Evaluation of Mechanical Properties of Glass Ionomer Cements Reinforced with Synthesized Diopside Produced via Sol–Gel Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Diopside Nanoparticles
2.2. Manufacture of Nanocomposite Samples
2.3. Mechanical Tests
2.4. Fluoride Release Assessment Test (ICP)
2.5. Characterizations
3. Results and Discussion
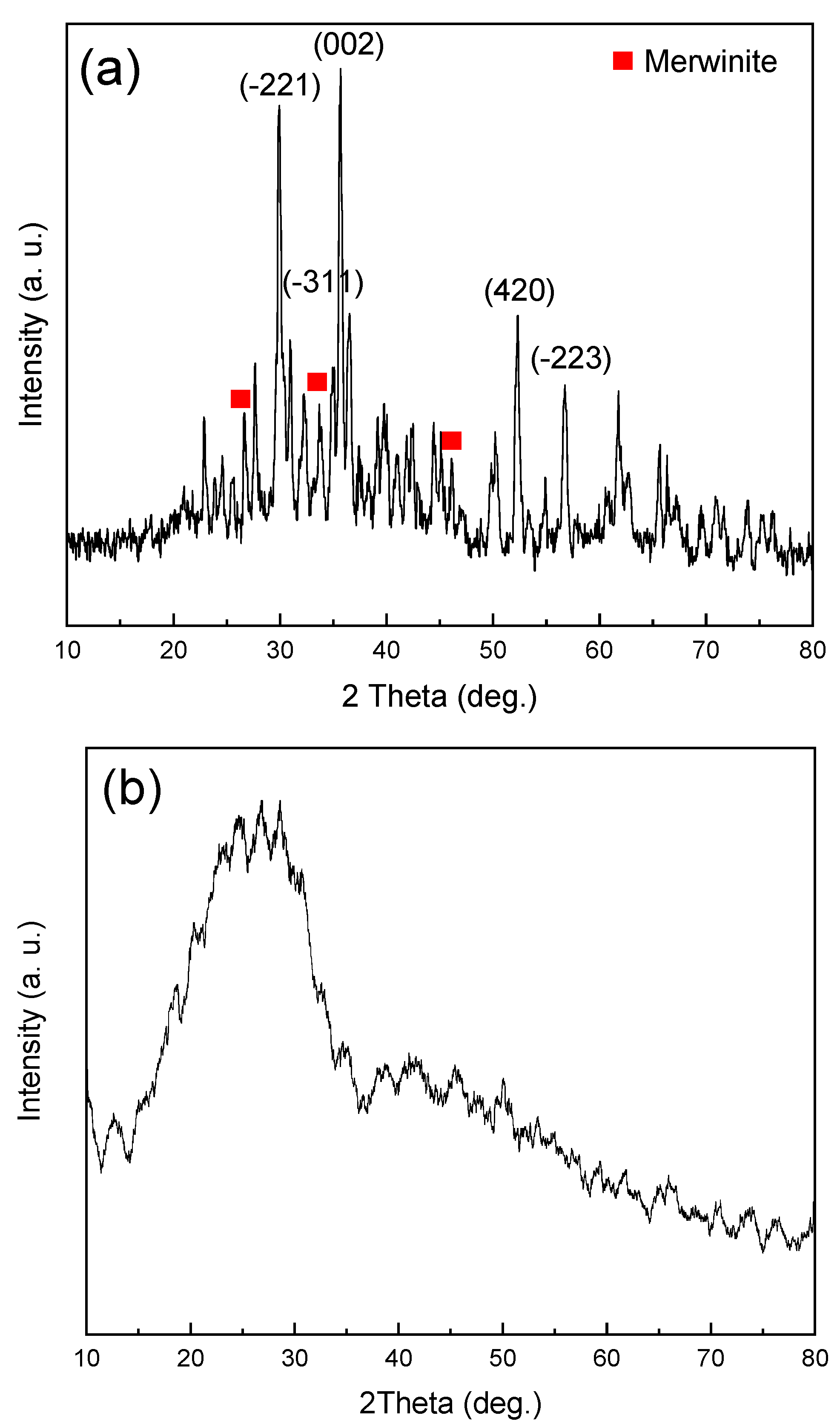
3.1. Characterization of Diopside (DIO) Nanoparticles
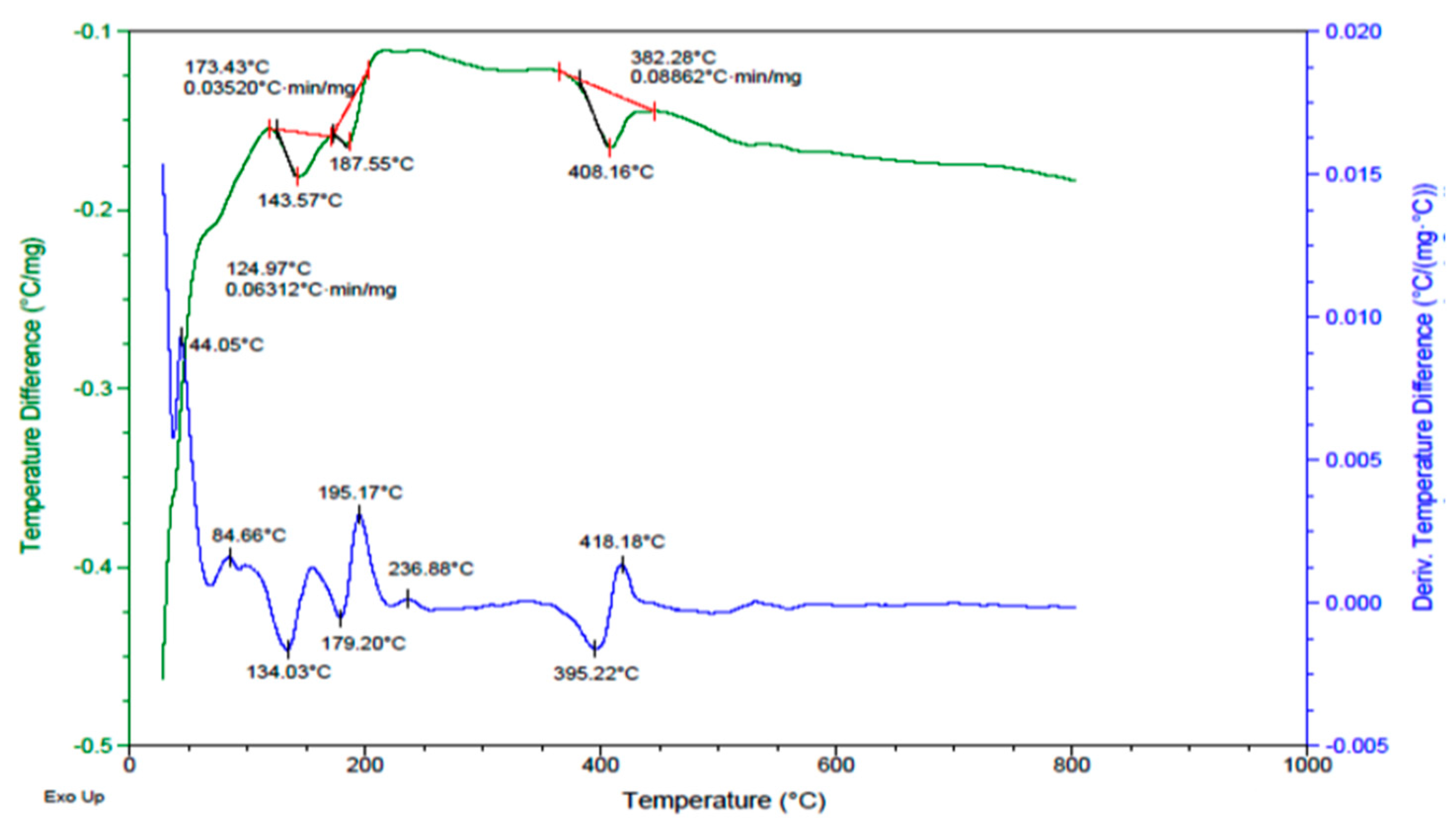
3.2. DTA of Synthesis Diopside (DIO)
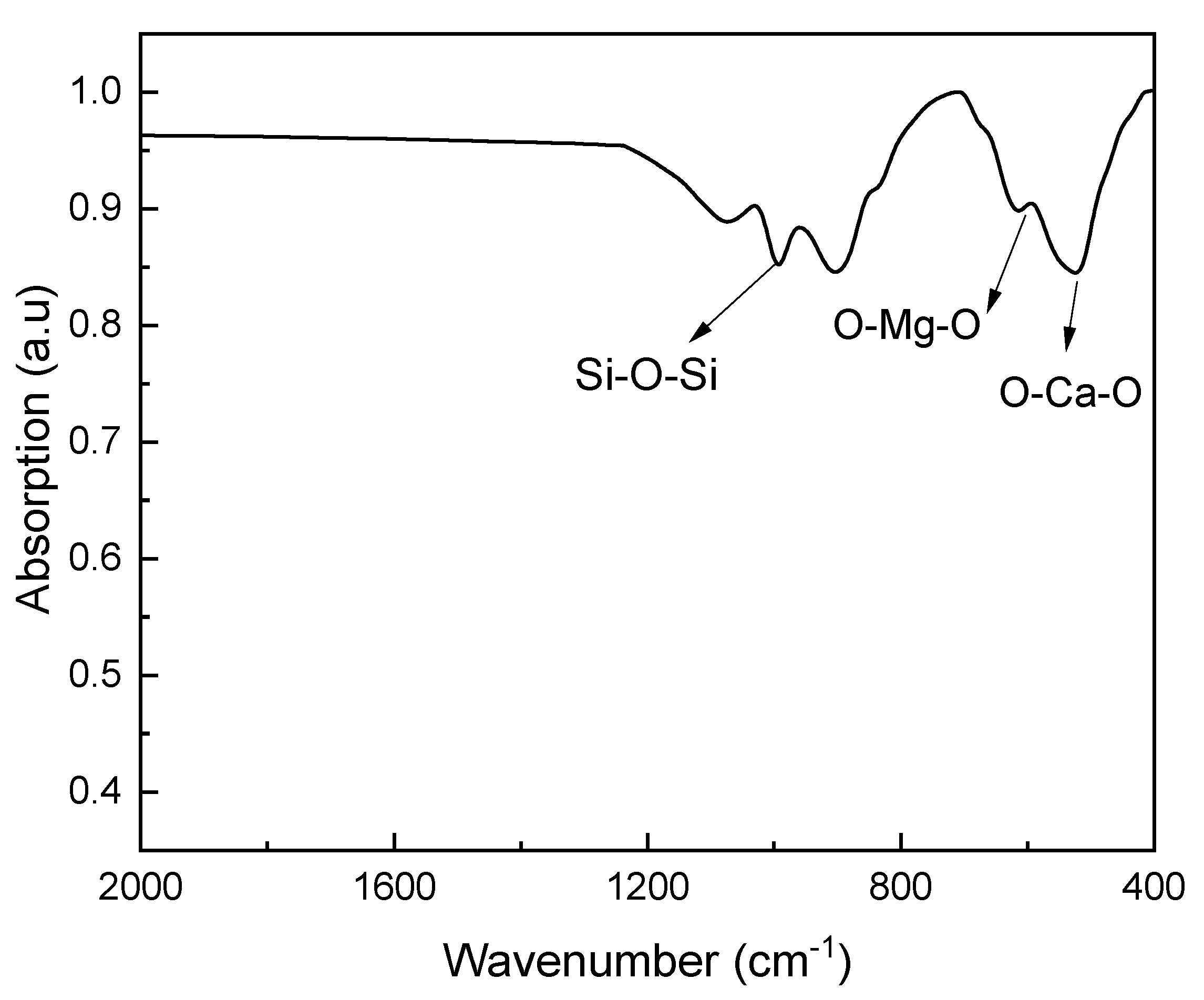
3.3. FTIR Analysis of Diopside Nanoparticles
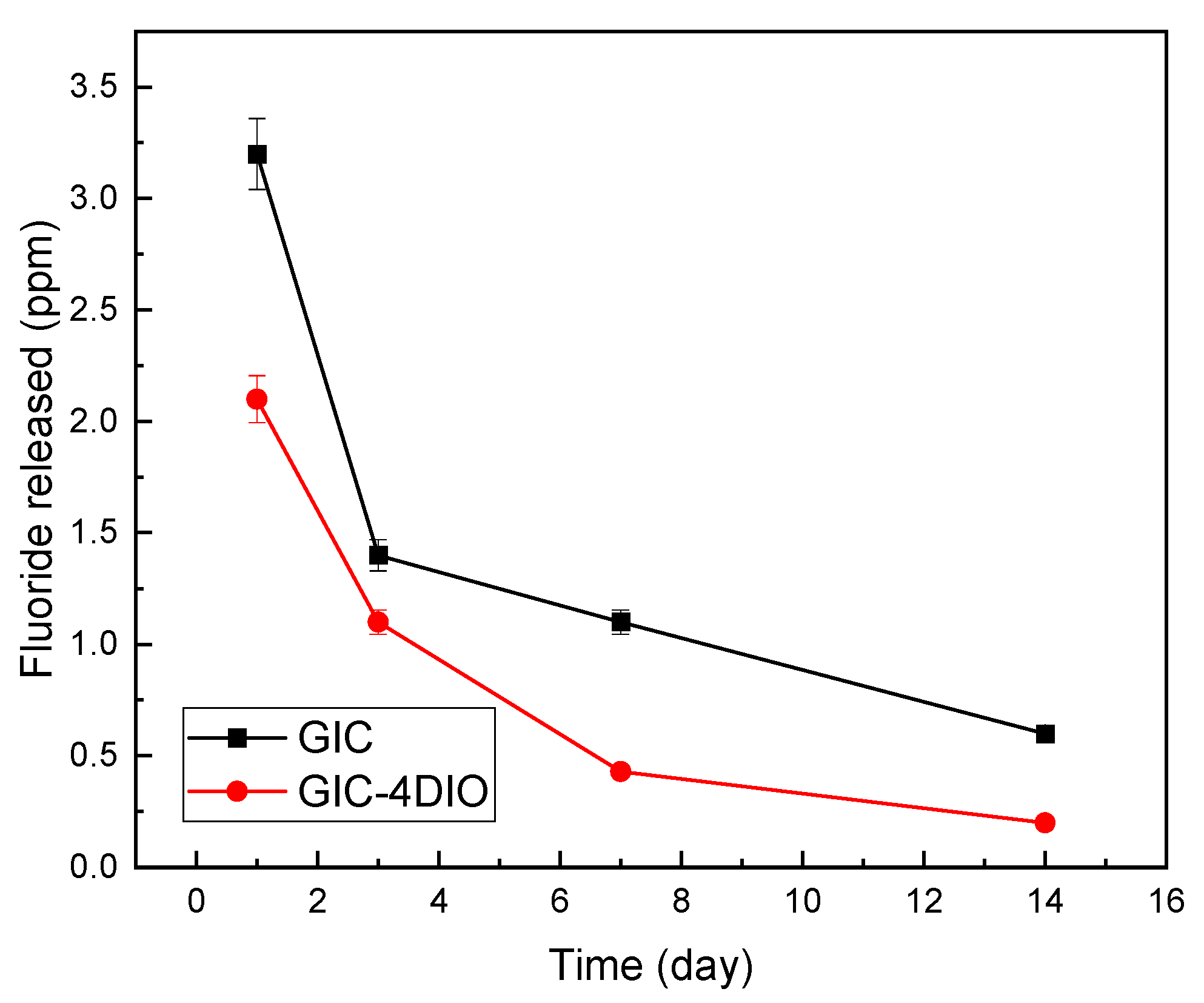
3.4. Evaluation of Fluoride Release
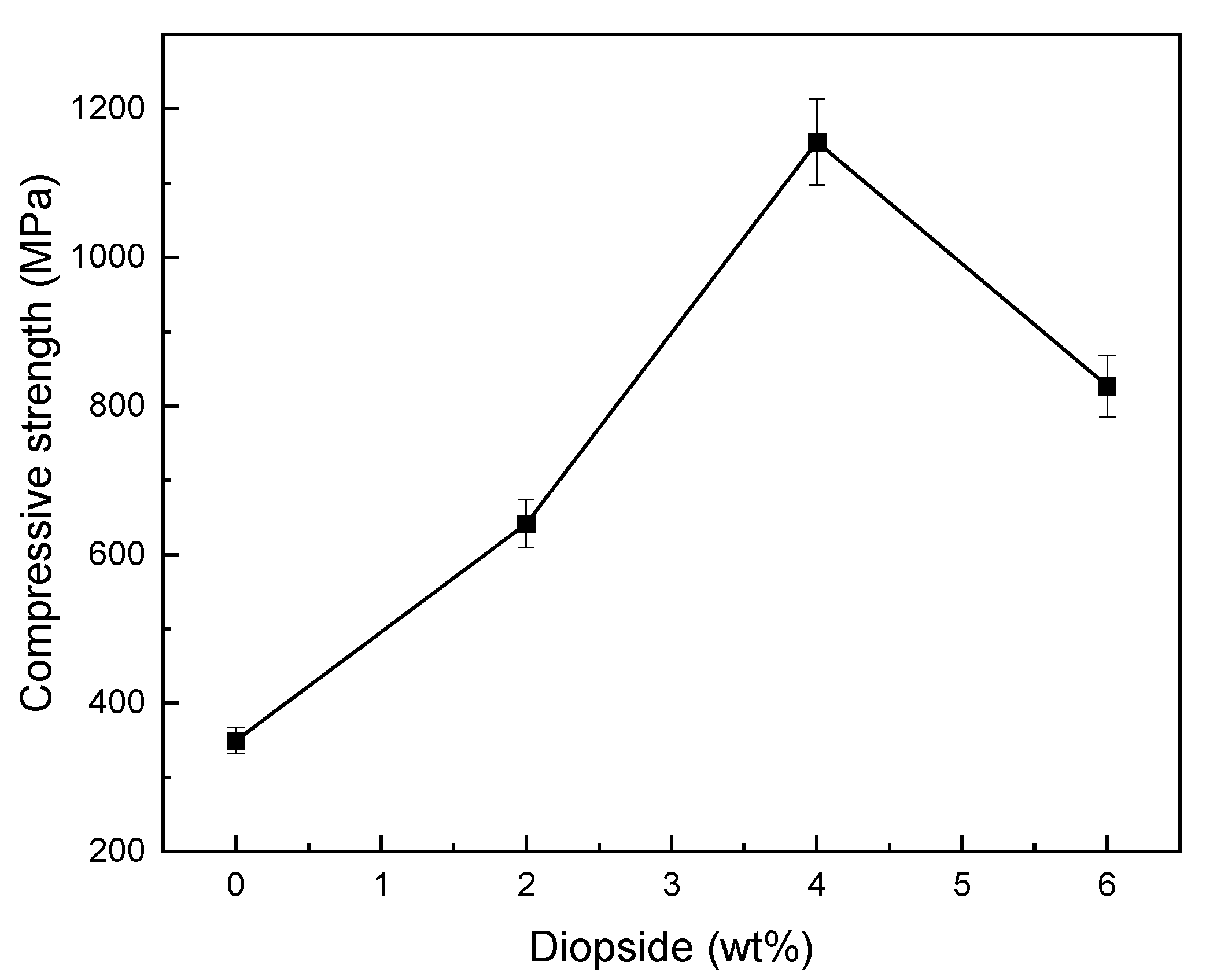
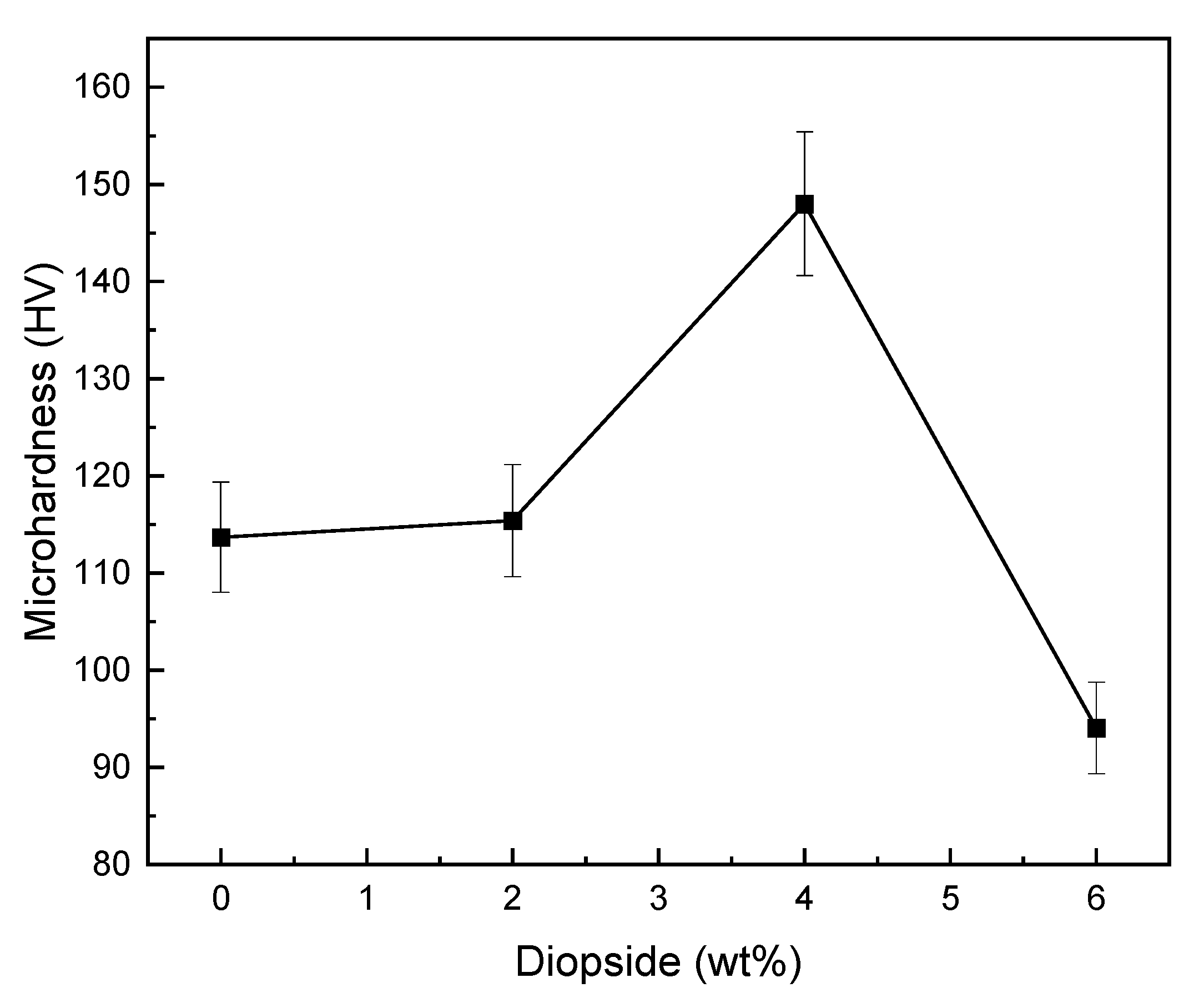
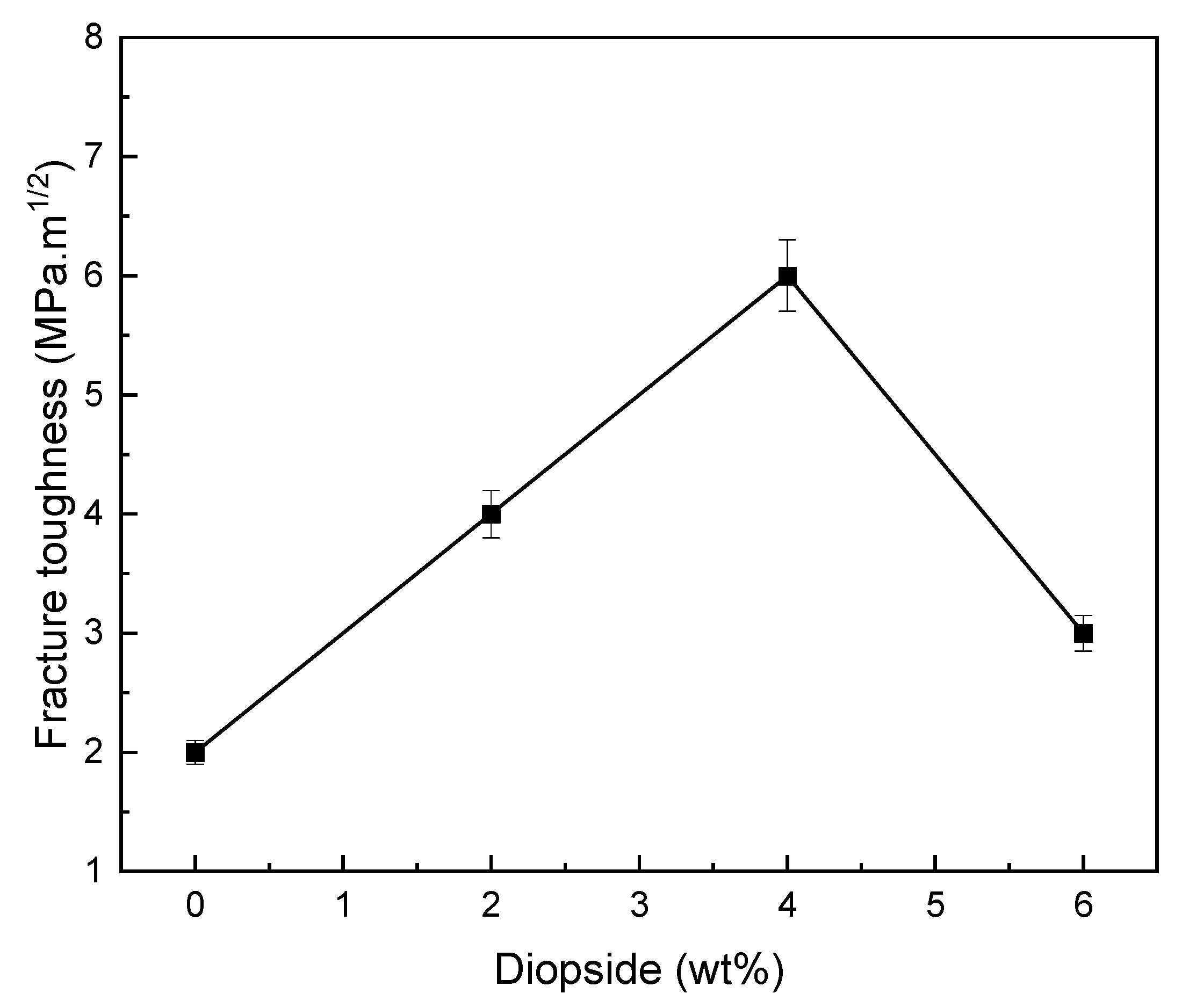
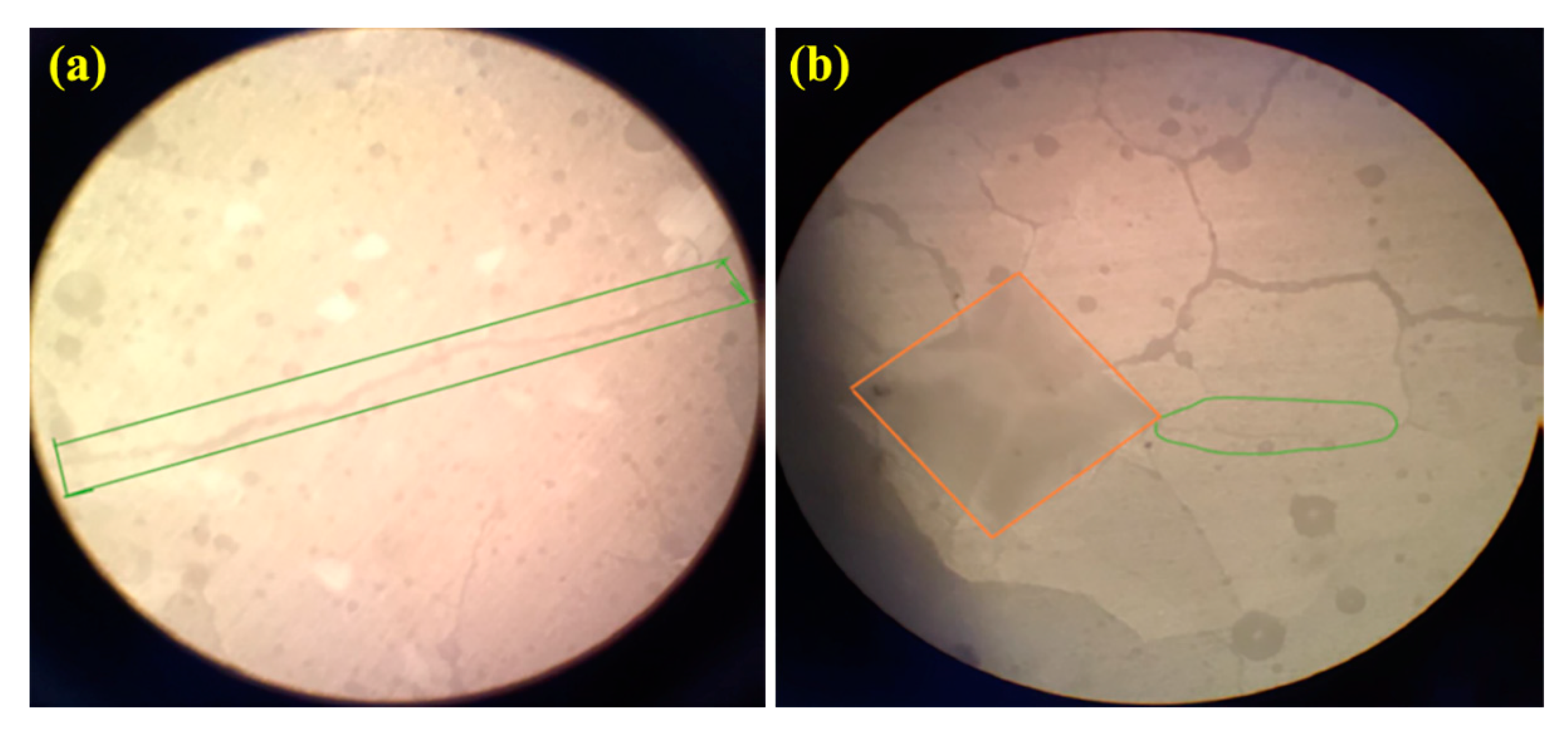
3.5. Mechanical Properties
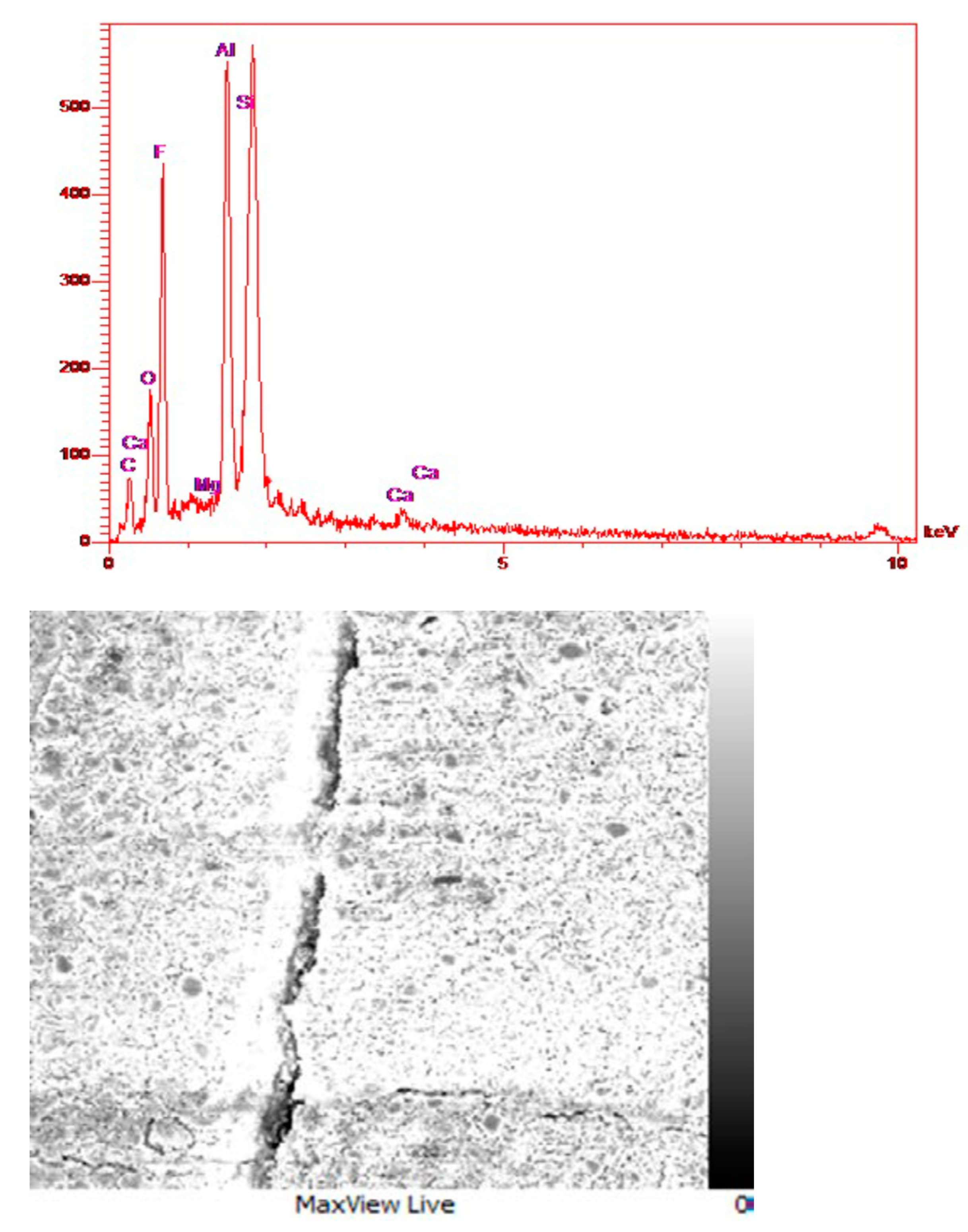
3.6. FESEM Images and EDS Analysis of Glass Ionomer and Glass Ionomer 4 wt% Diopside Nanocomposites
4. Conclusions
- Phase analysis confirms that pure and crystalline diopside (DIO) nanoparticles were synthesized by the sol–gel method;
- The optimal percentage of diopside (DIO) nanoparticles to increase the compressive strength, microhardness, and fracture toughness of glass ionomer cement (GIC) was 4 wt%, so the compressive strength, microhardness, and fracture toughness of glass ionomer cement (GIC) saw increases of about 230%, 30%, and 89%;
- Adding diopside (DIO) nanoparticles to the ceramic component of glass ionomer cement (GIC) causes a slight decrease in the amount of fluoride releases;
- The produced glass ionomer (GIC) diopside (DIO) cement nanocomposites, due to their mechanical properties, and favorable fluoride release, are suggested as a suitable option for dental restorations and orthopedic implants under load.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.S.; Khan, M.; Rehman, I.U. Nanoparticles, Properties, and Applications in Glass Ionomer Cements. Eur. J. Mol. Clin. Med. 2012, 7, 93–108. [Google Scholar] [CrossRef]
- Iaculli, F.; Salucci, A.; Di Giorgio, G.; Luzzi, V.; Ierardo, G.; Polimeni, A.; Bossù, M. Bond Strength of Self-Adhesive Flowable Composites and Glass Ionomer Cements to Primary Teeth: A Systematic Review and Meta-Analysis of In Vitro Studies. Materials 2021, 14, 6694. [Google Scholar] [CrossRef]
- Uzel, I.; Aykut-Yetkiner, A.; Ersin, N.; Ertuğrul, F.; Atila, E.; Özcan, M. Evaluation of Glass-Ionomer versus Bulk-Fill Resin Composite: A Two-Year Randomized Clinical Study. Materials 2022, 15, 7271. [Google Scholar] [CrossRef]
- Ratnayake, J.; Veerasamy, A.; Ahmed, H.; Coburn, D.; Loch, C.; Gray, A.R.; Lyons, K.M.; Heng, N.C.K.; Cannon, R.D.; Leung, M.; et al. Clinical and Microbiological Evaluation of a Chlorhexidine-Modified Glass Ionomer Cement (GIC-CHX) Restoration Placed Using the Atraumatic Restorative Treatment (ART) Technique. Materials 2022, 15, 5044. [Google Scholar] [CrossRef]
- Bilgrami, A.; Maqsood, A.; Alam, M.K.; Ahmed, N.; Mustafa, M.; Alqahtani, A.R.; Alshehri, A.; Alqahtani, A.A.; Alghannam, S. Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study. Materials 2022, 15, 4293. [Google Scholar] [CrossRef]
- Čulina, M.Z.; Rajić, V.B.; Šalinović, I.; Klarić, E.; Marković, L.; Ivanišević, A. Influence of pH Cycling on Erosive Wear and Color Stability of High-Viscosity Glass Ionomer Cements. Materials 2022, 15, 923. [Google Scholar] [CrossRef]
- Cheruvathoor, J.J.; Thomas, L.R.; Thomas, L.A.; Shivanna, M.M.; Machani, P.; Naik, S.; Al Kheraif, A.A. Push-Out Bond Strength of Resin-Modified Glass Ionomer Cement and Flowable Composite Luting Systems on Glass Fiber Post of Root Canal. Materials 2021, 14, 6908. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, A.M.; Moghaddam, F.; Hamedani, M.T. Mechanical properties of glass ionomer cement incorporating forsterite nanoparticles synthesized by the sol-gel method. J. Sol-Gel Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Werner, S.; Robert, P.; Peter, J.; Oswald, G. Mixing Component for Dental Glass Ionomer Cements. U.S. Patent US4360605A, 23 November 1979. [Google Scholar]
- Yli-Urpo, H.; Närhi, M.; Närhi, T. Compound changes and tooth mineralization effects of glass ionomer cements containing bioactive glass (S53P4), an in vivo study. Biomaterials 2005, 26, 5934–5941. [Google Scholar] [CrossRef]
- D’Orto, B.; Polizzi, E.; Nagni, M.; Tetè, G.; Capparè, P. Full Arch Implant-Prosthetic Rehabilitation in Patients with Type I Diabetes Mellitus: Retrospective Clinical Study with 10 Year Follow-Up. Int. J. Environ. Res. Public Health 2022, 19, 11735. [Google Scholar] [CrossRef]
- Rosso, M.; Blasi, G.; Gherlone, E.; Rosso, R. Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention of Mucositis in Head and Neck Cancer Patients Treated with Chemo-Radiotherapy. J. Chemother. 1997, 9, 382–385. [Google Scholar] [CrossRef]
- Tecco, S.; Grusovin, M.G.; Sciara, S.; Bova, F.; Pantaleo, G.; Cappare, P. The association between three attitude-related indexes of oral hygiene and secondary implant failures: A retrospective longitudinal study. Int. J. Dent. Hyg. 2018, 16, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Brantley, W.; Culbertson, B.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Azeem, P.A.; Reddy, K.V.; Chiranjeevi, P.; Bramanandam, M.; Rao, R.P. A novel cost-effective approach to fabricate diopside bioceramics: A promising ceramics for orthopedic applications. Adv. Powder Technol. 2021, 32, 875–884. [Google Scholar] [CrossRef]
- Nonami, T.; Tsutsumi, S. Study of diopside ceramics for biomaterials. J. Mater. Sci. Mater. Med. 1999, 10, 475–479. [Google Scholar] [CrossRef]
- Khandan, A.; Abdellahi, M.; Ozada, N.; Ghayour, H. Study of the bioactivity, wettability and hardness behaviour of the bovine hydroxyapatite-diopside bio-nanocomposite coating. J. Taiwan Inst. Chem. Eng. 2016, 60, 538–546. [Google Scholar] [CrossRef]
- Harandi, Z.J.; Nasab, S.G.; Teimouri, A. Synthesis and characterisation of magnetic activated carbon/diopside nanocomposite for removal of reactive dyes from aqueous solutions: Experimental design and optimisation. Int. J. Environ. Anal. Chem. 2019, 99, 568–594. [Google Scholar] [CrossRef]
- Choudhary, R.; Venkatraman, S.K.; Bulygina, I.; Senatov, F.; Kaloshkin, S.; Anisimova, N.; Kiselevskiy, M.; Knyazeva, M.; Kukui, D.; Walther, F.; et al. Biomineralization, dissolution and cellular studies of silicate bioceramics prepared from eggshell and rice husk. Mater. Sci. Eng. C 2021, 118, 111456. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawamura, N.; Nonami, T. Diopside Synthesized by Sol-gel Method as Phosphorus Adsorption Material: Evaluation of Apatite Deposition in Pseudo Body Solution. Trans. Mater. Res. Soc. Jpn. 2019, 44, 17–23. [Google Scholar] [CrossRef]
- Kemoli, A.M. The effects of ambient temperature and mixing time of glass ionomer cement material on the survival rate of proximal ART restorations in primary molars. Contemp. Clin. Dent. 2014, 5, 31–36. [Google Scholar] [CrossRef]
- Peez, R.; Frank, S. The physical–mechanical performance of the new Ketac™ Molar Easymix compared to commercially available glass ionomer restoratives. J. Dent. 2006, 34, 582–587. [Google Scholar] [CrossRef]
- Sayyedan, F.; Fathi, M.; Edris, H.; Doostmohammadi, A.; Mortazavi, V.; Hanifi, A. Effect of forsterite nanoparticles on mechanical properties of glass ionomer cements. Ceram. Int. 2014, 40, 10743–10748. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, K. Mechanical stability of dental materials. Appl. Nanocomposite Mater. Dent. 2019, 285–305. [Google Scholar] [CrossRef]
- Moradkhani, A.; Baharvandi, H.; Naserifar, A. Fracture Toughness of 3Y-TZP Dental Ceramics by Using Vickers Indentation Fracture and SELNB Methods. J. Kor. Ceram. Soc. 2018, 56, 37–48. [Google Scholar] [CrossRef]
- DeSchepper, E.J.; Berr, E.A., 3rd; Cailleteau, J.G.; Tate, W.H. A comparative study of fluoride release from glass-ionomer cements. Quintessence Int. 1991, 22, 215–219. [Google Scholar]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Answers to Problems: Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1978. [Google Scholar]
- Iwata, N.Y.; Lee, G.-H.; Tsunakawa, S.; Tokuoka, Y.; Kawashima, N. Preparation of diopside with apatite-forming ability by sol–gel process using metal alkoxide and metal salts. Colloids Surf. B Biointerfaces 2004, 33, 1–6. [Google Scholar] [CrossRef]
- Alecu, A.-E.; Costea, C.-C.; Surdu, V.-A.; Voicu, G.; Jinga, S.-I.; Busuioc, C. Processing of Calcium Magnesium Silicates by the Sol–Gel Route. Gels 2022, 8, 574. [Google Scholar] [CrossRef]
- Shoreh, S.K.H.; Ahmadyari-Sharamin, M.; Ghayour, H.; Hassanzadeh-Tabrizi, S.; Pournajaf, R.; Tayebi, M. Two- stage synthesis of SnO2-Ag/MgFe2O4 nanocomposite for photocatalytic application. Surf. Interfaces 2021, 26, 101326. [Google Scholar] [CrossRef]
- Fathi, M.; Kharaziha, M. Two-step sintering of dense, nanostructural forsterite. Mater. Lett. 2009, 63, 1455–1458. [Google Scholar] [CrossRef]
- Davoodi, D.; Emami, A.H.; Tayebi, M.; Hosseini, S.K. Rapid mechanochemical synthesis of nickel-vanadium carbide nanocomposite powder by magnesiothermic reaction. Ceram. Int. 2017, 44, 5411–5419. [Google Scholar] [CrossRef]
- Yamamoto, A.; Arita, K.; Lucas, M.; Shinonaga, Y.; Harada, K.; Abe, Y. Development of a New Hydroxyapatite-Ionomer Cement. In Proceedings of the 2009 World Congress on Preventive Dentistry, Phuket, Thailand, 7–10 September 2009. [Google Scholar]
- Gu, Y.; Yap, A.; Cheang, P.; Khor, K. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC). Biomaterials 2005, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, B.A.; Nilforoushan, M.R.; Talebian, N.; Tayebi, M. A comparative study on the self-cleaning behavior and antibacterial activity of Portland cement by addition of TiO2 and ZnO nanoparticles. Mater. Res. Express 2021, 8, 035403. [Google Scholar] [CrossRef]
- Khezrloo, A.; Tayebi, M.; Shafiee, A.; Aghaie, A. Evaluation of compressive and split tensile strength of slag based aluminosilicate geopolymer reinforced by waste polymeric materials using Taguchi method. Mater. Res. Express 2021, 8, 025504. [Google Scholar] [CrossRef]
- Goenka, S.; Balu, R.; Kumar, T.S. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J. Mech. Behav. Biomed. Mater. 2012, 7, 69–76. [Google Scholar] [CrossRef]
- McDonnell, R.D.; Spiers, C.J.; Peach, C.J. Fabrication of dense forsterite-enstatite polycrystals for experimental studies. Phys. Chem. Miner. 2002, 29, 19–31. [Google Scholar] [CrossRef]
- Ni, S.; Chou, L.; Chang, J. Preparation and characterization of forsterite (Mg2SiO4) bioceramics. Ceram. Int. 2007, 33, 83–88. [Google Scholar] [CrossRef]
- Hilton, T.J.; Ferracane, J.L.; Broome, J.C. Summitt’s Fundamentals of Operative Dentistry: A Contemporary Approach; Quintessence Publishing Company Incorporated: Surrey, UK, 2013. [Google Scholar]
- Wang, N.; Zhao, R.; Zhang, L.; Guan, X. Molecular insights into the adsorption of chloride ions in calcium silicate hydrate gels: The synergistic effect of calcium to silicon ratio and sulfate ion. Microporous Mesoporous Mater. 2022, 345, 112248. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Xiong, Q.-M.; Chen, Z.; Huang, J.-T.; Zhang, M.; Song, H.; Hou, X.-F.; Li, X.-B.; Feng, Z.-J. Preparation, structure and mechanical properties of Sialon ceramics by transition metal-catalyzed nitriding reaction. Rare Met. 2020, 39, 589–596. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y. Three-dimensional numerical investigation of mixed-mode debonding of FRP-concrete interface using a cohesive zone model. Constr. Build. Mater. 2022, 350, 128818. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, S.; Huang, Y.; Liu, X. Behavior of Reinforced Concrete Beams without Stirrups and Strengthened with Basalt Fiber–Reinforced Polymer Sheets. J. Compos. Constr. 2023, 27, 04023007. [Google Scholar] [CrossRef]
- Qi, B.; Gao, S.; Xu, P. The Application of Recycled Epoxy Plastic Sheets Waste to Replace Concrete in Urban Construction and Building. Processes 2023, 11, 201. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef]
- Khademolhosseini, M.; Barounian, M.; Eskandari, A.; Aminzare, M.; Zahedi, A.; Ghahremani, D. Development of New Al2O3/TiO2 Reinforced Glass-Ionomer Cements (GICs) Nano-Composites. J. Basic Appl. Sci. Res. 2012, 2, 7526–7529. [Google Scholar]
- Alobiedy, A.N.; Alhille, A.H.; Al-Hamaoy, A.R. Mechanical Properties Enhancement of Conventional Glass Ionomer Cement by Adding Zirconium Oxide Micro and Nanoparticles. J. Eng. 2018, 25, 72–81. [Google Scholar] [CrossRef]
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannan, T.P. Characterization and enhancement of physico-mechanical properties of glass ionomer cement by incorporating a novel nano zirconia silica hydroxyapatite composite synthesized via sol-gel. AIMS Mater. Sci. 2019, 6, 730–747. [Google Scholar] [CrossRef]




| Components | Amount (mg/L) |
|---|---|
| NaCl | 125.6 |
| KCl | 963.9 |
| KSCN | 189.2 |
| KH2PO4 | 654.5 |
| CaCl2.2H2O | 227.8 |
| NaHCO3 | 630.8 |
| Na2SO4.10H2O | 763.2 |
| NH4Cl | 178 |
| Urea | 200 |
| pH | 6.8 |
| Group | Fracture Toughness (MPa m1/2) | Compressive Strength (MPa) | Surface Microhardness (VHN) | Fluoride Release | Ref. |
|---|---|---|---|---|---|
| GIC+3%TiO2 | 1.29 | 176.27 | 48.34 | 14.96 (μg/cm2) | [51] |
| GIC+5%Al2O3 | _ | 190.57 | 96.23 | _ | [52] |
| GIC+3%ZrO2 | _ | _ | 88.8 | _ | [53] |
| GIC 5% nano ZrO2–SiO2–Hydroxyapatite | _ | 144.12 | _ | _ | [54] |
| GIC/DIO | 5.189 | 1155.7 | 148 | 2.1 (ppm) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojehdehi, A.M.; Moghaddam, F.; Hamawandi, B. Evaluation of Mechanical Properties of Glass Ionomer Cements Reinforced with Synthesized Diopside Produced via Sol–Gel Method. Materials 2023, 16, 2107. https://doi.org/10.3390/ma16052107
Nojehdehi AM, Moghaddam F, Hamawandi B. Evaluation of Mechanical Properties of Glass Ionomer Cements Reinforced with Synthesized Diopside Produced via Sol–Gel Method. Materials. 2023; 16(5):2107. https://doi.org/10.3390/ma16052107
Chicago/Turabian StyleNojehdehi, Ali Maleki, Farina Moghaddam, and Bejan Hamawandi. 2023. "Evaluation of Mechanical Properties of Glass Ionomer Cements Reinforced with Synthesized Diopside Produced via Sol–Gel Method" Materials 16, no. 5: 2107. https://doi.org/10.3390/ma16052107
APA StyleNojehdehi, A. M., Moghaddam, F., & Hamawandi, B. (2023). Evaluation of Mechanical Properties of Glass Ionomer Cements Reinforced with Synthesized Diopside Produced via Sol–Gel Method. Materials, 16(5), 2107. https://doi.org/10.3390/ma16052107







