Abstract
Perovskite solar cells (PSCs), one of the most promising photovoltaic technologies, have been widely studied due to their high power conversion efficiency (PCE), low cost, and solution processability. The architecture of PSCs determines that high PCE and stability are highly dependent on each layer and the related interface, where nonradiative recombination occurs. Conventional synthetic chemical materials as modifiers have disadvantages of being toxic and costly. Natural molecules with advantages of low cost, biocompatibility, and being eco-friendly, and have improved PCE and stability by modifying both functional layers and interface. In this review, we discuss the roles of natural molecules on PSCs devices in terms of the perovskite active layer, interface, carrier transport layers (CTLs), and substrate. Finally, the summary and outlook for the future development of natural molecule-modified PSCs are also addressed.
1. Introduction
Perovskite solar cells (PSCs) are generating enormous attention in the fields of renewable energy due to their brilliant optoelectronic properties, including high light absorption coefficient, tunable bandgap, and small exciton binding energy [1,2,3,4,5,6,7]. Based on these outstanding optoelectronic properties, scientists have made great advances, and 25.7% power conversion efficiency (PCE) has been achieved in the past ten years [8]. Moreover, the low-cost raw materials and low-temperature solution processability of perovskite has also made it a new star among many photovoltaic technologies, and even among whole renewable energy technology sector.
PSCs originated from dye-sensitized solar cells. Therefore, the architecture is similar to a sandwich, comprising the perovskite layer, charge transport layer and electrode. When the light through the transparent electrode enters the perovskite layer, the electron–hole pairs are generated then separated into free electrons and holes, i.e., photogenerated carriers. The photogenerated carriers will spontaneously diffuse to the corresponding charge transport layer under the concentration gradient, be extracted at the interface between the carrier transport layers (CTLs) and perovskite layer, and finally be collected by the electrode. This process has not been smooth sailing, because there are numerous defects at grain boundaries and the surface in the perovskite film owing to its ionic nature and the rapid crystal growth process. These defects will hinder carrier transport and cause nonradiative recombination. Furthermore, these defects are sensitive to environmental factors, such as moisture, oxygen, temperature, etc., affecting the optoelectronic properties of the perovskite, even leading to degradation. To solve these problems, many scientists have explored various strategies, such as additive engineering, [9,10] interface engineering, [11,12,13], and so on. Various materials with multiple functional groups have been used to assist these strategies. Among these materials, they can mainly divide into two types—synthetic chemical molecules and natural molecules. Synthetic chemical molecules may be toxic, environmentally unfriendly and costly. In contrast, natural molecules have the advantages of low cost, biocompatibility and being eco-friendly (Scheme 1). Therefore, natural molecules play a significant role in the field of PSCs.
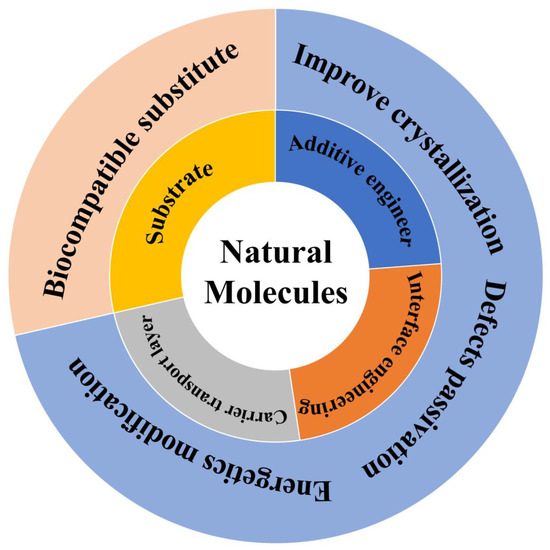
Scheme 1.
Illustration of roles and functions of natural molecules in PSCs.
Recently, many reviews of PSCs have been published and have provided more in-depth insights for researchers [14,15,16,17,18]. However, up to now, few reviews have focused on the development of PSCs modified by natural molecules. It is time to comprehensively summarize recent progress and provide a systematic understanding of natural molecule-modified PSCs. In this review, we first introduce perovskite additive engineering based on natural molecules, and then we discuss the interfacial engineering and CTLs modification and substrate. Finally, we give a conclusion and outlook of natural molecule-modified PSCs.
2. Natural Molecule-Based Additive Engineering for PSCs
Perovskite crystals have a soft lattice character and low formation energy; thus, either at grain boundaries or the surface, numerous defects could be formed during the crystallization process [19,20]. Furthermore, unfavorable ion migration and carrier recombination also occurred due to the ionic nature of the perovskite [21,22]. All of this deteriorates the performance of PSCs. To overcome these negative effects, many strategies including composition engineering, dimensionality engineering, nonstoichiometric approach and additive engineering have been intensively developed. Among them, additive engineering using natural molecules with the functional group has been reported to improve the crystallization, passivate the defects, and modify the energetics (Table 1).
1,3,7-trimethylxanthine, which is also named caffeine, is a common natural molecule in people’s daily life. Wang and co-authors added caffeine into perovskite through additive engineering [23]. As is shown in Figure 1a, the conjugated Lewis base with two different chemistry environment carboxyl groups, worked as the “molecular lock” that effectively coordinated with Pb2+. As a result, the increased growth activation energy of the perovskite retarded the perovskite crystal growth and induced a preferred orientation. As a result, a champion PCE of 20.25% with an open-circuit voltage of 1.143 V, a short-circuit current (Jsc) of 22.97 mA cm−2 and a fill factor of 77.13% has been realized (Figure 1b). Furthermore, the heat stability also improved due to the caffeine can interact with the perovskite again, suppressing the ion migration during the degradation process, leading to the device being stable at 85 °C over 1300 h.
Natural molecules with multiple functional groups provide more interaction with uncoordinated ions. Lin and co-authors employed the M13 bacteriophage as the crystal growth template of the perovskite [24]. As is shown in Figure 1c, the M13 contains four types of amino acids. The lone electron pair on the amino groups of N-terminal alanine and lysine and the negative charge on the carboxyl groups in aspartic acid and glutamic acid on the surface of M13 can effectively be coordinated with Pb2+ ions. After adding the M13 into the perovskite, the grains with a diameter from 300 nm to 1 μm are surrounded effectively due to the uniform length of the M13, which led to a homogenous and large crystal size. The M13-added PSC exhibited a champion PCE of 20.1% upon heating the precursor solution at 90 °C compares to the reference PSC with a champion PCE of 17.8%.
The obtained conventional two-step spin-coating lead iodide (PbI2) film is too smooth, which is not beneficial for achieving high-quality perovskite films. Liu and co-authors introduced choline chloride (vitamin B4, VB4) into PbI2 and constructed a novel heterogeneous PbI2 (HLI) structure to change this situation [25]. Through the addition of VB4, the (001) planes of HLI have two interplanar distances of 9.0 Å and 6.9 Å (Figure 1d), which alter the PbI2 crystal arrangement, leading to the rough and porous surface of PbI2 films that cause the organic precursor to diffuse into films, resulting in better morphology and large grains of the perovskite films. In addition, the negative charge cation vacancy defects of the perovskite films can be effectively passivated by the quaternary ammonium in VB4. With these synergistic effects of VB4, the champion PCE of PSCs is achieved at 22.13% with remarkably elevated Voc 1.17 V. They further fabricated PSCs using double-sided modification, and the PCE is up to 24.27% with enhanced Voc 1.21 V (Figure 1d).
As we all know, both UV light illumination and oxygen atmosphere extremely accelerate the rate of the degradation process. Lycopene (LCP), the strongest antioxidant, can eliminate both hydrogen peroxide radicals and singlet molecular oxygen produced during photooxidation [26]. Zhuang and co-authors introduced the LCP into the perovskite precursor to achieve efficient and fresh PSCs [27]. As shown in Figure 1e, the LCP molecule is a straight chain containing 11 conjugated double bonds and two non-conjugated double bonds. The long carbon chain makes the perovskite film more hydrophobic, and the LCP could prevent unstable chemical bonds in FA+ and MA+ from being attacked by oxygen through unsaturated bonds. Moreover, the LCP also can interact with the uncoordinated Pb2+ via the Lewis acid–base reaction, which effectively passivates the trap and facilitates a black phase to be formed at room temperature. Through the addition of LCP, the champion PCE was enhanced to 23.62% with a Voc of 1.21 V, a Jsc of 24.10 mA cm−2, and an FF of 81.00%. Due to the improved antioxidant ability, 91.7% of the initial average PCE was maintained for 1000 h in oxygen, and 84% was maintained under both UV irradiation and 21% oxygen atmosphere for 8 h compared to the control device of 5 h.
Various defects are generated during the crystallization process. among them, intrinsic site defects and surface defects significantly deteriorate the performance of the PSCs [28]. Guo and co-authors utilized the organic molecule Indigo as an efficient passivator for high-quality perovskite film [29]. The Indigo molecule containing the carbonyl group can interact with the perovskite surface uncoordinated Pb2+ and Pb-I antisite defects, and I#x2212; sites can interact with the amino group. Moreover, a hydrogen bond formed between the Indigo and the perovskite surface, which could restrain ion migration. After the treatment of Indigo, the champion PCE of the PSC improved to 23.22%, with a Voc of 1.16 V, a Jsc of 25.02 mA cm−2, and an FF of 80%. Furthermore, the Indigo-treated large-area device exhibited a champion PCE of 20.95% with a Jsc of 24.50 mA cm−2, a Voc of 1.14 V and an FF of 75%. Moreover, the moisture and thermal stability were enhanced by rich hydrogen bonds and the carbonyl group of Indigo, and 75% of the original PCE of Indigo-treated unencapsulated PSC was maintained after 60 °C aging for 1500 h, and only 15% of the PCE dropped in storage at the ambient environment after the 1500 h aging.
A suitable electronic structure is necessary for perovskite to form a favorable energy match with the adjacent layer [30,31]. Furthermore, the work function (WF) of the underlying layer determines the interface energetics [32]. Therefore, it is imperative to improve the optoelectronic properties of perovskite, including passivate bulk and interface defects, and modify interfacial energetics. Xiong and co-authors employed capsaicin in the perovskite precursor [33]. As is shown in Figure 1f, after the addition of capsaicin, the ultraviolet photoelectron spectra (UPS) exhibited a remarkable decrease in the WF from 4.95 to 4.48 eV, while the Fermi level position (EF) and the valance band maximum (VBM) increased by 0.48 eV, keeping a constant ionization potential. This means the surface energetics completely transformed from p-type to n-type. Furthermore, Kelvin probe force microscopy (KPFM) showed that a p–n homojunction with 100 nm thickness formed from the perovskite film surface. Therefore, a favorable interface with the electron transport layer (ETL) formed, improving charge transport. Moreover, the capsaicin-containing carbonyl group could effectively interact with the uncoordinated Pb2+, leading to both the interface nonradiative recombination and defect-assisted recombination being significantly suppressed. As a result, their p–i–n PSCs achieved a record efficiency of 21.88% with a Voc of 1.13 V, a Jsc of 23.10 mA cm−2 and an FF of 83.81%. The capsaicin also improved stability through enhanced hydrophobicity, and the unencapsulated device maintained 90% of initial PCE after storage for 800 h in the ambient environment.
Liu and co-authors introduced vitamin D2 (VD2) into the perovskite bulk [34], and 530 meV of the WF relative to the vacuum energy level (EVAC) increased, while the EF and VBM shifted from 1.09 to 0.51 eV, which indicated that the surface energetics transformed from n-type to p-type. KPFM exhibited that the thickness of the n–p homojunction formed accounts for about 80 nm. Based on the n–p homojunction, the interface barrier between the perovskite and the hole transport layer was minimized (Figure 1g), enhancing the interfacial charge transfer. In addition, the C=C bonds can interact with the uncoordinated Pb2+ using the Lewis acid–base reaction, which reduces the defects and improves the crystallization of perovskite. Meanwhile, vitamin C (VC) was introduced into the SnO2 ETL, and oxygen defects on the SnO2 film surface were reduced, enhancing electron mobility and reducing the interface energy-level offset; thus, the interfacial charge transfer was improved. As a result, this achieved a champion PCE of 24.20% and an FF of 81.01% due to the synergistic effect, and the hysteresis is negligible. Furthermore, 93.04% of the initial PCE of unencapsulated devices was retained after aging 5000 h at room temperature.
The above studies using natural molecules through additive engineering represent important breakthroughs either in crystallization or defect passivation and energetic modification of PSCs, promoting the development of PSCs even in the field of green energy. However, there are still some issues that should be addressed. For example, the PCE still lags behind synthetic chemical additives. In addition, the mechanism between natural molecules and perovskite is explored, while the distribution of natural molecules in perovskite and the interaction with the adjacent layer also should be considered systematically.
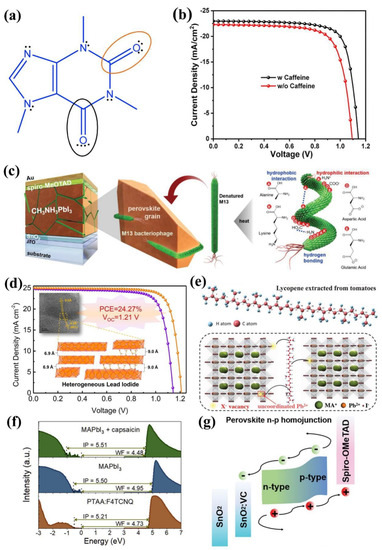
Figure 1.
Instances of natural molecule-based additive engineering for PSCs: (a) the Lewis chemical structure of caffeine, (b) J-V curves of the champion PSC of perovskite with and without caffeine in the reverse scan direction [23]. Copyright 2019, Elsevier. (c) Illustration of normal-type PSCs structure with the denatured M13 bacteriophage as the perovskite growth template [24]. Copyright 2020, Wiley-VCH. (d) J–V curves of the champion PSC of perovskite with ordered PbI2 films [25]. Copyright 2022, Elsevier. (e) The chemical structure of LCP and working as a passivator located at the grain boundary [27]. Copyright 2022, Wiley-VCH. (f) UPS spectra of secondary electron cutoff region and valence band region of PTAA: F4TCNQ, perovskite films with and without capsaicin grown on PTAA: F4TCNQ [33]. Copyright 2021, Elsevier. (g) Energy-level alignment schematics of the PSC comprising VD2 and VC [34]. Copyright 2022, Wiley-VCH.
3. Natural Molecule-Based Interface Engineering for PSCs
The interface is where the carrier extraction, transport and collection occurs, as well as photon transmission. Thus, the properties of the interface are significant for the performance of the PSC, even the stability of the PSC. A promising interface should have these properties, including no energy loss when carriers pass, strong enough to prevent the permeation of oxygen and humidity, as well as ion migration. With this requirement, scientists have put extensive effort into improving the properties of interfaces, including interface contact, interface energetics, and interface trap-states. In this section, some representative studies that have used natural molecules to modify the interface in terms of ETL and HTL are described.
Titanium dioxide (TiO2) is a commonly used ETL in PSCs, due to its advantages of high stability and excellent optoelectronic properties [35,36]. However, the ultraviolet photocatalysis effect and tremendous oxygen vacancies on the TiO2 surface can cause degradation of the perovskite. In addition, the energy level of TiO2 and perovskite are not well matched. All the above leads to poor performance of PSCs. To ameliorate the situation, You and co-authors used the biopolymer heparin sodium (HS) with multiple functional groups as the interlayer anchoring the TiO2 and perovskite [37]. After treatment with HS, interaction of the ionic functional groups, including -COO−, SO3− anionic in HS with uncoordinated Pb2+ in perovskite and Ti4+ in TiO2 occurred, with the Na+ cationic group prone to filling the vacancies of MA+ and interacting with the uncoordinated I−. All led to ameliorated morphology of the TiO2 surface with better hydrophilicity and no pinhole, and provided a homogeneous surface for the growth of perovskite, resulting in the perovskite film with fewer surface defects and better crystallinity. Furthermore, the strong anchoring effect of HS effectively hinders ion migration and suppresses hysteresis. As a result, this realized a champion PCE of 20.1% and improved long-term stability of 90% and 85% original PCE, which was maintained after 70 d storage at nitrogen and air, respectively.
Zhang and co-authors introduced dopamine (DA) to passivate the TiO2 surface using a chelating effect in n–i–p planar PSCs [38]. As is shown in Figure 2a, after the modification of DA, the enediol ligands of DA formed a conjugated structure with the surface Ti atoms, leading to a compact and dense TiO2 film, effectively suppressing deep trap-states and reducing oxygen vacancies. Moreover, the uncoordinated Pb2+ and the Pb-I/Br antisite defects on the surface of perovskite can be passivated by the terminal amino groups in DA. Therefore, the DA-modified TiO2 can be used as a crosslinker in the interface, effectively accelerating charge transfer and reducing charge accumulation at the surface between TiO2 and perovskite. As the result, this realized a champion PCE of 20.93% with negligible hysteresis, and 80% of the initial PCE of the unencapsulated device was maintained after 1200 h of continued full-sun illumination without any UV filter.
As for alternative ETL materials, SnO2 has the advantages of low-temperature manufacturing and good energy-level alignment. However, the inevitable surface defects and pinholes of the film significantly deteriorate the photovoltaic performance of PSCs. To overcome these shortages, Geng and co-authors employed L-aspartic acid (LAA) as the interlayer between ETL and perovskite [39]. As shown in Figure 2b, with the treatment of LAA, the carboxyl group interacts with uncoordinated Sn4+ and neutralizes the alkalinity of the hydroxyl group in SnO2, leading to a denser and smoother ETL layer with reduced defects, and negligible transmittance loss, and enhanced conductivity. Moreover, hydrogen bonds could be formed between the amino group of LAA and halogen ions of the perovskite, resulting in improved crystallization and inhibited ion migration. Thus, the ETL layer and the perovskite layer are connected chemically via LAA. In addition, the conduction band (ECB) of LAA-modified SnO2 decreased from −4.17 eV to −3.96 eV, which is more aligned with the perovskite layer, facilitating the charge transfer. As a result, a champion PCE of 22.63% with a Voc of 1.16 V, Jsc of 24.75 mA cm−2 and an FF of 79.32% was achieved, and the PCE only declined by 12.8% after aging 2184 h at 60 °C and 9.1% declined after aging 1680 h at a relative humidity of 40%.
The phenomenon of charge transport imbalance and charge accumulation at the interface result from the discrepant extraction rates, which is a bottleneck phenomenon of the PSC, and influences device performance [40]. To overcome this challenge, Kim and co-authors introduced 2-[carbamimidoyl(methyl)amino]acetic acid (creatine) into the interface between the SnO2 ETL and perovskite [41]. Creatine possesses high polarity and functional groups including the carbamimidoyl group and the carboxyl group. Amines and ammonium ions or an intermediate state between them could be converted from the carbamimidoyl group through a resonance structure. With the treatment of creatine, the WF of SnO2 decreased from 3.6 eV to 3.32 eV, and the conductivity increased by 0.44 × 10−3 mS cm−1. Furthermore, superfluous amines and ammonium ions can interact with halides and uncoordinated Pb2+ in the perovskite through electrostatic interaction, which facilitates charge transfer and prevents back-recombination. Furthermore, with the interlayer of creatine, the Urbach energy of the perovskite layer is slightly decreased, implying that a low level of energetic disorder resulted in a low defect density, and creatine passivates the defect states, improving the quality of the perovskite layer. As a result, a champion PCE of 20.8% with a Voc of 1.19 V, Jsc of 23.4 mA cm−2 and an FF of 75.9% was realized. The device with creatine exhibited better stability than the control, with over 90% of the original PCE of unencapsulated devices retained after storage for 50 days under ambient conditions.
PSCs with solely SnO2 ETL without any modification always exhibited poor electron extraction due to the appeared trap-states. To cover this shortage, 3-amino-4-pyrazolecarboxylic acid (APA) was employed by Chen and co-authors as the interlayer between the ETL and perovskite for ameliorating the optoelectronic properties of the buried perovskite film interface and ETL [42]. With the introduction of APA, the carboxyl group with lone pairs of electrons in APA could effectively coordinate with the uncoordinated Sn2+, leading to trap-states being reduced from 8.28 × 1016 cm−3 to 6.73 × 1016 cm−3, and electron mobility and conductivity are also improved. In addition, the difference between EF and VBM decreased by 0.12 eV, which means the APA-treated SnO2 is more n-type, facilitating charge transfer and collection. Moreover, carboxyl and pyrazole N are electron-rich units that could interact with uncoordinated Pb2+ through coordination and hydrogen bonding, which improves crystallization and regulates crystal growth. Therefore, APA works as a multifunctional bridge, chemically linked to the SnO2 and the buried perovskite interface, leading to an impressive champion PCE of 24.71% with a Voc of 1.159 V, Jsc of 25.51 mA cm−2 and an FF of 83.56%. In addition, over 83% of the original PCE was retained after aging in nitrogen for 2400 h at room temperature in the dark for the unencapsulated devices, and over 80% of the initial PCE was retained under 1 sun illumination of continuous maximum output power point (MPP) tracking after 500 h.
In inverted-structure (p–i–n) PSCs, nickel oxide (NiOX) is a promised HTL material due to the superiorities of high hole transport ability and excellent stability. However, imperfect film coverage and pinholes commonly arise during the solution fabrication process [43,44,45]. Meanwhile, the energy level of NiOX film is mismatched with the perovskite, leading to a decrease in carrier transport ability. To overcome these disadvantages, Xie and co-authors used adenine as the interlayer between NiOX and perovskite to regulate the energy level in PSCs [46]. Adenine is a kind of nucleobase that contains the easy-to-take redox reaction under low oxidation potential DNA and RNA polymers. With the modification of adenine, the WF and VBM dropped to 4.54 eV and 0.86 eV, respectively, leading to the highest occupied molecular orbital (HOMO) level shifted to 5.4 eV (Figure 2c), minimizing the energetic mismatch of HTL and perovskite, and increased the Voc. Furthermore, the Ni3+/Ni2+ ratio increased from 2.96 to 3.45, resulting in better hole conductivity. Moreover, the three main peaks of perovskite deposited on the NiOX/adenine were enhanced, indicating improved crystallization. Thus, the adenine worker as the surface modifier ameliorates the energy-level mismatch, improves the crystallization of perovskite, and enhances charge transport and extraction. As a result, a champion PCE of 18.96% with a Voc of 1.06, V, Jsc of 22.94 mA cm−2, and an FF of 77.76% was realized, and 90% of the initial PCE of the adenine-modified devices without encapsulation maintained after 600 h storage at room temperature under ambient conditions (50–60% relative humidity).
One of the Fullerene derivatives, [6,6]-phenyl-C61-butyric acid methyl ester (PCBM), in inverted-structure PSCs is widely used ETL material due to its additional function of passivating perovskite film surface defects and charge traps [47,48]. However, the problem of energy-level mismatch between the PCBM and metal electrode severely impedes electron extraction. To solve this problem, Xiong and co-authors inserted the natural molecules Isatin and Isatin-Cl between ETL and the aluminum (Al) cathode electrode [49]. After introducing the Isatin and Isatin-Cl, the WF of the Al electrode significantly decreased by 0.63 eV and 0.87 eV, respectively, which is attributed to the interfacial negative dipole formed between the ETL and electrode, leading to the lowest unoccupied molecular orbital (LUMO) of PCBM, higher than the WF of Al electrode, facilitating electron transport. As a result, the device with Isatin-Cl realized a champion PCE of 19.74% with a Voc of 1.086 V, Jsc of 22.27 mA cm−2 and an FF of 81.63%, while the device with Isatin had 19.25% with a Voc of 1.081 V, Jsc of 22.22 mA cm−2 and an FF of 80.15%.
These representative works show the application of natural molecules as interface engineering materials, promoting the development of the application of natural molecules in PSCs, and bring PSCs closer to commercial applications. Although the thermodynamics and dynamics of the carriers’ extraction, transport, and collection must be considered, the stability of the natural molecule before and after reacting with adjacent layers may be ignored, which may affect device performance and stability. Last, the cost of an extra interlayer should also be mentioned, which may cause a sharp rise in costs in commercial application.
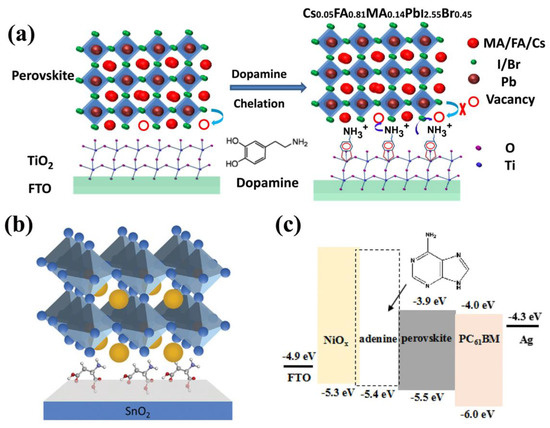
Figure 2.
Instances of natural molecule-based interface engineering for PSCs: (a) Schematic diagram of dopamine function between the perovskite and TiO2 interface [38]. Copyright 2019, Elsevier. (b) Passivation mechanism of LAA at the SnO2 ETL/PVK interface [39]. Copyright 2020, Wiley-VCH. (c) The energy diagram of PSCs and molecule structure of adenine [46]. Copyright 2020, Elsevier.
4. Natural Molecule-Modified Carrier Transport Layers
Both ETL and HTL are the CTLs. They separate and extract photogenerated carriers, while blocking counter carriers [50,51]. In addition, the overlying layer is directly affected by the chemical and surface properties of the CTL, such as the perovskite film, which further influences charge separation, transportation, and the final performance of the device. Thus, developing a high-quality CTL is significant for realizing high-performance PSCs. An ideal CTL should have high transmittance, low trap-state, suitable band alignment with adjacent layers, and proper conductivity that diminishes the interface charge accumulation and recombination. The commonly used CTL, including ETL (TiO2, SnO2, PCBM) and HTL (PEDOT: DSS, PTAA, NiOX) are suffering from their own disadvantages. To overcome these shortages, scientists have made efforts to improve the properties of the CTL, including dopants and substitutes (Table 1). Therefore, in this section, some representative work that uses natural molecules to improve the properties of HTL and ETL are described.

Table 1.
Summary of natural molecules applied in PSCs.
Table 1.
Summary of natural molecules applied in PSCs.
| Year | Natural Molecules | Methodology | PCE | Ref. |
|---|---|---|---|---|
| 2019 | Caffeine | Additive engineering | 19.8% | [23] |
| 2020 | M13 | Additive engineering | 20.1% | [24] |
| 2021 | Capsaicin | Additive engineering | 21.88% | [33] |
| 2022 | L-Theanine | Additive engineering | 24.58% | [52] |
| 2022 | Indigo | Additive engineering | 23.22% | [29] |
| 2022 | Vitamin B4 | Additive engineering | 24.27% | [25] |
| 2018 | Heparin Sodium | Interface engineering | 20.1% | [37] |
| 2019 | Dopamine | Interface engineering | 20.93% | [38] |
| 2020 | Creatine | Interface engineering | 22.1% | [41] |
| 2020 | Adenine | Interface engineering | 18.96% | [46] |
| 2023 | 3-amino-4-pyrazolecarboxylic acid | Interface engineering | 24.71% | [42] |
| 2019 | Isatin and Isatin-Cl | Interface engineering | 19.74% | [49] |
| 2018 | Dopamine | CTLs modification | 18.5% | [53] |
| 2017 | Dopamine | CTLs modification | 16.6% | [54] |
| 2018 | Cellulose paper | Substrate | 9.5% | [55] |
| 2019 | Bamboo-cellulose fibrils | Substrate | 11.68% | [56] |
TiO2 is a commonly used ETL material, but the electron conductivity, trap-state and mismatched band alignment level are still the challenges for high-performance PSCs. To change this situation, Peng and co-authors used DNA as a dopant into meso-TiO2 using the hydrothermal method [57]. After being doped by DNA, a positive charge in TiO2 particles could strongly interact with DNA-containing negative charges, therefore surrounding them with DNA, improving the crystallization and morphology of TiO2 film, and the high-quality meso-TiO2 film with better conductivity, increased hydrophilicity, and reduced trap-state. Furthermore, the EF of DNA-doped meso-TiO2 film is increased by 0.1 eV (Figure 3A), so the energy barrier between the perovskite and ETL is decreased. As a result, the device with DNA-doped meso-TiO2 exhibited a higher PCE of 17.59% with a Voc of 1.068 V, Jsc of 22.90 mA cm−2 and an FF of 71.90%.
Apart from the improvement of the PCE of PSCs, stability and potential Pb leakage should also be concerned. Mokhtar and co-authors indicated a bioinspired underlying solution to Pb isolation using hydroxyapatite (HAP) [58]. With the mixture of HAP nanoparticles, the two nanoparticles formed a scaffold for the PSCs. The HAP in the blended scaffold exhibited an average pore size of 36, 52, and 62 nm corresponding to 0%, 30%, and 70% HAP/TiO2 ratio, respectively. Therefore, there is more space for perovskite grain growth. Furthermore, the HAP is dispersed vertically within the percolated TiO2 nanoparticle network, and the phosphate (PO43−) group in HAP can interact with Pb2+, benefiting charge extraction and transport. As a result, this achieved a champion PCE of 20.98% with a Voc of 1.076 V, Jsc of 24.73 mA cm−2 and an FF of 78.85%, and similar stability of 0% to 70% HAP with over 85% of their initial PCEs maintained after storage under ambient conditions without encapsulation. Moreover, HAP exhibited excellent Pb2+ absorption capacity with 1350 mg g−1; the one-point broken devices with 70% HAP scaffold and 20 or 40 μm HAP encapsulation layer released Pb concentration in water are 0.75 and 0.55 ppm, respectively, lower than the 0% and HAP-free devices of 7.0 ppm (Figure 3B). Thus, HAP not only improves performance of PSCs but also impedes Pb leakage.
Compared to TiO2 ETL, SnO2 as ETL has advantages of simple processing, suitable energy-level alignment, and high carrier mobility [59,60]. However, an SnO2 ETL suffers from lattice mismatch with perovskite, which leads to interfacial stress and defects. In addition, oxygen vacancies are also generated during the low-temperature manufacturing process, which induces nonradiative recombination [61]. To solve these problems, Yu and co-authors treated SnO2 with tyrosine (Tyr) to improve the properties of SnO2 film, including passivating the interface defects and tuning the energy level [62]. After the SnO2 film was doped with Tyr, the oxygen vacancies were filled by Tyr through the interaction between the carboxyl group and Sn4+. Furthermore, the conduction band minimum (CBM) of Tyr-modified SnO2 increased from the pristine −4.34 eV to −4.15 eV, which diminishes the energy loss and promotes electron extraction. Moreover, the amino group in Tyr on the surface of the SnO2 film could interact with the I ion, and work as the bridge between perovskite and ETL, which not only eliminates the gap between the two layers facilitating charge transfer at the interface, but also improves the crystallization to render less grain boundary and preferable crystal orientation. As a result, the Tyr-modified device achieved a champion PCE of 22.17% with a Voc of 1.11 V, Jsc of 24.95 mA cm−2 and an FF of 80.05%, and 87% of the original PCE of the unencapsulated device was maintained after aging 864 h in ambient air (25 °C, 25% ± 5% relative humidity).
Although commercial SnO2 colloid solution was widely used for ETL preparation, the soft agglomerate phenomenon of the SnO2 colloid solution often appeared when under long-term storage due to van der Waals attraction, leading to incompletely covered film [63,64]. To overcome this shortage, Wang and co-authors employed two amino acids, glycine (GLY) and alanine (CA), and ammonium titanium fluoride (ATF) to ameliorate this situation [65]. As shown in Figure 3C, the fresh SnO2 exhibited its main peak at 9 nm, while the agglomerated SnO2 nanoparticle corresponded to 100 nm. In contrast, there is only a single peak at 10 nm after the addition of CLY or CA, which indicates that amino acids could effectively prevent soft agglomeration. When the ATF was added to the SnO2 colloid solution, the average size increased to 1.4 μm due to the fluoride ion decreasing the Zeta potential. After 30 d aging, the control and GLY SnO2 colloid solution observed two peaks. When the CA SnO2 colloid solution retained a single peak, this indicated that it was more stable for long-term storage. Furthermore, the carboxyl group interacted with the hydroxyl group on the surface of SnO2, and the carbonyl group passivated the oxygen vacancies via coordination with Sn4+, leading to an ameliorated morphology of SnO2 film. Meanwhile, the negatively charged defects of perovskite are effectively passivated by the amino group in GLY and CA, resulting in an improvement in crystallization. As a result, the PSC modified by GLY exhibited a champion PCE of 19.71%, with a Voc of 1.18 V, a Jsc of 22.83 mA cm−2 and an FF of 73%, and the encapsulated device maintained 83.58% of the original PCE after exposure to an 85% relative humidity environment for 360 h.
As the common HTL material in inverted PSCs, PEDOT:PSS has intrinsic properties including high conductivity, favorable transparency, and low-temperature solution processing [66,67,68,69]. However, there are still some disadvantages that should be considered, such as high acidity and low WF (5.1 eV). To overcome its shortages, Huang and co-authors obtained the copolymer DA-PEDOT:PSS by adding ammonium persulfate into the mixture of DA, PEDOT and PSS. With the doped DA, the WF of copolymer DA-PEDOT:PSS decreased by 0.23 eV, all the better for the alignment with the valence band (VB) of −5.4 eV, reducing the energy barrier and benefit charge transfer. Furthermore, the pH value of copolymer DA-PEDOT:PSS is 5.2, while PEDOT:PSS is 1.8. The decreased acidity means that the DA-PEDOT:PSS would generate less acid corrosion. Thus, the champion PCE improved to 16.6% better than the control of 15.2%. The unencapsulated DA-PEDOT:PSS-based device retained 85.4% of the initial PCE after aging 28 d under nitrogen conditions. Although the DA has improved the properties of PEDOT:PSS through copolymerization, the potential effect of DA on the HTL should be explored in depth. To clear these questions, Xue and co-authors investigated the effects of DA-doped PEDOT:PSS systematically [53]. As shown in Figure 3D, the DA-PEDOT:PSS film contact angle increased after annealing, which was attributed to the crosslinking reaction that occurred between DA and PEDOT. The surface with higher hydrophobicity of HTL is beneficial for the crystallization of perovskite and enhances waterproofness. Furthermore, the uncoordinated Pb2+ on the buried surface of the perovskite can be passivated by the amino and hydroxyl groups (Figure 3E), which effectively passivated surface defects and suppressed nonradiative recombination, promoting charge extraction. Moreover, the DA-PEDOT:PSS matched with the VB of perovskite well, due to deeper WF (5.33 eV), thus maximizing the built-in potential. As a result, a champion PCE of 18.5% with an increased Voc of 1.08 V was achieved. This exploration provides an essential guideline for designing a new HTL material of inverted PSCs with ameliorated performance.
For the improvement of the properties of HTLs, besides the modification approach, developing new substitute materials is another way. Yusoff and co-authors employed DNA–hexadecyl trimethyl ammonium chloride (CTMA) as the HTL in inverted PSCs [70]. Figure 3F shows that the HOMO and LUMO of DNA-CTMA are estimated at 5.4 eV and 1.1 eV, respectively. The HOMO energy level of DNA-CTMA is more matched with the VB of perovskite (−5.4 eV), facilitating hole extraction, and the higher LUMO level of DNA-CTMA could block electrons effectively. Furthermore, DNA-CTMA film exhibited excellent transparency (300–1100 nm) convenient for the light to pass through. Furthermore, an approximate 6% initial weight loss (the bound water) below 140 °C, which kept stable when temperature reached 225 °C, confirmed that DNA-CTMA has good thermal stability. As a result, a champion PCE of 15.86% with VOC of 1.04 V, JSC of 20.85 mA cm−2 and an FF of 73.15% was realized for the device with DNA-CTMA HTL.
CTLs are an important part of PSCs, and these works represent great progress in the use of natural molecule-modified CTLs in PSCs. However, most are concentrated on one question of CTLs, and cannot use a natural molecule to achieve defect passivation, energetics modification, and suppression migration simultaneously. Thus, there is still a long way to go for promising CTLs.
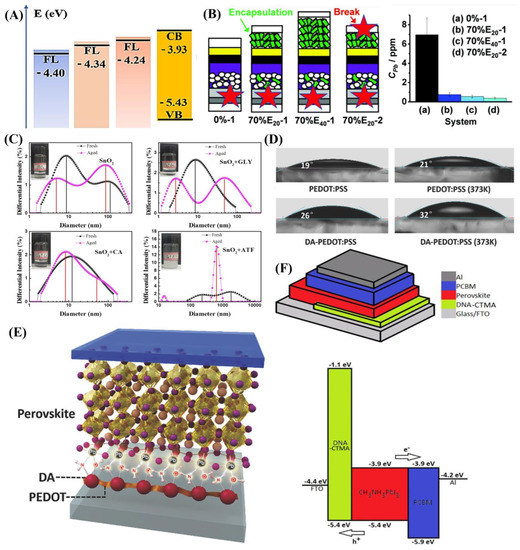
Figure 3.
Instances of natural molecule-modified CTLs. (A) Schematic illustration of the energy diagram [57]. Copyright 2020, Elsevier. (B) The breakage point in devices and Pb release after 24 h in water [58]. Copyright 2021, Royal Society of Chemistry. (C) DLS spectra of fresh and aged SnO2 solution with different additives [65]. Copyright 2021, Elsevier. (D) Temperature-dependent contact angles of PEDOT: PSS and DA-PEDOT: PSS. (E) Potential passivation effect [53]. Copyright 2018, Wiley-VCH. (F) Device structure and energy band alignment of DNA-CTMA-based PSCs [70]. Copyright 2016, Wiley-VCH.
5. Natural Molecule-Based Flexible Substrate
Flexible PSCs are commonly coated on plastic substrates including polyimide (PI), polyethylene terephthalate (PET), and polyethylene naphthalate (PEN) due to their advantages of excellent mechanical and chemical stability [71,72,73]. However, the extremely long time for degradation or decomposition in nature results in environmental pollution. Therefore, it is imperative to develop alternative biocompatible substrates for flexible PSCs.
In 2018, Gao and co-authors first employed biocompatible cellulose paper with low cost as the substrate of flexible PSCs [55]. The substrate modified by carbon not only exhibited good conductivity of 14.2 Ω sq−1 and a smoother surface, but also showed an aligned energy level with perovskite (Figure 4a). As a result, a champion PCE of 9.05% was achieved for the bio-substrate device without HTL, and good bending stability with 75% initial PCE was maintained after 1000 bending cycles (Figure 4b). Later, Zhu and co-authors reported a bamboo-cellulose nanofibril (b-CNF) substrate for flexible PSCs [56]. The b-CNF substrate exhibited brilliant transparency, and over 90% visible light (400–800 nm) could transmit through the paper. Combined with 150 nm indium zinc oxide (IZO), ultrahigh flexibility and good transmittance b-CNF/IZO electrodes with high conductivity were obtained. Moreover, the b-CNF/IZO electrode showed good mechanical stability with Young’s modulus of 7.3 GPa and tensile strength of 230 MPa (Figure 4c). In addition, the electrode remains a stable square resistance of 40 Ω sq−1 after 3000 bending tests at a 4 nm curvature radius, while the PEN/IZO electrode increased after 2400 bending tests (Figure 4d). As a result, the device fabricated on this substrate realized a champion PCE of 11.68% with VOC of 0.935 V, JSC of 16.92 mA cm−2 and an FF of 73.86%, and retained 70% of initial PCE after bending 1000 times at a 4 mm curvature radius.
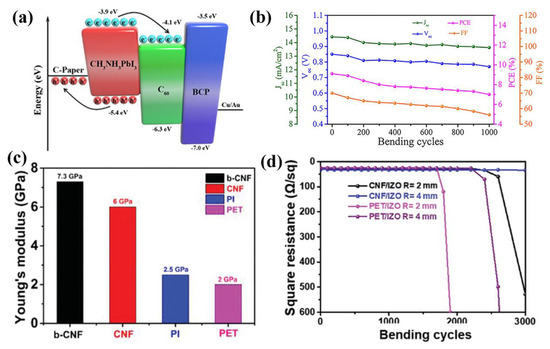
Figure 4.
Instances of natural molecule-based substrates for PSCs: (a) Energy-level diagram of the cellulose paper-based hole-free PSC. (b) The plot of photovoltaic parameters as a function of bending cycles [55]. Copyright 2018, Wiley-VCH. (c) The histogram of the average Young’s modulus for different films. (d) The square resistance variation curves of the b-CNF/IZO and the PET/IZO electrode bending at different curvature radii [56]. Copyright 2019, Wiley-VCH.
These studies represent great progress in flexible substrates, which means that environmentally unfriendly plastic substrates could be substituted by the biocompatible flexible substrate, and provide a new direction for flexible PSCs. However, there are still some problems to be solved. For example, although a biodegradable flexible substrate shows good flexibility and light transmittance, its device performance still needs to be greatly improved. In addition, the environmental stability of this type of substrate also should be addressed.
6. Summary and Outlook
This review concentrated on the recent progress of PSCs modified by natural molecules with high performance and stability. Compared to synthetic chemical molecules, Natural molecules are biocompatible and eco-friendly. More important, natural molecules play an important role either in perovskite or interface and CTL. Based on additive engineering, the introduced natural molecules improve the crystallization process and passivate the defects as well as the energetics modification. Through interface engineering, the employed natural molecules ameliorate interface contact and energy-level alignment, promoting charge transfer. Moreover, CTL doped by natural molecules improved the surface and chemical properties that benefit the overlying layer deposition, and enhanced charge separation and transportation. For the device substrate, natural molecule-based substrates exhibited brilliant flexible ability, excellent transmittance, and biocompatibility. In summary, natural molecules exhibited valuable functions in PSCs, and have a broader application potential. However, there are still some issues that should be considered.
First, the PCE of natural molecule-modified PSCs lags behind the PSCs modified by synthetic chemical materials. Thus, it is necessary to develop some natural molecules that can effectively promote the PCE of PSCs.
Second, when perovskite is modified with additive engineering, the distribution of natural molecules in it and the interaction with adjacent layers also need to be noted. The mechanism of natural molecules with perovskite and adjacent layers also should be investigated systematically.
Third, inspired by the natural molecule-based substrate, the CTL could also be substituted for natural molecules.
Funding
This work was financially supported by the National Natural Science Foundation of China (62274018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohd Yusoff, A.R.B.; Vasilopoulou, M.; Georgiadou, D.G.; Palilis, L.C.; Abate, A.; Nazeeruddin, M.K. Passivation and process engineering approaches of halide perovskite films for high efficiency and stability perovskite solar cells. Environ. Sci. Technol. 2021, 14, 2906–2953. [Google Scholar] [CrossRef]
- Tang, G.; Yan, F. Recent progress of flexible perovskite solar cells. Nano Today 2021, 39, 101155. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Seol, D.-J.; Cho, A.-N.; Park, N.-G. High-Efficiency Perovskite Solar Cells Based on the Black Polymorph of HC(NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef]
- Green, M.A.; Jiang, Y.; Soufiani, A.M.; Ho-Baillie, A. Optical Properties of Photovoltaic Organic–Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 4774–4785. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Wang, G.; Wang, W.; Bai, L.; Zhou, Q.; He, D.; Liu, B.; Zhao, P.; Tan, L.; Li, Y.; Wei, D.; et al. Grain Boundary Chemical Anchoring via Bidirectional Active Site Additive Enables Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2200904. [Google Scholar] [CrossRef]
- Chen, J.; Kim, S.-G.; Ren, X.; Jung, H.S.; Park, N.-G. Effect of bidentate and tridentate additives on the photovoltaic performance and stability of perovskite solar cells. J. Mater. Chem. A 2019, 7, 4977–4987. [Google Scholar] [CrossRef]
- Bi, H.; Liu, B.; He, D.; Bai, L.; Wang, W.; Zang, Z.; Chen, J. Interfacial defect passivation and stress release by multifunctional KPF6 modification for planar perovskite solar cells with enhanced efficiency and stability. Chem. Eng. J. 2021, 418, 129375. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zhang, C.; Gong, C.; Li, H.; Li, H.; Zhang, Z.; Yang, H.; Chen, J.; Zang, Z. Tailoring multifunctional anion modifiers to modulate interfacial chemical interactions for efficient and stable perovskite solar cells. Nano Energy 2022, 102, 107747. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J.; He, D.; Bai, L.; Zhou, Q.; Wang, W.; Xu, C.; Song, Q.; Lee, D.; Zhao, P.; et al. Simultaneous Passivation of Bulk and Interface Defects with Gradient 2D/3D Heterojunction Engineering for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 21079–21088. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Miah, M.H.; Rahman, M.B.; Nur-E-Alam, M.; Das, N.; Soin, N.B.; Hatta, S.F.W.M.; Islam, M.A. Understanding the Degradation Factors, Mechanism and Initiatives for Highly Efficient Perovskite Solar Cells. ChemNanoMat 2023, e202200471. [Google Scholar] [CrossRef]
- Yang, X.; Ni, Y.; Zhang, Y.; Wang, Y.; Yang, W.; Luo, D.; Tu, Y.; Gong, Q.; Yu, H.; Zhu, R. Multiple-Defect Management for Efficient Perovskite Photovoltaics. ACS Energy Lett. 2021, 6, 2404–2412. [Google Scholar] [CrossRef]
- Jeong, M.J.; Yeom, K.M.; Kim, S.J.; Jung, E.H.; Noh, J.H. Spontaneous interface engineering for dopant-free poly(3-hexylthiophene) perovskite solar cells with efficiency over 24%. Environ. Sci. Technol. 2021, 14, 2419–2428. [Google Scholar] [CrossRef]
- Wen, L.; Rao, Y.; Zhu, M.; Li, R.; Zhan, J.; Zhang, L.; Wang, L.; Li, M.; Pang, S.; Zhou, Z. Reducing Defects Density and Enhancing Hole Extraction for Efficient Perovskite Solar Cells Enabled by π-Pb2+ Interactions. Angew. Chem. Int. Edit. 2021, 60, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cui, J.; Chen, M.; Zhang, M.; Han, Y.; Qian, F.; Zhao, H.; Yang, S.; Yang, Z.; Bian, H.; et al. Multifunctional Enhancement for Highly Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2005776. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.-W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.-K.; et al. Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Lin, H.-S.; Lee, J.-M.; Han, J.; Lee, C.; Seo, S.; Tan, S.; Lee, H.M.; Choi, E.J.; Strano, M.S.; Yang, Y.; et al. Denatured M13 Bacteriophage-Templated Perovskite Solar Cells Exhibiting High Efficiency. Adv. Sci. 2020, 7, 2000782. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Z.; Li, Y.; Yan, G.; Huang, D.; Hou, S.; Zhou, W.; Wang, X.; Ren, J.; Xiang, Y.; et al. Heterogeneous lead iodide obtains perovskite solar cells with efficiency of 24.27%. Chem. Eng. J. 2022, 448, 137676. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and Protection against Solar UV Radiation. Skin Pharmacol. Physiol. 2002, 15, 291–296. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhou, D.; Liu, S.; Sun, R.; Shi, Z.; Liu, L.; Wang, T.; Liu, B.; Liu, D.; Song, H. Learning From Plants: Lycopene Additive Passivation toward Efficient and “Fresh” Perovskite Solar Cells with Oxygen and Ultraviolet Resistance. Adv. Energy Mater. 2022, 12, 2200614. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, S.; Li, N.; Huang, B.; Niu, X.; Li, L.; Sun, M.; Zhang, Y.; Zhang, X.; Zhu, C.; et al. Self-Elimination of Intrinsic Defects Improves the Low-Temperature Performance of Perovskite Photovoltaics. Joule 2020, 4, 1961–1976. [Google Scholar] [CrossRef]
- Guo, J.; Sun, J.; Hu, L.; Fang, S.; Ling, X.; Zhang, X.; Wang, Y.; Huang, H.; Han, C.; Cazorla, C.; et al. Indigo: A Natural Molecular Passivator for Efficient Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2200537. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, B.; Zhao, F.; Zheng, X.; Deng, Y.; Shao, Y.; Fang, Y.; Bai, Y.; Wang, C.; Huang, J. Matching Charge Extraction Contact for Wide-Bandgap Perovskite Solar Cells. Adv. Mater. 2017, 29, 1700607. [Google Scholar] [CrossRef]
- Schulz, P.; Edri, E.; Kirmayer, S.; Hodes, G.; Cahen, D.; Kahn, A. Interface energetics in organo-metal halide perovskite-based photovoltaic cells. Environ. Sci. Technol. 2014, 7, 1377–1381. [Google Scholar] [CrossRef]
- Olthof, S.; Meerholz, K. Substrate-dependent electronic structure and film formation of MAPbI3 perovskites. Sci. Rep. 2017, 7, 40267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hou, Z.; Zou, S.; Lu, X.; Yang, J.; Hao, T.; Zhou, Z.; Xu, J.; Zeng, Y.; Xiao, W.; et al. Direct Observation on p- to n-Type Transformation of Perovskite Surface Region during Defect Passivation Driving High Photovoltaic Efficiency. Joule 2021, 5, 467–480. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Wu, Y.; Zhang, Y.; Lyu, J.; Liu, Z.; Bian, S.; Bai, X.; Xu, L.; Zhou, D.; et al. Vitamin Natural Molecule Enabled Highly Efficient and Stable Planar n–p Homojunction Perovskite Solar Cells with Efficiency Exceeding 24.2%. Adv. Energy Mater. 2023, 13, 2203352. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- You, S.; Wang, H.; Bi, S.; Zhou, J.; Qin, L.; Qiu, X.; Zhao, Z.; Xu, Y.; Zhang, Y.; Shi, X.; et al. A Biopolymer Heparin Sodium Interlayer Anchoring TiO2 and MAPbI3 Enhances Trap Passivation and Device Stability in Perovskite Solar Cells. Adv. Mater. 2018, 30, 1706924. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, P.; Duan, Y.; Hu, X.; Li, F.; Song, Y. Dopamine-crosslinked TiO2/perovskite layer for efficient and photostable perovskite solar cells under full spectral continuous illumination. Nano Energy 2019, 56, 733–740. [Google Scholar] [CrossRef]
- Geng, Q.; Jia, X.; He, Z.; Hu, Y.; Gao, Y.; Yang, S.; Yao, C.; Zhang, S. Interface Engineering via Amino Acid for Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2201641. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Environ. Sci. Technol. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Kim, G.-W.; Choi, Y.; Choi, H.; Min, J.; Park, T.; Song, S. Novel cathode interfacial layer using creatine for enhancing the photovoltaic properties of perovskite solar cells. J. Mater. Chem. A 2020, 8, 21721–21728. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Tang, W.; Qiu, W.; Wu, Y.; Peng, Q. Heterocyclic amino acid molecule as a multifunctional interfacial bridge for improving the efficiency and stability of quadruple cation perovskite solar cells. Nano Energy 2023, 107, 108154. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Liu, X.; Cheung, S.H.; Chandran, H.T.; Li, H.-W.; Xu, X.; Xie, Y.-M.; So, S.K.; Yip, H.-L.; et al. Impact of surface dipole in NiOx on the crystallization and photovoltaic performance of organometal halide perovskite solar cells. Nano Energy 2019, 61, 496–504. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Zhao, G.; Zhao, K.; Wang, Z.-S. Pyridine-Terminated Conjugated Organic Molecules as an Interfacial Hole Transfer Bridge for NiOx-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 28960–28967. [Google Scholar] [CrossRef]
- Kaneko, R.; Kanda, H.; Sugawa, K.; Otsuki, J.; Islam, A.; Nazeeruddin, M.K. Perovskite Solar Cells Using Surface-Modified NiOx Nanoparticles as Hole Transport Materials in n-i-p Configuration. Sol. RLL 2019, 3, 1900172. [Google Scholar] [CrossRef]
- Xie, L.; Cao, Z.; Wang, J.; Wang, A.; Wang, S.; Cui, Y.; Xiang, Y.; Niu, X.; Hao, F.; Ding, L. Improving energy level alignment by adenine for efficient and stable perovskite solar cells. Nano Energy 2020, 74, 104846. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001. [Google Scholar] [CrossRef]
- Xiong, S.; Yuan, M.; Yang, J.; Song, J.; Guo, X.; Li, X.; Li, B.; Liu, X.; Duan, C.; Liu, F.; et al. Engineering of the Back Contact between PCBM and Metal Electrode for Planar Perovskite Solar Cells with Enhanced Efficiency and Stability. Adv. Opt. Mater. 2019, 7, 1900542. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Zheng, C.; Liu, Z.; Chen, L.; Yuan, N.; Ding, J.; Wang, D.; Liu, S. Plant-Derived L-Theanine for Ultraviolet/Ozone Resistant Perovskite Photovoltaics. Adv. Energy Mater. 2022, 13, 2203190. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, M.; Li, Z.; Yan, L.; Hu, Z.; Zhou, J.; Li, W.; Jiang, X.-F.; Xu, B.; Huang, F.; et al. Efficient and Stable Perovskite Solar Cells via Dual Functionalization of Dopamine Semiquinone Radical with Improved Trap Passivation Capabilities. Adv. Funct. Mater. 2018, 28, 1707444. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.-X.; Chang, J.-J.; Jiang, Y.-Y.; Xiao, Q.-S.; Li, Y. Improving the efficiency and stability of inverted perovskite solar cells with dopamine-copolymerized PEDOT:PSS as a hole extraction layer. J. Mater. Chem. A 2017, 5, 13817–13822. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, S.; Cui, K.; Qiu, Z.; Ge, S.; Cao, B.; Yu, J. Flexible and Biocompatibility Power Source for Electronics: A Cellulose Paper Based Hole-Transport-Materials-Free Perovskite Solar Cell. Sol. RLL 2018, 2, 1800175. [Google Scholar] [CrossRef]
- Zhu, K.; Lu, Z.; Cong, S.; Cheng, G.; Ma, P.; Lou, Y.; Ding, J.; Yuan, N.; Rümmeli, M.H.; Zou, G. Ultraflexible and Lightweight Bamboo-Derived Transparent Electrodes for Perovskite Solar Cells. Small 2019, 15, 1902878. [Google Scholar] [CrossRef]
- Peng, X.; Lu, H.; Zhuang, J.; Liu, X.; Ma, Z.; Wang, H.; Guo, Z.; Wang, Q.; Zhang, H.; Zhao, S. Enhanced performance of perovskite solar cells using DNA-doped mesoporous-TiO2 as electron transporting layer. Sol. Energy 2020, 206, 855–863. [Google Scholar] [CrossRef]
- Mokhtar, M.Z.; He, J.; Li, M.; Chen, Q.; Ke, J.C.R.; Lewis, D.J.; Thomas, A.G.; Spencer, B.F.; Haque, S.A.; Saunders, B.R. Bioinspired scaffolds that sequester lead ions in physically damaged high efficiency perovskite solar cells. Chem. Commun. 2021, 57, 994–997. [Google Scholar] [CrossRef]
- Baena, J.P.C.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Kandada, A.R.S.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Environ. Sci. Technol. 2015, 8, 2928–2934. [Google Scholar]
- Snaith, H.J.; Ducati, C. SnO2-Based Dye-Sensitized Hybrid Solar Cells Exhibiting Near Unity Absorbed Photon-to-Electron Conversion Efficiency. Nano Lett. 2010, 10, 1259–1265. [Google Scholar] [CrossRef]
- Han, S.; Zhang, H.; Li, Y.; Wang, R.; He, Q. Solution-processed amino acid modified SnO2 electron transport layer for carbon-based CsPbIBr2 perovskite solar cells. Mat. Sci. Semicon. Proc. 2021, 133, 105964. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.; Wang, G.; Wang, J.; Zhang, J.; Chen, P.; Li, C.; Duan, Y. Multifunctional tyrosine modified SnO2 to improve the performance of perovskite solar cells. Appl. Phys. Lett. 2022, 121, 073501. [Google Scholar] [CrossRef]
- You, S.; Zeng, H.; Ku, Z.; Wang, X.; Wang, Z.; Rong, Y.; Zhao, Y.; Zheng, X.; Luo, L.; Li, L.; et al. Multifunctional Polymer-Regulated SnO2 Nanocrystals Enhance Interface Contact for Efficient and Stable Planar Perovskite Solar Cells. Adv. Mater. 2020, 32, 2003990. [Google Scholar] [CrossRef]
- Bi, H.; Zuo, X.; Liu, B.; He, D.; Bai, L.; Wang, W.; Li, X.; Xiao, Z.; Sun, K.; Song, Q.; et al. Multifunctional organic ammonium salt-modified SnO2 nanoparticles toward efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2021, 9, 3940–3951. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Guo, W.; Ji, Y.; Zhou, Y.; Dang, J.; Wang, M. Functional molecule modified SnO2 nanocrystal films toward efficient and moisture-stable perovskite solar cells. J. Alloys Compd. 2022, 890, 161912. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly Conductive PEDOT:PSS Electrode with Optimized Solvent and Thermal Post-Treatment for ITO-Free Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The Origin of the High Conductivity of Poly(3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) (PEDOT−PSS) Plastic Electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Yip, H.-L.; Jen, A.K.Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Environ. Sci. Technol. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Yusoff, A.R.b.M.; Kim, J.; Jang, J.; Nazeeruddin, M.K. New Horizons for Perovskite Solar Cells Employing DNA-CTMA as the Hole-Transporting Material. ChemSusChem 2016, 9, 1736–1742. [Google Scholar] [CrossRef]
- Madaria, A.R.; Kumar, A.; Zhou, C. Large scale, highly conductive and patterned transparent films of silver nanowires on arbitrary substrates and their application in touch screens. Nanotechnology 2011, 22, 245201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Öberg, V.A.; Du, J.; Liu, J.; Johansson, E.M.J. Extremely lightweight and ultra-flexible infrared light-converting quantum dot solar cells with high power-per-weight output using a solution-processed bending durable silver nanowire-based electrode. Environ. Sci. Technol. 2018, 11, 354–364. [Google Scholar] [CrossRef]
- Oh, S.-J.; Jo, Y.; Lee, E.J.; Lee, S.S.; Kang, Y.H.; Jeon, H.-J.; Cho, S.Y.; Park, J.-S.; Seo, Y.-H.; Ryu, B.-H.; et al. Ambient atmosphere-processable, printable Cu electrodes for flexible device applications: Structural welding on a millisecond timescale of surface oxide-free Cu nanoparticles. Nanoscale 2015, 7, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).