Abstract
Indoor air quality has become a significant public health concern. The low cost and high efficiency of photocatalytic technology make it a natural choice for achieving deep air purification. Photocatalysis procedures have been widely investigated for environmental remediation, particularly for air treatment. Several semiconductors, such as TiO2, have been used for photocatalytic purposes as catalysts, and they have earned a lot of interest in the last few years owing to their outstanding features. In this context, this review has collected and discussed recent studies on advances in improving the photocatalytic activity of TiO2-based materials for indoor air treatment and bacterial inactivation. In addition, it has elucidated the properties of some widely used TiO2-based catalysts and their advantages in the photocatalytic process as well as improved photocatalytic activity using doping and heterojunction techniques. Current publications about various combined catalysts have been summarized and reviewed to emphasize the significance of combining catalysts to increase air treatment efficiency. Besides, this paper summarized works that used these catalysts to remove volatile organic compounds (VOCs) and microorganisms. Moreover, the reaction mechanism has been described and summarized based on literature to comprehend further pollutant elimination and microorganism inactivation using photocatalysis. This review concludes with a general opinion and an outlook on potential future research topics, including viral disinfection and other hazardous gases.
1. Introduction
Air pollution and the degradation of air quality are becoming severe issues to deal with, but these notions often remain abstract and complex to surround or identify [1]. The contamination of food and drink raises a lot of interest since they are linked to vital daily elements for everyone [2]. Understanding how airborne particles can affect food and beverage quality is the first step to understanding how air filtration systems can address this issue [3]. Therefore, it is natural that the agri-food sector has a significant challenge in protecting its employees and processes against harmful atmospheric pollutants [4].
The primary pollutants confronted in indoor air include carbon monoxide (CO), micro-organisms (fungi, bacteria, and viruses), nitrogen oxides (NOx), and a multitude of varieties of volatile organic compounds (VOCs) [5]. Given that in France, agri-food companies represent 15.3% of manufacturing industries with more than 17,647 companies [6], it is therefore essential and urgent to employ the purification system technology more effectively [7,8].
Numerous developing and encouraging technologies currently supply a solution to this issue [9,10]. Among them, heterogeneous photocatalysis in visible light proves its interest in compounds’ degradation and/or mineralization [11,12]. However, these technologies do not make it possible to effectively guarantee constant purification over time of the microorganisms without the need for frequent maintenance operations due to their excessive bulk [13,14]. Advanced Oxidation Processes (AOPs) are processes that produce highly oxidizing species such as hydroxyl radicals (•OH) and other reactive oxygen species (ROS), including the anion superoxide radical (•O2−) and hydrogen peroxide (H2O2) capable of degrading target pollutants present in effluents [15,16]. The semiconductor material TiO2 is considered a reference photocatalyst and an antibacterial agent due to its physicochemical properties [11,17,18]. However, TiO2 has a wide bandgap, which limits its practical application in environmental remediation under visible light irradiation, including a wide range of the solar spectrum [16,19]. Many strategies have been implemented to overcome this concern, such as doping TiO2 with metallic or non-metallic elements [20] and coupling with other semiconductors [21,22,23] to increase their absorption in the visible and improve the lifetime of electron-hole pairs [24]. It is possible to improve the redox process of pollutant degradation by doping TiO2 with a metal oxide, which produces photoexcited charge carriers [25].
Indoor air quality has emerged as a significant public health problem. Photocatalytic technology is a natural solution for deep air filtration due to its low cost and excellent efficiency. Photocatalysis methods have been extensively researched for environmental remediation, notably for air treatment. Several semiconductors, such as TiO2, have been used as photocatalytic catalysts, and they have gained a lot of attention in recent years due to their remarkable properties. For indoor air purification and bacterial inactivation, this review has compiled and evaluated current findings on improvements in the photocatalytic activity of TiO2-based photocatalytic materials. The characteristics of various popular TiO2-based catalysts and their benefits in the photocatalytic process have also been clarified, as well as how doping and heterojunction approaches might increase photocatalytic activity. Recent articles regarding diverse combined catalysts have been summarized and examined to underline the relevance of combining catalysts to boost efficiency. The studies that employ these catalysts to remove microorganisms and volatile organic compounds (VOCs) were also covered in this publication. Based on the literature, the reaction mechanism has also been defined and summarized to understand better pollutant removal and microorganism inactivation utilizing photocatalysis. Finally, this review’s conclusion includes a summary and prognosis on prospective future study areas, such as viral disinfection and other dangerous gases. To our knowledge, there are few studies on the catalytic activity of alternative materials for indoor air treatment by eliminating both pollutants types, microorganisms, and VOCs.
2. Photocatalysis and Mass Transfer
Heterogeneous photocatalytic oxidation (HPO) is one of the active investigations in environmental treatment and purification [26,27,28]. It is widely applied in air pollution treatment, especially volatile organic compounds [29,30]. The resourceful technology is reserved for decomposing gaseous contaminants by employing photocatalysts under UV or solar light free of additional energy expenses [13,31].
2.1. Principle of Photocatalysis
Photocatalysis is generally described as the process of employing light (UV or visible light) to activate a substrate (such as a semiconductor photocatalyst) so that photo-reaction can be accelerated or facilitated with the catalyst remaining unconsumed [5]. The process can be divided into five steps (Figure 1):
- (1)
- Transfer the reactants to the air phase.
- (2)
- Adsorption of the reactants on the surface of the catalyst.
- (3)
- Reaction in the adsorbed phase.
- (3.1)
- Absorption of a photon by the catalyst.
- (3.2)
- Generation of the electron-hole pairs.
- (3.3)
- Separation of the pair.
- (4)
- The oxidation and reduction with the adsorbed substrate.
- (5)
- Desorption of the intermediate product.
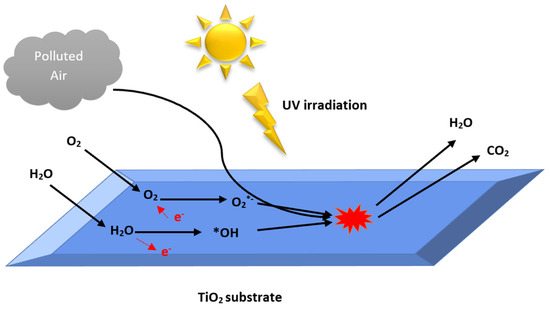
Figure 1.
Polluted air photocatalysis treatment by TiO2.
Among these five steps, the photocatalytic reaction is of crucial significance. It is initiated by the electron’s excitation from the filled valence band (VB) to the empty conduction band (CB) of the photocatalyst when the energy carried by the absorbed photon equals or exceeds the band gap of the photocatalyst (Figure 2). In addition, the reaction results in the creation of a negative electron in the CB and a positive hole in the VB is called an electron-hole pair [32,33,34]. The positive hole oxidizes the hydroxide ion to yield hydroxyl radical (•OH), a potent oxidant of organic pollutants. The photo-excited electron is reduced to form the superoxide radical anion (O2•−). These radicals are keys to the degradation of organic compounds [35].
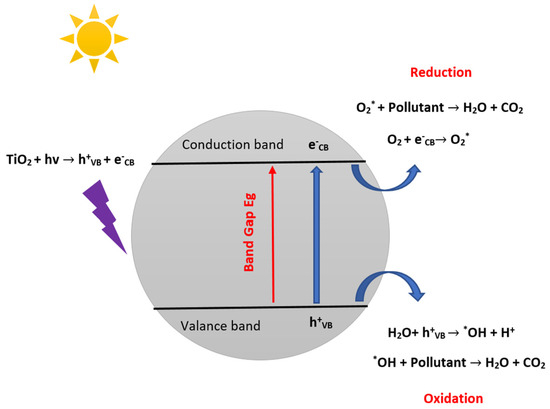
Figure 2.
Schematic illustration of the photocatalytic reaction mechanism.
2.2. Development of Heterogeneous Photocatalytic Oxidation
Among all semiconductors, Titanium dioxide (TiO2)-based materials have received particular attention in the photocatalysis field for their light absorption ability and high-efficiency treatment for both water and air; it was discovered by Fujishima and Honda in 1972 [36]. According to previous investigations, TiO2-based photocatalysts also provide the advantages of high stability, availability, nontoxicity, excellent photoactivity, and low cost [35]. The photocatalysis of TiO2 depends on variables such as specific surface area, crystallinity and surface hydroxyl groups of the TiO2 [37]. This particular material has relatively polar surfaces that allow easy adsorption of hydrophilic pollutants. Nevertheless, titanium dioxide (TiO2)-based materials have rapid recombination of electron-hole pairs, which, to some degree, suppresses the reaction efficiency [38,39].
Moreover, the band gap of materials is wide (3.0–3.2 eV), so the reaction is only activated with the irradiation of ultraviolet; hence the utilization of visible light irradiation is limited [14,40]. In order to improve the activity of photocatalysts under solar or artificial light at a lower energy cost and under more economic conditions, several strategies and investigations have been carried out to enhance the performance of TiO2 [41]. These strategies include chemical modification, dye sensitization, and coupling with other semiconductor materials by introducing impurity atoms into pure TiO2 to change electron-hole pairs concentrations in TiO2 [35].
Metal doping is a method in which traces of foreign elements are introduced within the crystal lattice, and researchers widely use this strategy to reduce the band gap of titanium dioxide-based materials [42]. Noble metallic metals such as Ag, Au, Pt, and Pd have been researched extensively for years because of their properties and contribution to visible light absorption [5].
Ag is of particular interest as it acts as an electron trap and leads to retard the recombination of the electron-hole pair through the improvement of the transfer of interfacial charge [43]. Yi et al. studied a composite of Ag–AgI–TiO2/CNFs; the Ag and I (Iodine) oxidation generated the reactive oxygen species (ROS) in the visible light range [44], doping TiO2 with Ag and I increase its light range, increasing the photocatalytic activity. Yangfeng Chen et al. proposed a composite of heterostructured g-C3N4/Ag/TiO2 microspheres by using the properties of Ag to delay the recombination of electron-hole pairs [45]. Apart from Ag, other metallic oxides can be composited with titanium dioxide to make the photo-reaction work under visible light. For example, halogens (X: Cl, Br, or I) bound to Bismuth oxide to form BiOX, as a new class of promising catalyst has also drawn significant attention due to their attractive physicochemical characteristics, such as unique micro/nanostructures, bandgaps, optical and electrical properties and many other physicochemical characteristics [46,47,48,49]. Wendong Zhang et al. [49] found that the nanoplate BiOBr was highly efficient under visible light for NO photoreduction. Those promising catalysts (halogens) can be used as heterostructured photocatalysts with TiO2 in order to enhance their photocatalytic activity. Actually, There is a lot of work done in heterogeneous photocatalysts with TiO2, such as TiO2/Ag [50], TiO2/SiO2 [51], TiO2/Fe2O3 [52,53], TiO2/Graphene [54] which have been shown to improve the photocatalytic performance of TiO2, especially in the degradation of organic pollutants. These are only a few examples of TiO2-based heterostructured photocatalysts. TiO2 may be mixed with a variety of different substances to improve its photocatalytic activity. Aguilera-Ruiz also stated that cuprous oxide (Cu2O), a visible-light-driven photocatalyst, has a band gap of about 2.07 eV [55]. Meanwhile, the conduction and valence band boundaries of BiVO4 are located at 0.11 V and 2.65 V NHE. Thus, the composite Cu2O/BiVO4 has a promising photocatalytic performance under visible light [56]. Those two interesting materials, CuO and BiVO4, can be used as the heterojunction or heterostructure to enhance the photocatalytic activity of the TiO2-based catalysts. Moreover, it has been shown that Ag- V- and Fe-doped TiO2 achieved by various routes are very efficient in the oxidation of VOCs (butyl acetate, hexane or gaseous toluene) [57].
Non-metal doping is another strategy established to increase titanium dioxide’s activity under solar or visible light. This technique takes advantage of the possible electronic transition from the induced new electronic states above TiO2 VB (2p or 3p orbitals of the dopant) to TiO2 CB (3d orbitals of Ti). Several researchers reported that the doped photocatalyst activity increases after non-metal doping, as the electronic structure has been modified to extend the absorption of the photocatalyst into the visible-light region [57]. Various studies have shown non-metal doping of TiO2, such as Nitrogen-doped TiO2 [58], carbon-doped TiO2 [59,60,61], sulfur-doped TiO2 [62,63], boron-doped TiO2 [64] and phosphorus-doped TiO2 [65]. Vaiano et al. studied recyclable visible-light active N-doped TiO2 photocatalysts coated on glass spheres using a simple sol-gel method. They obtained excellent photocatalytic activity with visible light irradiation [66].
2.3. Reactors and Configurations
The configuration of reactors for air treatment is a critical element in the efficiency of the process. It should promote effective contact between the catalyst and the photons on the one hand and between the catalyst and the pollutants on the other. Care must also be taken to limit pressure drops. This part will present different continuous-flow photoreactors used in the laboratory or on an industrial scale.
Usually, this type of reactor is made up of a perforated plate placed at the inlet to ensure the homogeneity of the airflow. The central box contains two fixing devices for the photocatalytic support on one hand and a UV lamp on the other. In this configuration, the polluted air, driven by a fan, passes through the photocatalytic support. Another reactor configuration is based on using porous monolithic supports with varying thicknesses. The structure is based on the successive use of several UV lamps and monolithic "honeycomb" type photocatalytic media. The lamps irradiate the front and back sides of the monolithic supports.
The photocatalytic medium is placed against the reactor’s internal wall and irradiated by a lamp set in a central tube. The particularity of this type of pilot is that the distance between the two plates or the diameters carrying the media is variable, which makes it possible to test the effect of the gap on the performance of the process.
Flat and cylindrical configurations:
A schematic representation of this rectangular configuration is used in the works of Assadi et his co-workers [3,5,17]. This reactor is formed by a chamber containing two glass plates at a variable distance. Each plate carries the photocatalytic media. Lamps are positioned along the length of the reactor at an equal distance in the inter-plate space [3,5,17].
The reactor is formed by two cylindrical tubes. The catalyst is installed on the inner wall of the outer cylinder. A UV lamp is installed in the inner tube in order to have uniform radiation from the catalytic surface. The gaseous effluent circulates between the outer tube’s inner wall and the inner tube’s outer wall. Note, To demonstrate the effect of material transfer, the diameter of the inner tube is studied to vary the thickness of the gas film [3,5,17].
Another planar configuration is based on surface-degraded fiber optic sheets on which titanium dioxide has been deposited. The latter replaced the bulky UV lamps. Optical fibers are used to activate the catalyst and further optimize the supply of UV radiation compared to lamps. This configuration will make it possible to inactivate the pollutants while offering compactness of the solution, i.e., lower pressure drops and easy handling in use. Figure 3 shows images of a fiber optic photocatalytic reactor and a fiber optic shee [3,5,17].
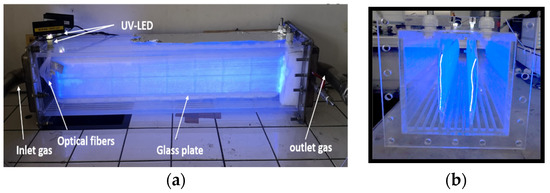
Figure 3.
(a) Images of a photocatalytic reactor based on optical fibers, (b) of a side view (Range of processed flow: from 5 at 20 m3/h with concentrations varying from 5 to 50 mg/m3) [5].
3. Volatile Organic Compounds (VOCs)
Volatile organic compounds are substances containing organic carbon which vaporize at significant rates [67,68]. They are the second-most widespread and various emissions classes after particulates [7]. Besides, we can state that as an approximate rule, VOCs are the organic liquids or solids whose vapor pressures at room temperature exceed 0.01 psi (=0.0007 atm) with atmospheric boiling points equal to or less than 480 °F estimated at 101.3 kPa, i.e., standard atmospheric pressure. Among hundreds of VOCs that have been qualitatively identified in the indoor environment, the main compounds are alkanes, alkenes, carboxylic acids and alcohols, esters, and aromatics [69]. Organic compounds are primarily found in home items such as wax, varnishes, and paints. All these chemicals can emit organic byproducts when utilized and stored in a non-controlled method. Studies have revealed that levels of numerous organic chemicals indoors are 2 to 5 times greater than outside. Therefore, with a specific level of time exposure, these organic compounds may have short or long term adverse health effects such as headaches, eye and respiratory tract irritation and even cancers [70].
VOCs in the atmosphere or the environment are relatively at low concentrations; hence, they are detectable based on interactions between the sensor component and the organic compounds. In addition, ventilation is also a conventional dilution method. Still, it is not firmly recommended in current practice because of its limitation on outdoor air quality (OAQ) and energy consumption [11,17,18]. Accordingly, researchers are still developing technologies and efficient approaches to meet IAQ standards and reduce energy costs to avail a secure, healthful, livable environment. During their research, Abidi and his collaborators studied the elimination of chloroform CHCl3 by using different catalysts and analyzing the removal efficiency under several initial concentrations of each catalyst type supported on polyester under certain conditions [5]. Many works have demonstrated the ability of some TiO2-based photocatalysts to remove VOCs from the air due to their high photocatalytic activity and stability [71]. Tobaldi et al., 2021 have reported that TiO2-graphene oxide composites exhibit enhanced photocatalytic activity for the removal of various VOCs, such as benzene, toluene, and formaldehyde [72]. Another work has shown that TiO2-carbon nanotube composite photocatalysts have efficient and improved photocatalytic activity for the removal of various VOCs, such as xylene and toluene [73]. The efficiency of the TiO2 can be enhanced in its photocatalytic activity for the elimination of VOCs in the air by doping it with metal oxides such as ZnO, Fe2O3, and WO3 [74], as this doping can increase the surface area and prevent electron-hole recombination. Table 1 summarizes several recent studies on the removal of VOCs using TiO2-metal.

Table 1.
List of some studies on using TiO2-metal for VOCs removal.
4. Microorganism Inactivation and Reactional Mechanisms
Understanding the mechanism of the bactericidal effect action of semiconductors is fundamental to improving its activity and, in particular, involves the analysis of the targets of TiO2 at the bacterial level [88]. TiO2 is a multifunctional photocatalyst that may be utilized to render microorganisms inactive [61]. The following steps are involved in the overall process for the inactivation of microorganisms using TiO2. Step 1: TiO2 is exposed to irradiation and undergoes a photocatalytic reaction that produces ROS like hydroxyl radicals (*OH) and superoxide radicals (O2*−). Step 2: ROS is formed in the photocatalytic reaction and interacts with bacterial cells and membranes and damaging DNA, proteins, and lipids. In addition, ROS can combine with water molecules to form more ROS, such as H2O2. Damages and harm caused by ROS interactions lead then to step 3 inactivation of the microorganism, in which the cell of the microorganism dies. The general microorganisms’ photocatalytic inactivation mechanisms of TiO2 can be summarized by the following equations and Figure 4:
TiO2 + hv → TiO2 (CB e−) + TiO2 (VB h+)
O2 + e− → O2−*
H2O + h+ → H+ + *OH
*OH + O2−* + Microorganism → Inactivated Microorganism
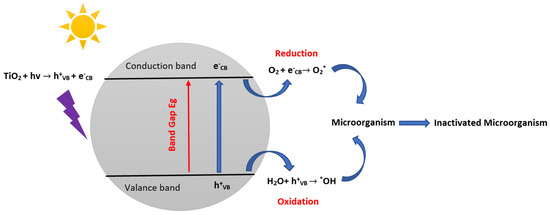
Figure 4.
Schematic illustration of the antibacterial photocatalytic mechanisms of TiO2 (inspired from ref. [89]).
Overall, because TiO2 is ecologically neutral and doesn’t produce toxic byproducts, using it as a photocatalyst to inactivate bacteria presents a viable substitute for conventional disinfection techniques that involve chemicals or heat [90]. The effectiveness of TiO2-based photocatalysis, however, is dependent on several variables, including the characteristics of the TiO2, the strength and wavelength of the light source, and the kind and quantity of bacteria present [91]. The inorganic semiconductors doping or adding a co-catalyst, such as TiO2, with metals such as Cu, mainly accelerates bacterial inactivation kinetics [76,92]. Different reactions will likely be generated when the copper oxides are in contact with the catalyst’s surface [76,92]. Indeed, CuO and Cu2O are spawned when there is an interaction between copper and O2 (air) under light irradiation. CuxO is found in two forms (CuO and Cu2O) and exhibits the Cu(+I) and Cu(+II) oxidation states, of which the main form that interacts with bacteria and VOCs is Cu2O, thus generating electrons at the level of the conduction band; Cu2O (CB e−) and holes in the valence band; Cu2O (VB h+) [78,92,93]. Under simulated sunlight, Cu2O (CB e−) enters a reduction reaction with TiO2 to reduce Ti4+ to Ti3+ and yields Cu(+I) at the VB h+ level, which may lead to bacterial inactivation and/or VOCs to form CO2, H2O, N, S and inactivated bacteria [17,76,92]. The main antibacterial photocatalytic mechanisms of TiO2 with Cu2O suggested by previous research papers cited above can be summarized by the following equations and Figure 5 [78,92,93]:
Cu2O + hv → Cu2O (CB e−) + Cu2O (VB h+)
Cu2O (CB e−) +TiO2 → TiO2− ou (Ti3+) + Cu2O
TiO2− + O2 → TiO2 +O2−*
°O2− + h+ → H2O*
H2O* + h+ + e−cb → H2O2
H2O2 + e−cb → OH + *OH
Cu2O (VB h+) + bacteria → CO2 and H2O
h+ + Bacteria → Inactivated Bacteria
H2O2 + Bacteria → Inactivated Bacteria
*OH + Bacteria → Inactivated Bacteria
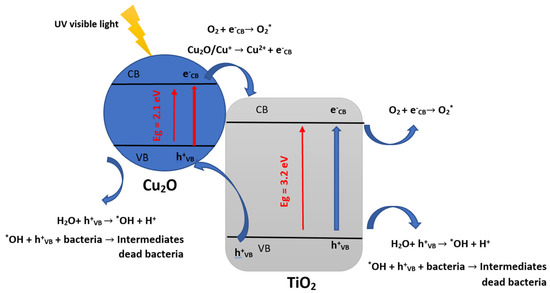
Figure 5.
The main antibacterial photocatalytic mechanisms of TiO2 with Cu2O (inspired from refs [78,92,93]).
The inactivation of bacteria cells can occur by many processes during the photocatalytic reactions, either the rupture of the cell membrane (Membrane disruption), the cell wall (Exposed cellular components), or the attack of the cells by ROS [19]. Where high levels of oxidative stress may be produced by ROS, which interacts with bacterial cells effectively and kills them by destroying the cell wall and a variety of bacterial cell components such as protein, lipids, carbohydrates, DNA, and amino acids [94]. Furthermore, when photocatalyst particles are deposited at the surface of bacterial cells, they can interact with them via diffusion and endocytosis mechanisms, which induce the destruction of membrane proteins or cell membranes owing to the phenomena of member permeability [19]. Moreover, both catalysts and generated ROS can interfere with the movement of electrons within the cell microorganisms, loss of protein motive force, depletion of intracellular ATP production with DNA replication disintegration, and intracellular outflow resulting in bacteria cell inactivation [95].
The hydroxyl radicals (*OH) produced on the surface of copper (Cu+) in contact with H2O with the holes generated at the level of VB h+ is the primary ROS involved in bacterial inactivation [29,96]. Cu2O exhibits high bacterial inactivation capacity when light irradiation stimulates electron transfer between copper and bacterial cells and produces reactive oxygen species (ROS), resulting in bacterial cell inactivation [78,97,98]. Abidi et al. investigated the effects of CuxO amounts at different sputtering times on the TiO2-Polyester (PES) photocatalyst in the inactivation of microorganisms [17]. For sputtering intensities ranging from 20 to 80 A, it was regarded that the CuxO/TiO2–PES catalyst sputtered at 80 A; the total inactivation of the bacteria was obtained after an hour of exposure to indoor light. Copper oxide showed high antibacterial activity, and the intrinsic activity of Cu(+I) can be enhanced by UV-vis illumination [17].
Additionally, Ag-NP is an excellent material used to improve the photocatalytic inactivation of microorganisms using TiO2, which has recently been proven in previous works [76]. Ag particles could inactivate bacteria as Ag-NP is an essential factor that controls and regulates antimicrobial activity [92].On contact of Ag with TiO2 under light irradiation, either Ag(0), Ag(+I), or Ag(+II) are yielded. The release of these different forms of Ag in contact with Escherichia coli induces bacterial inactivation [99]. The Ag used for TiO2-NT decoration showed +1 and +2 oxidation states (Ag+ and Ag2+) [78].
In its metallic state, silver is oxidized in the air (O2), breeding Ag2O; this substance yields Ag+ ions. This 4-electron process can be outlined by the following two equations [99]:
4 Ag0 + O2 → 2 Ag2O
2 Ag2O + 4H+ → 4 Ag+ + 2 H2O
Ag2O is at the origin of the inactivation of bacteria when it generates the production of reactive oxygen species in contact with TiO2-NTs. While Ag2O is in contact with TiO2 as a semiconductor, electrons (e−) are photo-generated by the semiconductor under the action of bandgap radiation as indicated by the chemical reaction (Equation (20)) and photo-generated holes (h+) react with H2O (Equation (18)) to yield hydroxyl radicals (OH°) [76,92]:
Ag2O + e− → 2 Ag+ + ½ O2−
h+ + H2O → *OH + H+
2H2O + O2 + 2e− → 2 *OH + 2 OH−
e− + O2 → O2*−
The suggested bacterial inactivation mechanism with Ag/TiO2 under light can be recapitulated in the following equations [76] and Figure 6:
Ag2O + hv → Ag2O (CB e−) + Ag2O (VB h+)
Ag2O (e− + h+) + TiO2 → Ag2O (VB h+) + TiO2 (CB e−)
TiO2 (CB e−) + O2 → TiO2 + O2
2 e− + O2 + 2H+ → H2O2
H2O2 + O2− → *OH + OH− + O2
Ag2O (VB h+) + Bacteria → Inactivated Bacteria
H2O2 + Bacteria → Inactivated Bacteria
*OH + Bacteria → Inactivated Bacteria
*OH + VOCs → CO2 + H2O
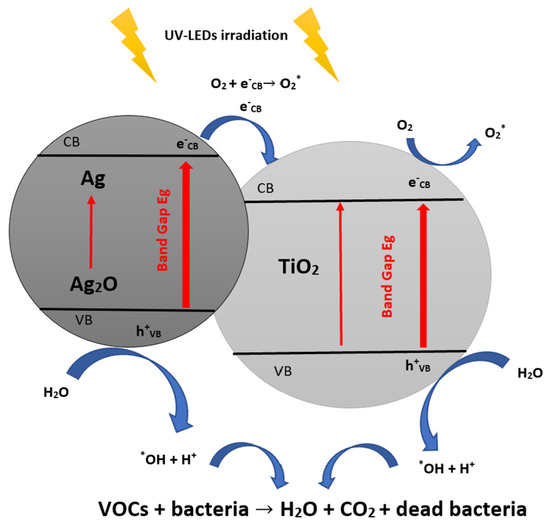
Figure 6.
The main antibacterial photocatalytic mechanisms of TiO2 with Ag2O (inspired from ref. [76]).
It is well known that the reaction of oxygen radicals in the cell causes its death [96]. Furthermore, different bacteria have different membrane structures [100]. For example, Gram − bacteria have peptidoglycan of the wall less thick than Gram + bacteria, which have an additional outer membrane composed of a double layer of lipids. This finding is in chains of different catalytic reactions and further disinfection efficiencies [101]. Accordingly, other bacteria’s survival rates will differ under identical disinfection conditions [102].
The most critical mechanism in antibacterial activity is cell membrane damage. Oxidative stress generated by ROS is a second mechanism involved in antibacterial activity [103,104,105]. This stress inhibits DNA replication, protein synthesis, and cellular metabolism, causing cell death [106]. In order to demonstrate the effect of ROS on cell death, a study was conducted in the absence and presence of L-cysteine, a natural antioxidant, with E. coli bacteria. Indeed, growth was inhibited by Cu-TiO2/GF with an efficiency of 79.4% in the absence of L-Cysteine compared to 65.1% in its presence. Similarly, Ag-TiO2/GF, where the efficiency was 100% without the antioxidant and diminished to 84.7% in its presence [92]. The experiment is conducted on the following bacteria: E. coli and Staphylococcus aureus (S. aureus) on copper-doped TiO2/GF and silver-doped TiO2/GF synthesized by sol-gel method, and at different relative humidities (Table 2).

Table 2.
List of some studies on using TiO2-metal for bacterial inactivation.
Overall, silver-doped TiO2/GF performed best on both bacteria, followed closely by copper TiO2/GF and [107] then TiO2/GF alone. The yield was better at a relative humidity of 60% than 80%. They were significantly lower at 40% humidity. E. coli is eliminated reasonably than S. aureus since the latter is a Gram + bacterium with a more complex wall [76,92].
5. Conclusions and Outlook
Recent studies on photocatalysis for indoor air purification and bacterial inactivation have shown promising results. Even though a variety of photocatalysts are available, TiO2-based materials are the most effective or, at the very least, effective option for practical and financial reasons. This review has collected and covered recent research that has improved the photocatalytic activity of materials based on TiO2 for VOC degradation in indoor air and bacterial inactivation. Coupling TiO2 materials with other methods has been increasingly explored. This paper also reviewed the literature on the material aspects of photocatalysis based on AgxO/TiO2 and CuxO/TiO2 to treat air-containing chemical and biological pollution. A bibliographical synthesis of the type of catalyst and the operating conditions was detailed concerning the decontamination of VOCs. Moreover, the different types of microorganisms treated by TiO2-based photocatalysts have been listed. In-depth explanations of the reaction mechanisms for photocatalytic degradation and inactivation have been provided. As a look ahead to future research, we believe more study and testing are needed to clarify and comprehend the benefits of TiO2-based materials on photocatalytic applications. There are only a few works on the combined treatment of chemical and biological pollution using photocatalysis at the same time. The investigations do not consider evaluating removal or if mineralization is complete, which is an essential criterion because it can generate more harmful intermediates than the pollutant. Finally, experiments in real cases of air pollution, such as hospital air pollution, are required to apply this process.
Author Contributions
Conceptualization, A.A.A. (Achraf Amir Assadi) and O.B.: methodology and writing—review, A.A.A. (Achraf Amir Assadi), N.B.H., L.K. and A.A.A. (Aymen Amine Assadi); writing—review and editing, A.A.A. (Achraf Amir Assadi), A.A.A. (Aymen Amine Assadi) and L.M.; conceptualization, funding acquisition, L.M.; methodology, L.K.; resources, project administration, supervision, A.A.A. (Aymen Amine Assadi): writing-review and editing, A.G. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for funding and supporting this work through Research Partnership Program no RP-21-09-66.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cincinelli, A.; Martellini, T. Indoor air quality and health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Capolongo, S.; Settimo, G.; Gola, M. (Eds.) Indoor Air Quality (IAQ) in Healthcare Facilities; Springer: Berlin/Heidelberg, Germany, 2017; Volume 4386. [Google Scholar]
- Assadi, I.; Guesmi, A.; Baaloudj, O.; Zeghioud, H.; Elfalleh, W.; Benhammadi, N. Review on inactivation of airborne viruses using non-thermal plasma technologies: From MS2 to coronavirus. Environ. Sci. Pollut. Res. 2021, 29, 4880–4892. [Google Scholar] [CrossRef]
- Heinsohn, R.J.; John, M.C. (Eds.) Indoor Air Quality Engineering: Enviromental Health and Control Indoor; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0-8247-4061-0. [Google Scholar]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Rtimi, S.; Wolbert, D.; Bessais, B.; Assadi, A.A. Modeling of indoor air treatment using an innovative photocatalytic luminous textile: Reactor compactness and mass transfer enhancement. Chem. Eng. J. 2022, 430, 132636. [Google Scholar] [CrossRef]
- Salmon, D.G. Annual Exporter Guide France. Available online: https://www.google.com.hk/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&ved=0CAQQw7AJahcKEwjw49TzpND9AhUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fapps.fas.usda.gov%2Fnewgainapi%2Fapi%2Freport%2Fdownloadreportbyfilename%3Ffilename%3DExporter%2520Guide_Paris_France_12-8-2014.pdf&psig=AOvVaw0bBETSpKjn6_G28dOcdT4j&ust=1678500724709337 (accessed on 25 December 2022).
- Erisman, J.W. Air Pollution Science for the 21st Century. Environ. Sci. Policy 2003, 6, 396. [Google Scholar] [CrossRef]
- Quyen, N.T.; Traikool, T.; Nitisoravut, R.; Onjun, T. Improvement of water quality using dielectric barrier discharge plasma. J. Phys. Conf. Ser. 2017, 860, 12031. [Google Scholar] [CrossRef]
- Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: Conceptualization of humidity effects, active oxygen species, problem of C1-C3 carbonyl pollutants. Appl. Catal. B Environ. 2010, 99, 428–434. [Google Scholar] [CrossRef]
- Ghezzi, S.; Pagani, I.; Poli, G.; Perboni, S.; Vicenzi, E. Rapid Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Tungsten Trioxide-Based (WO3) Photocatalysis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Elfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) with Ag Silver Nanoparticles (Ag-NPs): Photocatalytic Performance for Wastewater Treatment under Visible Light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef]
- Malayeri, M.; Haghighat, F.; Lee, C.S. Kinetic modeling of the photocatalytic degradation of methyl ethyl ketone in air for a continuous-flow reactor. Chem. Eng. J. 2021, 404, 126602. [Google Scholar] [CrossRef]
- Zhang, Z.; Gamage, J. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010, 764870. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV irradiation and TiO2-photocatalysis on airborne bacteria and viruses: An overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Al-hammadi, S.A.; Saleh, T.A. Simultaneous sorption ofdyes and toxic metals from waters using synthesized titania-incorporated polyamide. J. Mol. Liq. 2018, 269, 564–571. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Fu, S.; Lv, X.; He, Q.; Li, Y.; Ji, F.; Xu, X. Preparation and antibacterial activity of Ag/TiO2-functionalized ceramic tiles. Ceram. Int. 2022, 48, 4897–4903. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El, A.; Khezami, L. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Cao, J.; Zhu, Y.; Yuan, W.; Hu, Z.; Ao, Z.; Brudvig, G.W.; Tian, F.; Yu, J.C.; et al. Photocatalytically recovering hydrogen energy from wastewater treatment using MoS2@TiO2 with sulfur/oxygen dual-defect. Appl. Catal. B Environ. 2022, 303, 120878. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Study of synergetic effect by surface discharge plasma/TiO2 combination for indoor air treatment: Sequential and continuous configurations at pilot scale. J. Photochem. Photobiol. A Chem. 2015, 310, 148–154. [Google Scholar] [CrossRef]
- Karoui, S.; Ben Arfi, R.; Mougin, K.; Ghorbal, A.; Assadi, A.A.; Amrane, A. Synthesis of novel biocomposite powder for simultaneous removal of hazardous ciprofloxacin and methylene blue: Central composite design, kinetic and isotherm studies using Brouers-Sotolongo family models. J. Hazard. Mater. 2020, 387, 121675. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Amrane, A.; Djelal, H.; Elfalleh, W.; Assadi, A.A.; Rtimi, S. Photocatalytic performance of TiO2 impregnated polyester for the degradation of Reactive Green 12: Implications of the surface pretreatment and the microstructure. J. Photochem. Photobiol. A Chem. 2017, 346, 493–501. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; El Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500. [Google Scholar] [CrossRef]
- Kappadan, S.; Gebreab, T.W.; Thomas, S.; Kalarikkal, N. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process. 2016, 51, 42–47. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Almomani, F.; Rene, E.R.; Veiga, M.C.; Bhosale, R.R.; Kennes, C. Treatment of waste gas contaminated with dichloromethane using photocatalytic oxidation, biodegradation and their combinations. J. Hazard. Mater. 2021, 405, 123735. [Google Scholar] [CrossRef]
- Mohseni, M.; Prieto, L. Biofiltration of hydrophobic VOCs pretreated with UV photolysis and photocatalysis. Int. J. Environ. Technol. Manag. 2008, 9, 47–58. [Google Scholar] [CrossRef]
- Khezami, L.; Nguyen-Tri, P.; Saoud, W.A.; Bouzaza, A.; El Jery, A.; Duc Nguyen, D.; Gupta, V.K.; Assadi, A.A. Recent progress in air treatment with combined photocatalytic/plasma processes: A review. J. Environ. Manag. 2021, 299, 113588. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamashita, H. Efficient photocatalytic degradation of organics diluted in water and air using TiO2 designed with zeolites and mesoporous silica materials. J. Mater. Chem. 2011, 21, 2407–2416. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Serhane, Y.; Belkessa, N.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Continuous air purification by front flow photocatalytic reactor: Modelling of the influence of mass transfer step under simulated real conditions. Chemosphere 2022, 295, 133809. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Hodgson, A.T.; Destaillats, H.; Sullivan, D.P.; Fisk, W.J. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air 2007, 17, 305–316. [Google Scholar] [CrossRef]
- Muscetta, M.; Russo, D. Photocatalytic applications in wastewater and air treatment: A patent review (2010–2020). Catalysts 2021, 11, 834. [Google Scholar] [CrossRef]
- Riaz, N.; Fen, D.A.C.S.; Khan, M.S.; Naz, S.; Sarwar, R.; Farooq, U.; Bustam, M.A.; Batiha, G.E.S.; El Azab, I.H.; Uddin, J.; et al. Iron-zinc co-doped titania nanocomposite: Photocatalytic and photobiocidal potential in combination with molecular docking studies. Catalysts 2021, 11, 1112. [Google Scholar] [CrossRef]
- Malliga, P.; Pandiarajan, J.; Prithivikumaran, N.; Neyvasagam, K. Effect of film thickness on structural and optical properties of TiO2 thin films. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; Volume 2, pp. 488–491. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Rabhi, S.; Belkacemi, H.; Bououdina, M.; Kerrami, A.; Ait Brahem, L.; Sakher, E. Effect of Ag doping of TiO2 nanoparticles on anataserutile phase transformation and excellent photodegradation of amlodipine besylate. Mater. Lett. 2019, 236, 640–643. [Google Scholar] [CrossRef]
- Yi, J.; Huang, L.; Wang, H.; Yu, H.; Peng, F. AgI/TiO2 nanobelts monolithic catalyst with enhanced visible light photocatalytic activity. J. Hazard. Mater. 2015, 284, 207–214. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Single-crystalline 2D BiOCl nanorods decorated with 2D MoS2 nanosheets for visible light-driven photocatalytic detoxification of organic and inorganic pollutants. FlatChem 2021, 28, 100267. [Google Scholar] [CrossRef]
- Guan, Z.; Li, Q.; Shen, B.; Bao, S.; Zhang, J.; Tian, B. Fabrication of Co3O4 and Au co-modified BiOBr flower-like microspheres with high photocatalytic efficiency for sulfadiazine degradation. Sep. Purif. Technol. 2020, 234, 116100. [Google Scholar] [CrossRef]
- Raizada, P.; Thakur, P.; Sudhaik, A.; Singh, P.; Thakur, V.K.; Hosseini-Bandegharaei, A. Fabrication of dual Z-scheme photocatalyst via coupling of BiOBr/Ag/AgCl heterojunction with P and S co-doped g-C3N4 for efficient phenol degradation. Arab. J. Chem. 2020, 13, 4538–4552. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F. Visible-light photocatalytic removal of NO in air over BiOX (X = Cl, Br, I) single-crystal nanoplates prepared at room temperature. Ind. Eng. Chem. Res. 2013, 52, 6740–6746. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Wang, T. Preparation of porous TiO2/Ag heterostructure films with enhanced photocatalytic activity. Chem. Eng. J. 2015, 270, 418–427. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Wang, K.; Meng, Q.; Tang, Y.; Zhao, K. Facile fabrication of TiO2-SiO2-C composite with anatase/rutile heterostructure via sol-gel process and its enhanced photocatalytic activity in the presence of H2O2. Ceram. Int. 2022, 48, 9114–9123. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Jeon, B.H. Adsorption and Photodegradation Efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/Zeolite Nanophotocatalysts for the Removal of Cyanide. Ind. Eng. Chem. Res. 2019, 58, 2099–2112. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M.; Nogami, G. Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater. Chem. Phys. 1999, 59, 254–261. [Google Scholar] [CrossRef]
- Hou, F.; Lu, K.; Liu, F.; Xue, F.; Liu, M. Manipulating a TiO2-graphene-Ta3N5 heterojunction for efficient Z-scheme photocatalytic pure water splitting. Mater. Res. Bull. 2022, 150, 111782. [Google Scholar] [CrossRef]
- Li, H.; Hong, W.; Cui, Y.; Hu, X.; Fan, S.; Zhu, L. Enhancement of the visible light photocatalytic activity of Cu2O/BiVO4 catalysts synthesized by ultrasonic dispersion method at room temperature. Mater. Sci. Eng. B 2014, 181, 1–8. [Google Scholar] [CrossRef]
- Aguilera-Ruiz, E.; García-Pérez, U.M.; De La Garza-Galván, M.; Zambrano-Robledo, P.; Bermúdez-Reyes, B.; Peral, J. Efficiency of Cu2O/BiVO4 particles prepared with a new soft procedure on the degradation of dyes under visible-light irradiation. Appl. Surf. Sci. 2015, 328, 361–367. [Google Scholar] [CrossRef]
- Sun, S.; Ding, J.; Bao, J.; Gao, C.; Qi, Z.; Yang, X.; He, B.; Li, C. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent advances in synthesis and applications of carbon-doped TiO2 nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Ghumro, S.S.; Lal, B.; Pirzada, T. Visible-Light-Driven Carbon-Doped TiO2-Based Nanocatalysts for Enhanced Activity toward Microbes and Removal of Dye. ACS Omega 2022, 7, 4333–4341. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, N.; Huang, S.; Wu, N.L.; Wei, M. Sulfur-Doped Anatase TiO2 as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3791–3797. [Google Scholar] [CrossRef]
- Niu, P.; Wu, G.; Chen, P.; Zheng, H.; Cao, Q.; Jiang, H. Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 2020, 8, 172. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Magureanu, M.; Bogdan, N.; Hu, J.; Richards, R.; Florea, M.; Parvulescu, M. Plasma-assisted catalysis total oxidation of trichloroethylene over gold nano-particles embedded in SBA-15 catalysts. Catal. B Environ. 2007, 76, 275–281. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catalytic removal of typical volatile organic compound mixtures over Au-Pd/α-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Haghighat, F. Plasma-based indoor air cleaning technologies: The state of the art-review. Clean Soil Air Water 2014, 42, 1667–1680. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A review on catalytic nanomaterials for volatile organic compounds VOC removal and their applications for healthy buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranová, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Škapin, A.S.; Calvino, J.J.; Brezová, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Photocatalytic TiO2/carbon nanotube nanocomposites for environmental applications: An overview and recent developments. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 471–509. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Maachi, R. Indoor air treatment of refrigerated food chambers with synergetic association between cold plasma and photocatalysis: Process performance and photocatalytic poisoning. Chem. Eng. J. 2020, 382, 122951. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Kane, A.; Le Cann, P.; Gerard, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Innovative photocatalytic reactor for the degradation of VOCs and microorganism under simulated indoor air conditions: Cu-Ag/TiO2-based optical fibers at a pilot scale. Chem. Eng. J. 2021, 411, 128622. [Google Scholar] [CrossRef]
- Jia, Z.; Barakat, C.; Dong, B.; Rousseau, A. VOCs Destruction by Plasma Catalyst Coupling Using AL-KO PURE Air Purifier on Industrial Scale. J. Mater. Sci. Chem. Eng. 2015, 3, 19–26. [Google Scholar] [CrossRef]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S.; Assadi, A.A.; Bessais, B. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Vincent, G.; Queffeulou, A.; Marquaire, P.M.; Zahraa, O. Remediation of olfactory pollution by photocatalytic degradation process: Study of methyl ethyl ketone (MEK). J. Photochem. Photobiol. A Chem. 2007, 191, 42–50. [Google Scholar] [CrossRef]
- Vincent, G.; Schaer, E.; Marquaire, P.M.; Zahraa, O. CFD modelling of an annular reactor, application to the photocatalytic degradation of acetone. Process Saf. Environ. Prot. 2011, 89, 35–40. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Ren, X.; Liu, K.; Yang, J. Fine platinum nanoparticles supported on a porous ceramic membrane as efficient catalysts for the removal of benzene. Sci. Rep. 2017, 7, 16589. [Google Scholar] [CrossRef]
- Sedjame, H.J.; Fontaine, C.; Lafaye, G.; Barbier, J. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation. Appl. Catal. B Environ. 2014, 144, 233–242. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Jaroniec, M. Efficient catalytic removal of formaldehyde at room temperature using AlOOH nanoflakes with deposited Pt. Appl. Catal. B Environ. 2015, 163, 306–312. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K. ichi Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Tkachenko, O.P.; Kirichenko, O.A.; Kapustin, G.I.; Mishin, I.V.; Klementiev, K.V.; Ojala, S.; Kustov, L.M.; Keiski, R. Nanogold-containing catalysts for low-temperature removal of S-VOC from air. Top. Catal. 2009, 52, 351–358. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Vallet, C.; Wolbert, D. Use of DBD plasma, photocatalysis, and combined DBD plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—Synergetic effect and byproducts identification. Chem. Eng. J. 2014, 254, 124–132. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, L.C.; Chen, H.Y.; Chung, Y.C. Inactivation of Staphylococcus aureus and Escherichia coli in water using photocatalysis with fixed TiO2. Water. Air Soil Pollut. 2010, 212, 231–238. [Google Scholar] [CrossRef]
- Blanco-Galvez, J.; Fernández-Ibáñez, P.; Malato-Rodríguez, S. Solar photo catalytic detoxification and disinfection of water: Recent overview. J. Sol. Energy Eng. Trans. ASME 2007, 129, 4–15. [Google Scholar] [CrossRef]
- Magaña-López, R.; Zaragoza-Sánchez, P.I.; Jiménez-Cisneros, B.E.; Chávez-Mejía, A.C. The use of TiO2 as a disinfectant in water sanitation applications. Water 2021, 13, 1641. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Kane, A.; Jung, A.; Cann, P.L.; Bazantay, F.; Bouzaza, A.; Wolbert, D. Integrated process for the removal of indoor VOCs from food industry manufacturing Elimination of Butane-2,3-dione and Heptan-2-one by cold plasma-photocatalysis combination. Photochem. Photobiol. A Chem. 2020, 386, 112071. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Regmi, C.; Yamaguchui, T.; Lee, S.W. Inactivation of Staphylococcus aureus in visible light by morphology tuned α-NiMoO4. J. Photochem. Photobiol. A Chem. 2018, 350, 59–68. [Google Scholar] [CrossRef]
- Kőrösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic inactivation of plant pathogenic bacteria using TiO2 nanoparticles prepared hydrothermally. Nanomaterials 2020, 10, 1730. [Google Scholar] [CrossRef] [PubMed]
- Salavati-Niasari, M.; Davar, F. Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater. Lett. 2009, 63, 441–443. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Wang, W.; Cai, Y.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, J.; Kang, Y.; Chai, D.; Zhao, R.; Lei, Z. Sm2O3 embedded in nitrogen doped carbon with mosaic structure: An effective catalyst for oxygen reduction reaction. Energy 2017, 133, 115–120. [Google Scholar] [CrossRef]
- Gupta, S.B.; Bluhm, H. The potential of pulsed underwater streamer discharges as a disinfection technique. IEEE Trans. Plasma Sci. 2008, 36, 1621–1632. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014, 225, 2102. [Google Scholar] [CrossRef]
- Ado, A.; Tukur, A.I.; Ladan, M.; Gumel, S.M.; Muhammad, A.A.; Habibu, S.; Koki, I.B. A Review on Industrial Effluents as Major Sources of Water Pollution in Nigeria. Chem. J. 2015, 1, 159–164. [Google Scholar]
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Rtimi, S.; Kiwi, J.; Giannakis, S.; Pulgarin, C. Insights into the photocatalytic bacterial inactivation by flower-like Bi2WO6 under solar or visible light, through in situ monitoring and determination of reactive oxygen species (ROS). Water 2020, 12, 1099. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Palsaniya, S.; Manna, A.K.; Soni, R.K.; Bhattacharya, J. Evidence of oxygen defects mediated enhanced photocatalytic and antibacterial performance of ZnO nanorods. Colloids Surf. B Biointerfaces 2019, 184, 110541. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms. Water Res. 2019, 157, 106–118. [Google Scholar] [CrossRef]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ubonchonlakate, K.; Sikong, L.; Saito, F. Photocatalytic disinfection of P. aeruginosa bacterial Ag-doped TiO2 film. Procedia Eng. 2012, 32, 656–662. [Google Scholar] [CrossRef]
- Lee, M.; Shahbaz, H.M.; Kim, J.U.; Lee, H.; Lee, D.U.; Park, J. Efficacy of UV-TiO2 photocatalysis technology for inactivation of Escherichia coli K12 on the surface of blueberries and a model agar matrix and the influence of surface characteristics. Food Microbiol. 2018, 76, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus, P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).