Tuning the Red-to-Green-Upconversion Luminescence Intensity Ratio of Na3ScF6: 20% Yb3+, 2% Er3+ Particles by Changes in Size
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthetic Procedures
2.3. Characteristics
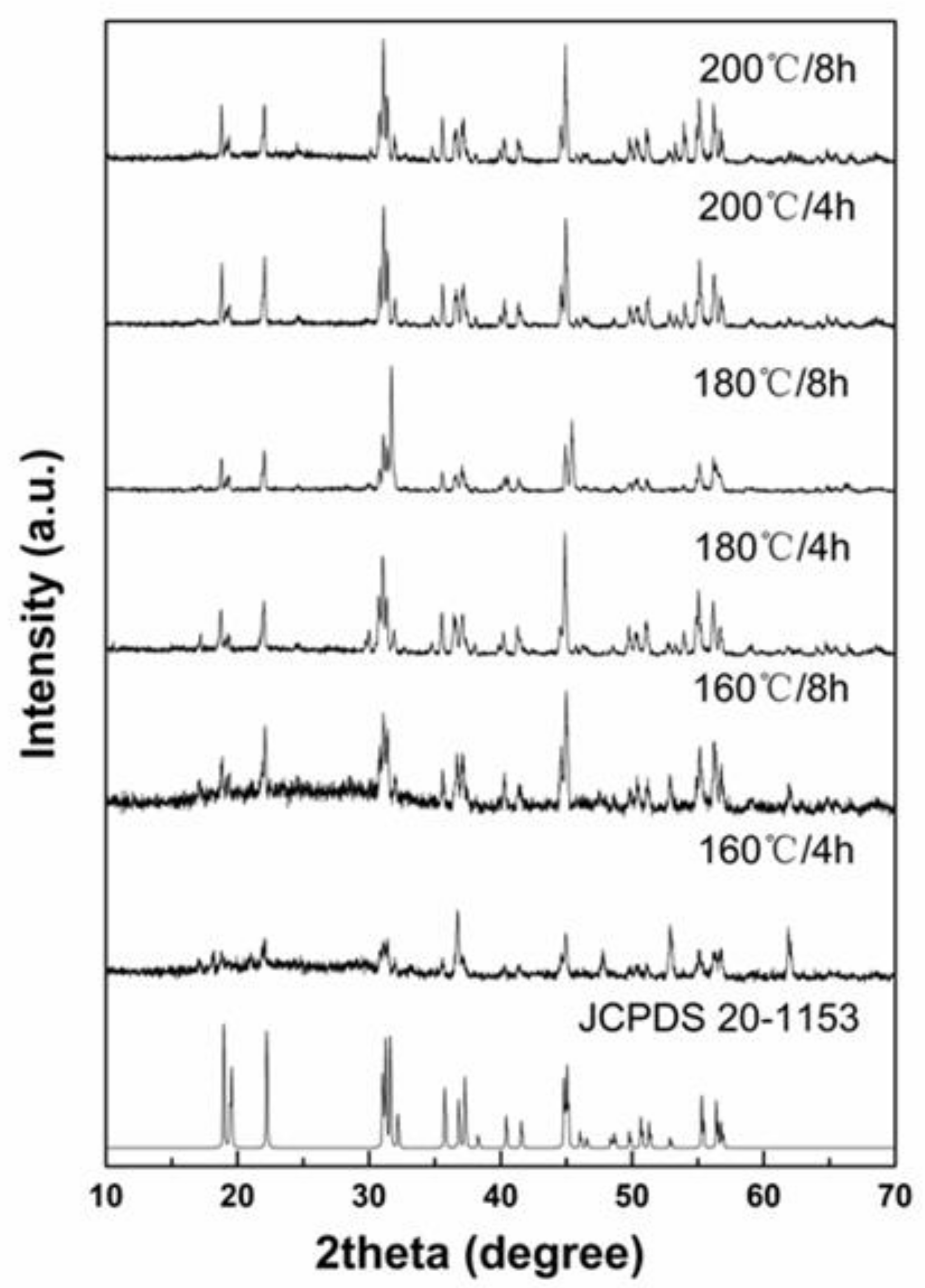
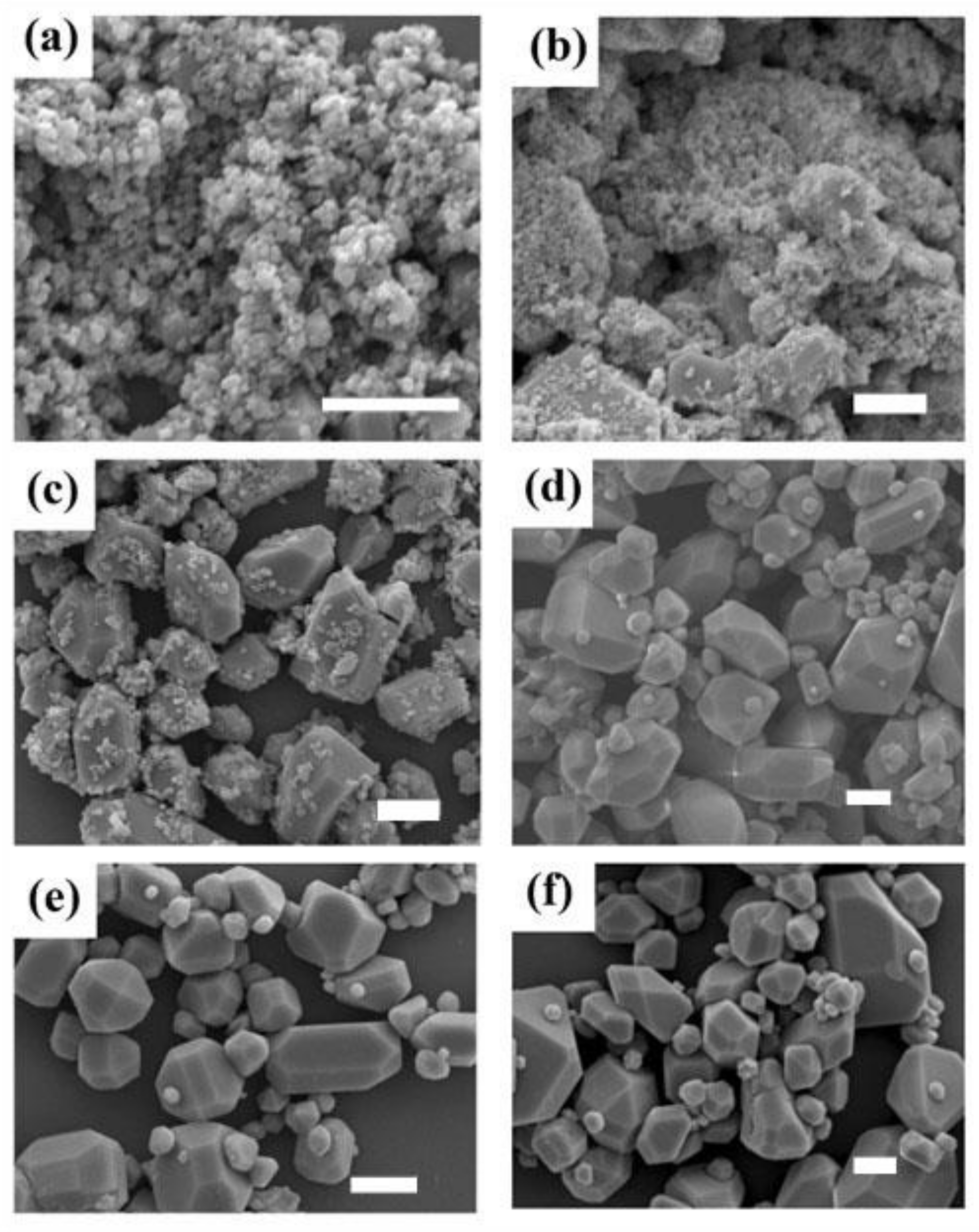
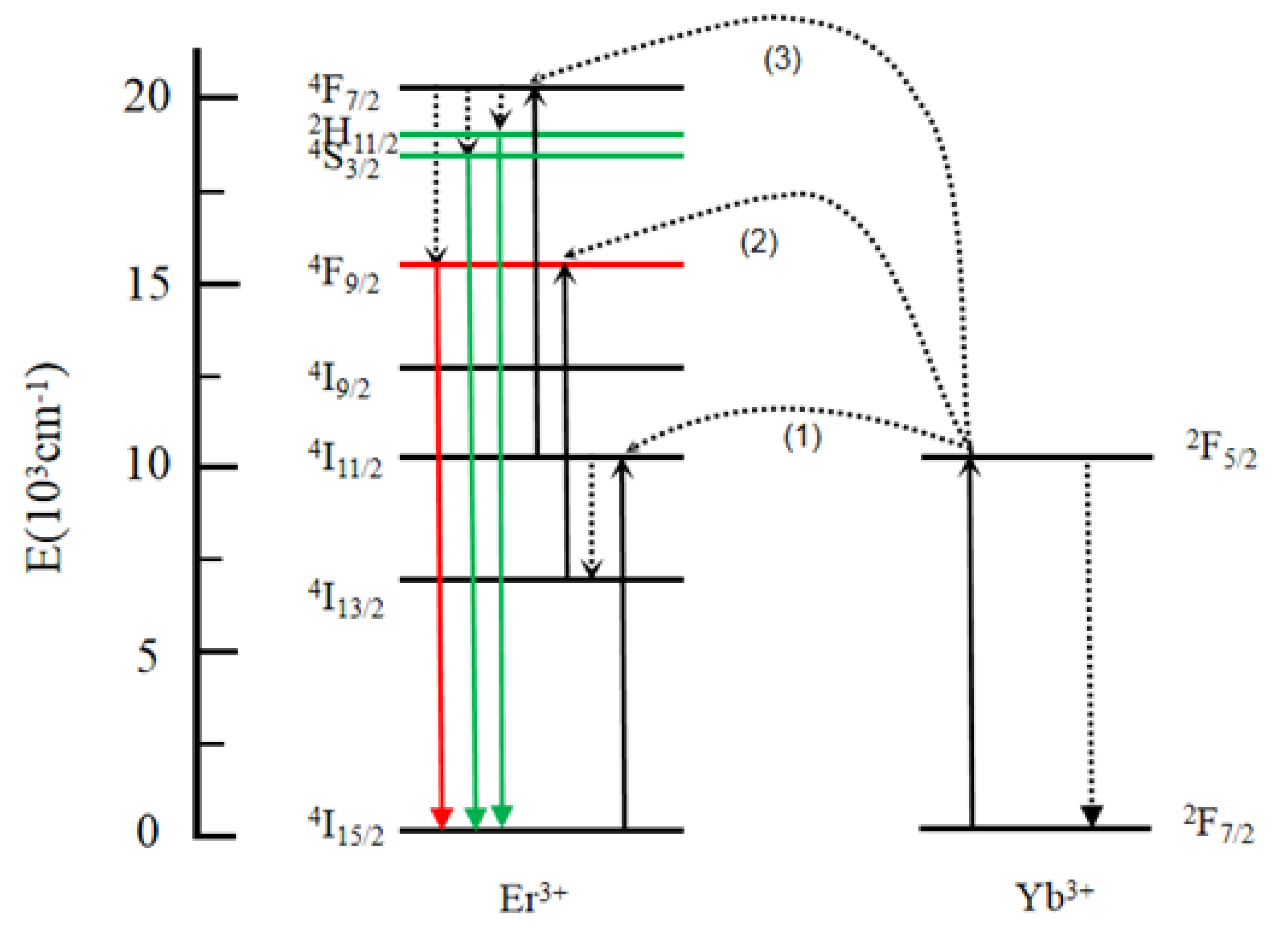
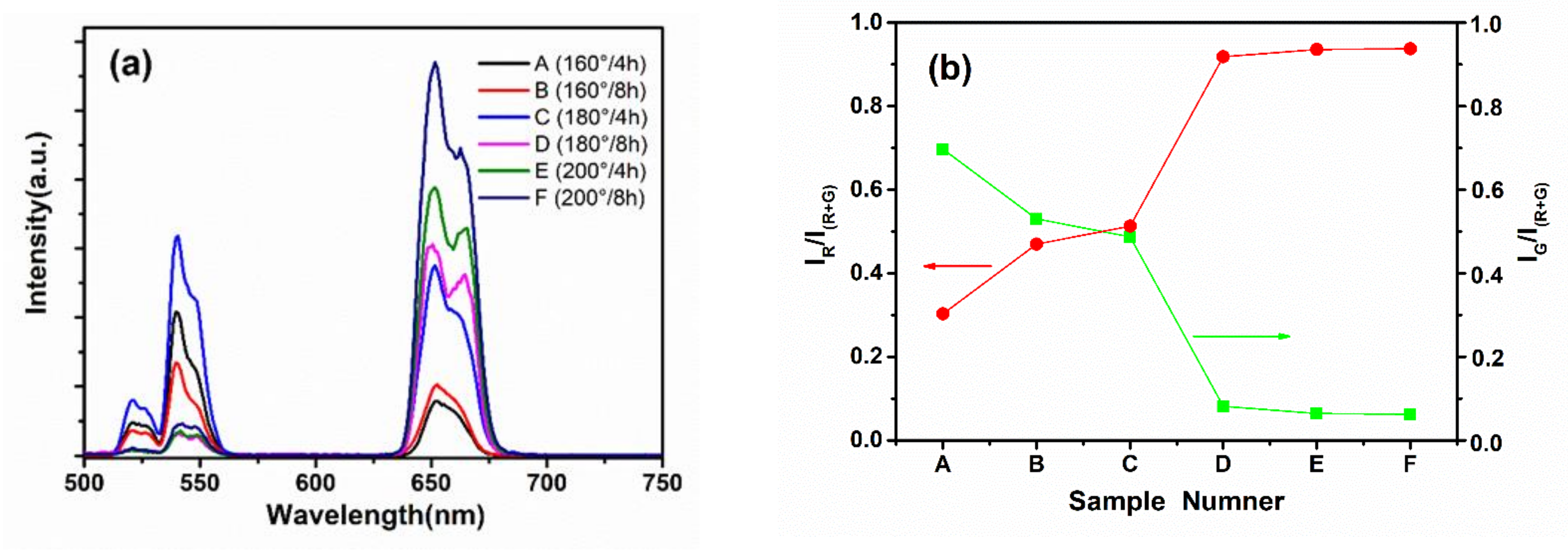
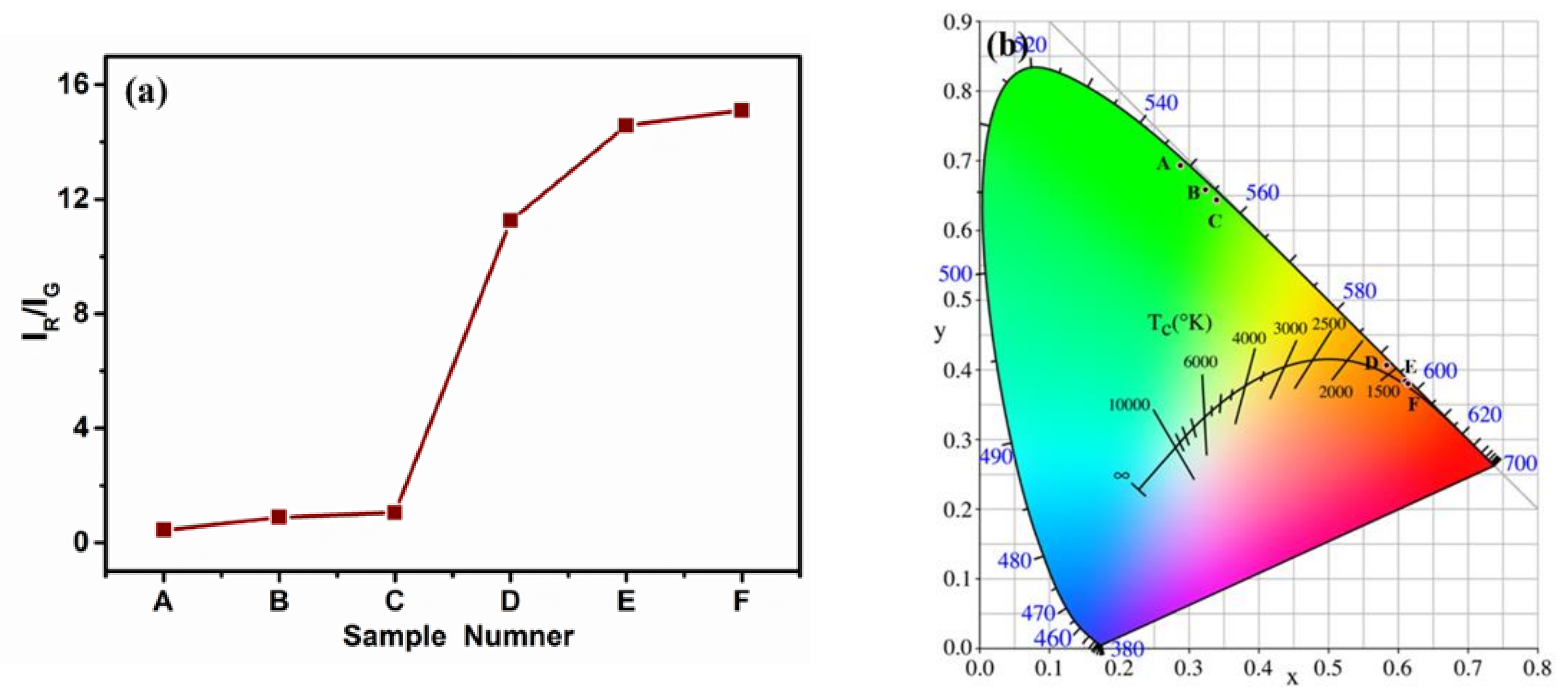
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.G.; Zhao, S.L.; Liang, Z.Q.; Zhang, J.J.; Zhu, W. The colour tuning of upconversion emission from green to red in NaScF4:Yb3+/Er3+ nanocrystals by adjusting the reaction time. J. Alloys Compd. 2017, 699, 1–6. [Google Scholar] [CrossRef]
- Anbharasi, L.; Gunaseelan, M.; Adusumalli, V.N.K.B.; Yamini, S.; Mahalingam, V.; Senthilselvan, J. Structural and Optical Studies of Strong Red Emitting NaxScF3+x: Yb3+, Er3+ Upconversion Nanoparticles. AIP Conf. Proc. 2019, 2115, 030179. [Google Scholar]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Zheng, X.L.; Zhang, J.; Camillis, S.D.; Jia, J.G.; Wang, H.; Liao, X.Z.; Piper, J.A.; Lu, Y.Q. Quantifying the Influence of Inert Shell Coating on Luminescence Brightness of Lanthanide Upconversion Nanoparticles. ACS Photonics 2022, 9, 758–764. [Google Scholar] [CrossRef]
- Pei, W.B.; Wang, L.L.; Wu, J.S.; Chen, B.; Wei, W.; Lau, R.; Huang, L.; Huang, W. Controlled Synthesis of Uniform NaxScF3+x Nanopolyhedrons, Nanoplates, Nanorods and Nanospheres Using Solvents. Cryst. Growth Des. 2015, 15, 2988–2993. [Google Scholar] [CrossRef]
- Pang, M.; Zhai, X.S.; Feng, J.; Song, S.Y.; Deng, R.P.; Wang, Z.; Yao, S.; Ge, X.; Zhang, H.J. One-step synthesis of water-soluble hexagonal NaScF4:Yb/Er nanocrystals with intense red Emission. Dalton Trans. 2014, 43, 10202. [Google Scholar] [CrossRef]
- Ai, Y.; Tu, D.T.; Zheng, W.; Liu, Y.S.; Kong, J.T.; Hu, P.; Chen, Z.; Huang, M.D.; Chen, X.Y. Lanthanide-doped NaScF4 nanoprobes: Crystal structure, optical spectroscopy and biodetection. Nanoscale 2013, 5, 6430. [Google Scholar] [CrossRef]
- Yang, M.S.; Ren, J.J.; Zhang, R.L. Preparation, upconversion luminescence, and solid-state NMR studies of water-soluble hexagonal NaScF4:Yb/Er microcrystals. J. Alloys Compd. 2017, 714, 160–167. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Liu, Z. Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 2013, 5, 23–37. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, P.W.; Hwang, K.; Lu, Y. An Exceptionally Simple Strategy for DNA-Functionalized Up-Conversion Nanoparticles as Biocompatible Agents for Nanoassembly, DNA Delivery, and Imaging. J. Am. Chem. Soc. 2013, 135, 2411–2414. [Google Scholar] [CrossRef]
- Qiao, X.F.; Zhou, J.C.; Xiao, J.W.; Wang, Y.F.; Sun, L.D.; Yan, C.H. Triple-functional core–shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro. Nanoscale 2012, 4, 4611–4623. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.S.; Chen, X.Y.; Liu, X.G. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, X.Y.; Zhai, X.S.; Li, Y.; Zhao, W.; Sun, W.; Zhang, Q.F.; Feng, J. Remarkably Enhanced Red Upconversion Emission in β-NaLuF4:Er,Tm Microcrystals via Ion Exchange. Inorg. Chem. 2022, 61, 10713–10721. [Google Scholar] [CrossRef]
- Zhai, X.S.; Chen, X.L.; Wang, S.Q.; Sun, W.; Du, J.Z.; Zhang, C.C.; Ren, T.; Zhang, Q.; Feng, J. Synthesis of small-sized hexagonal NaREF4 (RE = Yb, Lu) nanocrystals through accelerating phase transformation. J. Lumin. 2022, 244, 118694. [Google Scholar] [CrossRef]
- Zheng, X.L.; Zhu, X.J.; Lu, Y.Q.; Zhao, J.B.; Feng, W.; Jia, G.H.; Wang, F.; Li, F.Y.; Jin, D.Y. High-contrast visualization of upconversion luminescence in mice using time-gating approach. Anal. Chem. 2016, 88, 3449–3454. [Google Scholar] [CrossRef]
- Jakoby, M.; Beil, C.; Nazari, P.; Richards, B.S.; Seitz, M.; Turshatov, A.; Howard, I.A. Rare-earth coordination polymers with multimodal luminescence on the nano-, micro-, and milli-second time scales. iScience 2021, 24, 102207. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} Cluster Based Metal–Organic Framework with Enhanced Room-Temperature Phosphorescence and Optoelectronic Performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Del-Castillo, J.; Méndez-Ramos, J.; Acosta-Mora, P.; Yanes, A.C. Upconversion photonics in solvothermal Sr2YbF7: Tm3+@Sr2YF7 core-shell nanocrystals for enhanced photocatalytic degradation of pollutants. J. Lumin. 2022, 241, 118490. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.Y.; Li, G.H.; Tang, L. Friction and wear behavior of Ni-based alloy coatings with different amount of WC–TiC ceramic particles. J. Mater. Sci. 2023, 58, 1116–1126. [Google Scholar] [CrossRef]
- Yin, W.Y.; Esposito, D.V.; Yang, S.Z.; Ni, C.Y.; Chen, J.G.G.; Zhao, G.L.; Zhang, Z.J.; Hu, C.W.; Cao, M.H.; Wei, B. Controlling Novel Red-Light Emissions by Doping In2O3 Nano/Microstructures with Interstitial Nitrogen. Phys. Chem. C 2010, 114, 13234–13240. [Google Scholar] [CrossRef]
- Yin, W.Y.; Zhao, L.N.; Zhou, L.J.; Gu, Z.J.; Liu, X.X.; Tian, G.; Jin, S.; Yan, L.; Ren, W.L.; Xing, G.M.; et al. Enhanced Red Emission from GdF3:Yb3+,Er3+ Upconversion Nanocrystals by Li+ Doping and Their Application for Bioimaging. Chem. Eur. J. 2012, 18, 9239. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.B.; Wu, S.L.; Zhang, S.F. A facile and general approach for the multicolor tuning of lanthanide-ion doped NaYF4 upconversion nanoparticles within a fixed composition. J. Mater. Chem. 2010, 20, 9113–9117. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.D.; Wang, Y.F.; Ke, J.; Si, R.; Xiao, J.W.; Lv, G.M.; Shi, S.; Yan, C.H. Efficient Tailoring of Upconversion Selectivity by Engineering Local Structure of Lanthanides in NaxREF3+x Nanocrystals. J. Am. Chem. Soc. 2015, 137, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.J.; Zhou, L.J.; Yin, W.Y.; Liu, X.X.; Yan, L.; Jin, S.; Ren, W.L.; Xing, G.M.; Li, S.J.; et al. Mn2+ Dopant-Controlled Synthesis of NaYF4: Yb/Er Upconversion Nanoparticles for in vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef]
- Mao, Y.N.; Xian, P.F.; Jiang, L.; Hu, S.S.; Tang, J.F.; Yang, J. Temperature sensing performance based on up-conversion luminescence in hydrothermally synthesized Yb3+, Er3+ co-doped NaScF4 phosphors. Dalton Trans. 2020, 49, 7862–7871. [Google Scholar] [CrossRef]
- He, E.J.; Chen, S.F.; Zhang, M.L. Simultaneous morphology evolution and upconversion emission tuning of single Y-based fluoride microcrystal induced by Sc3+ co-doping. Mater. Res. Bull. 2017, 87, 61–71. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, J.; Shen, D.Y.; Hu, S.S.; Zhou, X.J.; Tang, J.F. Lanthanide-doped Sr2ScF7 nanocrystals: Controllable hydrothermal synthesis, growing mechanism and tunable up/down conversion luminescence properties. J. Mater. Chem. C 2017, 5, 3264–3275. [Google Scholar] [CrossRef]
- Teng, X.; Zhu, Y.H.; Wei, W.; Wang, S.C.; Huang, J.F.; Naccache, R.; Hu, W.B.; Tok, A.I.Y.; Han, Y.; Zhang, Q.C.; et al. Lanthanide-Doped NaxScF3+x Nanocrystals: Crystal Structure Evolution and Multicolor Tuning. J. Am. Chem. Soc. 2012, 134, 8340–8343. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Zhao, S.L.; Xu, Z.; Qia, B.; Yang, Y.X.; Zhu, W.; Xu, X.R. Controllable synthesis of tetragonal LiScF4:Yb3+, Er3+ nanocrystals and its upconversion photoluminescence properties Lithium scandium fluoride nanocrystals. Opt. Mater. 2016, 62, 255–260. [Google Scholar] [CrossRef]
- Chatterjeea, D.K.; Rufaihaha, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef]
- Ding, Y.J.; Teng, X.; Zhu, H.; Wang, L.L.; Pei, W.B.; Zhu, J.J.; Huang, L.; Huang, W. Orthorhombic KSc2F7:Yb/Er Nanorods: Controlled Synthesis and Strong Red Upconversion Emission. Nanoscale 2013, 5, 11928–11932. [Google Scholar] [CrossRef]
- Shan, J.N.; Ju, Y.G. A single-step synthesis and the kinetic mechanism for monodisperse and hexagonal-phase NaYF4: Yb, Er upconversion nanophosphors. Nanotechnology 2009, 20, 275603. [Google Scholar] [CrossRef]
- Zhai, X.s.; Liu, S.S.; Zhang, Y.L.; Qin, G.S.; Qin, W.P. Controlled synthesis of ultrasmall hexagonal NaTm0.02Lu0.98-xYbxF4 nanocrystals with enhanced upconversion luminescence. J. Mater. Chem. C 2014, 2, 2037–2044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Song, M.; Qin, Z. Tuning the Red-to-Green-Upconversion Luminescence Intensity Ratio of Na3ScF6: 20% Yb3+, 2% Er3+ Particles by Changes in Size. Materials 2023, 16, 2247. https://doi.org/10.3390/ma16062247
Zhang Y, Liu X, Song M, Qin Z. Tuning the Red-to-Green-Upconversion Luminescence Intensity Ratio of Na3ScF6: 20% Yb3+, 2% Er3+ Particles by Changes in Size. Materials. 2023; 16(6):2247. https://doi.org/10.3390/ma16062247
Chicago/Turabian StyleZhang, Yongling, Xiang Liu, Mingxing Song, and Zhengkun Qin. 2023. "Tuning the Red-to-Green-Upconversion Luminescence Intensity Ratio of Na3ScF6: 20% Yb3+, 2% Er3+ Particles by Changes in Size" Materials 16, no. 6: 2247. https://doi.org/10.3390/ma16062247
APA StyleZhang, Y., Liu, X., Song, M., & Qin, Z. (2023). Tuning the Red-to-Green-Upconversion Luminescence Intensity Ratio of Na3ScF6: 20% Yb3+, 2% Er3+ Particles by Changes in Size. Materials, 16(6), 2247. https://doi.org/10.3390/ma16062247





