Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2
Abstract
1. Introduction
2. Materials and Methods
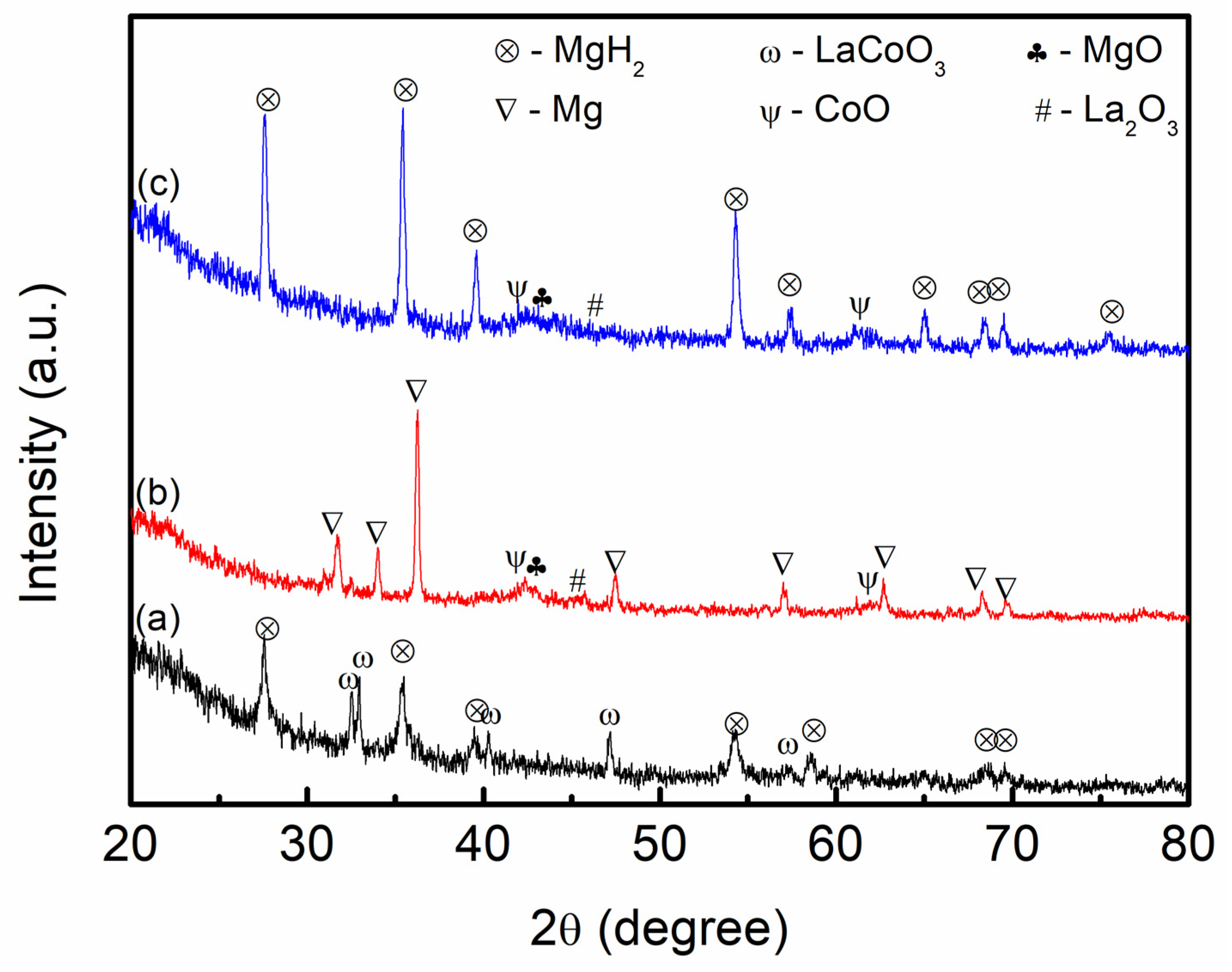
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, L.; Xuan, N.; Gu, G.; Lv, P.; Huang, N.; Song, C.; Zheng, M.; Wang, J.; Cui, P.; Gu, G.; et al. Power management and system optimization for high efficiency self-powered electrolytic hydrogen and formic acid production. Nano Energy 2023, 107, 108124. [Google Scholar] [CrossRef]
- Le, S.T.; Nguyen, T.N.; Linforth, S.; Ngo, T.D. Safety investigation of hydrogen energy storage systems using quantitative risk assessment. Int. J. Hydrogen Energy 2023, 48, 2861–2875. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Y.; Liu, W.; Zhao, X.; Xu, J.; Yuan, Z. Research progress of hydrogen energy and metal hydrogen storage materials. Sustain. Energy Technol. Assess 2023, 55, 102974. [Google Scholar] [CrossRef]
- Zhang, L.; Nyahuma, F.M.; Zhang, H.; Cheng, C.; Zheng, J.; Wu, F.; Chen, L. Metal organic framework supported niobium pentoxide nanoparticles with exceptional catalytic effect on hydrogen storage behavior of MgH2. Green Energy Environ. 2023, 8, 589–600. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Sulaiman, N.N.; Ismail, M. Effect of SrFe12O19 nanopowder on the hydrogen sorption properties of MgH2. RSC Adv. 2016, 6, 110004–110010. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Yartys, V.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Iyakutti, K.; Surya, V.J.; Lavanya, R.; Vasu, V.; Rajeswarapalanichamy, R.; Kawazoe, Y. Effects of nanostructures on the hydrogen storage properties of MgH2—A first principles study. Comput. Condens. Matter 2022, 30, e00643. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2–V nanocomposite. J. Alloys Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, W.; Zhang, Q.; Lu, C.; Sun, F.; Lin, X.; Zou, J. MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage. Chem. Eng. J. 2022, 434, 134701. [Google Scholar] [CrossRef]
- Sazelee, N.; Ali, N.A.; Yahya, M.S.; Mustafa, N.S.; Halim Yap, F.A.; Mohamed, S.B.; Ghazali, M.Z.; Suwarno, S.; Ismail, M. Recent advances on Mg–Li–Al systems for solid-state hydrogen storage: A Review. Front. Energy Res. 2022, 10, 875405. [Google Scholar] [CrossRef]
- Friedrichs, O.; Aguey-Zinsou, F.; Fernández, J.A.; Sánchez-López, J.; Justo, A.; Klassen, T.; Bormann, R.; Fernández, A. MgH2 with Nb2O5 as additive, for hydrogen storage: Chemical, structural and kinetic behavior with heating. Acta Mater. 2006, 54, 105–110. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, H.; Tang, Q.; Liu, Y.; Liu, J.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X.; Ba, Z. Ultra-fine TiO2 nanoparticles supported on three-dimensionally ordered macroporous structure for improving the hydrogen storage performance of MgH2. Appl. Surf. Sci. 2022, 585, 152561. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; Wang, X.; Wang, C.; Fan, X.; Tang, Z.; Wang, C.; Wang, Q.; Chen, L. Synergistic catalytic activity of porous rod-like TMTiO3 (TM= Ni and Co) for reversible hydrogen storage of magnesium hydride. J. Phys. Chem. C 2018, 122, 27973–27982. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Guo, X.; Yang, X. Achieve high-efficiency hydrogen storage of MgH2 catalyzed by nanosheets CoMoO4 and rGO. J. Alloys Compd. 2022, 911, 165153. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Chang, J.; Zhang, D.; Peng, Y.; Yang, X. Improvement of hydrogen storage performance of MgH2 by MnMoO4 rod composite catalyst. Solid. State Sci. 2021, 121, 106750. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Sazelee, N.A.; Idris, N.H.; Md Din, M.F.; Yahya, M.S.; Ali, N.A.; Ismail, M. LaFeO3 synthesised by solid-state method for enhanced sorption properties of MgH2. Results Phys. 2020, 16, 102844. [Google Scholar] [CrossRef]
- Soni, P.K.; Bhatnagar, A.; Shaz, M.A.; Srivastava, O.N. Effect of graphene templated fluorides of Ce and La on the de/rehydrogenation behavior of MgH2. Int. J. Hydrogen Energy 2017, 42, 20026–20035. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Liu, Y.; Ding, W.; Sun, C. Enhancement of hydrogen storage properties by in situ formed LaH3 and Mg2NiH4 during milling MgH2 with porous LaNiO3. Catal. Today 2018, 318, 113–118. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Sun, Z.; Yan, N.; Yu, H.; Lu, Z.; Zhu, X. Superior catalytic effect of facile synthesized LaNi4.5Mn0.5 submicro-particles on the hydrogen storage properties of MgH2. J. Alloys Compd. 2020, 844, 156069. [Google Scholar] [CrossRef]
- Juahir, N.; Mustafa, N.; Sinin, A.; Ismail, M. Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles. RSC Adv. 2015, 5, 60983–60989. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, J.; Li, P.; Zhai, F.; Wan, Q.; Liu, Z.; Qu, X. Dehydrogenation mechanism of ball-milled MgH2 doped with ferrites (CoFe2O4, ZnFe2O4, MnFe2O4 and Mn0.5Zn0.5Fe2O4) nanoparticles. J. Alloys Compd. 2015, 643, 174–180. [Google Scholar] [CrossRef]
- Cabo, M.; Garroni, S.; Pellicer, E.; Milanese, C.; Girella, A.; Marini, A.; Rossinyol, E.; Suriñach, S.; Baró, M.D. Hydrogen sorption performance of MgH2 doped with mesoporous nickel- and cobalt-based oxides. Int. J. Hydrogen Energy 2011, 36, 5400–5410. [Google Scholar] [CrossRef]
- Mandzhukova, T.; Khrussanova, M.; Grigorova, E.; Stefanov, P.; Khristov, M.; Peshev, P. Effect of NiCo2O4 additives on the hydriding properties of magnesium. J. Alloys Compd. 2008, 457, 472–476. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Chen, X.; Lv, Y.; Huang, H.; Yuan, J.; Lv, W.; Wu, Y. Remarkable enhancement and electronic mechanism for hydrogen storage kinetics of Mg nano-composite by a multi-valence Co-based catalyst. Mater. Today Nano 2022, 17, 100168. [Google Scholar] [CrossRef]
- Sarker, A.R. Synthesis of high quality LaCoO3 crystals using water based sol-gel method. Int. J. Mater. Sci. Appl. 2015, 4, 159–164. [Google Scholar]
- Ajmal, S.; Bibi, I.; Majid, F.; Ata, S.; Kamran, K.; Jilani, K.; Nouren, S.; Kamal, S.; Ali, A.; Iqbal, M. Effect of Fe and Bi doping on LaCoO3 structural, magnetic, electric and catalytic properties. J. Mater. Res. Technol. 2019, 8, 4831–4842. [Google Scholar] [CrossRef]
- Worayingyong, A.; Kangvansura, P.; Ausadasuk, S.; Praserthdam, P. The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 217–225. [Google Scholar] [CrossRef]
- Radev, L.; Pavlova, L.; Samuneva, B.; Kashchieva, E.; Mihailova, I.; Zaharescu, M.; Malic, B.; Predoana, L. Sol-gel synthesis and structure of La2O3-CoO-SiO2 powders. Process Appl. Ceram. 2008, 2, 103–108. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Gu, W.; Song, Y.; Wang, F. Toward synergy of carbon and La2O3 in their hybrid as an efficient catalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 77786–77795. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Schmidt, R.; Morán, E. Microwave assisted synthesis and characterization of perovskite oxides. ChemInform 2014, 45, 117–140. [Google Scholar] [CrossRef]
- Shokano, G.; Dehouche, Z.; Galey, B.; Postole, G. Development of a novel method for the fabrication of nanostructured Zr(x)Ni(y) catalyst to enhance the desorption properties of MgH2. Catalysts 2020, 10, 849. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bhatnagar, A.; Shahi, R.R.; Hudson, M.; Singh, M.K.; Srivastava, O. Effect of TiO2 nanoparticles on the hydrogen sorption characteristics of magnesium hydride. J. Nanosci. Nanotechnol. 2013, 13, 5493–5499. [Google Scholar] [CrossRef]
- Ranjbar, A.; Guo, Z.P.; Yu, X.B.; Wexler, D.; Calka, A.; Kim, C.J.; Liu, H.K. Hydrogen storage properties of MgH2–SiC composites. Mater. Chem. Phys. 2009, 114, 168–172. [Google Scholar] [CrossRef]
- Sulaiman, N.; Mustafa, N.; Ismail, M. Effect of Na3FeF6 catalyst on the hydrogen storage properties of MgH2. Dalton Trans. 2016, 45, 7085–7093. [Google Scholar] [CrossRef] [PubMed]
- Lozano, G.A.; Ranong, C.N.; Bellosta von Colbe, J.M.; Bormann, R.; Fieg, G.; Hapke, J.; Dornheim, M. Empirical kinetic model of sodium alanate reacting system (I). Hydrogen absorption. Int. J. Hydrogen Energy 2010, 35, 6763–6772. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Juahir, N.; Halim Yap, F.A. Catalytic effect of CeCl3 on the hydrogen storage properties of MgH2. Mater. Chem. Phys. 2016, 170, 77–82. [Google Scholar] [CrossRef]
- Yahya, M.S.; Sulaiman, N.N.; Mustafa, N.S.; Halim Yap, F.A.; Ismail, M. Improvement of hydrogen storage properties in MgH2 catalysed by K2NbF7. Int. J. Hydrogen Energy 2018, 43, 14532–14540. [Google Scholar] [CrossRef]
- Verma, S.K.; Shaz, M.A.; Yadav, T.P. Enhanced hydrogen absorption and desorption properties of MgH2 with graphene and vanadium disulfide. Int J Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Rahman, M.H.A.; Shamsudin, M.A.; Klimkowicz, A.; Uematsu, S.; Takasaki, A. Effects of KNbO3 catalyst on hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2019, 44, 29196–29202. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Tian, H.; Wang, Y. Effect of the hierarchical Co@C nanoflowers on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2017, 42, 28464–28472. [Google Scholar] [CrossRef]
- Czujko, T.; Oleszek, E.E.; Szot, M. New aspects of MgH2 morphological and structural changes during high-energy ball milling. Mater 2020, 13, 4550. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes Alloy 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Chawla, K.; Kumar Yadav, D.; Bajpai, A.; Kumar, S.; Jain, I.P.; Lal, C. Effect of PdCl2 catalyst on the hydrogenation properties and sorption kinetics of Mg. Sustain. Energy Technol. Assess 2022, 51, 101981. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Nicolaisen, T.; Ares Fernandez, J.R.; Klassen, T.; Bormann, R. Effect of nanosized oxides on MgH2 (de)hydriding kinetics. J. Alloys Compd. 2007, 434–435, 738–742. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.; Saremi-Yarahmadi, S.; Gregory, D.H. Facile preparation of β-/γ-MgH2 nanocomposites under mild conditions and pathways to rapid dehydrogenation. Phys. Chem. Chem. Phys. 2016, 18, 10492–10498. [Google Scholar] [CrossRef] [PubMed]
- Rahmaninasab, M.A.; Raygan, S.; Abdizadeh, H.; Pourabdoli, M.; Mirghaderi, S.H. Properties of activated MgH2+ mischmetal nanostructured composite produced by ball-milling. Mater. Renew. Sustain. Energy 2018, 7, 15. [Google Scholar] [CrossRef]
- Si, T.Z.; Zhang, X.Y.; Feng, J.J.; Ding, X.L.; Li, Y.T. Enhancing hydrogen sorption in MgH2 by controlling particle size and contact of Ni catalysts. Rare Met. 2021, 40, 995–1002. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Zang, L.; Guo, H.; Jiao, L.; Yuan, H.; Wang, Y. Highly dispersed MgH2 nanoparticle-graphene nanosheet composites for hydrogen storage. ACS Appl. Nano Mater. 2019, 2, 3828–3835. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, I.; Bobet, J.L.; Song, M.Y. Effects on the H2-sorption properties of Mg of Co (with various sizes) and CoO addition by reactive grinding. J. Alloys Compd. 2004, 366, 279–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. Mechanistic understanding of CoO-catalyzed hydrogen desorption from a LiBH4·NH3–3LiH system. Dalton Trans. 2015, 44, 14514–14522. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Agresti, F.; Russo, S.L.; Maddalena, A.; Palade, P.; Principi, G. Structure and hydrogen storage properties of MgH2 catalysed with La2O3. J. Alloys Compd. 2008, 450, 310–313. [Google Scholar] [CrossRef]
- Ares Fernández, J.R.; Aguey Zinsou, K.F. Superior MgH2 kinetics with MgO addition: A tribological effect. Catalysts 2012, 2, 330–343. [Google Scholar] [CrossRef]
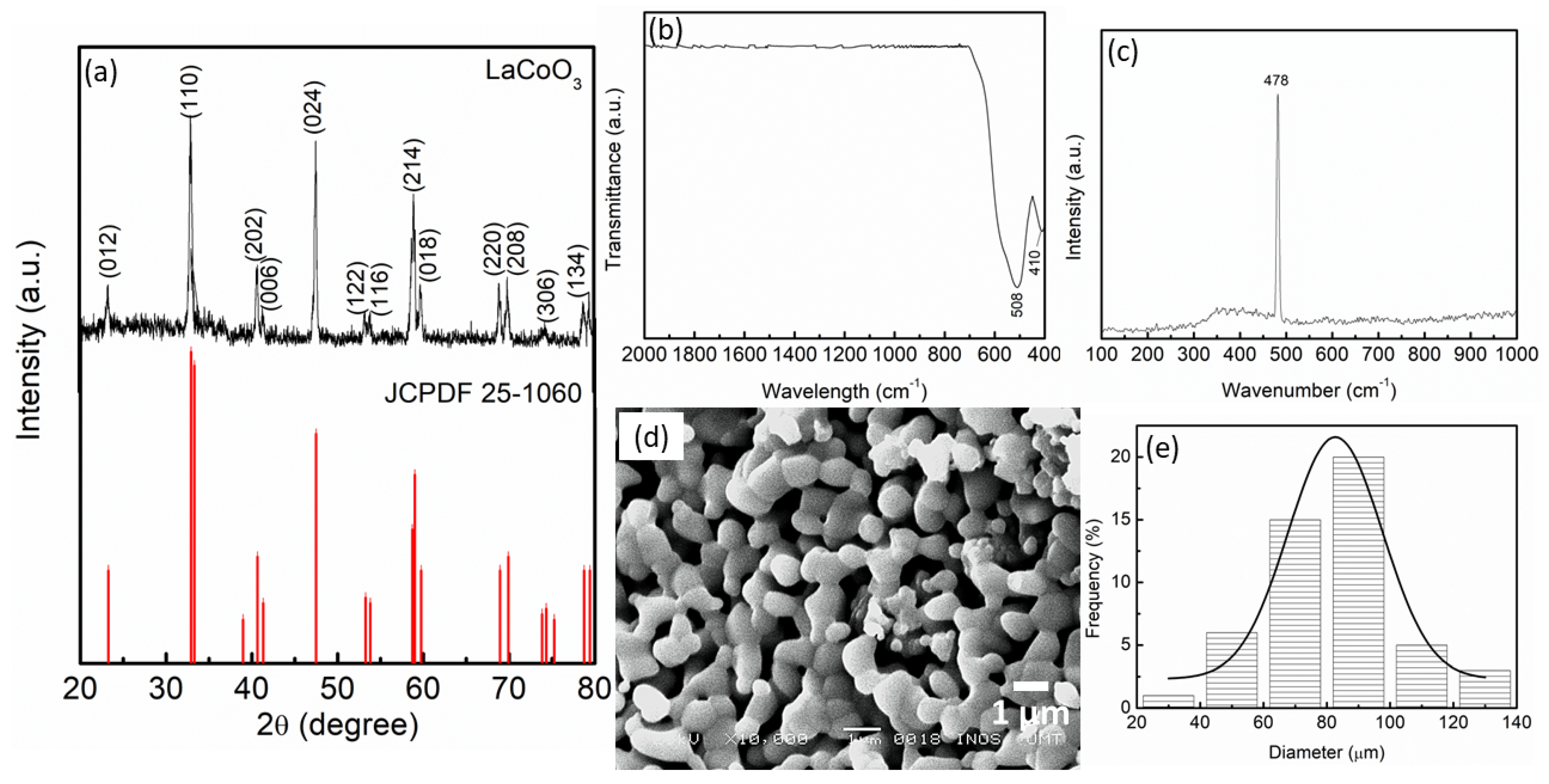
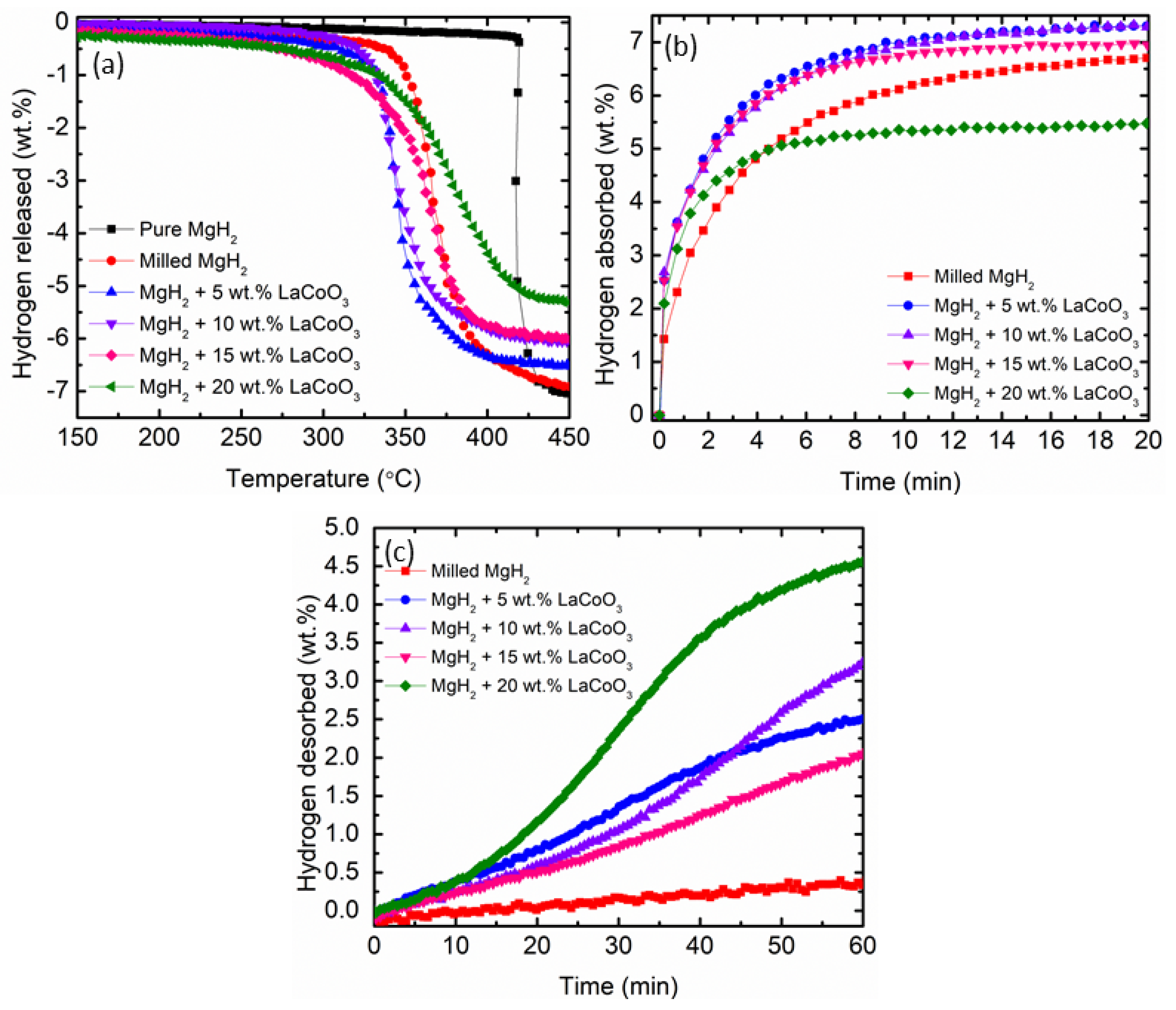
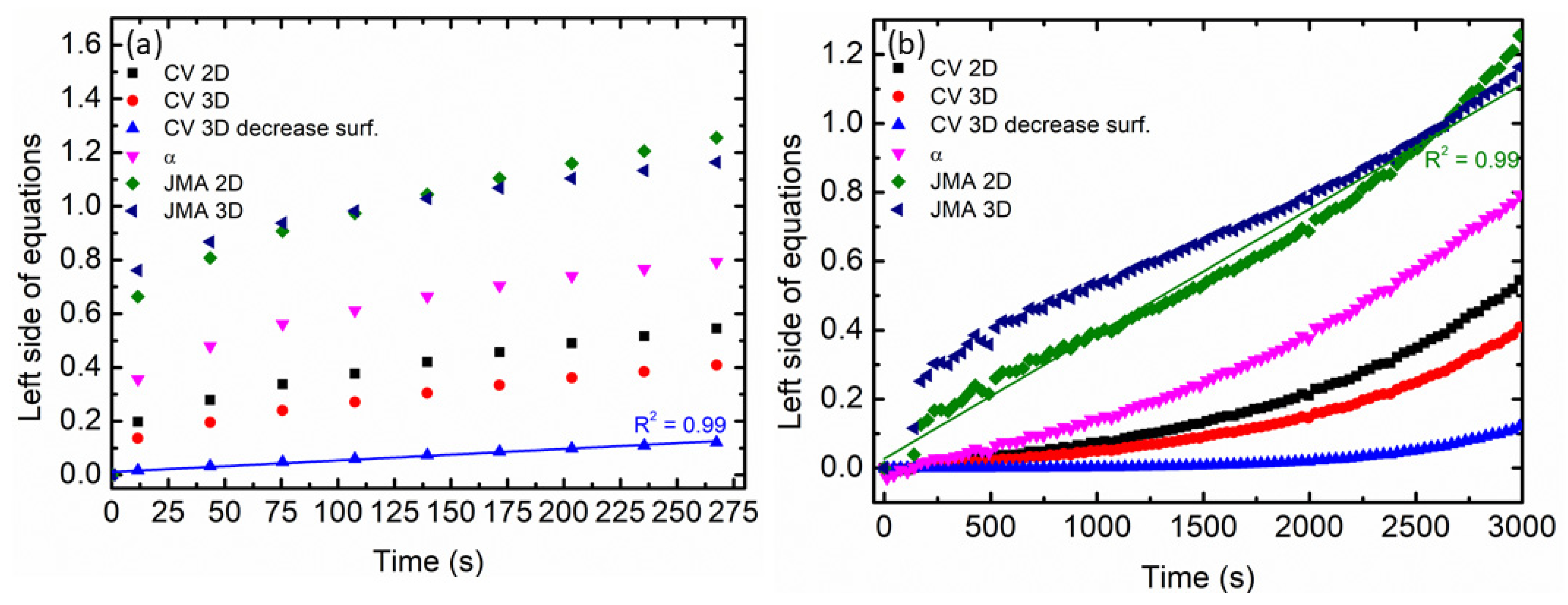
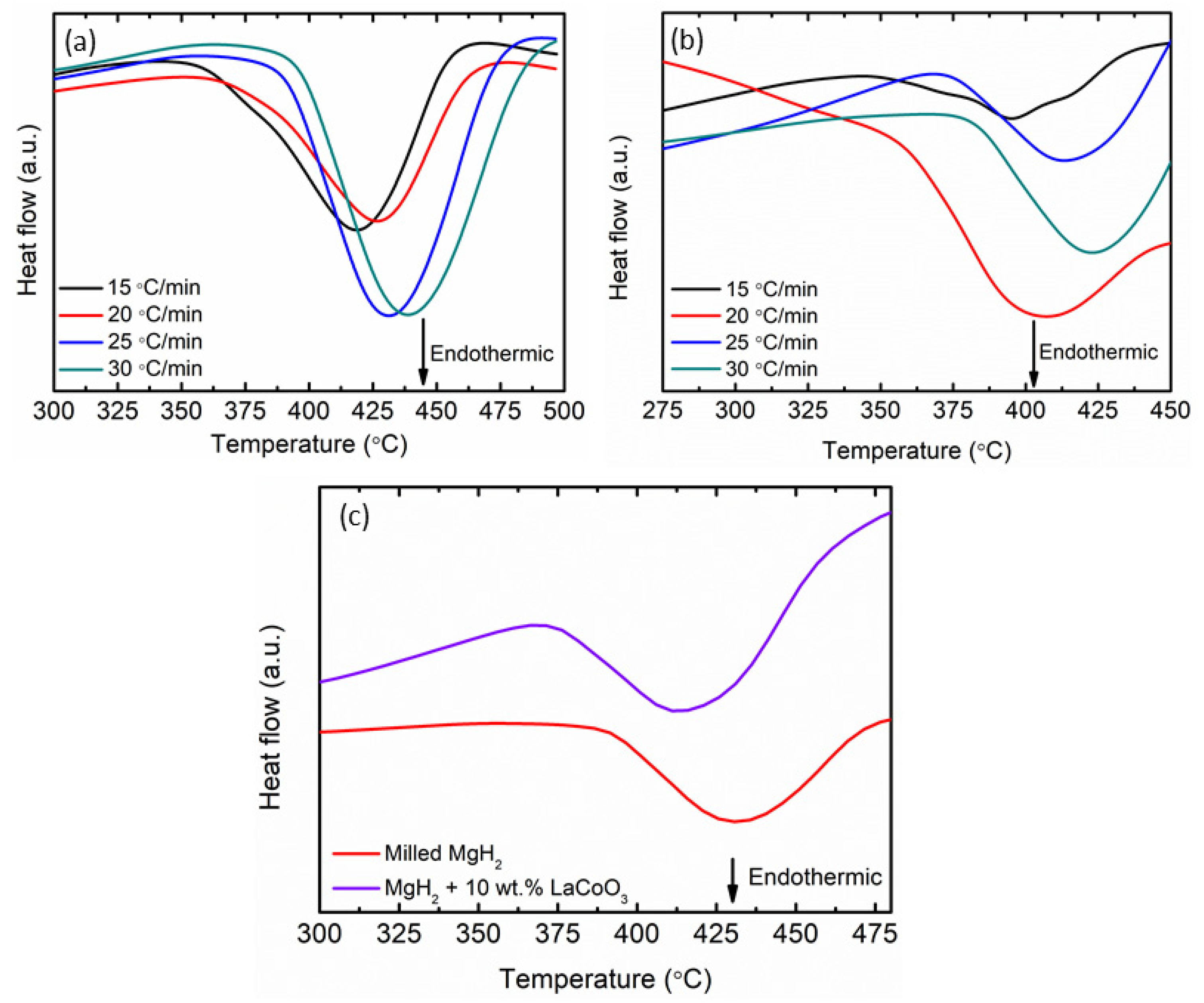
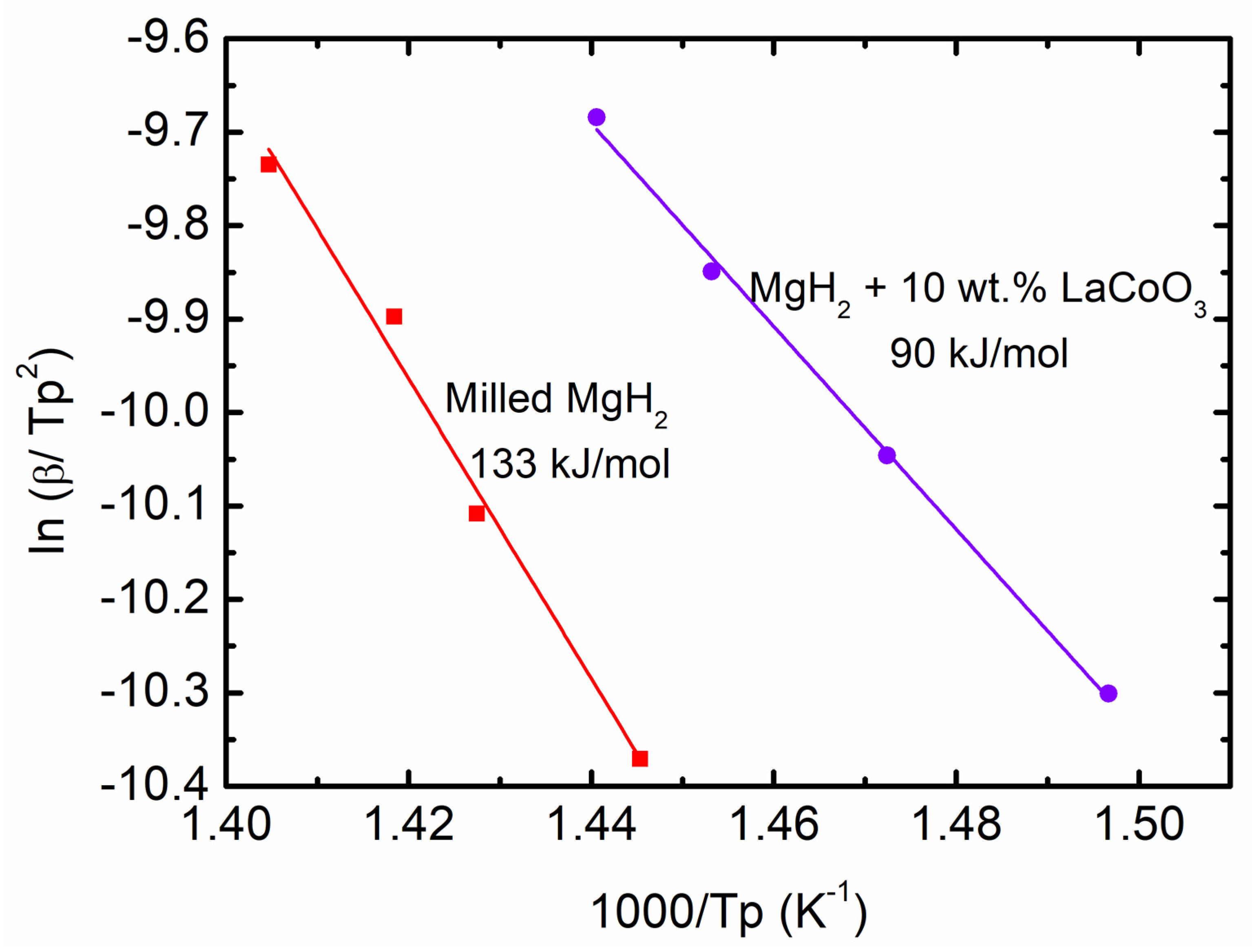
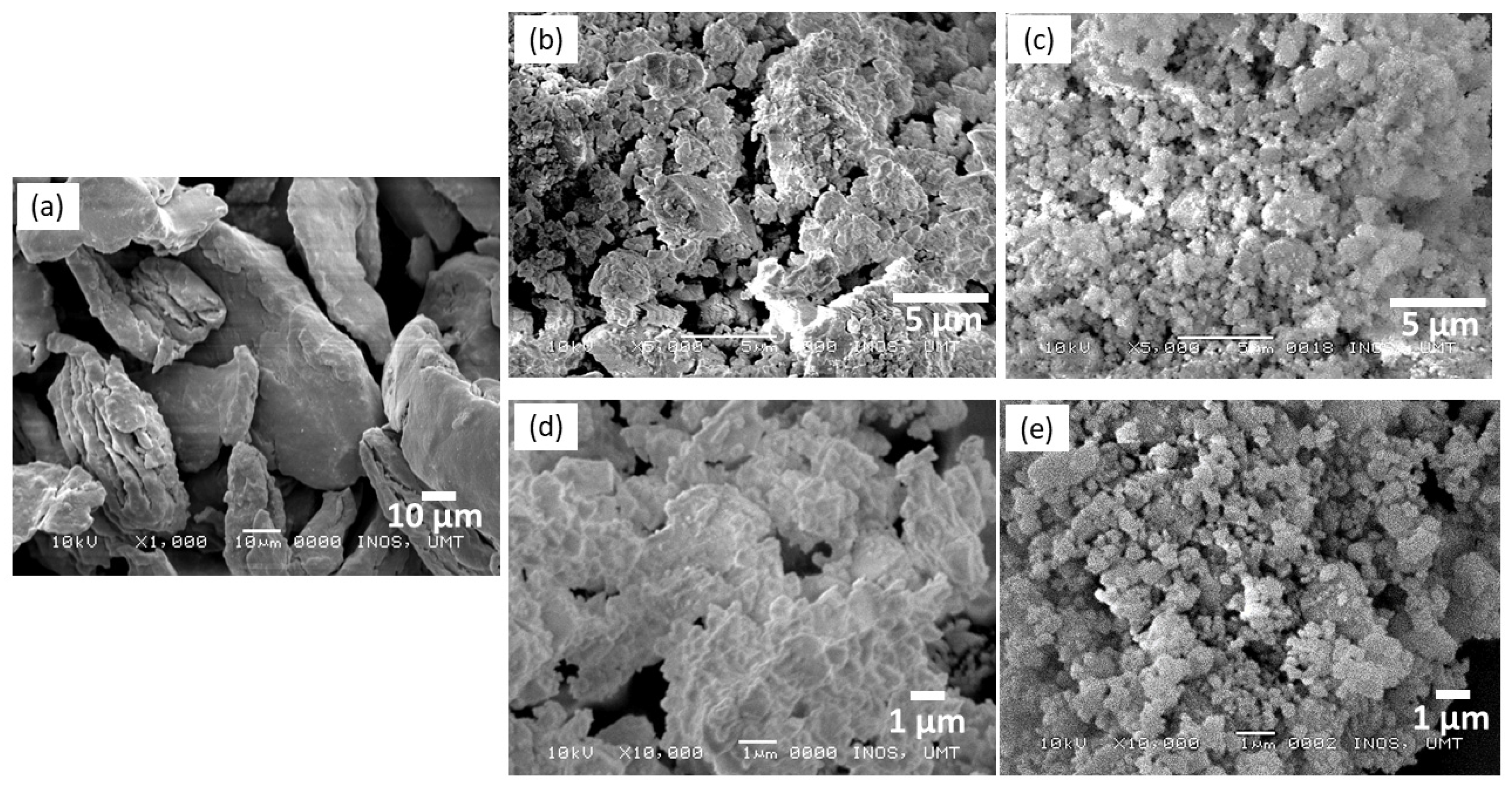
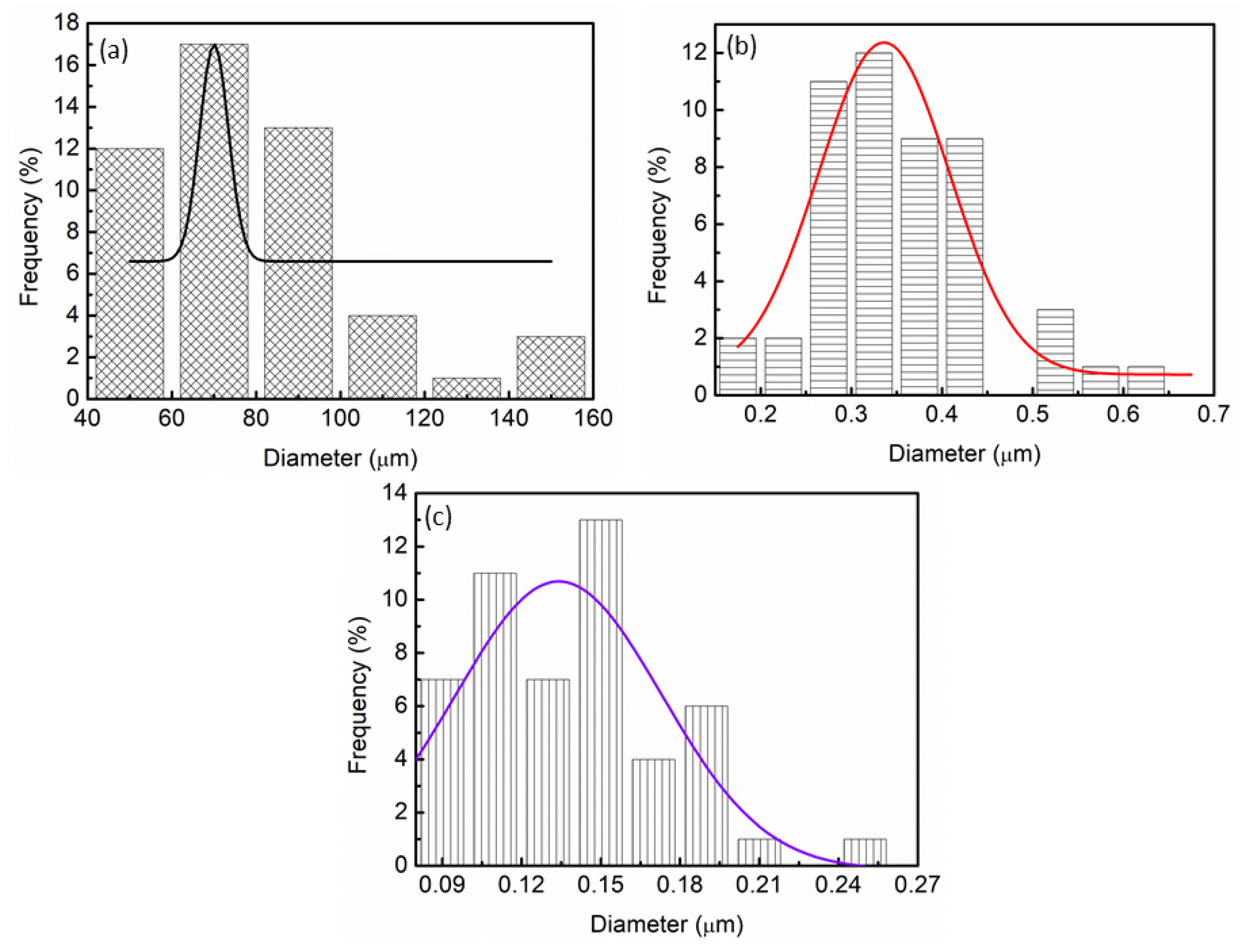
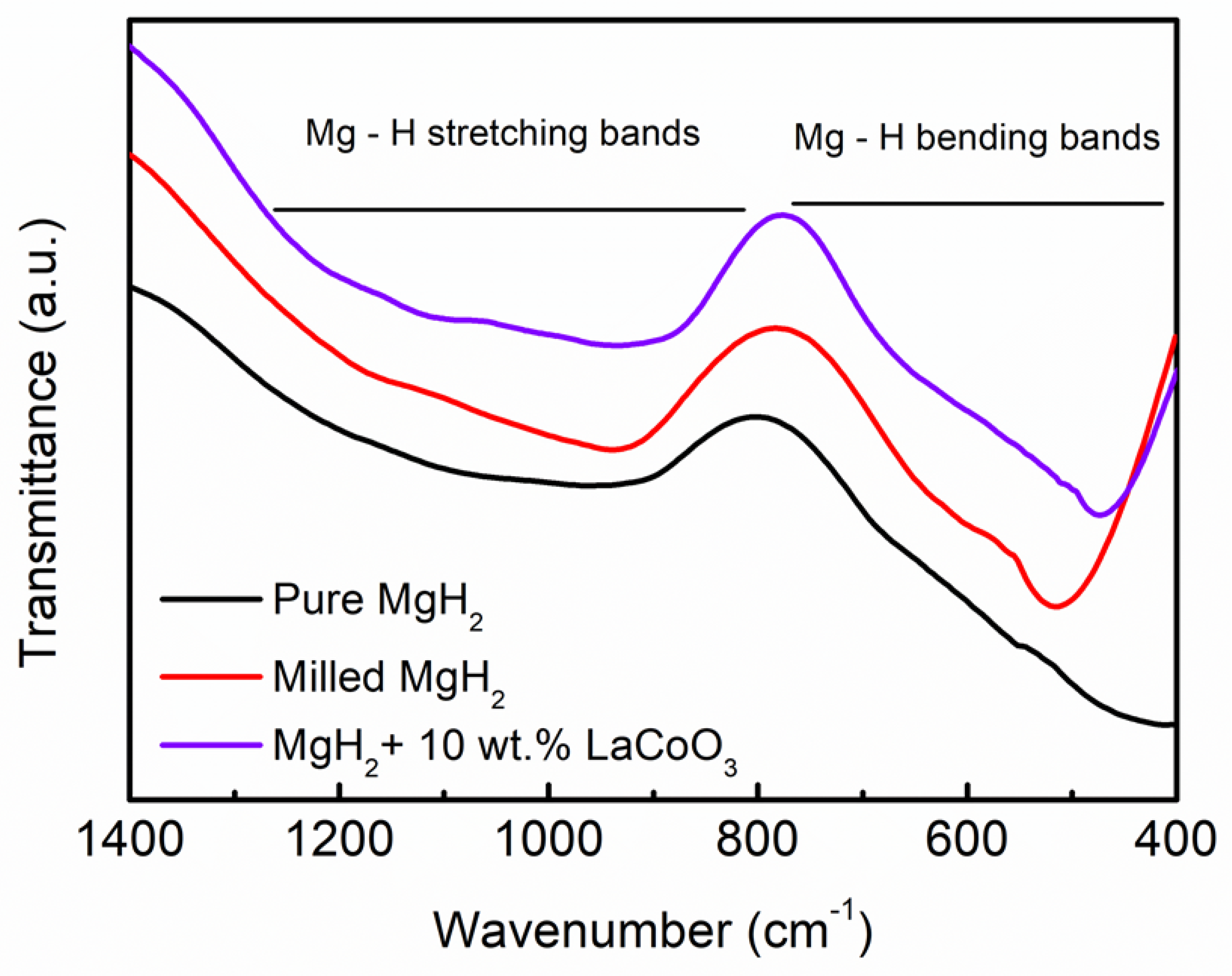
| Samples | Onset Desorption Temperature (°C) | Absorption Capacity (wt.%) | Desorption Capacity (wt.%) |
|---|---|---|---|
| Pure MgH2 | 420 | - | - |
| Milled MgH2 | 350 | 6.68 | 0.34 |
| 5 wt.% LaCoO3 with MgH2 | 316 | 7.30 | 2.46 |
| 10 wt.% LaCoO3 with MgH2 | 322 | 7.30 | 3.24 |
| 15 wt.% LaCoO3 with MgH2 | 310 | 6.99 | 2.04 |
| 20 wt.% LaCoO3 with MgH2 | 323 | 5.49 | 4.53 |
| Integrated Equation | Model |
|---|---|
| α = kt | Surface-controlled (chemisorption) |
| [−ln(1 − α)]1/2 = kt | JMA, n = 2 (e.g., two-dimensional growth of existing nuclei with constant interface velocity) |
| [−ln(1 − α)]1/3 = kt | JMA, n = 3 (e.g., two-dimensional growth of existing nuclei with constant interface velocity) |
| 1 − (1 − α)1/3 = kt | CV 2D: contracting volume, three-dimensional growth with constant interface velocity |
| 1 − (2α/3) − (1 − α)2/3 = kt | CV 3D: contracting volume, three-dimensional growth diffusion controlled with decreasing interface velocity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazelee, N.; Md Din, M.F.; Ismail, M.; Rather, S.-U.; Bamufleh, H.S.; Alhumade, H.; Taimoor, A.A.; Saeed, U. Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2. Materials 2023, 16, 2449. https://doi.org/10.3390/ma16062449
Sazelee N, Md Din MF, Ismail M, Rather S-U, Bamufleh HS, Alhumade H, Taimoor AA, Saeed U. Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2. Materials. 2023; 16(6):2449. https://doi.org/10.3390/ma16062449
Chicago/Turabian StyleSazelee, Noratiqah, Muhamad Faiz Md Din, Mohammad Ismail, Sami-Ullah Rather, Hisham S. Bamufleh, Hesham Alhumade, Aqeel Ahmad Taimoor, and Usman Saeed. 2023. "Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2" Materials 16, no. 6: 2449. https://doi.org/10.3390/ma16062449
APA StyleSazelee, N., Md Din, M. F., Ismail, M., Rather, S.-U., Bamufleh, H. S., Alhumade, H., Taimoor, A. A., & Saeed, U. (2023). Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2. Materials, 16(6), 2449. https://doi.org/10.3390/ma16062449











