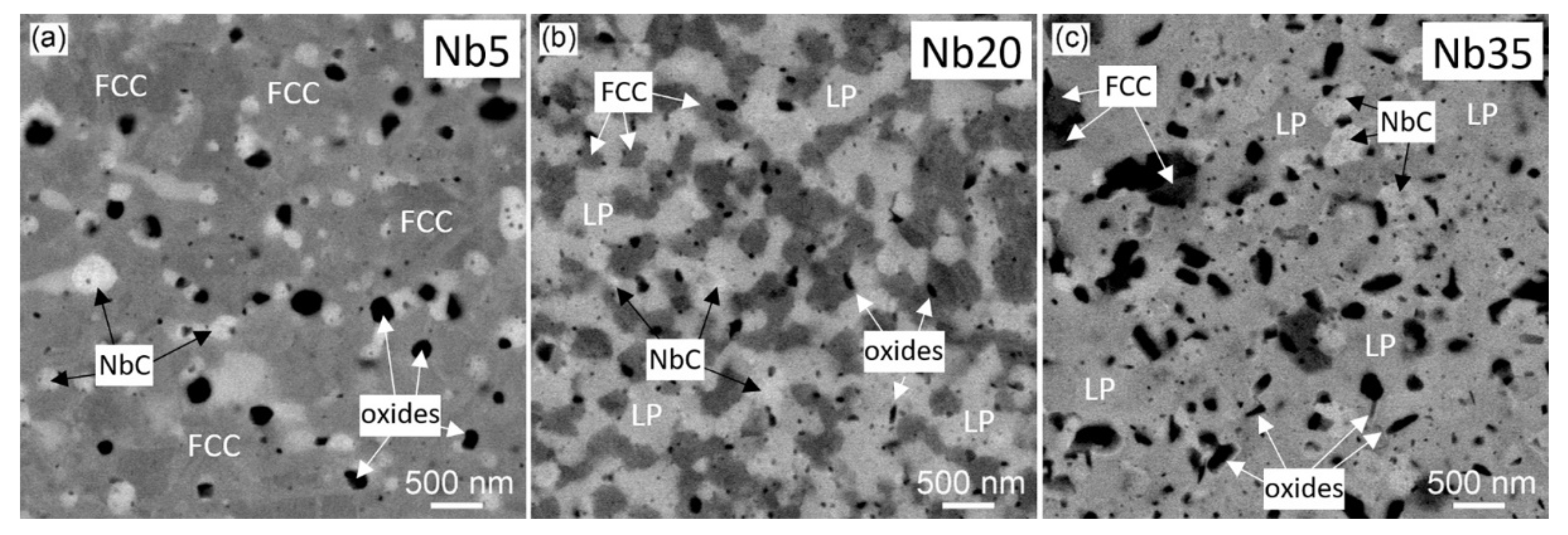

| Alloy | Co | Cr | Fe | Ni | Nb | Si |
|---|---|---|---|---|---|---|
| CoCrFeNiNb5 | 20.31 | 22.00 | 28.18 | 21.47 | 5.77 | 2.27 |
| CoCrFeNiNb20 | 17.44 | 19.68 | 22.32 | 18.65 | 19.70 | 2.21 |
| CoCrFeNiNb35 | 13.76 | 15.42 | 20.21 | 14.83 | 32.72 | 3.06 |
| Alloy | FCC Matrix | NbC | Cr7C3 | Laves Phase | NbxOy |
|---|---|---|---|---|---|
| CoCrFeNiNb5 | 75.7 ± 0.1 | 9.8 ± 0.2 | 14.5 ± 0.7 | - | - |
| CoCrFeNiNb20 | 51.9 ± 0.2 | 12.6 ± 0.1 | - | 34.5 ± 0.2 | 1.0 ± 0.5 |
| CoCrFeNiNb35 | 7.4 ± 0.4 | 17.4 ± 0.2 | - | 74.2 ± 0.1 | 0.9 ± 0.5 |
| Alloy | FCC Matrix | NbC | Cr7C3 | Laves Phase |
|---|---|---|---|---|
| CoCrFeNiNb5 | a = 0.35789 (0) | a = 0.44689 (0) | a = 0.70062 (7) b = 1.23547 (2) c = 0.45153 (0) | - |
| CoCrFeNiNb20 | a = 0.35918 (3) | a = 0.44531 (4) | - | a = 0.47989 (5) c = 0.78075 (8) |
| CoCrFeNiNb35 | a = 0.35885 (5) | a = 0.44503 (2) | - | a = 0.48033 (4) c = 0.78153 (1) |
| Alloy | CoCrFeNiNb5 | CoCrFeNiNb20 * | CoCrFeNiNb35 |
|---|---|---|---|
| Area fraction [%] | 0.03 ± 0.01 | 0.23 ± 0.14 | 0.25 ± 0.11 |
| Alloy | FCC Matrix | NbC | Cr7C3 | Laves Phase | Oxides |
|---|---|---|---|---|---|
| CoCrFeNiNb5 | 100–750 | 50–450 | 500–1500 | - | 10–500 |
| CoCrFeNiNb20 | 100–500 | 50–300 | - | 140–370 | 10–300 |
| CoCrFeNiNb35 | 100–350 | 50–600 | - | 130–370 | 10–350 |
| Alloy | Co | Cr | Fe | Ni | Nb | Si | Al | C | O |
|---|---|---|---|---|---|---|---|---|---|
| Nb5 | |||||||||
| Cr7C3 | 3.0 ± 1.6 | 52.2 ± 2.6 | 6.1 ± 1.1 | 0.4 ± 0.6 | 0.6 ± 0.3 | 0.6 ± 0.2 | - | 36.7 ± 2.5 | - |
| NbC | 1.4 ± 0.3 | 1.2 ± 0.4 | 1.3 ± 0.4 | 0.1 ± 0.3 | 65.5 ± 12.6 | 0.4 ± 0.5 | 0.3 ± 0.2 | 24.1 ± 7.8 | 5.5 ± 2.4 |
| matrix | 23.2 ± 0.3 | 14.7 ± 0.7 | 28.7 ± 0.7 | 23.2 ± 0.2 | 0.6 ± 0.3 | 1.1 ± 0.1 | 0.1 ± 0.1 | 7.8 ± 1.6 | 0.5 ± 0.6 |
| Nb20 | |||||||||
| NbC | 1.3 ± 0.6 | 1.3 ± 1.0 | 1.2 ± 0.4 | – | 74.6 ± 7.3 | 1.7 ± 2.8 | 0.3 ± 0.4 | 14.3 ± 7.8 | 6.3 ± 3.7 |
| Laves ph. | 18.9 ± 1.7 | 11.4 ± 0.5 | 17.8 ± 4.5 | 14.5 ± 1.0 | 31.5 ± 1.2 | 2.8 ± 0.4 | 0.1 ± 0.1 | 2.3 ± 2.0 | 2.7 ± 3.1 |
| matrix | 19.1 ± 1.6 | 24.9 ± 1.4 | 26.5 ± 1.2 | 21.7 ± 1.2 | 2.0 ± 0.3 | 0.4 ± 0.3 | 0.1 ± 0.2 | 5.0 ± 2.8 | - |
| Nb35 | |||||||||
| NbC | 1.1 ± 0.7 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.5 ± 0.4 | 72.2 ± 4.5 | 0.6 ± 0.3 | 0.4 ± 0.2 | 17.5 ± 5.2 | 5.3 ± 0.7 |
| Laves ph. | 14.7 ± 0.6 | 13.0 ± 0.8 | 17.8 ± 0.6 | 14.0 ± 0.5 | 33.9 ± 1.7 | 2.5 ± 0.2 | 0.3 ± 0.2 | 1.5 ± 1.6 | 2.2 ± 1.8 |
| matrix | 16.0 ± 0.6 | 24.7 ± 0.6 | 29.9 ± 1.4 | 21.5 ± 1.2 | 3.6 ± 0.8 | 0.4 ± 0.3 | 0.3 ± 0.2 | 3.4 ± 2.1 | - |
| Alloy | FCC Matrix | NbC | Ni6Nb6O | Laves Phase | NbO2 | Cr2O3 | CrNbO4 |
|---|---|---|---|---|---|---|---|
| CoCrFeNiNb5 | 88.1 ± 0.4 | 6.1 ± 0.1 | 5.2 ± 1.2 | - | 0.3 ± 0.2 | - | 0.3 ± 0.2 |
| CoCrFeNiNb20 | 48.9 ± 0.1 | 11.5 ± 0.2 | - | 39.1 ± 0.2 | 0.4 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| CoCrFeNiNb35 | 5.2 ± 0.2 | 17.4 ± 0.2 | - | 74.6 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 2.5 ± 0.2 |
| Alloy | CoCrFeNiNb5 | CoCrFeNiNb20 | CoCrFeNiNb35 |
|---|---|---|---|
| HV 1 | 398.4 ± 9.0 | 798.1 ± 14.0 | 1113.7 ± 11.6 |
| HVIT | 467.4 ± 10.2 | 880.2 ± 27.4 | 1309.8 ± 64.3 |
| EIT [GPa] | 208.4 ± 8.2 | 242.0 ± 11.1 | 259.1 ± 6.5 |
| Alloy | LT | LT after 100 h at 800 °C | at 800 °C | |||
|---|---|---|---|---|---|---|
| CYS | UCS | CYS | UCS | CYS | UCS | |
| CoCrFeNiNb5 | 953 ± 12 | * | 930 ± 16 | * | 453 ± 27 | 492 ± 18 |
| CoCrFeNiNb20 | ** | 2413 ± 89 | ** | 1518 ± 276 | 442 ± 17 | 503 ± 13 |
| CoCrFeNiNb35 | ** | 1821 ± 144 | ** | 1843 ± 468 | 1695 ± 110 | 1817 ± 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlík, M.; Průša, F.; Kratochvíl, P.; Thürlová, H.; Strakošová, A.; Čech, J.; Čapek, J.; Vronka, M.; Cabibbo, M.; Ekrt, O. Microstructure and Mechanical Properties of Spark Plasma Sintered CoCrFeNiNbX High-Entropy Alloys with Si Addition. Materials 2023, 16, 2491. https://doi.org/10.3390/ma16062491
Karlík M, Průša F, Kratochvíl P, Thürlová H, Strakošová A, Čech J, Čapek J, Vronka M, Cabibbo M, Ekrt O. Microstructure and Mechanical Properties of Spark Plasma Sintered CoCrFeNiNbX High-Entropy Alloys with Si Addition. Materials. 2023; 16(6):2491. https://doi.org/10.3390/ma16062491
Chicago/Turabian StyleKarlík, Miroslav, Filip Průša, Petr Kratochvíl, Hana Thürlová, Angelina Strakošová, Jaroslav Čech, Jiří Čapek, Marek Vronka, Marcello Cabibbo, and Ondřej Ekrt. 2023. "Microstructure and Mechanical Properties of Spark Plasma Sintered CoCrFeNiNbX High-Entropy Alloys with Si Addition" Materials 16, no. 6: 2491. https://doi.org/10.3390/ma16062491
APA StyleKarlík, M., Průša, F., Kratochvíl, P., Thürlová, H., Strakošová, A., Čech, J., Čapek, J., Vronka, M., Cabibbo, M., & Ekrt, O. (2023). Microstructure and Mechanical Properties of Spark Plasma Sintered CoCrFeNiNbX High-Entropy Alloys with Si Addition. Materials, 16(6), 2491. https://doi.org/10.3390/ma16062491








